Abstract
Background
COVID19 pandemic urged the need to take severe measures for reducing the epidemic spread. Lockdowns were imposed throughout countries and even Inborn errors of metabolism (IEMs) affected patients had to face it and adapt, with management strategies changes coming along. Phenylketonuria (PKU) is an inborn error of phenylalanine (Phe) metabolism causing, when not treated, blood Phe increases and consequent central nervous system (CNS) damage. Dietary intervention is the main recognized treatment and must be maintained long-life, however adherence is often suboptimal in adulthood. Aim of this study was to evaluate whether and how the pandemic had impacted PKUs metabolic control and what factors may have played a role as potential modifiers.
Methods
Patients ≥4 yo and in follow-up at our Metabolic Clinic were enrolled in this study, divided into subgroups according to age (GROUP A < 12 yo; GROUP B ≥ 12 yo). Videoconsults were conducted on a minimum monthly basis and collected DBS were studied and compared to previous year same time-period in order to evaluate possible changes.
Results
39% of patients (n = 121) increased the number of performed DBS. “Non-compliant” patients were reduced (11–3%) with a − 14% of patients with mean Phe levels >600 umol/l and a − 8% of patients with 100% DBS above same level. GROUP A maintained substantially unchanged metabolic control among two analyzed time-periods. On the contrary, GROUP B demonstrated significant reductions in mean blood Phe concentrations (p < 0.0001) during the pandemic (mean 454 umol/l, SD ± 252, vs. 556.4 umol/l, SD ± 301).
Discussion
COVID19 pandemic strongly impacted people's life with lifestyle habits changing consistently. PKU patients had to adapt their dietary restrictions to the new environment they were exposed to and, if younger patients could have been less exposed (meals strictly according to diet plan independently from setting), adolescent and adults strongly reflected the obligation to stay home by showing better metabolic control. Multiple factors could have played a role in that and the availability of teleconsultancy may have contributed allowing easier connections, but our data demonstrate how the pandemic and the environment can strongly impact PKUs adherence to treatment and how removing distance barriers can ameliorate and optimize metabolic compliance.
Keywords: COVID-19, PKU, Diet, Adherence, Metabolic control, Lockdown
Abbreviations: PKU, Phenylketonuria; PAH, phenylalanine hydroxylase; Phe, phenylalanine; DBS, Dried Blood Spot; Tyrosine, (Tyr); CNS, Central Nervous System; LD, Lockdown; IEM, Inborn errors of metabolism; MAM-2019, March–April-May 2019; MAM-2020, March–April-May 2020.
1. Introduction
Phenylketonuria (PKU; OMIM 261600) is an inborn error of metabolism caused by mutations in the PAH gene, coding for the liver enzyme phenylalanine hydroxylase (PAH, EC 1.14.16.1) and normally converting the aminoacid phenylalanine (Phe) into tyrosine (Tyr) [1]. Absence or decrease in PAH activity results in increased blood Phe concentrations, or its metabolites, with consequent toxic levels reaching mainly the CNS. Left untreated, symptoms can develop shortly after birth and include neurological impairment with possible psychomotor delay, seizures, autism and behavioral disorders. Mainstay treatment to date is a lifelong dietary intervention which can guarantee normal growth and neurodevelopment. Diet consists in the use of low protein foods, amino acid substitutes and micronutrient supplements. Palatability, flexibility and ease of use are primarily important to ensure patients' compliance to prescriptions [2].
Recently the world had to face the COVID19 pandemic and many countries announced quarantine measures (“lockdown”, LD) shortly after first reported cases. Italy was the first western country to deal with the spreading of the disease and imposing LD in order to delay and avoid community transmission, thus sudden lifestyle changes were undertaken. People had to cope and adapt switching to spending most of their time at home, moving from one place to another only for emergency reasons or work, working remotely and, more importantly, cooking themselves instead of consuming prepared meals in restaurants, pubs or canteens. All gathering places were also closed and younger people (e.g. students normally eating in the school-cafeteria), got back to eat at home.
