Abstract
Purpose:
The aim of this study was to compare coronary and carotid artery imaging and determine which one shows the strongest association with atherosclerotic cardiovascular disease (ASCVD) score.
Patients and Methods:
Two separate series patients who underwent either coronary computed tomography angiography (CTA) or carotid CTA were included. We recorded the ASCVD scores and assessed the CTA imaging. Two thirds were used to build predictive models, and the remaining one third generated predicted ASCVD scores. The Bland-Altman analysis analyzed the concordance.
Results:
A total of 110 patients were included in each group. There was no significant difference between clinical characteristics. Three imaging variables were included in the carotid model. Two coronary models (presence of calcium or Agatston score) were created. The bias between true and predicted ASCVD scores was 0.37 ± 5.72% on the carotid model, and 2.07 ± 7.18% and 2.47 ± 7.82% on coronary artery models, respectively.
Conclusions:
Both carotid and coronary artery imaging features can predict ASCVD score. The carotid artery was more associated to the ASCVD score than the coronary artery.
Keywords: 10-year ASCVD score, computed tomography angiography, head and neck CTA, calcium scoring, coronary CTA
In November 2013, the American College of Cardiology and the American Heart Association (ACC/AHA) released new recommendations for statin initiation based on patient risk profiles. These recommendations are based on the calculation of a 10-year risk of atherosclerotic cardiovascular disease (ASCVD) score using the pooled cohort equations.1–3 This equation relies on clinical risk factors including age, sex, race, cholesterol, treatment of hypertension, systolic blood pressure (SBP), diabetes, and smoking history.
Although the equation to calculate the ASCVD score does not include arterial imaging, many arterial imaging features are independently associated with the risk of ASCVD. More specifically, coronary artery computed tomography (CT) imaging including coronary artery calcium (CAC) score and carotid CT angiography (CTA)4 allows us to detect and characterize subclinical and clinical atherosclerotic changes.5,6 The CAC score is associated with the risk of clinical cardiovascular disease (CVD) events,7,8 as is carotid artery imaging.9,10 There has been much more research, attention, and clinical interest about coronary artery imaging than carotid artery imaging as it relates to the 10-year ASCVD risk.
Indeed, when the pooled cohorts equations were originally released, the ACC/AHA guidelines from 2013 suggested using adjunctive diagnostic tools such as the CAC score—or the ankle-brachial index (ABI) or high sensitivity C-reactive protein (hs-CRP)—to supplement the ASCVD score in selected individuals without endorsing carotid artery imaging.1 More recently, the 2018 ACC/AHA guidelines highlight that the pooled cohorts equations may substantially underestimate risk in certain populations (eg, patients with lower socioeconomic status or inflammatory diseases) and substantially overestimate risk in certain populations (eg, patients with higher socioeconomic status); accordingly, the 2018 guidelines emphasize the use of CAC scores in patients with borderline (5% to <7.5%) and intermediate (7.5% to <20%) risk.11,12 However, despite current emphasis on CAC scores in the latest ACC/AHA guidelines, the optimal adjunctive test to refine risk stratification is uncertain, with recent US Preventative Services Task Force (USPSTF) guidelines highlighting that there are “no trials evaluating the additional benefit of adding the ABI, hs-CRP or CAC score to traditional risk models.”13,14 Accordingly, the USPSTF found insufficient evidence to recommend use of the CAC score (or ABI or hs-CRP) and emphasized the need for further research.
In this study, we compared the association between coronary and carotid artery imaging features with 10-year ASCVD risk. Such comparisons are crucial because the optimal imaging modality (or modalities) to individualize risk assessment is still uncertain.
MATERIALS AND METHODS
Study Population
We retrospectively identified 2 separate consecutive series of patients: 1 series of patients who underwent a coronary CTA at our institution from January 2014 to July 2016 and 1 series of patients who underwent a head and neck CTA at our institution during the same period. We used the medical records to gather the clinical information required to calculate the 10-year ASCVD score using the pooled cohort equations from the 2013 ACC/AHA guidelines.2 We excluded patients for whom the 10-year ASCVD score could not be calculated [age outside the 40–79 range, total cholesterol outside the 130–320 mg/dL range, high-density lipoprotein (HDL)-cholesterol outside the 20–100 mg/dL range, SBP outside the 90–200 mm Hg range, and no smoking status record]. As the ASCVD score is specifically applicable for a primary prevention population, we also excluded patients who had a coronary or carotid artery stent placed, received a coronary artery bypass graft or carotid endarterectomy, or had a history of prior cardiac ischemia or stroke. We excluded patients for whom more than 6 months elapsed between the clinical visit/blood draw to measure the clinical variables and the imaging study of interest. Then, we applied a case-control design approach and randomly selected patients in each series so that we would get a set of coronary artery imaging patients and a set of carotid artery imaging patients with matching demographics, vascular risk factors, and 10-year ASCVD risk score. Our study was approved by our institutional review board.
