Abstract
Objective:
To report the clinical features and the treatment outcomes of patients with peripapillary choroidal neovascular membrane (CNVM) secondary to idiopathic intracranial hypertension (IIH).
Methods:
Retrospective, multicenter chart review of patients diagnosed with peripapillary CNVM in the course of the treatment and follow-up of IIH.
Results:
Records were reviewed from 7 different institutions between 2006 to 2016. 10 patients (13 eyes) with a diagnosis of IIH and at least 3 months of follow-up developed CNVM. Three of the total 10 patients developed bilateral CNVM. The mean time from the diagnosis of IIH to CNVM diagnosis was 41 months. Mean follow-up period was 8 months after diagnosis of CNVM was documented. All patients were treated with acetazolamide for IIH. Seven eyes were observed, and six eyes were given anti-vascular endothelial growth factor (anti-VEGF) injections, including bevacizumab, ranibizumab and aflibercept. All CNVMs regressed with subretinal fibrosis and visual acuity improved in most patients. Papilledema resolved in only one eye, while the other 12 eyes had persistent papilledema at the last follow-up.
Conclusions:
Peripapillary CNVM, a rare complication of IIH, often involves spontaneously with treatment of IIH. In vision-threatening and/or persistent cases, intravitreal anti-VEGF treatment may be a safe and effective therapeutic option.
Keywords: Idiopathic Intracranial Hypertension, Choroidal Neovascular Membrane, Papilledema, Neovascularization, Anti-Vascular Endothelial Growth Factor
Peripapillary choroidal neovascular membrane (CNVM) is an infrequent cause of vision loss in patients with idiopathic intracranial hypertension (IIH) (1–3). The prevalence of peripapillary CNVM in IIH has previously been reported as 0.53% (4). There are few reports on the management of IIH-associated peripapillary CNVM. The diagnosis of CNVM is made clinically based on the identification of a deep peripapillary hemorrhage and a greyish-white opacity merged within the area of optic disc edema. This greyish-white opacity indicates the presence of a membrane, which becomes increasingly more apparent on ophthalmoscopy as intracranial pressure is reduced and papilledema resolves. Diagnosis is assisted by subretinal fluid demonstrated on optical coherence tomography (OCT). Likewise, fluorescein angiography (FA) demonstrates hyperfluorescence secondary to CNVM leakage, along with an area of hypofluorescence as subretinal hemorrhage blocks choroidal transmission.
In most cases, medical or surgical treatment aimed at lowering the intracranial and/or optic nerve sheath pressure, results in progressive involution of peripapillary CNVM (20–23). For persistent or symptomatic CNVM, intravitreal anti-VEGF injection is the treatment of choice (4–7). We describe the management of 10 patients (13 eyes) who developed peripapillary CNVM in association with IIH.
Methods
Research conducted was in compliance with the Health Insurance Portability and Accountability Act (HIPAA) and Declaration of Helsinki, while abiding to all regional, national, and international laws of the institutions involved in this study. Every effort was made by the investigators to protect the rights of the patients. In addition, this study followed the tenets of the ethics committee of each contributing center and was approved by the Institutional Review Board of the University of Michigan.
Patients evaluated between January 1, 2006 and December 31, 2016 were reviewed and selected from the following institutions: University of Michigan, W.K. Kellogg Eye Center, Ann Arbor, Michigan, USA; Department of Ophthalmology and Visual Neurosciences, University of Minnesota, Minneapolis, Minnesota, USA; Department of Ophthalmology, Duke University Eye Center, Durham, North Carolina, USA; Department of Ophthalmology, University Hospital Zurich and University of Zurich, Zurich, Switzerland; Department of Ophthalmology, Washington University in St. Louis, St. Louis, Missouri, USA; Department of Ophthalmology, Indiana University School of Medicine, Indianapolis, Indiana, USA; Department of Ophthalmology, Hôpital Delafontaine, Saint-Denis, France. A diagnosis of IIH and a minimum of 3 months of follow up after CNVM treatment were required for study inclusion. Collected data included age, sex, body mass index (BMI), fundus examination findings, best-corrected visual acuity (BCVA) at the time of CNVM diagnosis and at last follow up, visual field mean deviation (VFMD), therapies for IIH, therapies for CNVM (if any), follow-up time, magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) results, lumbar puncture (LP) opening pressures, and status of papilledema and CNVM at the last follow-up. Patients with a diagnosis of CNVM before IIH diagnosis were excluded.
