Abstract
Context
Sagittal-plane cervical spine alignment has emerged as one of the most important clinical outcomes in health care. Nevertheless, the quantity and quality of research on the role that cervical sagittal alignment plays in improving sensorimotor and autonomic nervous functions are limited.
Objective
To investigate the immediate and long-term effects of cervical lordosis restoration and correction of anterior head translation (AHT) on pain, disability, autonomic nervous system function, and cervical sensorimotor control in athletes with chronic nonspecific neck pain.
Design
Randomized controlled clinical trial.
Setting
University research laboratory.
Patients or Other Participants
A total of 110 patients (59 males, 51 females) with chronic nonspecific neck pain and a defined hypolordotic cervical spine and AHT posture.
Intervention(s)
Patients were randomly assigned to the control or intervention group. Both groups received a multimodal program; the intervention group also received Denneroll cervical traction. Treatments were applied 3 times per week for 10 weeks.
Main Outcome Measure(s)
Outcome measures were cervical lordosis from C2 to C7, AHT, neck disability index, pain intensity, smooth-pursuit neck-torsion test, overall stability index, left- and right-rotation head repositioning accuracy, and amplitude and latency of skin sympathetic response. The measures were assessed 3 times: at baseline, after 10 weeks of treatment, and at 1-year follow-up.
Results
The general linear model with repeated measures indicated group × time effects in favor of the intervention group for the following management outcomes: cervical lordosis, AHT, neck disability index, pain intensity, smooth-pursuit neck-torsion test, overall stability index, left- and right-rotation head repositioning accuracy, and amplitude and latency of the skin sympathetic response (P values < .001).
Conclusions
Restoration of cervical sagittal alignment in the athletic population had a direct influence on pain, disability, autonomic nervous system dysfunction, and sensorimotor control. Our results should guide treatment planning for athletes and optimize their recovery time.
Trial Registration Number
Keywords: athletes, neck pain, autonomic nervous system, sensorimotor control
Key Points
Sagittal-plane cervical spine alignment influenced the skin sympathetic response and sensorimotor control.
Restoration of sagittal-plane cervical spine alignment in athletes directly influenced pain and disability.
Neck pain is among the most common musculoskeletal disorders requiring intervention, with an annual prevalence ranging from 30% to 50%.1 Chronic nonspecific neck pain (CNSNP) contributes a substantial proportion of the rising health care costs and workplace absenteeism for musculoskeletal disorders.1 Although the prevalence of neck pain among athletes is similar to that in the general population, sport-specific activities may put athletes at a higher risk of neck pain in some situations.2 Regardless of the origin of their symptoms, athletes with neck pain may have deficits in muscle recruitment, strength and endurance, repositioning acuity, postural stability, and oculomotor control.2
Conflicting views exist about the clinical importance of variations in the sagittal-plane cervical spine alignment in CNSNP. It is often asserted that abnormalities of the cervical curvature may represent a normal variant.3,4 Conversely, in clinical investigations, other authors5 indicated that sagittal-plane cervical spine alignment played an important role in neck pain, headaches, biomechanics, and neurophysiology. Regardless of the view one might favor, attention to the role of sagittal-plane cervical spine alignment in a variety of musculoskeletal disorders and consequent dysfunction that may lead to abnormal afferent information (dysafferentation) has increased.6 For example, in a recent retrospective study of athletes versus nonathletes, Oe et al7 found that male athletes had a better cervical lordosis and T1-slope relationship than nonathletic males, whereas female athletes had better overall global sagittal-plane cervical spine alignment than their nonathletic counterparts.
Irrespective of participation in sports, the cervical spine proprioceptive afferentation system is considered a major component of sensorimotor control.8 An intimate connection exists among afferent input from the proprioceptive, visual, and vestibular systems and a stable upright posture of the head and neck.8 Similarly, the abundance of mechanoreceptors in the cervical muscles, ligaments, and discs plays an important role, providing the necessary neurophysiological input in a feed-forward and feedback system for sensorimotor control via connections to the vestibular, visual, and central nervous systems.8 Of interest, a network of neurophysiological connections between the cervical spine mechanoreceptors and the sympathetic nervous system has been documented.9
Although the effects of autonomic system activity on musculoskeletal function have been studied extensively,10 little research supports the idea that the autonomic nervous system is intimately responsive to changes in the afferent articular input due to joint dysfunction.11 The assumption that restoring normal posture and cervical spine alignment is necessary for a better afferentation process has preliminary supporting evidence.12,13 Nevertheless, the quantity and quality of research on the role that sagittal-plane cervical spine alignment plays in improving sensorimotor and autonomic nervous functions have been limited. Accordingly, we aimed to investigate the immediate and long-term effects of cervical lordosis restoration and correction of anterior head translation (AHT) on pain, disability, autonomic nervous system function, and cervical sensorimotor control in athletes with CNSNP.
METHODS
Design
The investigation was a randomized, single-blind trial. Measurements were obtained before the randomization (baseline), after the 10-week intervention period, and at 1-year follow-up. The trial was registered with Clinicaltrials.gov (NCT04306640).