Coronary and Carotid Artery Imaging Protocol
The CT studies of the coronary and carotid arteries were performed on 16-slice and 64-slice CT scanners (GE or Siemens Healthcare) using spiral mode, 0.6- to 0.8-second gantry rotation and the following acquisition parameters: 100 to 120 kVp/240 mA.
For coronary artery imaging, patients whose heart rates (HRs) were too fast received oral β-blocker or sublingual nitroglycerine for a targeted HR around 60 ± 5 beats per minute (bpm). The calcium scoring imaging consisted of a noncontrast high-resolution CT, scan range: carina through the apex of the heart; slice thickness: 2.5 to 3 mm; imaging phase: for single source, end diastole for HR less than 63 bpm, end diastole and end systole for HR greater than 64 bpm; for dual source, end diastole for HR less than 79 bpm, end systole for HR greater than 80 bpm. For the coronary CTA, the contrast agent used was Isovue 370 (lopamidol; Bracco Diagnostics Inc, Monroe Township, New Jersey), 1.1 mL/kg, max no more than 200 mL, intravenous injection at a rate of 5 mL/s. The imaging acquisition protocol was as follows: scan range, 2 cm above the left anterior descending artery through the apex of the heart; slice thickness, 0.625 to 0.75 mm; imaging phase: for single source, end diastole for HR less than 65 bpm, end diastole and end systole for HR greater than 66 bpm; for dual source, end diastole for HR less than 65 bpm, end diastole and end systole for HR 66 to 75 bpm, end systole for HR greater than 86 bpm. Effective dose associated with the coronary artery CT protocol was about 4 to 6 mSv.
The imaging acquisition protocol for the carotid artery CTA was as follows: collimation, 16 or 64 × 0.5 to 1.25 mm, pitch around 1:1; slice thickness, 1 to 1.25 mm; reconstruction interval, 0.75 to 1 mm. A caudocranial scanning direction was selected, covering the mid-chest to the vertex of the brain. A bolus of 70 to 80 mL of Isovue 300 or 370 (lopamidol; Bracco Diagnostics Inc) was injected into an antecubital vein with a power injector at a rate of 4 to 5 mL/s. Optimal timing of the CTA acquisition was achieved using a test bolus technique. Effective dose associated with the carotid artery CTA protocol was 5 to 7 mSv.
Coronary and Carotid Artery Imaging Review
The left coronary artery and right coronary artery were assessed separately. The left coronary artery was divided into 3 segments: left main coronary artery, left anterior descending branch, and left circumflex branch. We formatted images perpendicular to the lumen of each of these segments and visually assessed each segment for maximal degree of stenosis, maximal atherosclerotic/calcium plaque thickness, and the presence or absence of calcified plaque. The degree of stenosis was calculated as the diameter of the smallest lumen divided by the diameter of the following normal lumen.
The Agatston coronary artery calcium score was computed for each of the coronary arteries based on the size and density of the regions identified to contain calcium15: 0 means no identifiable atherosclerotic plaque (a negative examination); 1 to 10 means minimal plaque burden; 11 to 100 means mild plaque burden; 101 to 400 means moderate plaque burden; and greater than 400 means extensive plaque burden.
Both left and right carotid arteries were divided into 3 segments: common carotid arteries, cervical internal carotid arteries, and intracranial internal carotid arteries. Vertebral arteries were divided into 3 segments: origins and proximal segments, cervical segments, and intracranial segments. We formatted images perpendicular to the lumen of each of these segments and manually assessed each segment for maximal degree of stenosis (using the North American Symptomatic Carotid Endarterectomy Trial criteria16), maximal atherosclerotic plaque thickness, presence of soft plaque (<60 HU), presence of calcified plaque (>130 HU), and presence of plaque ulceration.17
The neuroradiologist who reviewed the images was blinded to the ASCVD scores.
Statistical Analysis
We compared the demographics, vascular risk factors, and 10-year ASCVD risk scores for the 2 sets of patients (the group with carotid artery imaging and the group with coronary artery imaging). We used Mann-Whitney U tests to compare distributions of continuous variables (age, total cholesterol, HDL-cholesterol, SBP, and 10-year ASCVD risk score), chi-square tests for binary variables (sex, arterial hypertension, diabetes history, and smoking history), and Fisher exact tests for race/ethnicity.