The Statistical Package for Social Science v 22.0 software (SPSS, Chicago, Illinois, USA) was used to conduct the statistical analyses. Comparisons between the visits were made using the Wilcoxon test. All of the reported p-values were two-tailed, and those less than 0.05 were considered to be statistically significant.
Results
Based on data collected over a ten-year period from one of the study sites (University of Michigan, W.K. Kellogg Eye Center), we report a 0.96% prevalence of CNVM in patients with IIH associated papilledema and at least 3 months of documented follow-up.
Patient characteristics and treatment results are summarized in Table 1. A total of 13 eyes of 10 patients (8 women and 2 men) met the inclusion criteria, with a mean age of 35 years (range 15–54 years). The mean BMI was 35 kg/m2 (range: 19–48 kg/m2). Brain MRI and MRV were normal except for dilated optic nerve sheaths in all patients. LP opening pressures were over 30 cmH2O in all patients. All 13 eyes with CNVM also had papilledema. Peripapillary subretinal hemorrhage and subretinal fluid were found in 10 eyes, while 1 eye only had peripapillary subretinal fluid in association with CNVM (Fig 1). Fluorescein angiography (FA) performed in 4 of the 10 patients included in this study demonstrated hyperfluorescence in the mid-phase with late leakage, indicative of a peripapillary CNVM. The mean elapsed time between diagnosis of IIH and documentation of a diagnosis of CNVM was 41 months. The mean length of follow-up after CNVM diagnosis was 8 months (range: 3–54 months). All patients with IIH were treated with oral acetazolamide. Symptomatic or vision-threatening CNVMs were treated with intravitreal anti-VEGF injections in 6 eyes. Anti-VEGF injections were given at the discretion of the treating ophthalmologist guided by OCT findings. Three eyes received 1 bevacizumab (1.25 mg/0.05 ml) (Avastin, Genentech, Inc., South San Francisco, CA) injection, one eye received 3 bevacizumab injections, one eye received 2 bevacizumab and 4 aflibercept (2.0 mg/0.05 ml) (Eylea, Regeneron, Tarrytown, NY) injections, and one eye received 2 ranibizumab (0.5 mg/0.05 ml) (Lucentis, Genentech, Inc.) injections. Seven eyes with non-vision threatening CNVMs were observed (Table 2). In eyes managed with observation, the mean interval from time of diagnosis to time of resolution of CNVM was 248 days (range 132–435 days). The resolution of CNVM correlated with improvement of papilledema in these eyes, although persistent disc edema and elevation was noted at most recent follow-up in all eyes except one.
Table 1.
Demographics and clinical features of patients with IIH and CNVM.
| (n = 10 patients/ 13 eyes) | |
|---|---|
| Age | 35 (15–54) years |
| Gender (male/female) | 8/2 |
| BMI | 35 (19–48) kg/m2 |
| MRI/MRV | Normal |
| LP | > 30 cmH2O |
| Subretinal hemorrhage and/or fluid | 11 eyes |
| Time for CNVM formation | 41 (2–112) months |
| Follow-up time after CNVM | 8 (3–54) months |
| Treatment for IIH | Oral acetazolamide |
Data are expressed as the mean (range) of cases.
n = number
BMI = body mass index
MRI/MRV = magnetic resonance imaging/magnetic resonance venography
LP = lumber puncture
CNVM = choroidal neovascular membrane
IIH = idiopathic intracranial hypertension
Figure 1.