Participants
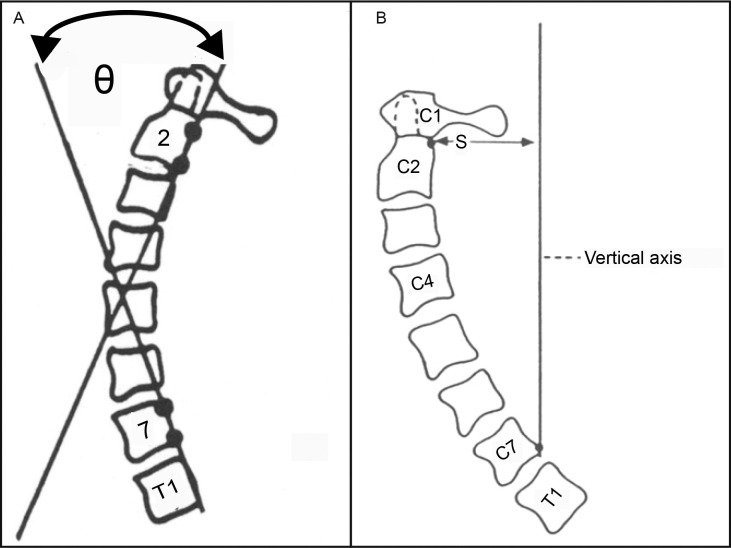
We recruited a convenience sample of 110 patients from our outpatient facility at the University of Cairo. Participants were recruited if they were aged 18 years or older, had chronic neck pain (ie, duration >3 months), and were able to attend a full course of 30 treatments (3 times per week for 10 weeks). Radiographic measurements of cervical lordosis and AHT were used to screen volunteers for inclusion. To measure cervical lordosis, we determined the absolute rotation angle (ARA) from C2 to C7 using the angle of intersection of 2 lines drawn along the posterior vertebral body margins of C2 and C7 (Figure 1).5 On upright lateral cervical spine radiographic images, AHT was measured as the horizontal offset of the posterior-superior body corner of C2 relative to a vertical line passing through the posterior-inferior body of C7 (Figure 1). Patients were included if they had an ARA from C2 of <20° and AHT distance of >25 mm.5 Participants were excluded if they presented with any of the following: (1) signs or symptoms of systemic pathology and inflammatory joint disease, (2) history of cervical spine trauma and musculoskeletal system surgery, or (3) disorder related to the spine and extremities. All participants provided written informed consent, and the study was approved by the University of Sharjah Research Ethics Committee.
Figure 1.
Radiographic measures. A, Measurement of cervical lordosis from C2 to C7. B, Measurement of anterior head translation. Abbreviation: S, horizontal offset of the posterior-superior body corner of C2 relative to the vertical line.
Procedures
Participants were randomly assigned to either the intervention (n = 55) or control (n = 55) group by an independent person who was not involved in the trial and was blinded to the research protocol. The randomization sequence (computer-generated permuted blocks of 4, 6, 8) was generated a priori for concealment. Each randomly permuted block was transferred to a sequence of consecutively numbered, sealed, opaque envelopes that were stored in a locked drawer. As each participant formally entered the trial, the researcher opened the next envelope in the sequence in the presence of the participant.
Both the intervention and control groups completed a 10-week multimodal program that consisted of physical pain-relief methods, including transcutaneous electrical nerve stimulation (TENS), thoracic spine mobilization and manipulation, and soft tissue mobilization. In addition to the physical pain-relief treatments, the intervention group used a cervical traction orthotic device (Denneroll Spinal Orthotics) to improve the altered sagittal-plane cervical spine alignment (AHT and ARA C2–C7). Therefore, the only difference in treatment applications between the 2 groups was the use of the traction device in the intervention group. The first follow-up evaluations were conducted at the end of the 10-week (30-session) multimodal program, and the second follow-up was conducted 1 year after the end of the 10-week intervention program.
Physical Pain-Relief Agents
Transcutaneous Electrical Nerve Stimulation
The participants in both groups received conventional TENS therapy (20 minutes). For the analgesic effects, they were treated with a frequency of 80 Hz, pulse width of 50 μs, intensity (in milliamperes) kept at the sensory threshold of each participant, symmetric rectangular biphasic waveform, and modulation up to 50% of variation frequency. Hot packs were applied over the shoulders and neck for 15 minutes before the TENS application to improve pain and any disability, optimizing the effectiveness of the intervention.14
Thoracic Spine Mobilization and Manipulation
All participants with hypomobile spinal segments, diagnosed using segmental mobility testing, received an initial treatment that included thrust manipulation techniques specific to the upper (T1–T4), middle (T5–T8), and lower (T9–T12) thoracic spine.15 Each participant received at least 1 intervention targeting a specific segment of the thoracic spine (lower, middle, or upper thoracic region) each session.
Soft Tissue Mobilization
Soft tissue mobilization was designed to address any soft tissue restrictions in the cervical and upper thoracic regions. Any taut and tender band within the following muscles was treated using deep tissue massage along the entire muscle length: infraspinatus, levator scapulae, splenius capitis and cervicis, supraspinatus, teres major and minor, and upper trapezius.16
Sagittal-Plane Cervical Spine Alignment Corrective Orthotic Device (Denneroll Extension Traction)
Participants in the intervention group underwent Denneroll extension traction. They were instructed to lie supine on the ground with their lower extremities straight, arms by their sides, and forearms gently folded across the trunk. The physiotherapist (I.M.) placed the apex of the Denneroll in either the midcervical region or the lower cervical region, depending on the apex of cervical curvature deformity in each participant. The treatment began with 3 minutes of sustained Denneroll extension traction and progressed to the goal of 20 minutes per session with 1 or 2 additional minutes per session. The Denneroll intervention was repeated 3 times per week for 10 weeks in the supervised setting (Figure 2). All interventions were delivered individually by the same physiotherapist (I.M.), who had 15 years of clinical experience and had received certified training in these manual techniques, to minimize intertherapist variation and enhance fidelity.
Figure 2.

Denneroll (Denneroll Spinal Orthotics) extension traction. Reprinted with permission from CBP Seminars, Inc.
Outcome Measures
The treatment effect was determined primarily based on radiographic changes in sagittal-plane cervical spine alignment and secondarily on the neck disability index (NDI), neurophysiological measures, and sensorimotor control outcomes. All outcome measures were obtained at 3 time points: at baseline, 10 weeks postintervention, and 1-year follow-up. The outcomes were measured in the same order for all participants.
Radiographic Measures of Cervical Alignment
Cervical lordosis measured as the ARA from C2 to C7 and AHT distance were obtained using standing lateral cervical radiographs as explained by Harrison et al.17 Both the cerivcal lordosis and AHT measurements are depicted in Figure 1.
