Abstract
Background
To investigate the combined effect of hypertension and hyperuricemia to the risk of ischemic stroke in a rural Chinese population.
Methods
The cross-sectional study was conducted from 2012 to 2013 in a rural area of China. After exclusion for missing data, we finally included 11,731 participants into analysis.
Results
After adjusting for age, current smoking, current drinking, BMI, TG, HDL-C and eGFR, hypertension was significantly associated with ischemic stroke in men (OR: 2.783, 95% CI: 1.793, 4.320) and in women (OR: 4.800, 95% CI: 2.945, 7.822). However, hyperuricemia was significantly associated with ischemic stroke only in women (OR: 1.888, 95% CI: 1.244, 2.864). After full adjustment, participants with both hypertension and hyperuricemia had 8.9 times higher risk than those without them. Finally, the interaction between hypertension and hyperuricemia was statistically significant only in women rather than in men after full adjustment.
Conclusions
This study demonstrated the positive correlations between hypertension, hyperuricemia and ischemic stroke. Our study also demonstrated the joint effect between hypertension and hyperuricemia towards ischemic stroke only in women, not in men.
Keywords: Hypertension, Hyperuricemia, Ischemic stroke
Background
Globally, stroke is a major cause of death and adult disability [1]. By 2013, 27 of China’s 33 provinces had stroke as the main cause of death [2]. In China, the annual stroke mortality rate is about 1.6 million people, which is about 157 deaths per 100,000 people due to stroke [3]. Among 100,000 people, strokes caused about 116 deaths in urban areas and 111 deaths in rural areas [3]. Therefore, stroke has emerged as a major health problem in China.
Hypertension (HTN) is one of the important risk factors of stroke. More than 60% of acute stroke patients had elevated blood pressure [4]. Furthermore, more than 70% of stroke patients had a history of hypertension, and nearly half of them had poor baseline blood pressure control [5–7]. The incidence of stroke increased proportionally with the increase in systolic and diastolic blood pressure, with a 3.1-fold increase in the relative risk for men and a 2.9-fold increase in women [8, 9]. Hyperuricemia is another potential important risk factor of stroke. The elevated level of serum uric acid was independently positively correlated with ischemic stroke in patients with aged < 60 years [10]. Previous prospective observational studies have showed that hyperuricemia was independently correlated with stroke incidence and mortality [11, 12]. On the other hand, hyperuricemia was also associated with hypertension, type 2 diabetes, dyslipidemia, chronic kidney disease, and cardiovascular events, particularly stroke [13–15].
Previous studies have clarified the potential correlation between hypertension, hyperuricemia and ischemic stroke. However, these studies only examined the independent effects of risk factors. So far, no studies have investigated the combined effect of hypertension and hyperuricemia to ischemic stroke. Thus, this study aimed to investigate the combined effect of HTN and hyperuricemia to the risk of ischemic stroke.
Methods
Study population
The present study was based on a cross-sectional epidemiological survey known as NCRCHS which conducted from January 2012 to August 2013. The detailed design and rationale of NCRCHS were fully discussed elsewhere [16]. A total number of 11,956 participants (age ≥ 35 years) were collected from Liaoning province, northeastern China. In the present work, 225 participants were further excluded for missing data. Eventually, 11,731 subjects were enrolled into the present work. Our study was approved by the Ethics Committee of China Medical University (Shenyang, China). All subjects provided written informed consent.
Data collection and measurements
The process about data collection and measurements was fully described in our previously published studies [17]. Before the survey, cardiologists and nurses participated a professional training, passed a standardized exam, and acquired the qualification to gather data. The data were collected through uniform questionnaires regarding demographic data, anthropometric parameters, health-related behaviors. The central steering committee with a subcommittee conducted the quality assurance process of data collection. The questionnaire was designed to collect detail information from participants. Smoking and drinking status were separated into current status and others according to participants’ self-reports. After participants rested for at least five minutes in a properly relaxed and sitting state, the blood pressure was taken three times and measured by two randomly selected workers. The mean reading of three consecutive values was determined as the final result of blood pressure. Before the measurement of anthropometric indices, lightweight clothes without shoes were required for the subjects. The weight of participants was quantified to 0.1 kg by calibrated digital scales and height was quantified to 0.1 cm by portable stadiometers. After individuals were fasting at least 12 h, blood samples were collected from the antecubital veins in the morning. For long-term storage, the serum of venous blood sample was isolated by calibrated centrifuge and frozen at − 20 °C. The fasting blood samples of individuals were tested by Olympus AU640 auto analyzers to measure the blood concentrations of FPG, TG, HDL-C, Scr and SUA.