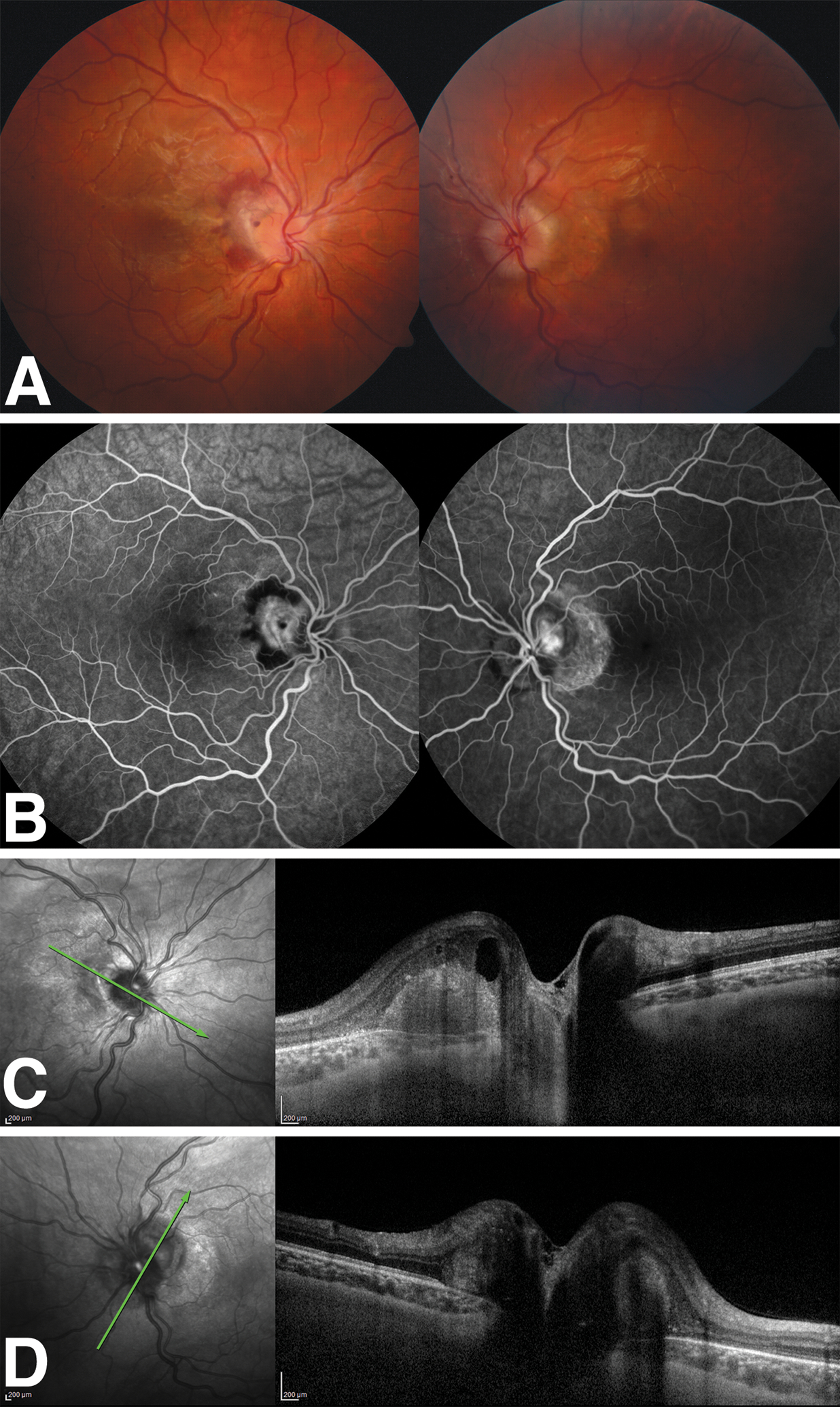
Patient 4. A. There is papilledema with peripapillary subretinal hemorrhage and fluid in the right eye and papilledema with peripapillary atrophy in the left eye. B. Mid-phase fluorescein angiogram reveals peripapillary hyperfluorescence surrounded by a ring of hypofluorescence in the right eye. In the left eye, there is peripapillary hyperfluorescence due to CNVM and an adjacent crescent-shaped hyperfluorescent window defect. C. OCT of the right eye shows intraretinal and subretinal fluid consistent with an active CNVM. D. OCT left eye demonstrates peripapillary thickening with outer retinal atrophy. OCT, optical coherence tomography; CNVM, choroidal neovascular membrane.
Table 2.