Of all 110 cases in each group, about two thirds of the cases (75/110) were randomly selected using “sample” functions in RStudio Desktop (Mac OS Version 1.1.456, Boston, Massachusetts).18 These cases were used to build linear models incorporating carotid or coronary artery imaging. The last third of the cases in each group (35/110) were introduced into the linear models to evaluate the efficacy of the linear models.
For the group that received coronary artery imaging, we developed 2 predictive models using Im steps with backward in R18 for multiple linear regression analysis to predict the 10-year ASCVD risk score. The imaging features included in the first linear model (model #1) were degree of stenosis, maximal plaque thickness, and presence or absence of calcium. The second linear model (model #2) included degree of stenosis, maximal plaque thickness, and total Agatston score.
For the group that received carotid artery imaging, we used the same process in R. The imaging features included in the linear model were degree of stenosis, maximal plaque thickness, presence or absence of plaque ulceration, presence or absence of soft plaque, presence or absence of superficial calcium, and presence or absence of deep calcium.
After generating the linear models, the final 35 cases were introduced into the linear models to generate a predicted ASCVD risk score. Mann-Whitney U tests were used to analyze the differences between true ASCVD risk score and the predicted ASCVD risk score. The Bland-Altman analysis was used to analyze the biases between true ASCVD risk score and the predicted ASCVD risk score, respectively. The biases between the observed and predicted ASCVD risk scores for the carotid model and the coronary models were also compared using Mann-Whitney U tests.
All statistical processes, including the Mann-Whitney U tests, χ2, and Bland-Altman analysis, were conducted with RStudio Desktop (Mac OS Version 1.1.456, Boston). Adjusted R2 and Akaike information criterion were used to validate the efficacities of the linear models. For all analyses, statistical significance was set at α = .05.
RESULTS
Study Patients
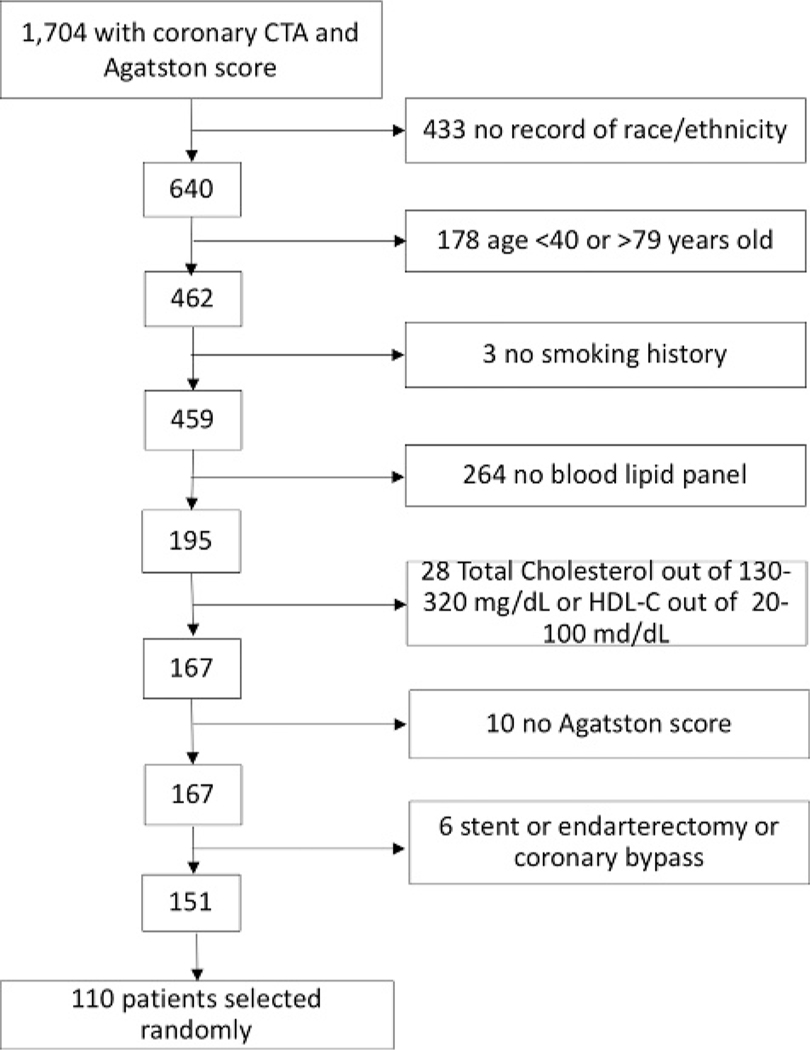
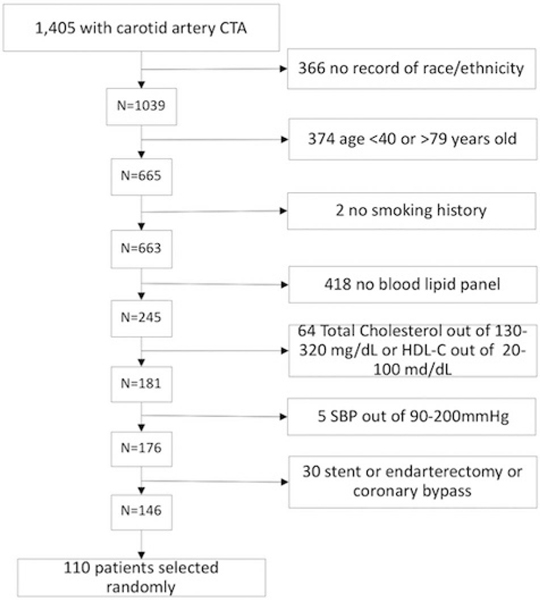
For this study, we used a set of 110 patients with carotid artery imaging and a separate set of 110 patients with coronary artery imaging. The selection of these patients is illustrated in Figures 1 and 2.
FIGURE 1.