Definition
The body mass index (BMI) was determined as: weight (kg)/height (m2). The estimated glomerular filtration rate (eGFR) was defined according to the CKD-EPI equation [18]. The definition of hyperuricemia was serum uric acid (SUA) ≥357 μmol/L for females and ≥ 417 μmol/L for males [19]. Hypertension was determined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg [20]. Ischemic stroke was determined as a history of cerebrovascular events, which was demonstrated by either cranial CT or MR scan within the past 2 years.
Statistical analysis
The results were displayed as mean values ± standard deviation (SD) or median (interquartile) for continuous variables. The following category variables were presented as frequencies (percentages). Students’ t-test or Mann-Whitney test was applied to compare continuous variables between groups. The chi-square test was performed to compare category variables between groups. Additionally, the rank-sum test was used to compare ordinal category variables between groups. Multivariate logistic regression analyses were performed to evaluate the relationship of hypertension and hyperuricemia to ischemic stroke. The results were displayed as odds ratio (OR) and 95% confidence interval (95% CI). All of the analyses were performed by SPSS 25.0 software (IBP corp). A two-tailed P value < 0.05 was considered as significant.
Results
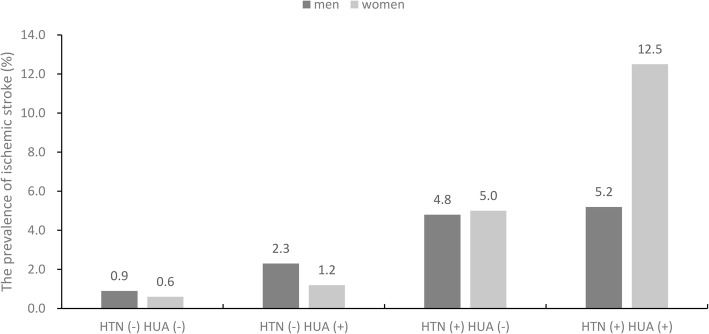
Table 1 shows the characteristics of study population divided by ischemic stroke and sex. The prevalence of ischemic stroke was 3.16% in men and 3.12% in women. Population with ischemic stroke had higher age, SBP and DBP in both sexes, and had higher BMI, TG and SUA only in women. The percentage of current smoking and current drinking was dramatically lower in patients group than those in normal group in both sexes. The percentage of hyperuricemia was higher only in women and hypertension was higher in both genders. As shown in Fig. 1, the prevalence of ischemic stroke was greater in HTN (+) and HUA (+) in both gender than in HTN (−) and HUA (−) (0.9% vs. 5.2% for male; 0.6% vs. 12.5% for female).
Table 1.