Demographic, clinical and treatment features of patients with IIH and CNVM.
| Patient | Sex | Age (years) |
Laterality | Imaging modality used in diagnosis | Initial VA | VA last visit | Treatment | Follow up time (months) | Last status CNVM | Last status papilledema |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 32 | Left | FA | 20/20 | 20/60 | Bevacizumab x 3 | 56 | Resolved | Improved |
| 2 | F | 26 | Right | OCT | 20/500 | 20/200 | Ranibizumab x 2 | 8 | Resolved | Improved |
| 3 | M | 21 | Right | FA + OCT | 20/30 | 20/40 | Observation | 6 | Resolved | Improved |
| 4 | F | 51 | Right | Fundus photography | 20/20 | 20/20 | Observation | 8 | Resolved | Improved |
| Left | Fundus photography | 20/25 | 20/25 | Observation | 8 | Resolved | Improved | |||
| 5 | F | 15 | Right | OCT | 20/40 | 20/40 | Observation | 5 | Resolved | Resolved |
| 6 | F | 54 | Right | FA + OCT | CF | 20/250 | Bevacizumab x 1 | 21 | Resolved | Improved |
| 7 | F | 45 | Right | OCT | 20/50 | 20/40 | Observation | 8 | Resolved | Improved |
| Left | OCT | Hand motion | 20/100 | Bevacizumab x 2, Aflibercept x 4 | 8 | Resolved | Improved | |||
| 8 | F | 23 | Right | Fundus photography | 20/250 | 20/250 | Bevacizumab x 1 | 18 | Resolved | Improved |
| 9 | M | 48 | Left | OCT | 20/50 | 20/200 | Bevacizumab x 1 | 3 | Resolved | Improved |
| 10 | F | 30 | Right | FA | 20/40 | 20/32 | Observation | 14 | Resolved | Persistent |
| Left | FA | 20/400 | 20/400 | Observation | 14 | Resolved | Persistent |
VA, visual acuity; FA, fluorescein angiography; OCT, optical coherence tomography; IIH, idiopathic intracranial hypertension; CNVM, choroidal neovascular membrane
Mean BCVA at the time of CNVM diagnosis and at the last visit were 0.80±0.91 logMAR and 0.60±0.44 logMAR, respectively (p= 0.62). At the end of the follow-up period, most untreated and treated eyes either had stable or improved BCVA (Table 2). Mean initial and last BCVA in observed group were 0.32±0.42 logMAR and 0.37±0.40 logMAR, respectively (p= 0.63). Mean initial and last BCVA in treated group were 1.58±0.98 logMAR and 0.98±0.16 logMAR (p= 0.37). Mean improvement in VA was 0.05±0.19 logMAR in observed group and −.60±1.10 logMAR in treated group (p= 0.258).
Eight of the thirteen eyes included in this investigation underwent automated (Humphrey) visual field testing at time of CNVM diagnosis and at most recent follow-up, which included three patients in the treatment group and five in the observation group. The average VFMD for both treated and untreated eyes at time of CNVM diagnosis and at most recent follow-up were −8.59±5.60 dB and −5.91±5.45 dB, respectively (p= 0.19). Mean initial and last VFMD in the observed group were −13.38±7.08 and −8.43±6.07 respectively (p= 0.50). Mean initial and last VFMD in the treated group were 1.58±0.98 logMAR and 0.98±0.16 respectively, logMAR (p= 0.37). Mean change in VFMD was 01.82±4.20 in the observed group and 2.68±2.37 (p= 0.54) in the treatment group.
All CNVMs regressed with residual peripapillary subretinal fibrosis either following treatment (Fig 2) or observation (Fig 3). Papilledema resolved completely in only one eye, while the other 12 eyes had improved but persistent disc edema and elevation at the last follow-up.
Figure 2.