Patients affected by IEMs did not differ and had to face LD measures the same way, switching their “managing the diet” habits from a known and usual setting to a total new one within just some days. Clinical monitors and health supervisors had to adapt too in order to encounter new needs, and “telehealth” became the main tool doing so assuring the need of care besides the pandemic spread.
PKU is a chronic disease mainly affecting nutritional life aspects, thus can be used as a proficient IEM example to reflect possible LD's induced changes in IEM affected patients' life. Accordingly, aim of this study was to evaluate whether and how PKU patients' metabolic control may have changed during the pandemic.
2. Aims of the study
Aim of this study was to analyze and identify possible significant changes in blood Phe concentrations and in monitoring frequencies (number of DBS made) during lockdown (2020) compared to same time-period during the previous year (2019).
3. Patients and methods
Patients affected by PKU (confirmed by molecular analysis) requiring dietary intervention and in follow up at our Metabolic Clinic (San Paolo Hospital, ASST Santi Paolo e Carlo, University of Milan, Italy) were enrolled in this study and subjected to videoconsults on at least a monthly basis. Study population was divided into subgroups with regards to age: GROUP A, age 4–12 yo (“pediatric population”) and GROUP B ≥ 12 yo (“adolescent and adult population”). Patients <4 yo were excluded due to dietary restrictions expected to be strictly observed independently from environment. We identified as “lost at follow up” all patients who, before lockdown period, weren't getting back to clinic for at least 2 consecutive years as suggested by literature [3]. As first lockdown in Italy began at the end of February 2020 and finished at the end of May 2020, study periods were identified as March–April-May 2019 (MAM-2019) and March–April-May 2020 (MAM-2020). As dried blood spots (DBS) have been proven to be a powerful sampling method to monitor PKUs metabolic control (in this study identified by mean of blood Phe values during analyzed time periods), they were reviewed and analyzed considering both monitoring frequencies and single obtained Phe value results [4]. Patients with less than 1 DBS performed per month were excluded due to possible related bias, as suggested by literature. All analyzed DBS were collected during the morning after overnight fasting, as suggested by current guidelines [5]. Metabolic control was considered “insufficient” for identified Phe levels above set “safe ranges” depending on age (>360 umol/L if age <12 yo and >600 umol/L for age ≥ 12 yo) and patients consequently identified as “non-compliant” for Phe levels >1000 umol/l. Pregnancy and Kuvan treatment starting along analyzed time periods were considered as exclusion criteria due to possible related bias.
4. Statistical analysis
Data were analyzed category related. Wilcoxon paired sample t-test was used to compare sets of data acquired from patients during MAM 2019 and MAM 2020; confidence intervals (95% CIs) were used to examine differences in blood Phe concentrations between the two identified time periods. For normal distribution of individual characteristics, Pearson's correlations were used for comparison; in other cases Spearman's correlations were used. Data were analyzed using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean (standard deviation, SD) and median (min-max). Significant values were considered for p < 0.05.
5. Results
5.1. Demographics
A total of 310 PKU patients in actual follow up at our Clinic were screened and reviewed for enrollment purposes. Among those, 192 patients were considered eligible for this study as meeting all inclusion criteria (n = 192, sample size). Mean identified age for study population was 21.9 y.o. (age range 4–65 yo). Gender resulted equally distributed among GROUP B and nearly equally distributed among GROUP A [Table 1].
Table 1.
PKU sample demographic characteristics.
| Demographics (n = 192) |
||
|---|---|---|
| GROUP A (n = 51) | GROUP B (n = 141) | |
| Age (y) | ||
| Mean (SD) | 8.4 (±2.0) | 26.8 (± 11.7) |
| Median (min-max) | 9.1 (4–11) | 24.0 (12–65) |
| Gender (n) | ||
| M/F | 31/20 | 70/71 |
5.2. Monitoring frequencies
Follow up visits didn't differ as much in numerical terms comparing 2019–2020 (+2.9% in 2020). Major change was, during 2020, how they've been done: only 9% of visits were performed in person while in 91% they were done remotely.