Flowchart detailing the selection of the patients who underwent coronary CTA for this study.
FIGURE 2.
Flowchart detailing the selection of the patients who underwent carotid CTA for this study.
Clinical Characteristics
There were no significant differences between the 2 patient groups’ demographics, clinical characteristics, and 10-year ASCVD risk score (Table 1).
TABLE 1.
Comparison of the Demographic, Clinical Characteristics, and 10-Year ASCVD Risk Score Between the Patients With Coronary Artery Imaging and the Patients With Carotid Artery Imaging
| Patients With Coronary Artery Imaging (n = 110) | Patients With Carotid Artery Imaging (n = 110) | P | |
|---|---|---|---|
| Age, median (IQR) | 60.00 (53.25–67.00) | 59.50 (52.00–66.00) | 0.645 |
| Sex (female) | 52 (47.3%) | 58 (52.7%) | 0.252 |
| Race | 0.910 | ||
| Caucasian | 71 (64.5%) | 66 (60.0%) | |
| Asian | 18 (16.4%) | 20 (18.2%) | |
| Hispanic/Latino | 16 (14.5%) | 19 (17.3%) | |
| African American | 5 (4.5%) | 5 (4.5%) | |
| Arterial hypertension treatment | 62 (56.4%) | 56 (50.9%) | 0.252 |
| Diabetes | 22 (20.0%) | 18 (16.4%) | 0.303 |
| Smokers | 11 (10.0%) | 12 (10.9%) | 0.760 |
| Total cholesterol, median (IQR) | 176.5 (153.7–206.7) | 179.0 (163.2–210.0) | 0.412 |
| HDL-C, median (IQR) | 51.5 (43.0–62.7) | 51.0 (40.0–61.0) | 0.389 |
| Systolic blood pressure, median (IQR) | 127.0 (117.25–142.0) | 126.5 (118.0–140.0) | 0.865 |
| 10-Year ASCVD score, median (IQR) | 7.1 (3.7–14.6) | 7.85 (3.4–15.7) | 0.667 |
IQR indicates interquartile range.
Imaging Characteristics
Among the 110 patients with coronary artery CT, 40 (36.3%) had stenosis, and 53 (52.7%) had calcified plaque; the average plaque thickness was 2.31 mm (Table 2).
TABLE 2.
Coronary Artery Imaging Characteristics
| Overall | |
|---|---|
| Total no. patients | 110 |
| Patients with stenosis, n (%) | 40 (36.3) |
| Patients with >50% stenosis, n (%) | 14 (12.7) |
| Patients with >70% stenosis, n (%) | 3 (2.7) |
| Maximum stenosis, % mean (IQR) | 40.5 (24.1–54.7) |
| No. arterial segments showing stenosis | |
| 0 | 70 |
| 1 | 23 |
| 2 | 4 |
| 3 | 11 |
| 4 | 2 |
| Maximum plaque thickness, mean (SD), mm | 2.31 (0.83) |
| Patients with calcified plaque, n (%) | 53 (52.7) |
| No. arterial segments showing calcified plaque | |
| 0 | 47 (42.7) |
| 1 | 24 (21.8) |
| 2 | 12 (10.9) |
| 3 | 15 (13.6) |
| 4 | 12 (10.9) |
| Agatston score, mean (IQR) | 85.36 (13.02–378.76) |
IQR indicates interquartile range.