The NDI was used to measure disability. It consists of 10 items that inquire about standard daily activities. The responsiveness to change, construct validity, and reliability have been adequately investigated in a variety of patient populations.18
Numeric Pain-Rating Scale
The average intensity of neck pain over the preceding week was assessed using a numeric rating scale from 0 (no pain) to 10 (worst pain). The reliability and validity of the numeric rating scale have been reported as good.19
Sensorimotor Control
Assessment of sensorimotor function consisted of joint position testing of the cervical spine, coordination of eye and head motor control using the smooth-pursuit neck-torsion test (SPNT), and an evaluation of postural stability in upright stance on a dynamic-balance platform.
Cervical Joint Position Sense Testing
Head-repositioning accuracy was assessed using a cervical range-of-motion device as described by previous investigators.20,21 With participants seated in an upright neutral posture on a stool with no back rest and their feet touching the ground, we determined their perceived natural head position and used it as the reference neutral point. The cervical range-of-motion device was positioned at 0, 0, and 0 for x, y, and z rotational displacements, respectively. With their eyes closed, participants were instructed to remember their natural head position as the starting posture, actively rotate the head 30° to the left about the vertical y-axis, and then return to the natural head position.
Participants were given an oral signal by the investigator (A.A.) to stop when they reached approximately 30° of cervical rotation and then repositioned the head to the starting position. They were encouraged to strive for accuracy rather than speed in returning to the natural head position. This process was repeated at 30° to the right side about the vertical y-axis. They completed 3 repetitions within 60 seconds in each rotation direction, for a total of 6 trials. We calculated head-repositioning accuracy using the difference (°) between the primary rotational plane of movement between the natural head position reference and the return to neutral; this protocol has been validated previously.20
Eye and Head Motor Control
The SPNT was administered using an electro-oculography device to quantify any alteration and improvement in visual motor control.22 Participants performed the SPNT with the head and trunk in neutral, forward-facing posture. Next, while keeping the head in the natural position, the torso was rotated 45° (about a vertical y-axis) to each side in a consecutive manner. Participants performed 3 eye blinks and were instructed to follow the path of a light source as perfectly as they could with their eyes. The accuracy of the SPNT was determined as the difference between the average increase and decrease in the participant's natural head positions compared with the torsioned positions.
Assessment of Postural Stability
Postural stability characteristics were evaluated using a Biodex Balance System SD (BBS; model 950-440; Biodex Medical Systems). Balance testing was performed on the unlocked platform to allow free concurrent movement in the anterior-posterior and medial-lateral directions. The platform permits variable levels of resistance to movement perturbation ranging from 1 to 8, with 1 being the most restrictive, as established by the manufacturer. The BBS measures the deviation of each axis during dynamic-balance assessments instead of measuring the center-of-gravity deviation during static conditions. Based on these data, the BBS software measures balance indices for anterior-posterior and medial-lateral stability and general stability (overall stability index [OSI]) according to tilt variance, whereby reduced balance is associated with a large variance. For each participant, the balance indices were calculated during three 10-second trials, with a 20-second rest period between trials. Analysis was based on the average of the 3 trials. Dynamic- and static-balance testing was assessed following the same procedure, with the BBS set to a dynamic position of 4 out of 8. This position represents the available range of resistance of the middle level of the platform (Figure 3).23
Figure 3.
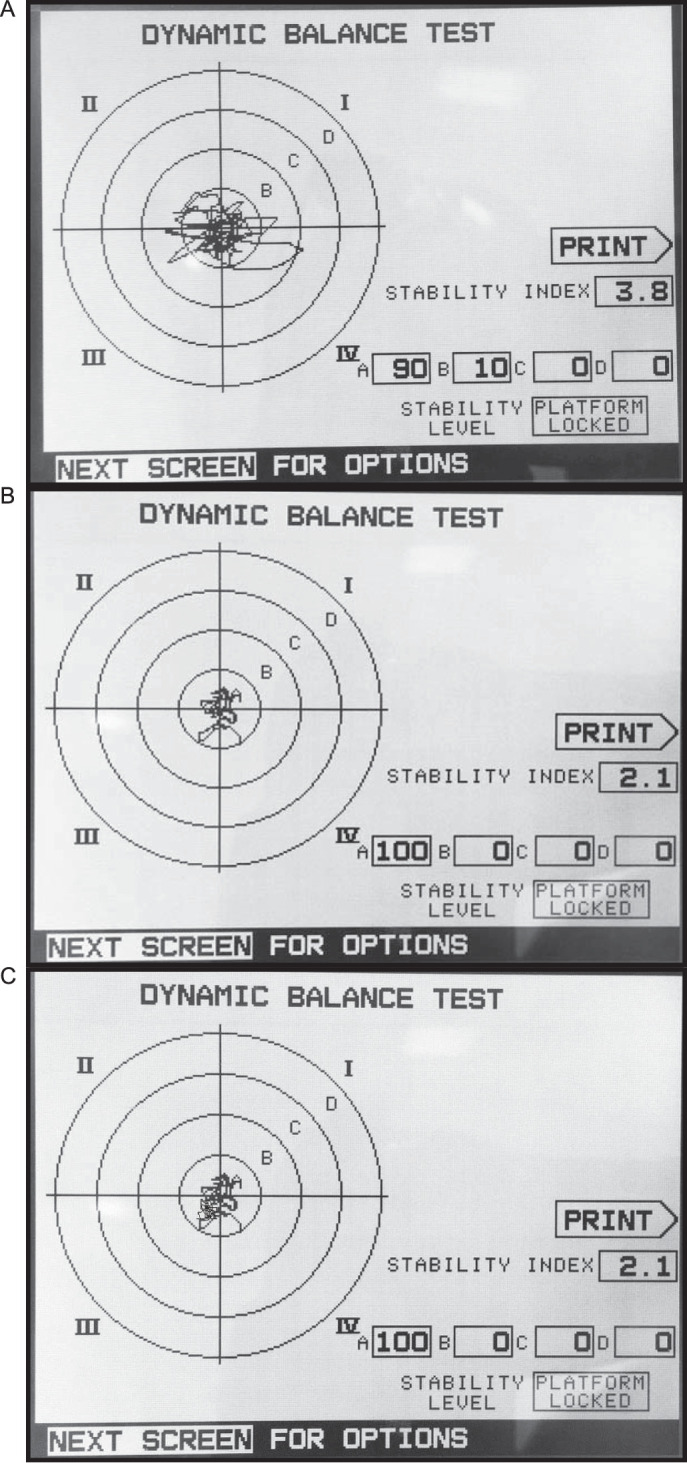
Biodex (Biodex Medical Systems, Inc) balance outcomes at A, baseline, B, 10 weeks, and C, 1 year. The distance from the center of the body (COB) was also calculated using the standardized zones A through D defined in the system, where each zone represents an increment of 5° in tilt of the platform, from 5° in zone A to a maximum 20° in zone D. The percentage of the COB trace in each zone at each time is shown: A, 90% for zone A, 10% for zone B, and 0% for zones C and D; B and C, 100% for zone A and 0% for zones B through D.