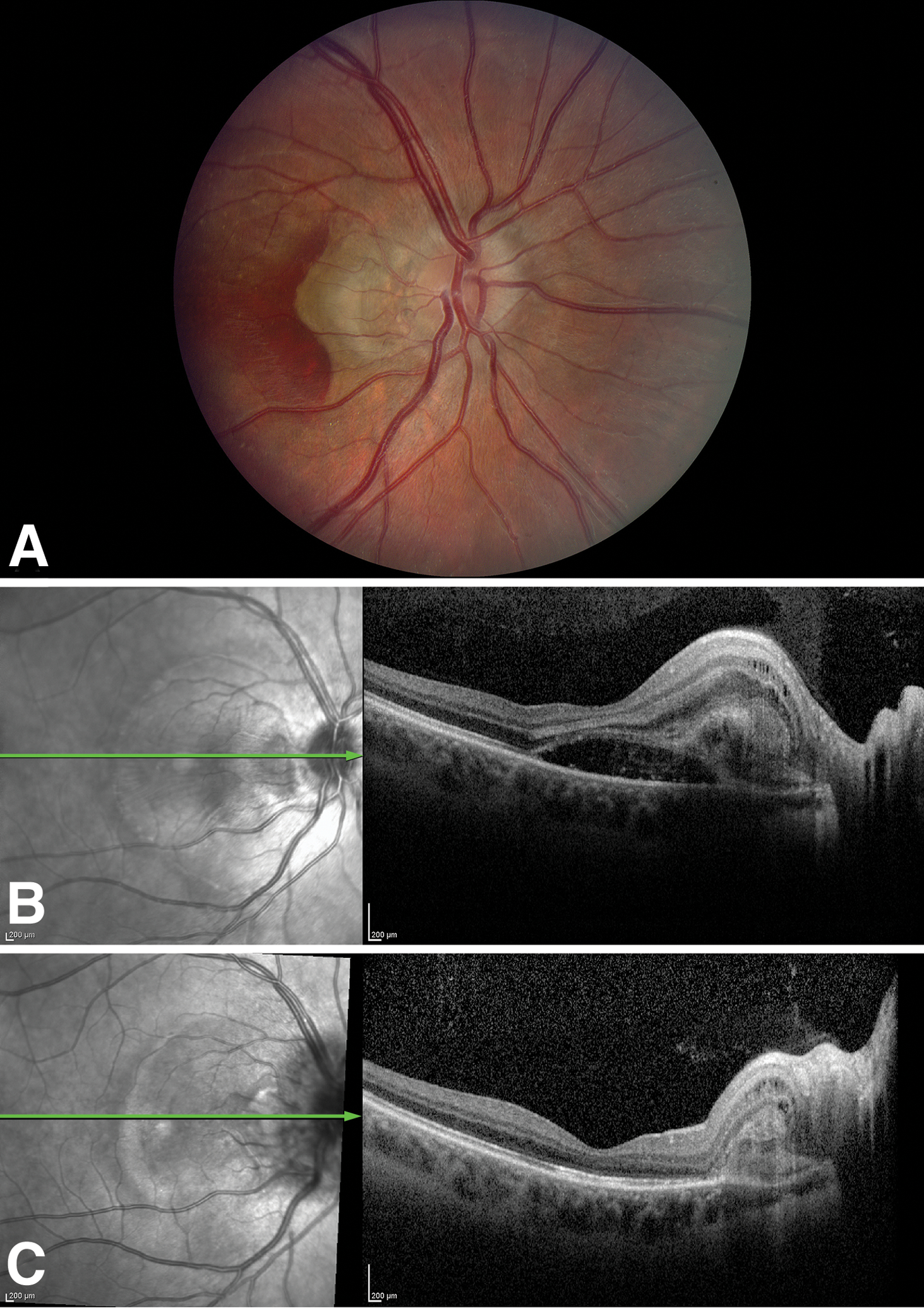
Patient 2.A. The right fundus shows papilledema with peripapillary subretinal hemorrhage and intraretinal and subretinal fluid due to CNVM. B. Pre-treatment OCT shows subretinal fluid extending into the fovea. C. OCT shows resolution of subretinal fluid after treatment following 2 intravitreal ranibizumab injections. CNVM, choroidal neovascular membrane; OCT, optical coherence tomography.
Figure 3.
