Abstract
Gold nanoparticles are a kind of nanomaterials that have received great interest in field of biomedicine due to their electrical, mechanical, thermal, chemical and optical properties. With these great potentials came the consequence of their interaction with biological tissues and molecules which presents the possibility of toxicity. This paper aims to consolidate and bring forward the studies performed that evaluate the toxicological aspect of AuNPs which were categorized into in vivo and in vitro studies. Both indicate to some extent oxidative damage to tissues and cell lines used in vivo and in vitro respectively with the liver, spleen and kidney most affected. The outcome of these review showed small controversy but however, the primary toxicity and its extent is collectively determined by the characteristics, preparations and physicochemical properties of the NPs. Some studies have shown that AuNPs are not toxic, though many other studies contradict this statement. In order to have a holistic inference, more studies are required that will focus on characterization of NPs and changes of physical properties before and after treatment with biological media. So also, they should incorporate controlled experiment which includes supernatant control Since most studies dwell on citrate or CTAB-capped AuNPs, there is the need to evaluate the toxicity and pharmacokinetics of functionalized AuNPs with their surface composition which in turn affects their toxicity. Functionalizing the NPs surface with more peculiar ligands would however help regulate and detoxify the uptake of these NPs.
Keywords: Cell lines, Gold nanoparticles, In vivo, In vitro, Toxicity
Highlights
-
•
In vitro and in vivo indicate oxidative damage to tissues and cell lines.
-
•
The liver, spleen and kidney tissues and cells were most affected.
-
•
Some controversy exist, however, the primary toxicity is collectively determined by the properties of the NPs.
-
•
Characterization of NPs and changes in physicochemistry before and after treatment with bio-media should be evaluated.
-
•
Functionalizing the NPs surface with more peculiar ligands would help regulate and detoxify the uptake of these NPs.
1. Introduction
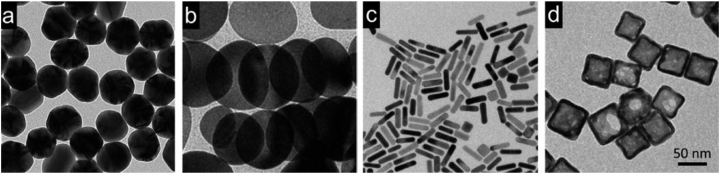
Nanomaterials such as engineered gold nanoparticles (AuNPs), possess promising applications due to their mechanical, electrical, thermal, chemical and optical properties [1,2] which vary from bulk gold. Bulk gold is regarded a biological inert, a feature that is seen only at macroscopic level (Fig. 1), however at the size of nanoscale, gold (Au) possess various attributes because of its surface plasmon resonance excitation features [3,4]; [5].
Fig. 1.
Transmission electron microscope (TEM) images for various kinds of Au nanostructures (a) nanospheres, (b) nanodisks, (c) nanorods, and (d) cubic nanocages (Black et al., 2014).
Gold nanoparticles (AuNPs) have received high interest in the field of biomedicine and its applications [[6], [7], [8], [9]].
The speedy development of AuNPs technology presents a great potential for later applications because of their large volume specific surface areas and a very diverse surface properties than bulk gold. These features have transform AuNPs to a status of great relevance in the emergence of excellent nanoelectronic chips, and potentials for a wide variety of environmental and biomedical applications.
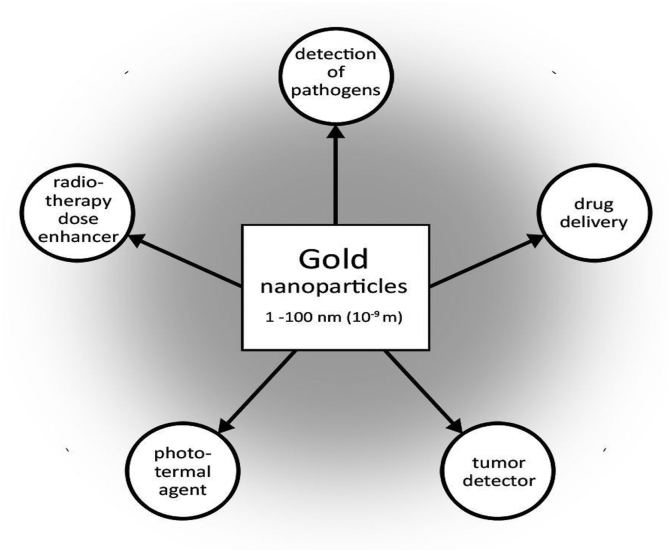
They possess great potentials as presented in Fig. 2 in diagnostic tracers, drug delivery, photothermal and radio-therapy, gene therapy, biosensing, as well as counter cancer agents, enzymes immobilization and cell imaging [2,7,10]; [[11], [12], [13]].