Sympathetic Skin Response
Participants were instructed to avoid using medications and cosmetics (on the hands) and engaging in physical activity on the day of the study. They were also instructed to avoid smoking, eating, and drinking coffee during the 2 hours before the recordings. Before the measurements, participants spent 20 minutes in a room with a controlled temperature of 22°C to 24°C.
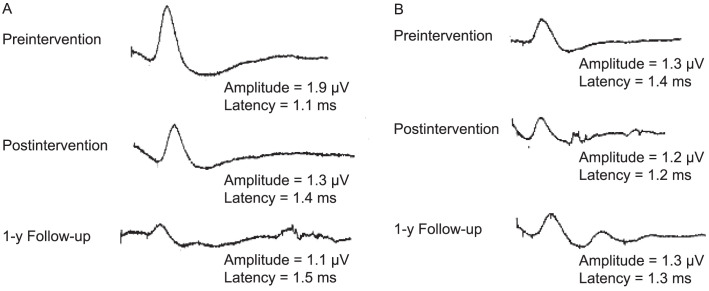
The electromyography equipment (model Neuro-MEP-Micro; Neurosoft Company) was used to determine the sympathetic skin response (SSR). With skin at a constant temperature of 32°C, surface electrodes were placed on the palmar side of the hand, and reference electrodes were placed on the dorsum. Measurements were obtained bilaterally using a stimulus to the contralateral wrist. To prevent participant habituation, a nonuniform interval of >1 minute with an intensity of 20 to 30 mA was used. If or when habituation did occur, the stimulation procedure was ceased for 3 to 5 minutes. Using a 10-second time frame, skin potentials were recorded, and the mean values for both the latency and peak-to-peak amplitude were calculated using a sweep speed set at 500 ms per division. An absent SSR was identified as no response after 10 consecutive stimuli24 (Figure 4).
Figure 4.
Skin sympathetic responses at each time point for A, the intervention group and B, the control group. Mean peak-to-peak amplitude (amplitude) and mean latency (latency) are provided.
All outcome assessments were carried out by 2 assessors blinded to group allocation. The ARA from C2 to C7, AHT, NDI, pain intensity, left and right head-repositioning accuracy, OSI, and SSR amplitude and latency were performed by a physiotherapist with 18 years of experience in these measurement techniques (A.A.). The SPNT was conducted by an ophthalmologist (not an author) with 5 years of experience (Remon Wasim).
Sample-Size Calculation
Study sample-size estimates of means and SDs were obtained from a pilot investigation in which 10 participants underwent a similar protocol. The mean difference and SD of the ARA from C2 to C7 were estimated to be 13° ± 15° from this pilot study. Accordingly, given a significance level of 5% and statistical power of 80%, at least 50 participants for each treatment arm were needed. To compensate for potential participant withdrawal, we increased the sample size by 10%.
Data Analysis
The statistical procedure depended on the principle of intention to treat for between-groups comparisons. To manage any missing data, multiple imputations were used. Parametric methods for significance testing were determined using the Levene test for equality of variances and Kolmogorov-Smirnov test for expressing continuous data as means with SDs.
To follow up and compare the effects of the 2 alternative treatments over 1 year, we examined the results using 2-way analysis of variance with repeated measures. If interactions were present, we performed post hoc paired and independent t tests. The model worked as follows: group was included as a single independent factor; time, as an interaction factor; and group × time, as an interaction factor. The minimal clinically important difference of the NDI was set at 10 points, and effect sizes for all variables were measured using the Cohen d, where d ≈ 0.2 indicated a limited effect; d ≈ 0.5, a moderate clinical effect; and d ≈ 0.8, a large effect with very significant clinical relevance. The α was set at .05. We used SPSS (version 20.0; IBM Corp) to analyze the data, with normality and equal-variance assumptions ensured before the analysis.
RESULTS
We screened 190 volunteers and included data from 110. Five participants in the intervention group and 6 in the control group did not finish the study. The flow of participants during the study period is shown in Figure 5. The 2 groups were comparable in age, mass, sex, marital status, pain duration, and smoking status (Table 1).
Figure 5.
Participant flow chart.
Table 1.
Baseline Participant Characteristics
| Characteristic |
Intervention Groupa (n = 55) |
Control Groupb,c (n = 55) |
| Age, mean ± SD (range), y | 20 ± 3 (47–65) | 21 ± 4 (45–64) |
| Mass, mean ± SD, kg | 59 ± 9 | 60 ± 8 |
| Sex, No. (%) | ||
| Males | 30 (54.5) | 29 (52.7) |
| Females | 25 (45.5) | 26 (47.3) |
| Pain duration, No. (%), y | ||
| 1–5 | 20 (36.4) | 17 (30.9) |
| >5 | 35 (63.6) | 38 (69.1) |
| Sport,d No. (%) | ||
| Baseball | 11 (20.0) | 13 (23.6) |
| Football | 27 (49.1) | 24 (43.6) |
| Handball | 12 (21.8) | 13 (23.6) |
| Other | 5 (9.1) | 5 (9.1) |
The group received standard care plus the Denneroll device (Denneroll Spinal Orthotics).
The group received standard care only.