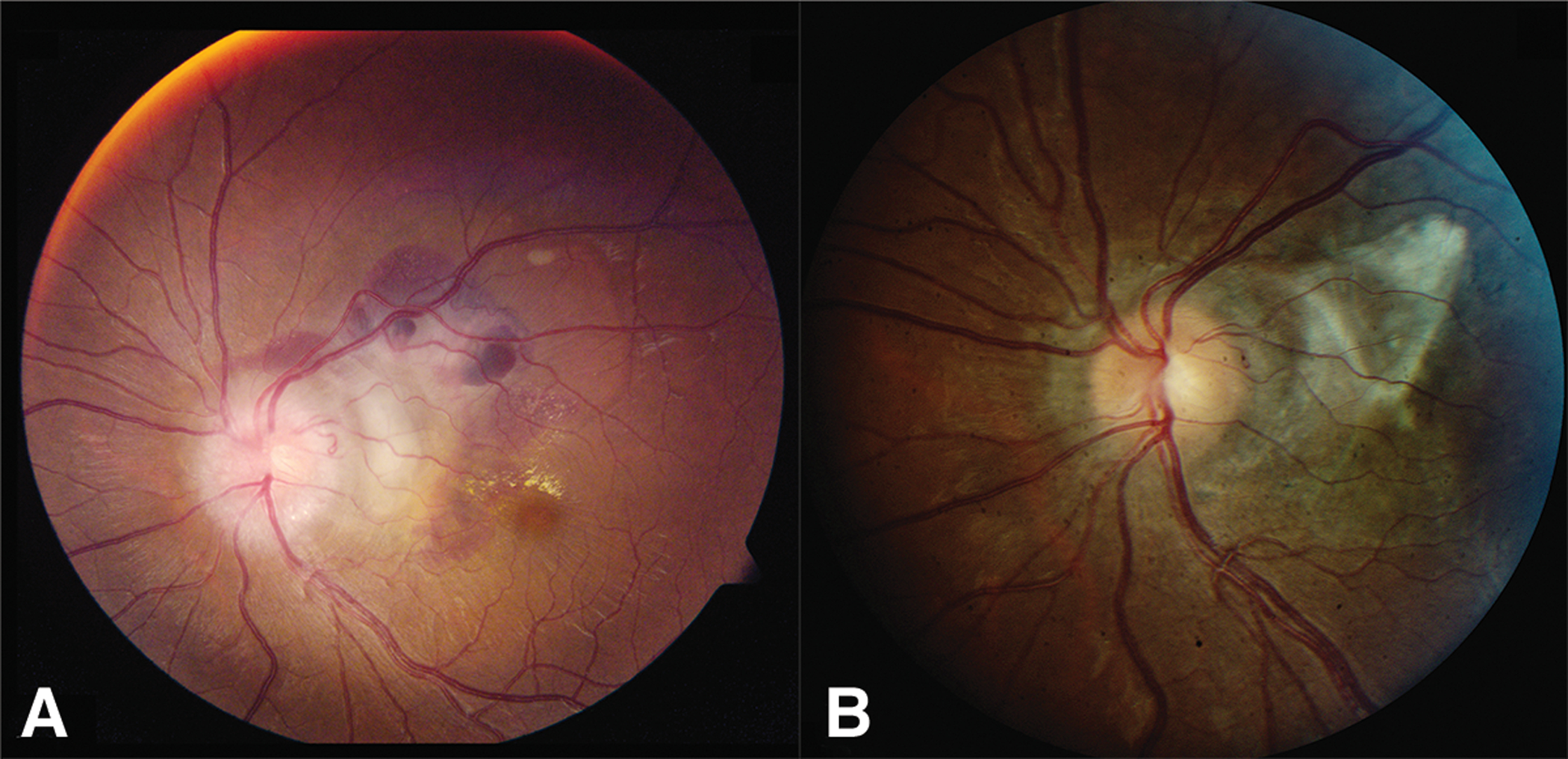
Patient 10. A. Peripapillary CNVM and papilledema is present. B. Following 8 months of observation, there is regression of CNVM with subretinal fibrosis and diminished optic disc edema.
Discussion
In our multicenter retrospective chart review of patients who developed peripapillary CNVM secondary to IIH, 10 patients (13 eyes) from 7 different institutions were included, spanning a 10-year period from the beginning of the anti-VEGF era in 2006. All patients with IIH were treated with acetazolamide. Six eyes were treated with intravitreal anti-VEGF injections, including bevacizumab, ranibizumab, and aflibercet. The remaining seven eyes were managed with close follow-up. Regression of peripapillary CNVM was documented in all eyes.
Peripapillary CNVM is an infrequent complication of chronic papilledema and can cause significant vision loss in patients with IIH. Data on this rare disorder are limited to few case reports and small case series (8–14). The diagnosis of CNVM is based on a combination of clinical examination findings, and features observed on OCT and FA. There is deep peripapillary hemorrhage associated with SRF detected on OCT. While fluid around the disc is common in patients with IIH, the presence of a greyish-white opacity within this area of edema indicates the presence of a CNVM. On fluorescein angiography, there is hyperfluorescence in the area of leakage from the CNVM, and hypofluorescence in the area where subretinal hemorrhage is present.