Fig. 2.
Gold nanoparticles (AuNPs) potentials in biomedical fields [14].
In addition, they have been used in food and drink industries [9].
Furthermore, AuNPs approval by the Food and Drug Administration (FDA) for different biomedical applications has resulted in increased areas of applications such as cancer therapy, as drug carriers and other biological applications [[15], [16], [17], [18]].
AuNPs are recently employed to improve solar cells and as liquid crystals that are used as flash memory devices [19,20]. Other applications include control of pollution, purification of water and hydrogen, and as catalysts in oxidation of carbon monoxide [[21], [22], [23], [24]].
The great daily utilization of AuNPs could increase the probability of the human body exposure to these NPs. Many studies on the toxicological impact of AuNPs from academic and commercial sources have been revealed [[25], [26], [27]].
Most recently, many dietary supplements in form of drinks that contain AuNPs (SD-AuNPs) have been produced. The companies that produced them claimed that such addition of AuNPs has health benefits, which include enhancement of mood, acting as an anti-inflammatory agent and improvements of cognitive function [28].
However, even though they have great potential uses in aspect of biomedical, environmental, and industrial applications, there is very few information about the short and long term health effects in organisms and the environment at large.
Reports have indicated that synthesized NPs can circulate for a longer period in the body without rejection by the immune system of the body. These features are in fact sharpened by their surface charges, small size and such are of great concern especially during syntheses and applications. AuNPs of health risks are generated in various shapes, sizes and surface charges. However, the huge influence that comes from the physiochemical properties presents fresh issues regarding future health status. Recently, there is little information on health effects of AuNPs and no regulatory safety and guidelines associating their properties to toxicities [29].
Although many studies described low toxicity of AuNPs compared to other NPs which are metal-based, but their wide usage makes it imperative to determine their effects in consumer products.
1.1. Routes of exposures to gold nanoparticles (AuNPs)
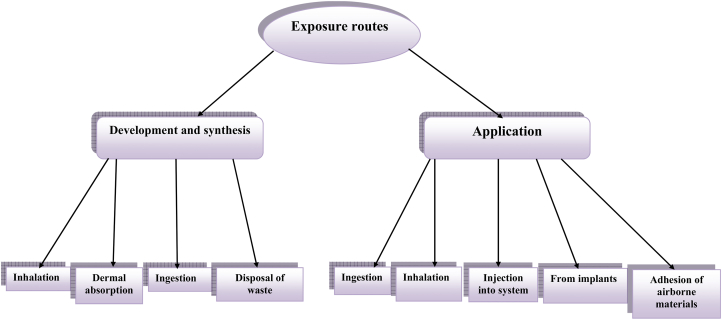
Exposure to AuNPs could happen either during development and synthesis or applications through direct ingestion or injection into the system, and disposal of waste [30,31]. Other exposure routes include dermal absorption, inhalation, from implants, adherence of airborne and surface materials which becomes difficult to detect [15,31,32] (Fig. 4). It can also happen as a result of AuNP-composite attached to consumer products in homes, markets and other outdoor places [33].
Fig. 4.
Exposure routes and patterns to gold nanoparticles (AuNPs). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
These exposures could result to their persistence and accumulation in the environment thereby possessing the ability to get into the food chain which finally affects abiotic and biotic aspects [35]. This enhances the uptake of AuNPs by other environmental organisms such as algae and fish which can further be consumed by animals and humans.