Characteristics of study population divided by ischemic stroke and sex
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Ischemic stroke | Ischemic stroke | |||||
| No (n = 5206) | Yes (n = 170) | p value | No (n = 6104) | Yes (n = 197) | p value | |
| Age (years) | 54.0 ± 10.7 | 63.7 ± 9.1 | < 0.001 | 53.1 ± 10.3 | 62.9 ± 8.2 | < 0.001 |
| Current smoking (%) | 3000 (57.0) | 81 (47.6) | 0.015 | 1002 (16.4) | 30 (15.2) | 0.658 |
| Current drinking (%) | 2405 (45.7) | 44 (25.9) | < 0.001 | 183 (3.0) | 0 (0.0) | 0.014 |
| BMI (kg/m2) | 24.7 ± 3.5 | 25.0 ± 3.4 | 0.362 | 24.8 ± 3.8 | 26.0 ± 3.7 | < 0.001 |
| FPG (mmol/L) | 5.6 (5.2–6.1) | 5.8 (5.4–6.4) | < 0.001 | 5.5 (5.1–6.0) | 5.7 (5.3–6.7) | < 0.001 |
| TC (mmol/L) | 5.2 ± 1.0 | 5.2 ± 1.0 | 0.586 | 5.3 ± 1.1 | 5.6 ± 1.0 | < 0.001 |
| TG (mmol/L) | 1.2 (0.9–1.9) | 1.4 (1.0–2.1) | 0.055 | 1.2 (0.9–1.9) | 1.9 (1.2–2.8) | < 0.001 |
| HDL-C (mmol/L) | 1.4 ± 0.4 | 1.3 ± 0.4 | 0.012 | 1.4 ± 0.3 | 1.3 ± 0.3 | < 0.001 |
| LDL-C (mmol/L) | 2.9 ± 0.8 | 3.0 ± 0.8 | 0.004 | 3.0 ± 0.8 | 3.3 ± 0.8 | < 0.001 |
| eGFR (ml/min/1.73 m2) | 94.5 ± 15.3 | 85.5 ± 15.3 | < 0.001 | 92.4 ± 16.1 | 82.6 ± 18.7 | < 0.001 |
| SBP (mmHg) | 143.2 ± 22.2 | 159.8 ± 26.9 | < 0.001 | 139.5 ± 23.6 | 161.3 ± 27.4 | < 0.001 |
| DBP (mmHg) | 83.6 ± 11.8 | 87.7 ± 11.8 | < 0.001 | 80.4 ± 11.5 | 85.8 ± 12.2 | < 0.001 |
| SUA (μmol/L) | 333.3 ± 83.0 | 344.4 ± 97.5 | 0.088 | 254.6 ± 66.7 | 289.8 ± 88.6 | < 0.001 |
| Hyperuricemia (%) | 779 (14.8) | 33 (19.4) | 0.098 | 432 (7.1) | 41 (20.8) | < 0.001 |
| Hypertension (%) | 2787 (53.0) | 143 (84.1) | < 0.001 | 2889 (47.3) | 177 (89.8) | < 0.001 |
| Anti-hypertensive drug | 612 (11.6) | 89 (52.4) | < 0.001 | 956 (15.6) | 127 (64.5) | < 0.001 |
Data are expressed as mean ± standard deviation (SD) or median (interquartile range) and numbers (percentage) as appropriate
Abbreviations: BMI body mass index, FPG fasting plasma glucose, TC total cholesterol, TG triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate, SBP systolic blood pressure, DBP diastolic blood pressure, SUA serum uric acid
Fig. 1.
Prevalence of ischemic stroke according to the hypertension and hyperuricemia stratified by sex
Logistic regression was performed to display the association of SBP, DBP and SUA with ischemic stroke by sex (Table 2). After adjusting for age, current smoking, current drinking, BMI, FPG, TC, TG, HDL-C, LDL-C and eGFR, SBP was significantly associated with ischemic stroke in men (OR: 1.017, 95% CI: 1.011, 1.024) and in women (OR: 1.018, 95% CI: 1.013, 1.024). Similarly, DBP and SUA was significantly associated with ischemic stroke in both men and women.
Table 2.