Percentages were rounded, so the sum may not equal 100%.
The athletic activity in which the participant was involved.
The general linear model using repeated measurements identified group × time effects favoring the intervention group for the following management outcomes: NDI, pain intensity, SPNT, postural stability measured as the OSI using the BBS software, right- and left-rotation head- repositioning accuracy, and SSR amplitude and latency. At the first follow-up evaluation (10 weeks postintervention), we calculated unpaired t test analyses and identified no differences between the intervention and control groups for most of the management outcomes: NDI (t108 = 4.114, P = .11), pain intensity (t108 = −1.609, P = .07), SPNT (t108 = 0, P = .48), OSI (t108 = −4.311, P = .12), right-rotation repositioning accuracy (t108 = 1.609, P = .31), left-rotation repositioning accuracy (t108 = −2.358, P = .07), and SSR latency (t108 = 4.114, P = .71). In contrast, differences favoring the intervention group were identified for ARA C2 to C7 cervical lordosis (t108 = 17.5, P < .001), AHT (t108 = 15.5, P < .001), and SSR amplitude (t108 = −4.114, P = .005). Tables 2 through 5 detail these findings.
Table 2.
Changes in Sagittal-Plane Alignment Management Outcomes in the Intervention and Control Groups Over Time
| Variable |
Outcome, Mean ± SDa |
Cohen d Effect Size r |
P Value |
|||||
| Baseline |
10 wk |
1 y |
10 wk versus Baseline |
1 y versus Baseline |
Group |
Time |
Group × Time |
|
| Absolute rotation angle,b ° | <.001d | <.001d | <.001d | |||||
| Intervention group | 5.3 ± 5.1 | 20.0 ± 2.9 | 19.4 ± 2.1 | −0.9 | −0.8 | |||
| Control group | 5.8 ± 4.9 | 6.9 ± 4.7 | 5.7 ± 4.9 | −0.1 | 0.01 | |||
| P value (95% CI) | .43 (−2.6, 1.1) | <.001d (11.5, 14.5) | <.001d (12.2, 15.1) | |||||
| Anterior head translation,c cm | <.001d | <.001d | <.001d | |||||
| Intervention group | 3.6 ± 0.6 | 1.1 ± 0.5 | 1.3 ± 0.6 | 0.9 | 0.8 | |||
| Control group | 3.0 ± 0.5 | 2.9 ± 0.7 | 2.9 ± 0.8 | 0.1 | 0.1 | |||
| P value (95% CI) | .53 (−0.2, 0.3) | <.001c (−2.2, −1.6) | <.001c (−1.9, −1.5) | |||||
Except where indicated otherwise.
Cervical lordosis measurement from the C2 to C7 posterior body lines.
Horizontal offset of C2 relative to C7.
Indicates difference (P < .05).
Table 5.
Changes in Neurophysiological Outcomes in the Intervention and Control Groups Over Time
| Sympathetic Skin Resistance |
Outcome, Mean ± SDa |
Cohen d Effect Size r |
P Value |
|||||
| Baseline |
10 wk |
1 y |
10 wk versus Baseline |
1 y versus Baseline |
Group |
Time |
Group × Time |
|
| Amplitude, mV | <.001b | <.001b | <.001b | |||||
| Intervention group | 1.9 ± 0.2 | 1.4 ± 0.3 | 1.2 ± 0.2 | 0.7 | 0.9 | |||
| Control group | 1.8 ± 0.3 | 1.6 ± 0.2 | 1.7 ± 0.6 | 0.3 | 0.1 | |||
| P value (95% CI) | .43 (−0.06, 0.15) | .005b (−0.28, −0.05) | <.001b (−0.74, −0.52) | |||||
| Latency, ms | <.001b | <.001b | <.001b | |||||
| Intervention group | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | −0.5 | −0.7 | |||
| Control group | 1.2 ± 0.1 | 1.3 ± 0.9 | 1.1 ± 0.1 | −0.07 | 0.4 | |||
| P value (95% CI) | .42 (−0.06, 0.02) | .71 (−0.03, 0.04) | <.001b (0.13, 0.21) | |||||
Except where indicated otherwise.
Indicates difference (P < .05).
Table 3.
Changes in Pain and Disability Outcomes in Intervention and Control Groups Versus Time
| Variable |
Outcome, Mean ± SDa |
Cohen d Effect Size r |
P Value |
|||||
| Baseline |
10 wk |
1 y |
10 wk versus Baseline |
1 y versus Baseline |
Group |
Time |
Group × Time |
|
| Neck disability index | <.001c | <.001c | <.001c | |||||
| Intervention group | 33.7 ± 3.2 | 20.6 ± 1.9 | 10.9 ± 4.2 | 0.9 | 0.9 | |||
| Control group | 32.2 ± 3.5 | 21.0 ± 1.6 | 26.1 ± 3.6 | 0.8 | 0.6 | |||
| P value (95% CI) | .02c (0.28, 2.83) | .11 (−1.15, 0.22) | <.001c (−16.62, −13.82) | |||||
| Pain intensityb | <.001c | <.001c | <.001c | |||||
| Intervention group | 5.5 ± 1.2 | 1.9 ± 0.7 | 1.3 ± 0.5 | 0.8 | 0.9 | |||
| Control group | 5.3 ± 0.9 | 2.1 ± 0.6 | 4.2 ± 0.7 | 0.9 | 0.5 | |||
| P value (95% CI) | .24 (−0.16, 0.63) | .07 (−0.46, 0.18) | <.001c (−4.08, −3.61) | |||||
Table 4.