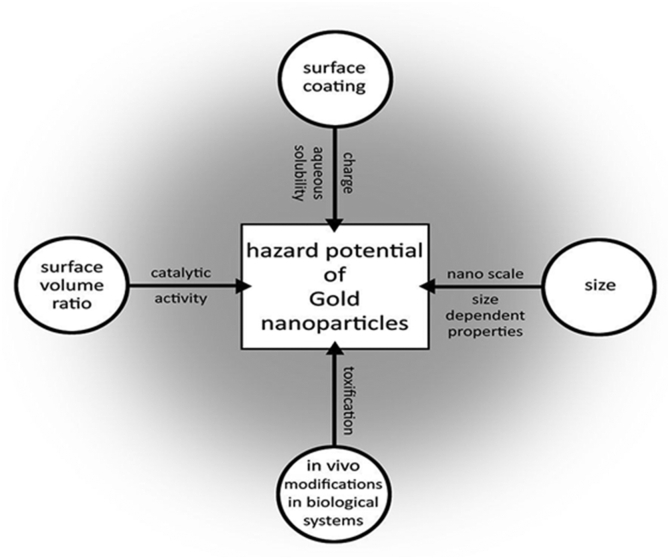
The shape and size of AuNPs have affected their toxicity in some situations. These peculiar features have resulted in various chemical attributes transforming into different cellular studies in which some were observed to be toxic while others as non toxic.
The toxicity of some was found to be size dependent because of the presence of their surface coated with ligands [1,36,37]. Meanwhile, in others it was due to their large surface area to volume ratio which gives avenue for increased surface particle activity [17]. Therefore, this has contributed to provision of a very easy flexible route for reactivity and penetration in biological system than bulk gold particle as presented in Fig. 3.
Fig. 3.
Hazardous nature and influence of Gold nanoparticles (AuNPs) [14].
The interactions of AuNPs with biological systems are mostly associated with their physiochemical properties which internalize them within the cells, a condition that is not likely with larger particles. Such is among the reasons why AuNPs could be more toxic than larger particles when compared on a mass dosage. This emphasizes the relevance of their size in the large surface area to volume ratio that foresees their applications in biomedical systems [38,39].
1.2. Synthesis and preparation of gold nanoparticles (AuNPs)
Methods of preparation of AuNPs include chemical, physical, and biological [40].
1.2.1. Chemical reduction
Such method comprises of two stages which include the reduction by agents (sugars, hydrogen, sulphites, hydroxylamine, hydrogen peroxide, formaldehyde, acetylene, oxalic acids and citric acid) and stabilization by agents such as phosphorous ligands, sulphur ligands, polymers and surfactants to prevent its aggregation [[41], [42], [43]].
-
•
Green methods: The paths for synthesis through green chemistry are eco-friendly and toxic-free. A Formation of AuNPs with sizes of 25 + 7 nm via a simple green biosynthesis was revealed using natural biomaterial egg shell membrane (ESM) [[44], [45], [46]]. So also, ref. [47] synthesized AuNPs using Pterocarpus marsupium.
-
•
Citrate reduction (Turkevich method): This is the most prominent method for preparation of AuNPs that was developed in 1951. However, some adjustments were made by the Frens' group where it involved reduction of citrate by Au particles to generate NPs of 20 nm [[48], [49], [50], [51]].
-
•
Brust-Schiffrin method: The process is a reaction pathway that comprises forming a thermally and air-stable AuNPs that have a reduced dispersion values [[52], [53], [54], [55], [56]].
-
•
Polymer-based synthesis: The shape and size of AuNPs are recognized as importat elements in formation of colloidal Au. The association of polymers with AuNPs affect the diversity of size and stability of the particles.
1.2.2. Physical method
-
•
Electrochemical method: This involves the electrochemical preparation of AuNPs by a simple two-electrode cell, with reduction and oxidation of the cathode and anode respectively. It was first observed by Reetz, in 1994. The process has been known to be superior to other procedures as a result of its low cost, modest equipment, lower processing temperature and simple process managing [[57], [58], [59]].
-
•
Seeding growth method: AuNPs with diameters of 5–40 nm having narrow size dispersity are prepared. The size of particles can be well arranged by the change ratio of seed to metal salt. Trisodium citrate is used as the source of OH ions in the seeding step and sodium borohydrate (NaBH4) as reducing agent [[60], [61], [62]]. In addition, AuNPs were formed on high elevated plateau in the selective solution under the UV solar radiation [63].
-
•
Ultraviolet-induced photochemical synthesis: Many researchers have reported that photoreduction allows the preparation of single crystallite AuNPs with controlled sizes which is achieved efficiently by photochemistry [64,65,66,67].