Multivariate logistic regression of SBP, DBP and SUA for ischemic stroke by sex
| Odds Ratio (95%CI) | ||||||
|---|---|---|---|---|---|---|
| Model 1 | P value | Model 2 | P value | Model 3 | P value | |
| Men | ||||||
| SBP | 1.027 (1.021, 1.033) | < 0.001 | 1.020 (1.013, 1.026) | < 0.001 | 1.017 (1.011, 1.024) | < 0.001 |
| DBP | 1.027 (1.015, 1.039) | < 0.001 | 1.035 (1.022, 1.048) | < 0.001 | 1.032 (1.018, 1.045) | < 0.001 |
| SUA | 1.002 (1.000, 1.003) | 0.087 | 1.002 (1.001, 1.004) | 0.008 | 1.002 (1.000, 1.004) | 0.032 |
| Women | ||||||
| SBP | 1.030 (1.025, 1.035) | < 0.001 | 1.021 (1.016, 1.027) | < 0.001 | 1.018 (1.013, 1.024) | < 0.001 |
| DBP | 1.037 (1.025, 1.048) | < 0.001 | 1.035 (1.023, 1.047) | < 0.001 | 1.028 (1.016, 1.040) | < 0.001 |
| SUA | 1.006 (1.005, 1.008) | < 0.001 | 1.004 (1.002, 1.006) | < 0.001 | 1.003 (1.000, 1.005) | 0.018 |
Model 1: no adjust
Model 2: adjust for age, current smoking, current drinking
Model 3: adjust for age, current smoking, current drinking, BMI, FPG, TC, TG, HDL-C, LDL-C, eGFR
Abbreviations: BMI body mass index, FPG fasting plasma glucose, TC total cholesterol, TG triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate, SBP systolic blood pressure, DBP diastolic blood pressure, SUA serum uric acid
Multivariate logistic regression was performed to reveal the association of hypertension and hyperuricemia with ischemic stroke by sex (Table 3). After adjusting for age, current smoking, current drinking, BMI, FPG, TC, TG, HDL-C, LDL-C and eGFR, hypertension was significantly associated with ischemic stroke in men (OR: 2.783, 95% CI: 1.793, 4.320) and in women (OR: 4.800, 95% CI: 2.945, 7.822). However, hyperuricemia was significantly associated with ischemic stroke only in women (OR: 1.888, 95% CI: 1.244, 2.864).
Table 3.
Multivariate logistic regression of hypertension and hyperuricemia for ischemic stroke by sex
| Odds Ratio (95%CI) | ||||||
|---|---|---|---|---|---|---|
| Model 1 | P value | Model 2 | P value | Model 3 | P value | |
| Men | ||||||
| Hypertension | 4.700 (3.104, 7.116) | < 0.001 | 3.245 (2.119, 4.969) | < 0.001 | 2.783 (1.793, 4.320) | < 0.001 |
| Hyperuricemia | 1.386 (0.940, 2.042) | 0.099 | 1.627 (1.092, 2.424) | 0.017 | 1.431 (0.919, 2.230) | 0.113 |
| Women | ||||||
| Hypertension | 9.849 (6.186, 15.680) | < 0.001 | 5.933 (3.680, 9.566) | < 0.001 | 4.800 (2.945, 7.822) | < 0.001 |
| Hyperuricemia | 3.451 (2.413, 4.934) | < 0.001 | 2.529 (1.748, 3.661) | < 0.001 | 1.888 (1.244, 2.864) | 0.003 |
Model 1: no adjust
Model 2: adjust for age, current smoking, current drinking
Model 3: adjust for age, current smoking, current drinking, BMI, FPG, TC, TG, HDL-C, LDL-C, eGFR
Abbreviations: BMI body mass index, FPG fasting plasma glucose, TC total cholesterol, TG triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate
Table 4 shows logistic regression of the joint effect of hypertension and hyperuricemia for ischemic stroke by sex. Participants without both hypertension and hyperuricemia were defined as the reference group. For men, participants with hypertension and hyperuricemia had 5.9 times higher risk of ischemic stroke than those without them in model 1. After adjusting for age, current smoking, current drinking, BMI, FPG, TC, TG, HDL-C, LDL-C and eGFR (model 3), participants with both of them had 4.1 times higher risk than those without them in men. For women, participants with hypertension and hyperuricemia had 24.3 times higher risk of ischemic stroke than those without them in model 1. After full adjustment of covariates, participants with both of them had 8.9 times higher risk than those without them. Finally, the interaction between hypertension and hyperuricemia was statistically significant only in women rather than in men after full adjustment.
Table 4.