Changes in Postural-Control Outcomes in the Intervention and Control Groups Over Time
| Variable |
Outcome, Mean ± SDa |
Cohen d Effect Size r |
P Value |
|||||
| Baseline |
10 wk |
1 y |
10 wk versus Baseline |
1 y versus Baseline |
Group |
Time |
Group × Time |
|
| Smooth-pursuit neck torsion, ° | <.001c | <.001c | <.001c | |||||
| Intervention group | 0.3 ± 0.07 | 0.2 ± 0.07 | 0.1 ± 0.07 | 0.6 | 0.8 | |||
| Control group | 0.4 ± 0.04 | 0.2 ± 0.06 | 0.3 ± 0.05 | 0.8 | 0.5 | |||
| P value (95% CI) | .51 (−0.03, 0.02) | .48 (−0.04, 0.02) | <.001c (−0.22, −0.17) | |||||
| Biodexb balance test, ° | <.001c | <.001c | <.001c | |||||
| Intervention group | 0.7 ± 0.07 | 0.4 ± 0.1 | 0.5 ± 0.06 | 0.9 | 0.85 | |||
| Control group | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.07 | 0.3 | −0.2 | |||
| P value (95% CI) | .007 (0.05, 0.12) | .12 (−0.09, 0.11) | <.001c (−0.16, −0.11) | |||||
| Head-repositioning error in rotation, right side, ° | <.001c | <.001c | <.001c | |||||
| Intervention group | 3.4 ± 0.7 | 2.6 ± 0.6 | 1.8 ± 0.8 | 0.5 | 0.7 | |||
| Control group | 3.2 ± 0.9 | 2.4 ± 0.7 | 3.4 ± 1.1 | 0.4 | −0.1 | |||
| P value (95% CI) | .06 (0.03, 0.66) | .31 (−0.15, 0.38) | <.001c (−1.9, −1.24) | |||||
| Head-repositioning error in rotation, left side, ° | <.001c | <.001c | <.001c | |||||
| Intervention group | 3.7 ± 1.1 | 2.5 ± 0.8 | 1.5 ± 0.5 | 0.5 | 0.8 | |||
| Control group | 3.8 ± 0.9 | 2.8 ± 0.5 | 3.7 ± 0.7 | 0.5 | 0.1 | |||
| P value (95% CI) | .32 (−0.58, 0.21) | .07 (−0.53, 0.02) | <.001c (−2.33, −1.71) | |||||
Except where indicated otherwise.
Biodex Medical Systems, Inc.
Indicates difference (P < .05).
At the 1-year follow-up, differences were present for all management variables. These findings indicated larger improvements in the intervention group for all variables. Tables 2 through 5 document these results.
DISCUSSION
The differences between our intervention and control groups for the sensorimotor-control measures and SSR indicated that restoration of sagittal-plane cervical spine alignment did, in fact, alter pain, disability, autonomic nervous system function, and sensorimotor control. Therefore, our primary hypothesis was confirmed. To our knowledge, we are the first to provide objective evidence that the sagittal-plane cervical spine alignment curve influenced these specific management outcomes in patients with chronic neck pain.
Cervical Lordosis Improvements
The improvements in the forward head posture and cervical lordotic curve in our intervention group are consistent with the findings of other investigators25–27 who identified the effectiveness of cervical Denneroll traction and 3-point bending traction in reducing this abnormal posture. It is probable that cervical-extension traction devices, such as the Denneroll, cause longitudinal tension on the anterior longitudinal ligament and a generalized unloading of the intervertebral disc due to a shift in the axis of cervical-extension rotation posteriorly toward the facets because they are nearer the apex of the traction-load application. The application of sustained extension traction loading likely causes viscoelastic deformation of the cervical spine soft tissue, resulting in a more normal load sharing.25–27 The outcome of such sustained application of extension traction was apparent in the increased cervical curve and reduction in AHT present only in the intervention group. Our rehabilitation protocol of 3 sessions per week over 10 weeks was consistent with previous reports25–27 and is generally considered the standard for achieving adequate improvement in patients with sagittal-plane curvature.
Pain Intensity and Disability
Our multimodal program, alone or in conjunction with Denneroll traction, was roughly equally successful in improving participants' pain and disability levels after 30 treatment sessions over 10 weeks. In contrast, our 1-year follow-up evaluation demonstrated regressions in the pain and functional index measurements for the control group.
The transient relief from our multimodal program alone in the control participants was in general agreement with other investigations,25–27 indicating that this may be a trend for people with chronic cervical spine disorders. For instance, Masaracchio et al28 reported on the short-term effects of spinal manipulation and exercises, and Langevin et al29 provided evidence to support only the short-term effects of manual therapy treatment in individuals with chronic cervical dysfunction.
Overall, our findings revealed improvements that were stable and different in the pain and disability measures of the intervention group. This longer-lasting improvement in the group receiving Denneroll seems attributable to the restoration of normal cervical alignment and is consistent with previous assessments of individuals with chronic cervical spine disorders.25–27
Sensorimotor Control
A detailed interplay exists between proprioception and postural control. The novel result of our investigation was that correction of sagittal-plane cervical spine alignment was essential for sensorimotor control, likely attributable to restoring a more efficient afferentation process. This concept is supported by researchers30 who observed that more efficient afferentation processes were important substrates for sensorimotor control.
The assumption that restoring normal posture and cervical spine alignment is important for a better afferentation process has some preliminary evidence. For instance, it has been proposed that as the position of the head migrates forward, increased strain is placed on the muscles and ligaments of the head, neck, and shoulders. This abnormal head posture results in altered joint positions and dysfunction that may lead to abnormal afferent information (dysafferentation).31
Additionally, altered cervical lordosis and forward head translation cause both a reduced range of movement and an altered segmental cervical spine kinematic pattern.32 Therefore, altered sagittal-plane cervical spine alignment could result in abnormal sensorimotor integration via changes in afferent input as a direct consequence of altered cervical spine kinematics and soft tissue strains.6,31,32
Previous evidence reflected that sagittal-plane cervical spine alignment affected normalization of the afferentation processes. Thus, it is surprising that, at the first follow-up (10 weeks postintervention), the intervention group that received Denneroll cervical traction did not show improvements in many variables compared with the control group across all of the cervical motor-control outcomes. However, we observed improvement at long-term follow-up (1 year).