Wendel et al (4) reported the largest series of peripapillary CNVM in patients with IIH-associated papilledema, prior to the development of VEGF inhibitors. The authors retrospectively reviewed 1140 patients with IIH and identified 6 cases with peripapillary CNVM, for a prevalence of 0.53%. There were 5 females and 1 male with a mean age of 46.3 years. All patients had unilateral peripapillary CNVM and were treated with acetazolamide or furosemide to lower the intracranial pressure. They elected observation for 3 patients while 2 were treated with argon laser photocoagulation and 1 with photodynamic therapy. At the end-point of their study, 3 of 6 CNVMs had regressed, 2 were smaller and inactive, and 1 was smaller but with a limited area of presumed activity. No recurrence of CNVM was noted.
In our study we elected observation in 7 eyes, and intravitreal anti-VEGF injections were administered in 6 eyes. The decision to treat was based on the identification of a CNVM with persistent subretinal fluid threatening the fovea, expanding retinal exudation, increasing deep peripapillary hemorrhage, or a decline in visual acuity out of proportion with the amount of optic disc edema. All but one eye in our study showed either stable or improved vision at the end of follow-up and all CNVMs completely regressed.
The causal relationship between developing a peripapillary CNVM and papilledema is controversial. Kaeser and Borruat (15) reported a patient with bilateral papilledema and unilateral peripapillary CNVM with spontaneous involution of the membrane along with improvement of the patient’s vision from 20/200 to 20/30 after the treatment of IIH with acetazolamide for 1 year. However, Sathornsumettee et al (2) described a patient with IIH whose peripapillary CNVM did not regress and visual acuity that did not improve after optic nerve sheath fenestration, despite dramatic improvement in the degree of papilledema. In our study, all 13 peripapillary CNVMs regressed with concurrent improvement in papilledema. It is unclear whether our findings are related to the medical therapy of IIH, the treatment of the CNVM, or simply due to the natural course of the CNVM.
The exact pathogenesis of peripapillary CNVM in patients with papilledema due to IIH is unclear but it seems to be different from CNVMs that occur in other posterior segment diseases such as exudative age-related macular degeneration (16–19). In macular degeneration, retinal pigment epithelium (RPE) dysfunction and Bruch’s membrane breaks occur as a result of retinal drusen deposition and inflammation, which subsequently promotes hypoxia and angiogenesis (19, 20). Peripapillary CNVM in IIH might result from a pressure deformity of Bruch’s membrane at the level of the optic nerve head (16). This combined with persistent hypoxic environment created by axonal swelling may initiate the angiogenesis cascade, leading to the neovascular membrane formation (21,22).
Management options for peripapillary CNVM in patients with IIH include observation, subretinal surgery, laser photocoagulation, photodynamic therapy or intravitreal injection of anti-VEGF agents (3, 6, 16, 23, 24). While therapeutic decisions must be made on a case-by-case basis, the use of anti-VEGF agents currently is the method of choice (1, 7, 23, 24).
Our study has several limitations, including the method of diagnosis of CNVM. In each case the diagnosis of CNVM was made clinically with supporting features observed on retinal imaging. Not all patients underwent both OCT and fluorescein angiography. Other limitations of our report include retrospective design and small sample size.
In conclusion, peripapillary CNVM in association with papilledema due to IIH may regress spontaneously with no vision loss with adequate medical treatment of IIH. In vision threatening cases, intravitreal injection of bevacizumab, ranibizumab or aflibercept may be an effective treatment option to preserve visual function.
Acknowledgement:
The authors of this manuscript would like to acknowledge Leslie M. Niziol, statistician at the University of Michigan Kellogg Eye Center for contributing the statistical analysis and interpretations inherent to this manuscript.
The authors have not received grant support or funding to support this research. The authors do not have any proprietary interests in the materials described in the article, and there are no conflicts to disclosure. This manuscript has not been previously published nor is under consideration for publication elsewhere.