Multivariate logistic regression of the joint effect of hypertension and hyperuricemia for ischemic stroke by sex
| Odds Ratio (95%CI) | ||||||
|---|---|---|---|---|---|---|
| Model 1 | P value | Model 2 | P value | Model 3 | P value | |
| Men | ||||||
| HTN (−) HUA (−) | 1 (reference) | 1 (reference) | 1 (reference) | |||
| HTN (−) HUA (+) | 2.516 (1.055, 6.000) | 0.037 | 3.133 (1.299, 7.556) | 0.011 | 2.890 (1.173, 7.121) | 0.021 |
| HTN (+) HUA (−) | 5.498 (3.409, 8.866) | < 0.001 | 3.777 (2.317, 6.157) | < 0.001 | 3.296 (1.998, 5.437) | < 0.001 |
| HTN (+) HUA (+) | 5.917 (3.276, 10.688) | < 0.001 | 4.827 (2.642, 8.819) | < 0.001 | 4.059 (2.126, 7.751) | < 0.001 |
| P value for interaction | 0.007 | 0.011 | 0.073 | |||
| Women | ||||||
| HTN (−) HUA (−) | 1 (reference) | 1 (reference) | 1 (reference) | |||
| HTN (−) HUA (+) | 2.136 (0.491, 9.284) | 0.312 | 1.694 (0.387, 7.421) | 0.484 | 1.353 (0.306, 5.991) | 0.691 |
| HTN (+) HUA (−) | 8.956 (5.465, 14.677) | < 0.001 | 5.492 (3.309, 9.114) | < 0.001 | 4.592 (2.740, 7.696) | < 0.001 |
| HTN (+) HUA (+) | 24.254 (13.688, 42.976) | < 0.001 | 12.689 (7.027, 22.912) | < 0.001 | 8.913 (4.726, 16.809) | < 0.001 |
| P value for interaction | < 0.001 | < 0.001 | < 0.001 | |||
Model 1: no adjust
Model 2: adjust for age, current smoking, current drinking
Model 3: adjust for age, current smoking, current drinking, BMI, FPG, TC, TG, HDL-C, LDL-C, eGFR
Abbreviations: BMI body mass index, FPG fasting plasma glucose, TC total cholesterol, TG triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate
Discussion
This study demonstrated the independent and positive correlations between hypertension, hyperuricemia and ischemic stroke in a rural Chinese population. More importantly, our study for the first time implicated the joint effect between hypertension and hyperuricemia towards ischemic stroke only in women, not in men. Our results suggest the joint effect of hypertension and hyperuricemia on ischemic stroke in women may be greater than the sum of their individual effects. Therefore, our research may provide a simple explanation for the public to understand the harm of hypertension and hyperuricemia on ischemic stroke.
In this study, hypertension was positively associated with the risk of ischemic stroke in both men and women, which was consistent with previous studies [21–23]. For a long time, elevated blood pressure has been associated with cardiovascular outcomes, and the correlation between hypertension and increased risk of stroke may be the strongest and easiest to recognize. Previous randomized controlled trials have suggested that lowering high blood pressure was positive treatment in patients with acute ischemic stroke [24, 25]. Evidence has shown that 80% of patients with acute ischemic stroke have hypertension, which is independently associated with poor prognosis [26–28].
Uric acid is a product of human purine metabolism and is known to be related to many risk factors for strokes, such as high blood pressure, obesity, and diabetes [13, 15, 29]. In our study, elevated uric acid was also positively associated with the risk of ischemic stroke but only in women. Consistently, the Rotterdam Study showed that elevated uric acid was a positive risk factor for stroke only in women [30]. However, some previous studies revealed the correlation between hyperuricemia and stroke in both men and women, though stronger in women than in men [31, 32]. In addition, elevated uric acid may modestly increase the risk of stroke morbidity and mortality [11, 12]. In this study, we adopted the classic threshold of 6 and 7 mg/dL SUA for women and men to define hyperuricemia. However, many recent studies indicate that a cardiovascular SUA threshold was significantly lower than that used for the classic definition of hyperuricemia [33, 34]. Thus, further researches are warranted to explore the combination of hypertension and hyperuricemia on ischemic stroke.