-
•
Ultrasound aided synthesis: This involves the ultrasound reduction of Au precursor in the presence of 2-propanol. The stabilizing agents used include citrate, disulphide and several dendrimers [68,69,70]; [71].
-
•
Laser ablation synthesis: Better results with accuracy and reproducibility have been obtained through laser ablation method, by virtue of size and shape. In view of that, the procedure of pulsed laser which requires constant evaporation and condensation occurrences for Au showcase a complex physical approach that can be efficiently applied to generate AuNPs with tuneable qualities. The process involves the HAuCl4 reduction by laser beam (wavelength: 532 nm), thereby yielding AuNPs of 5 nm and lower in size [[72], [73], [74], [75]].
1.2.3. Biological method
AuNPs are synthesized by enzymes, microorganism and plants.
-
•
Microbial synthesis of AuNPs: AuNPs can be generated by using microorganisms due to the need for environmental-friendly and low cost procedures. The method involves the using of enzymes such as reductases, ligninases and laccases in the nucleation and development of AuNPs. Some reported that Klebsiella pneumonia mediated the synthesis of AuNPs. Other Au mediated synthesis is the isolation of soil fungus Penicillium crustosum for preparation of AuNPs by extracellular proteins [76,77].
-
•
Plant mediated synthesis of AuNPs: Recently, adoption of plants in preparation of AuNPs received so much attention due to their low cost, non-toxic property, environmental-friendly and availability. Some studies have reported the synthesis of AuNPs using Aloe vera, Cinnamomum camphora, Azadirachta indica and Coriandrum sativum [[78], [79], [80]]. In addition, some phytochemicals of Salacia chinensis have been usd in the synthesis of AuNPs (Jadhav et al., 2018)
1.3. In vivo toxicity studies of AuNPs
While there exist much literature about in vitro studies, there are few toxicological reports of AuNPs in animal models, which are the preferred system for toxicologic evaluation of a novel agent and should be useful to clarify the toxicity of AuNPs [12].
It has become paramount to evaluate the in vivo profile of nanomaterials prior to any potential therapeutic applications [81]. Some studies conducted to evaluate the in vivo toxicity have been presented in Table 1.
Table 1.
Some in vivo toxicity studies of gold nanoparticles (AuNPs).
| Organism | Particle | Effects | Ref. |
|---|---|---|---|
| Wistar rats | AuNPs | Traces of AuNPs in kidney, spleen, liver, intestine, urine and feces. Smaller NPs induced greater effects in DNA damage | [82] |
| Fetal organs | AuNPs | No indication of toxicity in fetus and placenta | [83] |
| Pregnant C57BL/6 mice | AuNPs | Non crossing of maternal-fetal barrier | [84] |
| Female and male mice | AuNPs | Liver and kidney damage whose effects were sex dependent | [85,86] |
| Zebrafish embryo | AuNPs (functionalized with TMATeAuNPs) | Delay in development of eyes and pigmentation | [87] |
| Rats | AuNPs | Changes in gene expression | [88,89] |
| Mice | AuNSs on GSNPs | Lungs, kidney hemorrhage, lymphocytic infiltration and inflammatory response | [[90], [91], [92]] |
| BALB/c mice | AuNPs | Apoptosis and inflammation of liver tissue | [93,94] |
| Mice | PEG-coated AuNPS | Liver damage | [95] |
| Male WU wistar rats | AuNPs | Large particles of spherical AuNPs were observed in blood, spleen and liver while smaller particles were seen in spleen, blood, thymus, lungs, liver, kidney, testis, heart, and brain | [16] |
| Female mice | AuNPs | Spherical AuNPs in live and macrophages | [96] |
| Mice (ddy) | AuNPs | AuNPs of all sizes were noticed in spleen, liver and lungs | [97] |
| Male wistar rats | AuNPs | AuNPs persist and accumulate in spleen and liver | [88,89] |
| Wistar rats | Citrate coated-AuNPs | Accumulate in neurons, liver, spleen, kidney and cross the blood brain barrier; no toxicity | [98] |
| Rats | PEG-coated AuNPs | Accumulation in spleen and liver | [99] |
| Mice | PEG-coated AuNPs | Apoptosis and acute inflammation | [100] |
| Rats | PEG-coated AuNPs | ROS-induced cytotoxicity that is size-dependent | [101] |