We see no obvious unifying explanation for our findings other than to speculate that sustained postural imbalances can result in a state of continuous asymmetric and increased mechanical loading. Empirically, when the asymmetry is reversed, and the unbalanced loading is corrected by restoration of normal posture, load sharing and kinematics become more normal, and improvements in measurement outcomes are revealed over time. Although direct empirical support for this explanation is lacking, the belated improvement in participants after spinal or postural correction (or both) is supported by the results of Diab and Moustafa,27 who noted continued improvement in pain scores at 6 months posttreatment compared with those documented after a 10-week treatment regimen. Similarly, continued improvements in pain intensity, disability, and function at long-term follow-up in treatment groups after measurable spinal and postural correction have been described in several randomized trials.25,26
Autonomic Function: SSR
The differences between our intervention and control groups at the 2 postintervention intervals exemplify the important role of sagittal-plane cervical spine alignment in maintaining the normal function of the autonomic nervous system. As we observed, pain cannot be considered the only determinant for sympathetic system dysfunction in patients with CNSNP. The increase in latency and decrease in amplitude in our intervention group after spinal correction make sense and agree with the findings of Welch and Boone, who concluded that “cervical adjustments could manifest a shift to parasympathetic dominance.”33(p92) Considering the close proximity of the cervical spine to the brainstem region, restoration of a more normal anterior head posture and cervical lordosis may improve parasympathetic activation and decrease overall sympathetic tone. A reduction in adverse mechanical tension acting on the brainstem, cranial nerves 5 through 12, and specifically cranial nerve 10, may be one of the underlying mechanisms that explains the improved SSR in our intervention group because improved head posture and increased lordosis would result in reduced longitudinal stress and strain on the neural elements.34
Limitations and Summary
Our investigation had a few limitations that should lead to future work. First, we did not blind participants or treatment providers. Second, the participants were a convenience sample from our outpatient facility and may not be entirely representative of all people with CNSNP disorders. Third, the condition-specific outcome measures that we used (NDI, numeric pain rating, cervical range of motion, balance testing, SSR) to verify if improved sagittal-plane cervical spine alignment variables were related to improved sensorimotor and neurophysiological responses may not be the ideal assessments for CNSNP outcomes. Fourth, we identified findings indicative of sagittal-plane cervical spine alignment influence on sensorimotor control and SSRs. Therefore, variables other than cervical posture that were not identified or accounted for herein were likely related to motor control and autonomic function.
CONCLUSIONS
Improvement in cervical lordosis and reduction in AHT distance using the Denneroll extension traction orthotic device had positive effects on pain, disability, autonomic nervous system dysfunction, and sensorimotor control. These results have important implications for the assessment and rehabilitation of patients with CNSNP.
ACKNOWLEDGMENTS
We thank Chiropractice BioPhysics NonProfit (Eagle, ID) for supplying the Denneroll devices used in this study.
REFERENCES
- 1. .Haldeman S, Carroll L, Cassidy JD. Findings from the Bone and Joint Decade 2000 to 2010 Task Force on Neck Pain and Its Associated Disorders. J Occup Environ Med. 2010;52(4):424–427. doi: 10.1097/JOM.0b013e3181d44f3b. [DOI] [PubMed] [Google Scholar]
- 2. .Durall CJ. Therapeutic exercise for athletes with nonspecific neck pain: a current concepts review. Sports Health. 2012;4(4):293–301. doi: 10.1177/1941738112446138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. .Côté P, Cassidy JD, Yong-Hing K, Sibley J, Loewy J. Apophysial joint degeneration, disc degeneration, and sagittal curve of the cervical spine: can they be measured reliably on radiographs? Spine (Phila Pa 1976) 1997;22(8):859–864. doi: 10.1097/00007632-199704150-00007. [DOI] [PubMed] [Google Scholar]
- 4. .Haas M, Taylor JA, Gillette RG. The routine use of radiographic spinal displacement analysis: a dissent. J Manipulative Physiol Ther. 1999;22(4):254–259. doi: 10.1016/s0161-4754(99)70053-9. [DOI] [PubMed] [Google Scholar]
- 5. .Harrison DD, Harrison DE, Janik TJ, et al. Modeling of the sagittal cervical spine as a method to discriminate hypolordosis: results of elliptical and circular modeling in 72 asymptomatic subjects, 52 acute neck pain subjects, and 70 chronic neck pain subjects. Spine (Phila Pa 1976) 2004;29(22):2485–2492. doi: 10.1097/01.brs.0000144449.90741.7c. [DOI] [PubMed] [Google Scholar]
- 6. .Artz NJ, Adams MA, Dolan P. Sensorimotor function of the cervical spine in healthy volunteers. Clin Biomech (Bristol Avon) 2015;30(3):260–268. doi: 10.1016/j.clinbiomech.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. .Oe S, Yamato Y, Hasegawa T, et al. Spinal sagittal alignment, hospital anxiety and depression scale scores, and patient-reported outcome among people with sporting activity. Asian Spine J. 2020;14(3):341–349. doi: 10.31616/asj.2019.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. .Riemann BL, Lephart SM. The sensorimotor system, part II: the role of proprioception in motor control and functional joint stability. J Athl Train. 2002;37(1):80–84. [PMC free article] [PubMed] [Google Scholar]
- 9. .Hellström F, Roatta S, Thunberg J, Passatore M, Djupsjöbacka M. Responses of muscle spindles in feline dorsal neck muscles to electrical stimulation of the cervical sympathetic nerve. Exp Brain Res. 2005;165(3):328–342. doi: 10.1007/s00221-005-2309-7. [DOI] [PubMed] [Google Scholar]