References
- 1.Belliveau MJ, Xing L, Almeida DRP, Gale JS, ten Hove MW. Peripapillary choroidal neovascular membrane in a teenage boy: presenting feature of idiopathic intracranial hypertension and resolution with intravitreal bevacizumab. J Neuroophthalmol. 2013;33:48–50. [DOI] [PubMed] [Google Scholar]
- 2.Sathornsumetee B, Webb A, Hill DL, Newman NJ, Biousse V. Subretinal hemorrhage from a peripapillary choroidal neovascular membrane in papilledema caused by idiopathic intracranial hypertension. J Neuroophthalmol. 2006;26:197–199. [DOI] [PubMed] [Google Scholar]
- 3.Tewari A, Shah GK, Dhalla MS. Combination photodynamic therapy and juxtascleral triamcinolone acetonide for the treatment of a peripapillary choroidal neovascular membrane associated with papilledema. Br J Ophthalmol 2006;90:1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wendel L, Lee AG, Boldt HC, Kardon RH, Wall M. Subretinal neovascular membrane in idiopathic intracranial hypertension. Am J Ophthalmol. 2006;141:573–574. [DOI] [PubMed] [Google Scholar]
- 5.Davis AS, Folk JC, Russell SR, et al. Intravitreal bevacizumab for peripapillary choroidal neovascular membranes. Arch Ophthalmol. 2012;130:1899–1075. [DOI] [PubMed] [Google Scholar]
- 6.Castellarin AA, Sugino IK, Nasir M, Zarbin MA. Clinicopathological correlation of an excised choroidal neovascular membrane in pseudotumour cerebri. Br J. Ophthalmol 1997;81:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamerson SC, Arunagiri G, Ellis BD, Leys MJ. Intravitreal bevacizumab for the treatment of choroidal neovascularization secondary to pseudutomor cerebri. Int Ophthalmol. 2009;29:183–185. [DOI] [PubMed] [Google Scholar]
- 8.Andrews LE, Liu GT, Ko MW. Idiopathic intracranial hypertension and obesity. Horm Res Paediatr. 2014;81:217–225. [DOI] [PubMed] [Google Scholar]
- 9.Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri) A case-control study. Neurology. 1991;41:244. [DOI] [PubMed] [Google Scholar]
- 10.Randhawa S, Van Stavern GP. Idiopathic intracranial hypertension (pseudotumor cerebri). Curr Opin Ophthalmol. 2008;19:445–453. [DOI] [PubMed] [Google Scholar]
- 11.Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayreh MS, Hayreh SS. Optic disc edema in raised intracranial pressure. I. Evolution and resolution. Arch Ophthalmol. 1977;95:1247–1312. [DOI] [PubMed] [Google Scholar]
- 13.Orcutt JC, Page NG, Sanders MD. Factors affecting visual loss in benign intracranial hypertension. Ophthalmology. 1984;91:1303–1312. [DOI] [PubMed] [Google Scholar]
- 14.Gospe SM 3rd, Bhatti MT, El-Dairi MA. Anatomic and visual function outcoes in pediatric idiopathic intracranial hypertension. Br J Ophthalmol. 2016;100:505–509 [DOI] [PubMed] [Google Scholar]
- 15.Kaesar P-F, Borruat F-X. Peripapillary neovascular membrane: a rare cause of acute vision loss in pediatric idiopathic intracranial hypertension. J AAPOS. 2011;15:83–86. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Ferrucci S, Peripapillary subretinal neovascular membranes: a review. Optometry. 2011; 82:681–688. [DOI] [PubMed] [Google Scholar]
- 17.Lopez PF, Green WR. Peripapillary subretinal neovascularization. A review. Retina. 1992;12:147–171. [PubMed] [Google Scholar]
- 18.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bressler SB. Introduction: Understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology. 2009;116(10 Suppl):S1–S7. [DOI] [PubMed] [Google Scholar]
- 20.Blasiak J, Petrovski G, Veréb Z, Facskó A, Kaarniranta K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed Res Int. 2014;2014:768026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503. [DOI] [PubMed] [Google Scholar]
- 22.Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee I-J, Maccheron LJ, Kwan AS. Intravitreal bevacizumab in the treatment of peripapillary choroidal neovascular membrane secondary to idiopathic intracranial hypertension. J Neuroophthalmol. 2013;33:155–157. [DOI] [PubMed] [Google Scholar]
- 24.Más-Ramirez AM, Villegas VM, Garcia JM, Acevedo S, Serrano L. Intravitreal bevacizumab for peripapillary subretinal neovascular membrane associated to papilledema: a case report. P R Health Sci J. 2012;31:341–351. [PubMed] [Google Scholar]