| Rats | AuNPs | Distribution of AuNPs were observed in testis liver and kidney. However, no effects on testis whereas mild changes were noticed in kidney and liver sections | [102] |
| Mice | GSH- and BSA-coated AuNCs | Affects kidnay function and produce toxicity | [103] |
| Broiler chicken | AuNPs | Caused recognizable oxidative damage to blood, histopathological changes, up-regulation of IL-6, expression of Nrf2 gene, fragmentation of DNA, significant decrease in antibody titer against avian influenza (AI) and newcastle disease (ND) | [104] |
| Mice | AuNPs | Damage to neuronal system | [85,86] |
| Male CD1 mice | Functionalized AuNPs | Accumulation at various parts of the brain | [105] |
| Drosophila melanogaster | Citrate capped-AuNPs | Caused transmissible mutagenic effects | [106] |
| Drosophila melanogaster | Citrate capped-AuNPs | Sharp decline in fertility and life span, presence of DNA fragments, and strong over-expression of stress proteins | [107] |
| Mice | Citrate capped-AuNPs | Greatest toxicity and affecting organ index | [12] |
| Mice | AuNPs capped with BSA and HSePEGeCOOH | Produced no effect on normal growth | [108] |
| Mice | AuNPs | Induced reduction in RBC, spleen index and body weight | [12] |
| Mice | Naked colloidal AuNPs | Caused loss of weight and appetite. However, smaller AuNPs did not produce any sickness | [109] |
| Female mice | AuNHsd | Complete survival was evident across all concentrations | [110] |
| D. magna | HAuCl4 | LC50 was reported as 2 mg/l after 48hrs | [[111], [112], [113]] |
| D. magna | HAuCl4 | LC50 was reported as 0.64 mg/l after 48hrs | [114] |
| M. macrocopa | HAuCl4 | LC50 was reported as 0.62 mg/l after 48hrs | [[111], [112], [113]] |
| T. arcticus | LC50 was 14.4 mg/l after 96hrs | [114] |
It is widely conceived that AuNPs have high-level accumulation in the liver and spleen leading to further damages on the organism [103]. Gold nanorods (AuNRs) were subcutaneously injected into mice. Most of it stayed within the injection site. However, the Au ions released into the system produced tissue oxidative damage in the site [149]. Studies have been performed by various researchers to assess the distribution and accumulation in the liver and other organs.
Lopez-Chaves et al. (2018) exposed HT-29 and HepG2 cells, and wistar rats to 10, 30 or 60 nm AuNPs to evaluate their location and distribution in subcells and tissues including other effects. They found traces of AuNPs in liver, intestine, urine, feces, kidney and spleen. Moreover, transmission electron microscopy revealed the particles in colon cells and liver samples. Their size has played a major role in determining the differences in biodistribution and route of excretion. The smallest NPs induced greater deleterious effects as supported by the DNA damage and their location inside the cell nucleus. This is possible because the ultra small AuNPs exhibits better circulation times and different biodistribution when compared to larger AuNPs as stated by Schmid et al., [150]. It has been illustrated that ultra small AuNPs could possess cytotoxic properties when the ligands for its stabilization permit direct access to the surface of Au either for catalytic activity of the uncovered surface or for direct association with biological molecules [150]. Thus, the physicochemical properties of nanomaterials, such as size, shape, and surface coating, also play an important role in this sense. Fraga et al. [151], reported that the surface coating of AuNPs had much influence on toxicity instead of having on biodistribution. To support this, another study revealed that there was a surface charge and size dependent distribution of NPs in rats after exposure to AuNPs at 5.3 μg/rat [152]. Similarly, AuNPs had size-dependent distribution in which the smallest showed highest widespread and crossing the blood brain barrier [16,97]. Despite that the blood brain barrier happens to prevent the central nervous system from AuNPs as stated by Sadauskas et al. [84], but the ultra sized particles were able to cross. Thus, further confirming the size-dependent influence of AuNPs.