Among the 110 patients with carotid artery CTA, 42 (38.2%) had stenosis, 40 (36.4%) had soft plaque, 57 (51.8%) had superficial calcium, 16 (14.5%) had deep calcium, and 8 (7.3) had plaque ulceration. The average plaque thickness was 3.36 mm (Table 3).
TABLE 3.
Carotid Artery Imaging Characteristics
| Overall | |
|---|---|
| Total no. patients | 110 |
| Patients with stenosis, n (%) | 42 (38.2) |
| Patients with >50% stenosis, n (%) | 25 (22.7) |
| Patients with >70% stenosis, n (%) | 17 (15.5) |
| Maximum stenosis, % mean (IQR) | 53.9 (30.4–82.7) |
| Maximum plaque thickness in mm, mean (SD) | 3.36 (1.55) |
| Patients with soft plaque, n (%) | 40 (36.4) |
| No. arterial segments showing soft plaque | |
| 0 | 70 (63.6) |
| 1 | 10 (9.1) |
| 2 | 14 (12.7) |
| 3 | 9 (8.2) |
| 4 | 7 (6.4) |
| Patients with superficial calcium, n (%) | 57 (51.8) |
| No. arterial segments showing superficial calcium | |
| 0 | 53 (48.2) |
| 1 | 17 (15.5) |
| 2 | 11 (10.0) |
| 3 | 8 (7.3) |
| 4 | 10 (9.1) |
| 5 | 4 (3.6) |
| 6 | 4 (3.6) |
| 7 | 1 (0.9) |
| 8 | 2 (1.8) |
| Patients with deep calcium, n (%) | 16 (14.5) |
| No. arterial segments showing deep calcium | |
| 0 | 94 (85.5) |
| 1 | 10 (9.1) |
| 2 | 3 (3.0) |
| 3 | 2 (1.8) |
| 4 | 0 (0) |
| 5 | 1 (0.9) |
| 6 | 0 (0) |
| Patients with plaque ulceration, n (%) | 8 (7.3) |
| No. arterial segments showing plaque ulceration | |
| 0 | 102 (92.7) |
| 1 | 7 (6.4) |
| 2 | 0 (0) |
| 3 | 0 (0) |
| 4 | 1 (0.9) |
IQR indicates interquartile range.
Predictive Models
We included 75 patients out of each group to build the models using the coronary and carotid artery imaging features to predict the 10-year ASCVD risk score (Table 4). For the carotid artery imaging, the model that best predicted the 10-year ASCVD risk score included 3 features: maximal wall thickness, presence or absence of soft plaque, and presence or absence of ulceration. For the coronary artery imaging, the best model included only the presence of calcification. The total number of arterial segments with calcification was calculated for each patient. We tested a second model including only the Agatston score instead of the presence of calcification, but the latter performed less well in predicting the 10-year ASCVD risk score compared with the one using the presence of calcification.
TABLE 4.
Models Built From the Coronary and Carotid Imaging Features to Predict the 10-Year ASCVD Risk Score (n = 75)
| 95% CI |
||||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower | Upper | SE | t | P | Adjust R2 | AIC | |
| Coronary artery imaging features | ||||||||
| Model #1 | ||||||||
| Intercept | 8.35 | 5.01 | 11.70 | 1.68 | 4.98 | <0.001 | 0.10 | 568.3 |
| Calcium | 2.54 | 0.84 | 4.23 | 0.85 | 2.98 | 0.004 | ||
| Model #2 | ||||||||
| Intercept | 10.27 | 7.56 | 12.99 | 1.36 | 7.54 | <0.001 | 0.071 | 570.4 |
| Agatston score | 0.01 | 0.002 | 0.016 | 0.003 | 2.58 | 0.011 | ||
| Carotid artery imaging features | ||||||||
| Intercept | ||||||||
| 6.73 | 4.47 | 8.98 | 1.13 | 5.95 | <0.00 | 0.30 | 517.3 | |
| Maximal plaque thickness | ||||||||
| 1.41 | 0.75 | 2.05 | 0.32 | 4.36 | <0.001 | |||
| Soft plaque | ||||||||
| 1.61 | 0.12 | 3.34 | 0.87 | 1.85 | 0.068 | |||
| Ulceration | ||||||||
| 11.29 | 5.29 | 17.29 | 3.01 | 3.76 | <0.001 | |||
AIC indicates Akaike information Criterion; OR, odds ratio.