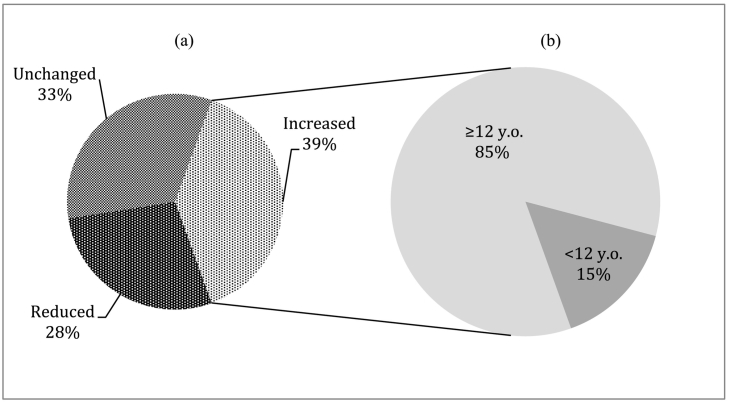
In terms of blood monitoring frequencies, a total number of 1068 dB were received by our Clinic during MAM 2020, vs 1131 during MAM 2019 (− 5.9%). 39% of patients (n = 121) increased the number of performed DBS, moving from at least 1 dB to a maximum of 5 per month. Among those who increased monitoring frequencies, 85% (n = 103) were adolescents and adults thus frequency increase was more evident among older population (Fig. 1).
Fig. 1.
Percentages of patients changing monitoring frequencies among two analyzed time periods (MAM 2019 vs. MAM 2020) (a), also divided per age (b).
Furthermore we could observe 37 patients (12%) who weren't performing any DBS in MAM 2019 and started doing so in MAM 2020 (at least one DBS per month).
5.3. Metabolic control
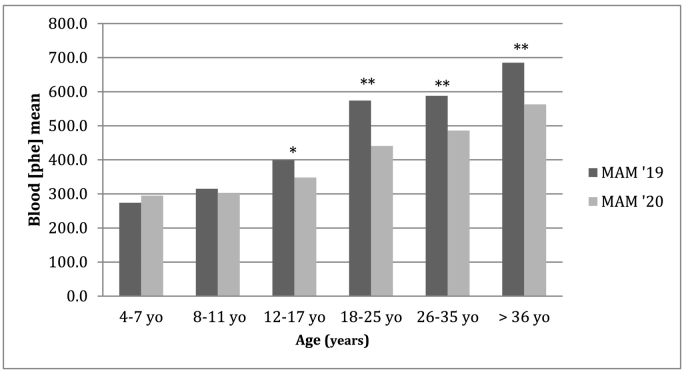
Metabolic control did not differ significantly among pediatric population (GROUP A) during MAM 2019 vs MAM 2020. On the contrary, adolescent and adult population (GROUP B) demonstrated a significant improvement in metabolic control showing reduced Phe values during MAM 2020 compared to MAM 2019, as shown in Table 2.
Table 2.
Metabolic control expressed by mean of Phe levels assessed with DBS among two analyzed time periods (MAM 2020 vs MAM 2019).
| Blood Phe values (umol/l) |
|||
|---|---|---|---|
| MAM 2019 | MAM 2020 | p | |
| GROUP A | |||
| Mean (SD) | 315.4 (114) | 309.2 (134) | 0.717 |
| Median (min-max) | 311.5 (85–586) | 287.8 (86–645) | |
| GROUP B | |||
| Mean (SD) | 556.4 (301) | 454 (252) | 0.000⁎ |
| Median (min-max) | 478 (113–1612) | 409 (35–1525) | |
Significant values were considered for p < 0.05.
An increase in the percentage of patients with mean Phe blood concentrations below the upper recommended threshold could also be observed analyzing both age groups, as shown in Fig. 2.