It has been reported by Rattanapinyopituk et al. [83] that Au were absent in fetal organs but present in the placenta following exposure to 20 and 50 nm AuNPs with no indication of toxicity to the fetus or placenta. The study has not revealed increase of endocytic vesicle in syncytiotrophoblasts and fetal endothelial cells in the maternal-fetal barrier. Thus, proposing that clathrin and caveolin-mediated endocytosis played a major role in crossing of AuNPs in the placenta. A similar study involving pregnant C57BL/6 mice has indicated non transfer of AuNPs [84]. They found out that the AuNPs do not cross the placenta barrier which could be due to their inability to penetrate the cell membranes by non-endocytic processes.
Significant differences were observed in some liver enzymes after intraperitoneal injection of AuNPs to rats [153]. Meanwhile those AuNPs capped with trisodium citrate dihydrate had produced slight nephrotoxicity and hepatotoxicity [119]. AuNPs serving as biolabel, biosensor and drug carriers induced hepatotoxicity, cytotoxicity and toxicity to the spleen and lung [[154], [155], [156]]. Apoptosis and inflammation of liver tissue was observed in mice (BALB/c) after intravenous administration of AuNPs [93,94]. Specifically that liver and spleen help in detoxification make them becomes the first target organs.
There was a slight liver damage in mice after injection with PEG-coated AuNPs [95]. So also, only large particles of spherical AuNPs were observed in blood, spleen and liver while smaller particles of about 10 nm were seen in all spleen, blood, thymus, lungs, liver, kidney, testis, heart, and brain of in male WU wistar rats [16].
Spleen and liver were observed to be the major organs for such NPs accumulation via the reticuloendothelial system and could lead to their toxicity [109,157]. Sadauskas et al. [96], found spherical AuNPs in the liver and macrophages in female mice. In addition, AuNPs of all sizes were noticed in spleen, liver and lungs of mice (ddy) [97].
However, AuNPs did not yield signs of toxicity in hepatocytes following 24 h of exposure. 5 nm AuNPs stabilized with PVP were simply absorbed by endothelial cells of blood vessels, hepatocytes and kupffer cells either in vitro or in vivo. Sadauskas et al. [84], has earlier reported that AuNPs irrespective of their size are absorbed by Kupffer cells of the liver primarily and secondarily by macrophages at other sites.
The up and down genes regulated were expressed in male wistar rats exposed to AuNPs. They also persist and accumulate in the spleen and liver [88,89].
Oral, tail vein and intraperitoneal injection of citrate-coated AuNPs to mice revealed greatest toxicity and affects organ index [12]. Thus, functionalization and capping could present potentiality of toxicity in AuNPs.
Many AuNPs toxicological studies have used AuNPs with various capping, conjugated or stabilizing agents. To enhance their activity in various applications, AuNPs are stabilized, coated, conjugated or functionalized with various organic moieties to enhance their stability, which then forms a layer of protection on the particles surface [158]. Majority of these molecules exhibit lower cytotoxic property and great biodistribution [159].
Varying levels of toxicities have been revealed which involved the use of some stabilizing, capping, or conjugating agents for AuNPs which include sodium borohydride, hydrazinium hydroxide, citrate [160,161], polyelectrolyte poly (allylamine) hydrochloride [162], and CTAB [163].
High molecular weight PEG of 5000 Da induced more stability to the coated AuNPs than low molecular weight (2000 Da) PEG as reported by Zhang et al. [164]. HMW PEG-stabilized NPs were observed to be the less toxic [165]. Glutathione (GSH) was then adopted as an alternative to PEG in the construction of AuNPs for therapeutics purposes. GSH exhibits biocompatibility and low immunogenicity [166].
It was stated earlier that 1.1 nm GSH capped-AuNPs produced low toxicity than 1.4-nm triphenylphosphine monosulfonate–AuNPs which upregulate stress-related genes [167].
To this end, Vijayakumar et al. [160,161], conducted a study to compare the cytotoxic effects of 3 stabilizing agents (citrate, starch and gum Arabic [GA]) on the PC-3 and MCF-7 cell lines. They observed that citrate-AuNPs were more cytotoxic at higher concentrations when compared to starch- and GA-AuNPs which could possibly be due to the acidic nature of citrate.
Colloids citrate coated AuNPs crossed the blood brain barrier and accumulate in neurons, liver, spleen and kidney. However, there was no toxicity [98]. Though the blood-brain barrier seems to prevent the CNS from AuNPs, their crossing in this case could be attributed to the citrate coat.