Both hypertension and hyperuricemia were positively associated with the risk of ischemic stroke. Results of our study also show that the combination of hypertension and hyperuricemia had more than nine-fold higher risk than those without hypertension and hyperuricemia in women. However, the joint effect of hypertension and hyperuricemia was not observed in men. These findings showed that the interaction between hypertension and hyperuricemia was statistically significant only in women rather than in men after adjustment for age, current smoking, current drinking, BMI, TG, HDL-C and eGFR. The potential mechanism related to ischemic stroke might be arterial stiffness caused by the combination of hyperuricemia and hypertension [35, 36].
There are some reasons that may explain the gender differences in the joint effect of hypertension and hyperuricemia to ischemic stroke. Previous studies have shown that hyperuricemia was more relevant with hypertension in women than men [37, 38]. In addition, the Apolipoprotein Mortality Risk Study suggested that uric acid was more strongly associated to stroke in women than in men [32]. Furthermore, according to a systematical review, individuals with moderate hypertension had a higher risk of stroke in women than in men [39]. Therefore, the gender differences in the joint effect of hypertension and hyperuricemia to ischemic stroke were reasonable.
This study still has several limitations that need to be noticed. Firstly, the cross-sectional design cannot prove the causality between hypertension, hyperuricemia, and ischemic stroke. Secondly, this study was conducted in rural areas of northeast China, which may produce selection bias. Thirdly, although a recent study showed that diuretic-related hyperuricemia had the same cardiovascular risk as nondiuretic-related hyperuricemia, the lack of information on diuretics may influence the outcome of serum uric acid and ischemic stroke in hypertensive patients [40]. Finally, we did not collect information about the history of stroke and related medicine.
Conclusions
This study demonstrated the positive correlations between hypertension, hyperuricemia and ischemic stroke in a rural Chinese population. More importantly, our study demonstrated the joint effect between hypertension and hyperuricemia towards ischemic stroke only in women, not in men. Our results suggest the joint effect of hypertension and hyperuricemia on ischemic stroke in women may be greater than the sum of their individual effects.
Acknowledgements
We would like to express our gratitude to all those who exert their effects in achieving this study.
Abbreviations
- SD
Standard deviation
- BMI
Body mass index
- FPG
Fasting plasma glucose
- TC
Total cholesterol
- TG
Triglyceride
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- eGFR
Estimated glomerular filtration rate
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- SUA
Serum uric acid
Authors’ contributions
In this study, P Sun and M Chen did the study design, statistical analyses and results interpretation. Z Li, Y Zhou, S Yu, H Yang, G Sun, L Zheng and Y Sun participated as analyzing and resolving difficulties of analytic strategies and results discussion. Finally, X Guo functioned as final reviewer and corresponding author. All authors read and approved the final manuscript.
Funding
This study was funded by National Natural Science Foundation of China (Grant # 81800361), National Key R&D Program of China (Grant #2017YFC1307600), and the National Key Research and Development Program from the Ministry of Science and Technology of China (Project Grant # 2018YFC1312400, Sub-project Grant # 2018YFC1312403).
Availability of data and materials
The datasets generated during and analysed during the current study are not publicly available due to individual privacy concerns but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in compliance with the ethical principle of the Declaration of Helsinki. All participants provided written informed consent and all procedures were performed in accordance with the ethical standards. The study protocol was approved by the Ethics Committee of China Medical University (Shenyang, China).
Consent for publication
All co-authors and participants have been informed and given their consent for publication of this article.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peng Sun and Mengqi Chen contributed equally to this work.