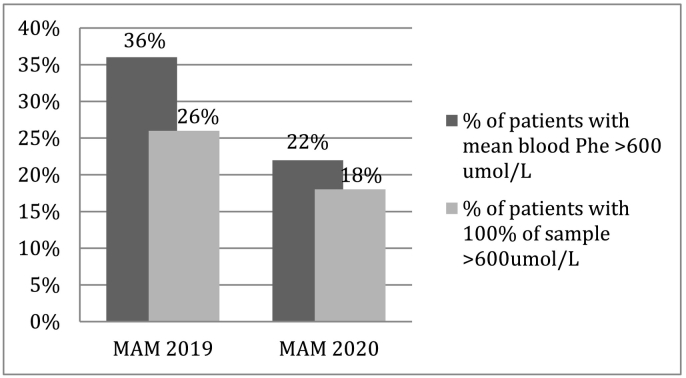
Fig. 3.
Variations between percentages of PKU non-compliant patients during MAM 2019 vs MAM 2020.
No significant differences could be observed on a gender basis.
Fig. 2.
Changes in blood Phe values according to age, comparing MAM 2019 vs. MAM 2020 time-period. ⁎ = statistically significant (p values <0.05); ⁎⁎ = statistically significant (p values <0.01).
“Non-compliant” patients were also reduced (lowering from 11% to 3%) with also a finding of −14% of patients with mean Phe levels >600 umol/l and a − 8% of patients with 100% DBS above same level (results shown in Fig. 3).
5.4. Patients' stories
-
-
PATIENT 1: M., 19 yo male PKU, lost at follow-up, no DBS sent to our Centre since 2017. Last Phe value at plasma aminoacids profile = 1680 umol/L (year 2017). At the beginning of MAM 2020 M. could be recalled offering telehealth services and advised to get back to the Centre for monitoring purposes. He thus performed a preliminary DBS which demonstrated non-compliant Phe levels (1613 umol/L). Strict diet was restarted and M. was compliant to follow new dietary recommendations. At the end of April 2020, DBS showed mean blood Phe levels of 244 umol/L, meaning excellent metabolic control. Such results are still confirmed at present.
-
-
PATIENT 2: S., 30 yo male PKU, poor cognition of being PKU thus non-compliant to recommendations and difficult to identify in terms of Phe tolerance. During MAM 2019 he had sent to our Clinic just n.2 dB. After recall he could be again involved in adherence and began to be interested and participating. During MAM 2020 n.13 dB were received (4.33 dB/month) and tolerance could be again identified thus dietary intervention could be optimize.
-
-
PATIENT 3: G., 22 years old female PKU, developmentally delayed for a genetic condition, showing insufficient metabolic control since accepted on a daily basis in a controlled community where consuming meals. During MAM 2019, her mean blood Phe levels were 649.2 ± 243.3 umol/L (min: 303 umol/L; max: 998 umol/L). After LD measures enforcement, G. couldn't attend her habitual community and was obliged to get back at home with her parents that were asked again to take care of the diet. Average metabolic control during MAM 2020 changed strongly: her Phe levels drop down to 289.5 ± 182.2 3 umol/L (min:74 umol/L; max: 571 umol/L).
6. Discussion
Transition from childhood to adulthood can be demanding. Studies suggest that children are more independent with their food choices as they become adolescents, thus more likely to be influenced by peers and less likely to choose healthy foods among others [6]. Same scenario can be expected among IEMs affected patients, among whom PKUs can best represent the scenario of a strongly recommended dietary intervention, challenging since the very beginning of life and more and more so every other year. PKUs are known to experience difficulties when dealing with social environments to which they can be exposed (i.e. social life, job activities, etc.) and consequent insufficient metabolic control is often describe. This may also be linked to an altered perception and awareness of the disease: about 40% of PKUs do not consider PKU as a disease and consequently don't fully understand the importance of the dietary intervention [7].