Lipka et al. [99], also an accumulation of PEG Coated AuNPs in liver and spleen of rats intratracheally exposed. Furthermore, PEG-coated AuNPs have caused apoptosis and acute inflammation in mice liver [100].
So also, the PEG coated AuNPs produced a ROS-induced cytotoxicity that is size-dependent. Furthermore, 42.5 and 61.2 nm sized accumulates primarily in spleen and liver.
Thus, the authors revealed that the in vitro toxicity is size and dose dependent whereby the higher levels and smaller sized AuNPs lead to cytotoxicity. So also, smaller sizes produce more damage to cells by production of ROS. The biodistribution has been testified by the authors to be dependent on size and displayed some elements of accumulation and clearance [101].
The activity of AuNPs in vivo and in rat liver sections backed up by histological examinations prefers the latter as a credible and sound technique for investigating the toxicity of NPs in liver [168].
In a study, different effects and distribution of AuNPs were observed after histological examinations of testis, liver and kidney. There were no effects on testis whereas mild changes were noticed in kidney and liver sections [102].
GSH-coated AuNPs do not induce toxicity by passing via the kidney as contrary stated in another study with tiopronin monolayer protected clusters (TMPC) using the same concentrations.
So also, GSH- and BSA-protected Au nanoclusters (AuNCs) affects kidney function in mice and produce toxicity responses that could be removed after 28 days [103]. This could primarily be due to the role the kidney plays in filtration of the NPs in the renal glomeruli into the urine [84].
A study involving the immune organs of broiler chickens has confirmed that addition of 15 ppm AuNPs to drinking water has caused recognizable oxidative damage to blood, histopathological changes, up-regulation of IL-6, expression of Nrf2 gene, fragmentation of DNA, significant decrease in antibody titer against avian influenza (AI) and newcastle disease (ND) [104].
AuNPs in mice has caused damage to the neuronal system [85,86]. However, Porphyran-reduced AuNPs have not yield any anomaly [110]. Even though, functionalized AuNPs accumulate at various parts of the brain in male CD1 mice [105]. Citrate-capped AuNPs have caused transmissible mutagenic effects in Drosophila melanogaster [106].
In addition, Drosophila melanogaster was used to determine the effects of 15-nm citrate-capped AuNPs by Pompa et al. [107]. They noticed a Sharp decline in fertility and life span, presence of DNA fragments, and strong over-expression of stress proteins after administration of 12 μg/g AuNPs daily by ingestion. This illustrates how NPs modify the functioning of a complex biological system once introduced.
Large amount of AuNPs had caused a decline in red blood cells and body weight of mice. However, following oral administration it induced reduction in RBC, spleen index and body weight [12]. A large proportion of the mice died within 21 days after exposure to 8–37 nm naked colloidal AuNPs and caused loss of weight and appetite [109]. Toxicity studies have been undergone also on some lower model organisms such as fishes where LC50 were reported. The same have been done for AuNPs in assessing their toxicity and the LC50 of HAuCl4 was reported as 2 mg/l after 48hrs in D. magna by Li et al. (2010), 0.64 mg/l by Nam et al. [114] and 0.62 mg/l in M. macrocopa by Li et al. [[111], [112], [113]]. LC50 was 14.4 mg/l in T. arcticus after 96hrs as stated by Nam et al. [114]. Botha et al. [169] stated that exposure to AuNPs had produced a response in a dose dependent manner. Though, presenting NPs hazard potentials and the complete picture can only be seen once their characteristics and behaviours have been studied.
1.4. In vitro toxicity studies of AuNPs
Majority of nanotoxicological evaluations are performed in vitro since it is simple to conduct.
Though the outcome may not predict the in vivo toxicity accurately [170], it however provide the background for understanding the uptake and mechanism of toxicity which can be seen in Table 2 by some key studies.
Table 2.