Testing, Validation, and Comparison of the Models
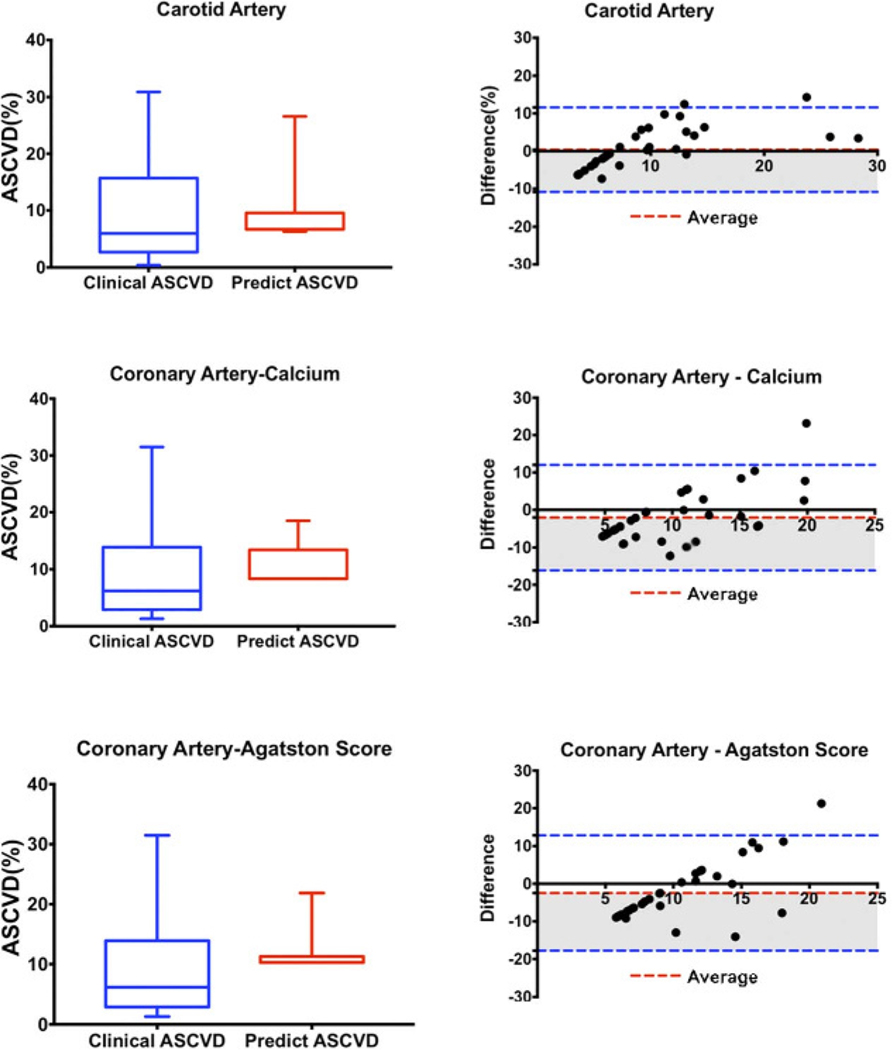
We used the remaining 35 patients out of each group into the linear models, respectively, to generate their predicted ASCVD risk scores, and then compared the predicted ASCVD risk scores with the true ASCVD risk scores (Table 5, Fig. 3). The true ASCVD risk scores of the coronary and carotid artery imaging patients were 9.04 ± 7.60% and 9.48 ± 8.50%, respectively. The true ASCVD risk score was best predicted by the model built from the carotid artery imaging features. The bias between the observed and predicted ASCVD risk scores was 0.37 ± 5.72 [95% confidence interval (CI), −10.86 to 11.60]. The model using the presence or absence of coronary calcium performed less well, with a bias of −2.07 ± 7.18 (95% CI, −16.15 to 12.02), followed by the model using the Agatston score, with a bias of −2.47 ± 7.82 (95% CI, −17.79 to 12.86). The biases of the model built from the carotid artery imaging features were significantly less than the biases of the models from coronary artery imaging features (P = 0.027 using the model using coronary calcium, and 0.012 for the model using the Agatston score). There was no significant difference between the biases from the 2 coronary models (P = 0.580).
TABLE 5.