COVID-19 world pandemic imposed a new set of challenges for IEMs affected patients and PKU population can represent a valuable example of that. During the pandemic, patients were asked to stay home and avoid contact with other people, thus severe repercussions on both food access and utilization were evident with direct effects on lifestyle habits, including changes in social environments and physical activity patterns to which they were exposed. Efforts to keep choosing healthy dietary habits and maintain adherence to treatment had to be made, same for clinical monitors and health providers that had to adapt trying to find new ways for improving metabolic outcomes besides social distancing. The use of “telehealth” was one of the most important improvements and adaptation to that, with virtual care becoming a consistent part of clinical care [8,9]. Our study investigated how all that can have possibly affected PKU patients, analyzing possible changes in metabolic control and monitoring frequencies over same time-periods (2020 vs. 2019). Differences could be found and were mostly evident for adolescent and adult population. If among child population (GROUP A) metabolic control did not differ significantly during MAM 2019 vs MAM 2020, adolescent and adult patients (GROUP B) demonstrated a significant decrease in blood Phe concentrations with significant improvements in metabolic control (556.4 ± 301 umol/l in MAM 2019 vs. 454 ± 252 umol/l in MAM 2020). This may have been related to the different social and familiar context: younger patients maybe already used to be “controlled” and kept on track by parents, while adolescent and adult patients are more used to managing diet alone thus are more prone to change lifestyle habits when pushed to. Moreover, even if a slight decrease in the total number of performed DBS could be observed among two analyzed time periods, a total of 121 (39%) patients increased the number of DBS performed, maybe reflecting an improvement in illness awareness and the will to improve dietary adherence (Fig. 1). We found this evidence relatively uncommon considering how we are normally used to read in literature about adolescent and adult patients demonstrating poor dietary adherence with increasing age [[10], [11], [12]]. Our results could demonstrate instead how adolescent and adult patients, if exposed to a more favorable social environment with less external influences, can concentrate more on diet and significantly improve metabolic control reducing out of range Phe-values.
Being exposed not to strangers but only to familiar/parents, having more spare time to spend cooking and to advocate to improve adherence to the regular consumption of aminoacids substitutes played a major role in that and can thus be identified as main modifiers optimizing compliance, as already previously suggested by literature [13]. Also, as follow up visits didn't differ as much in terms of numbers comparing 2019–2020 (+2.9% in 2020), these results add support to the fact that it wasn't the changes in clinical management that changed metabolic control of our patients, but themselves.
Psychological issues and potential QoL/neurological changes weren't assessed in this study as not considered first aims of evaluation and this can be a limit to fully determine how the pandemic affected PKU patients' lives. Even if strongly impacting lives, lockdown may have created the ideal situation to encourage PKU patients to take care of themselves, pushing them to adapt the diet/supplements to their lives and not only the other way round. This could be furthermore demonstrated with the evidence of reducing percentages of non-compliant patients, both considering having Phe values >600 umol/l and >1000 umol/l, and for the finding of increasing monitoring frequency over MAM 2020.
On the healthcare providers perspective, we strongly believe that the empowerment of interventions that can promote access to care and assistance services (such as “telehealth”) played a major role in that. Such assumption is even more supported considering that associations between higher staffing intensity and improvements in patient's adherence to clinics' recommendations have been already described in literature [14]. As we strongly used telehealth services during MAM 2020, we speculate that this was definitely linked to the better outcomes observed.