References
- 1.Krishnamurthi R, Feigin V, Forouzanfar M, Mensah G, Connor M, Bennett D, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet Glob Health. 2013;1(5):e259–81. 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed]
- 2.Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet (London, England). 2016;387(10015):251–72. [DOI] [PubMed]
- 3.Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42(12):3651–3654. doi: 10.1161/STROKEAHA.111.635755. [DOI] [PubMed] [Google Scholar]
- 4.Miller J, Kinni H, Lewandowski C, Nowak R, Levy P. Management of hypertension in stroke. Ann Emerg Med. 2014;64(3):248–255. doi: 10.1016/j.annemergmed.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Niska R. Blood pressure measurements at emergency department visits by adults: United States. NCHS data brief. 2007;2011(72):1–8. [PubMed] [Google Scholar]
- 6.Anderson C, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355–65. 10.1056/NEJMoa1214609. [DOI] [PubMed]
- 7.Ishitsuka K, Kamouchi M, Hata J, Fukuda K, Matsuo R, Kuroda J, et al. High blood pressure after acute ischemic stroke is associated with poor clinical outcomes: Fukuoka Stroke Registry. Hypertension (Dallas, Tex : 1979). 2014;63(1):54–60. [DOI] [PubMed]
- 8.Kannel W, Wolf P, Verter J, McNamara P. Epidemiologic assessment of the role of blood pressure in stroke. The Framingham study. JAMA. 1970;214(2):301–310. doi: 10.1001/jama.1970.03180020021004. [DOI] [PubMed] [Google Scholar]
- 9.Kannel W, Wolf P, McGee D, Dawber T, McNamara P, Castelli W. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham study. JAMA. 1981;245(12):1225–1229. doi: 10.1001/jama.1981.03310370017013. [DOI] [PubMed] [Google Scholar]
- 10.L W, W H, D M, Q Z, C W, E P, M W Relationship between serum uric acid and ischemic stroke in a large type 2 diabetes population in China: A cross-sectional study. J Neurol Sci. 2017;376:176–180. doi: 10.1016/j.jns.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Guevara J, Kim K, Choi H, Heitjan D, Albert D. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(7):885–892. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Hou W, Zhang X, Hu L, Tang Z. Hyperuricemia and risk of stroke: a systematic review and meta-analysis of prospective studies. Atherosclerosis. 2014;232(2):265–270. doi: 10.1016/j.atherosclerosis.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41(6):1183–90. 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed]
- 14.Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18(6):523–530. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 15.Feig D, Kang D, Johnson R. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X, Li Z, Guo L, Zheng L, Yu S, Yang H, et al. An update on overweight and obesity in rural Northeast China: from lifestyle risk factors to cardiometabolic comorbidities. BMC Public Health. 2014;14(1):1046. 10.1186/1471-2458-14-1046. [DOI] [PMC free article] [PubMed]
- 17.Chen M-Q, Shi W-R, Shi C-N, Zhou Y-P, Sun Y-X. Impact of monocyte to high-density lipoprotein ratio on prevalent hyperuricemia: findings from a rural Chinese population. Lipids Health Dis. 2020;19(1):48. doi: 10.1186/s12944-020-01226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed]
- 19.PHF G, ERM S. Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane Database Syst Rev. 2017;4:Cd008652. doi: 10.1002/14651858.CD008652.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. 10.1001/jama.289.19.2560. [DOI] [PubMed]
- 21.Gąsecki D, Coca A, Cunha P, Hering D, Manios E, Lovic D, et al. Blood pressure in acute ischemic stroke: challenges in trial interpretation and clinical management: position of the ESH working group on hypertension and the brain. J Hypertens. 2018;36(6):1212–21. 10.1097/HJH.0000000000001704. [DOI] [PubMed]
- 22.Alloubani A, Saleh A, Abdelhafiz I. Hypertension and diabetes mellitus as a predictive risk factors for stroke. Diabetes & metabolic syndrome. 2018;12(4):577–584. doi: 10.1016/j.dsx.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Lackland DT, Voeks JH, Boan AD. Hypertension and stroke: an appraisal of the evidence and implications for clinical management. Expert Rev Cardiovasc Ther. 2016;14(5):609–616. doi: 10.1586/14779072.2016.1143359. [DOI] [PubMed] [Google Scholar]