Comparison of the True and Predicted 10-Year ASCVD Risk Scores by the Coronary and Carotid Artery Imaging Models (n = 35)
| True ASCVD Risk Score, % | Predicted ASCVD Risk Score, % | Bias, % | 95% Limits of Agreement |
|||
|---|---|---|---|---|---|---|
| Lower, % | Upper, % | |||||
| Coronary artery imaging patients | 9.04 ± 7.60 | Model #1: calcium | 11.11 ± 3.56 | −2.07 ± 7.18 | −16.15 | 12.02 |
| Model #2: Agatston score | 11.51 ± 2.88 | 2.47 ± 7.82 | −17.79 | 12.86 | ||
| Carotid artery imaging patients | 9.48 ± 8.50 | 9.11 ± 4.72 | 0.37 ± 5.72 | −10.86 | 11.60 | |
FIGURE 3.
The differences between true ASCVD and predicted ASCVDs. Figure 3 can be viewed online in color at www.jcat.org.
DISCUSSION
Our results show both coronary and carotid artery imaging findings are associated with the 10-year ASCVD risk score. The Bland-Altman analyses suggest that our models do not correlate well across the entire range of clinical ASCVD scores. Our models tended to overestimate the ASCVD risk in the low-risk population and to underestimate the ASCVD risk for the subjects with high ASCVD scores. It seems that the imaging features that we identified in our models (maximal plaque thickness, soft plaque, and ulceration for carotid arteries; calcium for coronary arteries) are in agreement with other studies testing the association between imaging biomarkers and vascular events.7,19,20
The existing literature has focused mostly on coronary artery imaging, especially the calcium scoring, as it relates to the 10-year ASCVD risk. The calcium score is correlated with traditional coronary risk factors.21 A number of research studies have demonstrated that adding the calcium score to the ASCVD risk score improves the stratification between subjects at high versus low risk for coronary events. Patients with a low calcium score have a low 10-year CVD event rate.22 Other studies have shown that a coronary CTA adds incremental discriminatory power over the calcium scoring for discrimination of individuals at risk of myocardial infarct and cardiovascular death.23 In our study, instead of focusing on how arterial imaging could add discrimination to the ASCVD risk score, we tried to determine how arterial imaging correlates with the ASCVD risk score and which imaging (coronary or carotid) better correlates with the ASCVD risk score.
Carotid artery imaging features can also predict ASCVD events. A meta-analysis of 11 population-based studies (N = 54,336) concluded that imaging the carotid plaque and its characteristics had significantly higher diagnostic accuracy for future incident myocardial infarction compared with carotid intima media thickness (IMT).24 In another study where 874 patients without ASCVD history were followed for a total 1402 days, 119 ASCVD events were recorded and could be predicted by the presence of carotid artery plaques.25
Previous studies have compared coronary and carotid artery imaging to predict ASCVD events. One study showed coronary calcium scoring to be a superior predictor of incident CVD compared with carotid IMT (area under the curve 0.81 vs 0.78, respectively).26 For coronary heart disease, the hazard ratios per 1 SD increment increased 2.5-fold (95% CI, 2.1–3.1) for calcium scoring and 1.2-fold (95% CI, 1.0–1.4) for carotid IMT.26 Another study showed that calcium scoring improved prediction, discrimination, and reclassification of cardiovascular events better than carotid ultrasound measures, and the prediction and discrimination were similar for stroke/transient ischemic attack.27 A last study found that carotid ultrasound seems to be a better predictive method for assessing ASCVD events compared with calcium scoring.20 Actually, another study found that carotid plaque burden and maximum carotid plaque thickness can predict ASCVD, but IMT did not.28 Our study focused on 10-year ASCVD risk as calculated by the pooled equations instead of ASCVD events themselves and found that carotid artery CT imaging findings paralleled ASCVD risk more closely than the coronary artery imaging findings.29
We acknowledge several limitations to our study. Our study was retrospective in design. We were not able to identify 1 single population of patients who underwent both coronary and carotid artery imaging. Instead, we used 2 separate groups of patients selected from consecutive series of patients with well-matched demographics, similar clinical risk factors, and similar 10-year ASCVD risks scores. In addition, we only assessed CT and not other imaging modalities that allow imaging of both carotid and coronary arteries, including magnetic resonance imaging and conventional angiography. We did not explore whether the addition of imaging would improve the prediction value of pooled equations, because this would have required a prospective study to assess the incidence of vascular events. Rather, we assessed in a retrospective study whether there was an association between the results of the pooled equations and the results of imaging as an initial step to determine if more advanced imaging than the calcium scoring should even be considered as a potential addition to the pooled equations. We clarified this in our discussion.