7. Conclusion
The world scenario in which we have recently found ourselves operating as health care providers has strongly impacted on standard clinical practice as well as, on the other hand, on people suffering from chronic diseases and in need of follow up and treatment. Emerged data from this study can demonstrate how much the social context to which this type of patients are normally exposed to can have a strong influence on the ability to keep metabolic control on track, but how much this can actually also lead to positive outcomes, obtaining a significant improvement in metabolic trends when correctly assessed and addressed. PKU population represent an excellent example of how this can be possible. Our results highlight positive and statistically significant changes in metabolic control and monitoring frequencies in the population of adolescent and adult PKU during lockdown, thus strongly suggest keeping implementations made during lockdown even on the long term, in order to be able to enhance as much as possible the positive effects achieved and continue to reach patients more effectively and more quickly, facilitating them in their treatment processes and in the possibility of receiving assistance regardless of their geographical location and/or possibilities related to the social context to which they are exposed to.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.OMIM® and Online Mendelian Inheritance in Man . PHENYLKETONURIA; PKU. 2020. OMIM Entry – # 261600 – PHENYLKETONURIA; PKU. [Google Scholar]
- 2.MacDonald A. Diet and compliance in phenylketonuria. Eur. J. Pediatr. Suppl. 2000;159(2):136–141. doi: 10.1007/pl00014375. [DOI] [PubMed] [Google Scholar]
- 3.Beazer J., Breck J., Eggerding C., Gordon P., Hacker S., Thompson A. Strategies to engage lost to follow-up patients with phenylketonuria in the United States: best practice recommendations. Mol. Genet. Metab. Rep. 2020;23(January) doi: 10.1016/j.ymgmr.2020.100571. p. 100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li K., Naviaux J.C., Monk J.M., Wang L., Naviaux R.K. Improved dried blood spot-based metabolomics: a targeted, broad-spectrum, single-injection method. Metabolites. 2020;10(3) doi: 10.3390/metabo10030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Wegberg A.M.J. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J. Rare Dis. 2017;12(1):1–56. doi: 10.1186/s13023-017-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour M., Hoerr S.L., Li Huang Y. Inappropriate dieting behaviors and related lifestyle factors in young adults: are college students different? J. Nutr. Educ. Behav. 1997;29(1):21–26. doi: 10.1016/s0022-3182(97)70142-0. [DOI] [Google Scholar]
- 7.Cazzorla C. Living with phenylketonuria in adulthood: the PKU ATTITUDE study. Mol. Genet. Metab. Rep. 2018 doi: 10.1016/j.ymgmr.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadir M.A. Role of telemedicine in healthcare during COVID-19 pandemic in developing countries. Telehealth Med. Today. 2020:1–5. doi: 10.30953/tmt.v5.187. [DOI] [Google Scholar]
- 9.Golinelli D., Boetto E., Carullo G., Nuzzolese A.G., Landini M.P., Fantini M.P. How the COVID-19 pandemic is favoring the adoption of digital technologies in healthcare: a literature review. medRxiv. 2020 doi: 10.1101/2020.04.26.20080341. pp. 0–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter J.H. How practical are recommendations for dietary control in phenylketonuria? Lancet. 2002;360(9326):55–57. doi: 10.1016/S0140-6736(02)09334-0. [DOI] [PubMed] [Google Scholar]
- 11.Walter J.H., White F.J. Blood phenylalanine control in adolescents with phenylketonuria. Int. J. Adolesc. Med. Health. 2004;16(1):41–45. doi: 10.1515/IJAMH.2004.16.1.41. [DOI] [PubMed] [Google Scholar]
- 12.Crone M.R., Spronsen F.J., Oudshoorn K., Bekhof J., van Rijn G., Verkerk P.H. Behavioural factors related to metabolic control in patients with phenylketonuria. J. Inherit. Metab. Dis. 2005;28(5):627–637. doi: 10.1007/s10545-005-0014-0. [DOI] [PubMed] [Google Scholar]
- 13.Borghi L., Moreschi C., Toscano A., Comber P., Vegni E. The PKU & ME study: a qualitative exploration, through co-creative sessions, of attitudes and experience of the disease among adults with phenylketonuria in Italy. Mol. Genet. Metab. Reports. 2020;23(January) doi: 10.1016/j.ymgmr.2020.100585. p. 100585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurecki E.R. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017;120(3):190–197. doi: 10.1016/j.ymgme.2017.01.001. [DOI] [PubMed] [Google Scholar]