- 24.Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, et al. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol. 2009;8(1):48–56. 10.1016/S1474-4422(08)70263-1. [DOI] [PubMed]
- 25.Schrader J, Lüders S, Kulschewski A, Berger J, Zidek W, Treib J, et al. The ACCESS study: evaluation of acute candesartan Cilexetil therapy in stroke survivors. Stroke. 2003;34(7):1699–703. 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed]
- 26.Wallace JD, Levy LL. Blood pressure after stroke. Jama. 1981;246(19):2177–2180. doi: 10.1001/jama.1981.03320190035023. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the international stroke trial. Stroke. 2002;33(5):1315–1320. doi: 10.1161/01.STR.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 28.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension. 2004;43(1):18–24. doi: 10.1161/01.HYP.0000105052.65787.35. [DOI] [PubMed] [Google Scholar]
- 29.Chu NF, Wang DJ, Liou SH, Shieh SM. Relationship between hyperuricemia and other cardiovascular disease risk factors among adult males in Taiwan. Eur J Epidemiol. 2000;16(1):13–17. doi: 10.1023/A:1007654507054. [DOI] [PubMed] [Google Scholar]
- 30.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 31.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS) J Intern Med. 2009;266(6):558–570. doi: 10.1111/j.1365-2796.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Iso H, Murakami Y, Miura K, Nagai M, Sugiyama D, et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-JAPAN study. J Atheroscler Thromb. 2016;23(6):692–703. 10.5551/jat.31591. [DOI] [PMC free article] [PubMed]
- 33.Maloberti A, Giannattasio C, Bombelli M, Desideri G, Cicero AFG, Muiesan ML, et al. Hyperuricemia and risk of cardiovascular outcomes: the experience of the URRAH (uric acid right for heart health) project. High Blood Press Cardiovasc Prev. 2020;27(2):121–8. 10.1007/s40292-020-00368-z. [DOI] [PubMed]
- 34.Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, et al. Identification of the uric acid thresholds predicting an increased Total and cardiovascular mortality over 20 years. Hypertension. 2020;75(2):302–8. 10.1161/HYPERTENSIONAHA.119.13643. [DOI] [PubMed]
- 35.Rebora P, Andreano A, Triglione N, Piccinelli E, Palazzini M, Occhi L, et al. Association between uric acid and pulse wave velocity in hypertensive patients and in the general population: a systematic review and meta-analysis. Blood Press. 2020;29(4):220–31. 10.1080/08037051.2020.1735929. [DOI] [PubMed]
- 36.Maloberti A, Rebora P, Andreano A, Vallerio P, De Chiara B, Signorini S, et al. Pulse wave velocity progression over a medium-term follow-up in hypertensives: Focus on uric acid. J Clin Hypertension (Greenwich, Conn). 2019;21(7):975–83. [DOI] [PMC free article] [PubMed]
- 37.Kivity S, Kopel E, Maor E, Abu-Bachar F, Segev S, Sidi Y, et al. Association of serum uric acid and cardiovascular disease in healthy adults. Am J Cardiol. 2013;111(8):1146–51. 10.1016/j.amjcard.2012.12.034. [DOI] [PubMed]
- 38.Babio N, Martínez-González MA, Estruch R, Wärnberg J, Recondo J, Ortega-Calvo M, et al. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr Metab Cardiovasc Dis. 2015;25(2):173–80. 10.1016/j.numecd.2014.10.006. [DOI] [PubMed]
- 39.Gorgui J, Gorshkov M, Khan N, Daskalopoulou SS. Hypertension as a risk factor for ischemic stroke in women. Can J Cardiol. 2014;30(7):774–782. doi: 10.1016/j.cjca.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Maloberti A, Bombelli M, Facchetti R, Barbagallo CM, Bernardino B, Rosei EA, et al. Relationships between diuretic-related hyperuricemia and cardiovascular events: data from the URic acid right for heArt health study. J Hypertens. 2021;39(2):333–40. 10.1097/HJH.0000000000002600. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analysed during the current study are not publicly available due to individual privacy concerns but are available from the corresponding author on reasonable request.