In conclusion, both carotid and coronary artery imaging findings are associated with the 10-year ASCVD risk score. The association with the carotid artery imaging findings is stronger. This finding may have particular relevance given existing controversies about how carotid imaging should be addressed in the ACC/AHA guidelines.30 In addressing research gaps recently highlighted by the USPSTF,13,14 future clinical trials focused on individualizing ASCVD risk assessment should consider studying carotid imaging in addition to calcium scoring and coronary artery imaging.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 2.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63: 2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63: 2889–2934. [DOI] [PubMed] [Google Scholar]
- 4.Wintermark M, Jawadi SS, Rapp JH, et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol. 2008;29: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaid M, Fujiyoshi A, Kadota A, et al. Coronary artery calcium and carotid artery intima media thickness and plaque: clinical use in need of clarification. J Atheroscler Thromb. 2017;24: 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Divakaran S, Cheezum MK, Hulten EA, et al. Use of cardiac CT and calcium scoring for detecting coronary plaque: implications on prognosis and patient management. Br J Radiol. 2015; 88:20140594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur Heart J. 2018;39:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66: 1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell CC, Korcarz CE, Tattersall MC, et al. Carotid artery ultrasound texture, cardiovascular risk factors, and subclinical arterial disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Br J Radiol. 2018; 91:20170637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klarin D, Cambria RP, Ergul EA, et al. Risk factor profile and anatomic features of previously asymptomatic patients presenting with carotid-related stroke. J Vasc Surg. 2018;68:1390–1395. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2019; 73:3153–3167. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73: e285–e350. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins JT, Lloyd-Jones DM. USPSTF recommendations for assessment of cardiovascular risk with nontraditional risk factors: finding the right tests for the right patients. JAMA. 2018;320:242–244. [DOI] [PubMed] [Google Scholar]
- 14.Lin JS, Evans CV, Johnson E, et al. Nontraditional risk factors in cardiovascular disease risk assessment: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018; 320:281–297. [DOI] [PubMed] [Google Scholar]
- 15.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 16.North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke. 1991;22:711–720. [DOI] [PubMed] [Google Scholar]
- 17.Miskolczi L, Guterman LR, Flaherty JD, et al. Depiction of carotid plaque ulceration and other plaque-related disorders by intravascular sonography: a flow chamber study. AJNR Am J Neuroradiol. 1996;17:1881–1890. [PMC free article] [PubMed] [Google Scholar]
- 18.Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 19.McEvoy JW, Martin SS, Dardari ZA, et al. Coronary artery calcium to guide a personalized risk-based approach to initiation and intensification of antihypertensive therapy. Circulation. 2017;135:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim GH, Youn HJ, Choi YS, et al. Carotid artery evaluation and coronary calcium score: which is better for the diagnosis and prevention of atherosclerotic cardiovascular disease? Int J Clin Exp Med. 2015;8: 18591–18600. [PMC free article] [PubMed] [Google Scholar]
- 21.Sung J, Lim SJ, Choe Y, et al. Comparison of the coronary calcium score with the estimated coronary risk. Coron Artery Dis. 2008;19:475–479. [DOI] [PubMed] [Google Scholar]
- 22.Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. JACC Cardiovasc Imaging. 2017;10:143–153. [DOI] [PubMed] [Google Scholar]
- 23.Al-Mallah MH, Qureshi W, Lin FY, et al. Does coronary CT angiography improve risk stratification over coronary calcium scoring in symptomatic patients with suspected coronary artery disease? Results from the prospective multicenter international CONFIRM registry. Eur Heart J Cardiovasc Imaging. 2014;15:267–274. [DOI] [PubMed] [Google Scholar]
- 24.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. [DOI] [PubMed] [Google Scholar]
- 25.Kim G, Youn HJ, Choi YS, et al. Is carotid artery evaluation necessary for primary prevention in asymptomatic high-risk patients without atherosclerotic cardiovascular disease? Clin Interv Aging. 2015;10: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gepner AD, Young R, Delaney JA, et al. Comparison of carotid plaque score and coronary artery calcium score for predicting cardiovascular disease events: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6:e005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sillesen H, Sartori S, Sandholt B, et al. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur Heart J Cardiovasc Imaging. 2018;19:1042–1050. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell C, Korcarz CE, Gepner AD, et al. Ultrasound carotid plaque features, cardiovascular disease risk factors and events: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2018;276:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein JH, Tattersall MC. Carotid intima-media thickness and cardiovascular disease risk prediction. J Am Coll Cardiol. 2014;63:2301–2302. [DOI] [PubMed] [Google Scholar]