- 10. .Elefteriou F. Impact of the autonomic nervous system on the skeleton. Physiol Rev. 2018;98(3):1083–1112. doi: 10.1152/physrev.00014.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. .Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–S226. [PubMed] [Google Scholar]
- 12. .Yong MS, Lee HY, Lee MY. Correlation between head posture and proprioceptive function in the cervical region. J Phys Ther Sci. 2016;28(3):857–860. doi: 10.1589/jpts.28.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. .Lee MY, Lee HY, Yong MS. Characteristics of cervical position sense in subjects with forward head posture. J Phys Ther Sci. 2014;26(11):1741–1743. doi: 10.1589/jpts.26.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. .French SD, Cameron M, Walker BF, Reggars JW, Esterman AJ. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD004750.pub2. CD004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. .Vasudevan JM, Plastaras C, Becker S. Cervical radiculopathy. In: Wyss JF, Patel A, editors. Therapeutic Programs for Musculoskeletal Disorders. New York, NY: Demos Medical;; 2013. pp. 279–288. [Google Scholar]
- 16. .Mintken PE, Derosa C, Little T, Smith B. American Academy of Orthopaedic Manual Physical Therapists. A model for standardizing manipulation terminology in physical therapy practice. J Man Manip Ther. 2008;16(1):50–56. doi: 10.1179/106698108790818567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. .Harrison DE, Harrison DD, Cailliet R, Troyanovich SJ, Janik TJ, Holland B. Cobb method or Harrison posterior tangent method: which to choose for lateral cervical radiographic analysis. Spine (Phila Pa 1976) 2000;25(16):2072–2078. doi: 10.1097/00007632-200008150-00011. [DOI] [PubMed] [Google Scholar]
- 18. .MacDermid JC, Walton DM, Avery S, et al. Measurement properties of the neck disability index: a systematic review. J Orthop Sports Phys Ther. 2009;39(5):400–417. doi: 10.2519/jospt.2009.2930. [DOI] [PubMed] [Google Scholar]
- 19. .Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10(4):390–392. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 20. .Loudon JK, Ruhl M, Field E. Ability to reproduce head position after whiplash injury. Spine (Phila Pa 1976) 1997;22(8):865–868. doi: 10.1097/00007632-199704150-00008. [DOI] [PubMed] [Google Scholar]
- 21. .Wibault J, Vaillant J, Vuillerme N, Dedering Å, Peolsson A. Using the cervical range of motion (CROM) device to assess head repositioning accuracy in individuals with cervical radiculopathy in comparison to neck-healthy individuals. Man Ther. 2013;18(5):403–409. doi: 10.1016/j.math.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 22. .Tjell C, Tenenbaum A, Sandström S. Smooth pursuit neck torsion test—a specific test for whiplash associated disorders? J Whiplash Relat Disord. 2002;1(2):9–24. doi: 10.3109/J180v01n02_02. [DOI] [Google Scholar]
- 23. .Schmitz R, Arnold B. Intertester and intratester reliability of a dynamic balance protocol using the Biodex Stability System. J Sport Rehabil. 1998;7(2):95–101. doi: 10.1123/jsr.7.2.95. [DOI] [Google Scholar]
- 24. .Kucera P, Goldenberg A, Kurca E. Sympathetic skin response: review of the method and its clinical use. Bratisl Lek Listy. 2004;105(3):108–116. [PubMed] [Google Scholar]
- 25. .Moustafa IM, Diab AA, Harrison DE. The effect of normalizing the sagittal cervical configuration on dizziness, neck pain, and cervicocephalic kinesthetic sensibility: a 1-year randomized controlled study. Eur J Phys Rehabil Med. 2017;53(1):57–71. doi: 10.23736/S1973-9087.16.04179-4. [DOI] [PubMed] [Google Scholar]
- 26. .Moustafa IM, Diab AA, Taha S, Harrison DE. Addition of a sagittal cervical posture corrective orthotic device to a multimodal rehabilitation program improves short- and long-term outcomes in patients with discogenic cervical radiculopathy. Arch Phys Med Rehabil. 2016;97(12):2034–2044. doi: 10.1016/j.apmr.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 27. .Diab AA, Moustafa IM. The efficacy of forward head correction on nerve root function and pain in cervical spondylotic radiculopathy: a randomized trial. Clin Rehabil. 2012;26(4):351–361. doi: 10.1177/0269215511419536. [DOI] [PubMed] [Google Scholar]
- 28. .Masaracchio M, Cleland JA, Hellman M, Hagins M. Short-term combined effects of thoracic spine thrust manipulation and cervical spine nonthrust manipulation in individuals with mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2013;43(3):118–127. doi: 10.2519/jospt.2013.4221. [DOI] [PubMed] [Google Scholar]
- 29. .Langevin P, Desmeules F, Lamothe M, Robitaille S, Roy JS. Comparison of 2 manual therapy and exercise protocols for cervical radiculopathy: a randomized clinical trial evaluating short-term effects. J Orthop Sports Phys Ther. 2015;45(1):4–17. doi: 10.2519/jospt.2015.5211. [DOI] [PubMed] [Google Scholar]
- 30. .Prochazka A, Gritsenko V, Yakovenko S. Sensory control of locomotion: reflexes versus higher-level control. Adv Exp Med Biol. 2002;508:357–367. doi: 10.1007/978-1-4615-0713-0_41. [DOI] [PubMed] [Google Scholar]
- 31. .Moustafa IM, Youssef A, Ahbouch A, Tamim M, Harrison DE. Is forward head posture relevant to autonomic nervous system function and cervical sensorimotor control? Cross sectional study. Gait Posture. 2020;77:29–35. doi: 10.1016/j.gaitpost.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 32. .Miyazaki M, Hymanson HJ, Morishita Y, et al. Kinematic analysis of the relationship between sagittal alignment and disc degeneration in the cervical spine. Spine (Phila Pa 1976) 2008;33(23):E870–E876. doi: 10.1097/BRS.0b013e3181839733. [DOI] [PubMed] [Google Scholar]
- 33. .Welch A, Boone R. Sympathetic and parasympathetic responses to specific diversified adjustments to chiropractic vertebral subluxations of the cervical and thoracic spine. J Chiropr Med. 2008;7(3):86–93. doi: 10.1016/j.jcm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. .Harrison DE, Cailliet R, Harrison DD, Troyanovich SJ, Harrison SO. A review of biomechanics of the central nervous system–part II: spinal cord strains from postural loads. J Manipulative Physiol Ther. 1999;22(5):322–332. doi: 10.1016/s0161-4754(99)70065-5. [DOI] [PubMed] [Google Scholar]