Some in vitro toxicity studies involving AuNPs.
| Organism | Particle | Effects | Ref. |
|---|---|---|---|
| Human colorectal adenocarcinoma cells (HT29) | AuNPs | Significant reduction in viability of cells. However, no gnotoxic effects | [115] |
| HepG2 cells | AuNPs | Indicated tails moment similar to those from positive control in which cells were exposed to hydrogen peroxide | [82] |
| HepG2 cells | AuNPs | AuNPs do not change the concentration of inflammatory markers when compared to the control | [82] |
| MG63 cells | AuNPs | Low long term toxicity | [116] |
| BALB/c 3T3 fibroblast cells | Coated and uncoated spherical AuNPs | DNA damage results via indirect oxidative stress | Guglielmo et al., 2012 |
| Epithelial cells of airways | AuNPs | Elevation of lipid peoxidase as well as DNA damage and cytotoxicity | [117] |
| L5178Y cells | AuNPs | No damage to the DNA at 60 nm but there was damage at 100 nm | [118] |
| MRC-5 cells | AuNPs capped with GNPC and GNPB | Slight hepatotoxic and nephrotoxic | [119] |
| MRC-5 human lung fibroblasts | AuNPs | High lipid peroxidation, upregulation of antioxidants, expressions of protein and gene of stress response | [[111], [112], [113]] |
| Rat liver | AuNPs | Yield a great lipid peroxidation | [120] |
| Human leukemia (HL-60) and hepatoma (HepG2) cell lines | AuNPs | Cytotoxicity effects associated with reduction of GSH and increase in ROS | [121] |
| 3T3 cells | Plain and GSH-capped AuNPs | Produce more reactive oxygen species than plain AuNPs | [122] |
| HeLa and U937 cells | Citrate-capped AuNPs | Cytotoxic | [123,124] |
| HepG2 and PBMC cells | AuNPs capped with either sodium citrate or polyamidoamine dendrimers | In vitro cytotoxicity and genotoxicity effects at low concentrations | [125] |
| Balb/3T3 cells | AuNPs uncoated and coated with hyaluronic acid | Oxidative stress was reflected in DNA damage but with reduced cytotoxicity | [126] |
| C17.2 and PC12 cells | AuNPs | Caused oxidative stress by cell viability and deformations of actin and tubulin | [127] |
| Balb/3T3 cells | Citrate-stabilized AuNPs | Cytotoxicity by disruption of actin cytoskeleton | [25] |
| MRC-5 cells | AuNPs | Autophagy and oxidative stress | [[111], [112], [113]] |
| Human keratinocyte cell line HaCaT | AuNPs | Cell death by apoptosis and necrosis | [128] |
| Vero, MRC-5, and NIH/3T3 cells | AuNPs | Reduction in growth and was related with apoptosis and autophagy | [129] |
| Granulose cells of the ovary | AuNPs | Induced an elevation in estrogen accumulation | Stelzer et al. [130] |
| Human spermatozoa | AuNPs | Affects viability and motility | [131] |
| A549 cells | AuNPs coated with serum proteins | Intrinsic and extrinsic apoptotic pathways reflected in cell damage | [132] |
| Human fetal lung fibroblasts | AuNPs | Destabilized the expression of 19 genes in the cells | [117] |
| A549 cells | AuNPs | Assumed circular shape because of the induced stress | [133] |
| Hman liver cell lines (HL7702 cells) | AuNPs | Early decrease in cytosolic GSH, depolarization of mitochondrial transmembrane potential and subsequently apoptosis | [134] |
| MG63 osteoblast-like cells | AuNPs | Cell death | [116] |
| A549 cells | AuNPs | Cytotoxicity by substantial changes in nuclear morphology and nuclear condensation | [133] |
| Polymer-modified AuNPs | Hemocompatibility with human RBCs | [135,136] | |
| A549 cells | AuNPs | An inflammatory response | [137] |
| AGS, A549, NIH3T3, PK-15, and Vero cells | AuNPs | Suppression of growth of cells in a dose-dependent manner by delay of cell cycle and induction of apoptosis | [138] |
| Vero cells | AuNPs | Reduction in cell growth and related to apoptosis | [129] |
| NIH3T3 cells | AuNPs | Autophagy | [129] |
| MRC-5 cells | AuNPs | DNA damage | [129] |
| Breast cells (MDA-MB-231) | AuNPs | Reduction in proliferation | [139] |
| Human cell lines | AuNRs coated with methoxypolyethylene glycol thiol | Alterations in viability of cells with the exception of thyroid papillary carcinoma cells | [137] |
| Human cells | AuNPs | Little or no immunotoxic, cytotoxic, and genotoxic effects | [140] |
| CHO, BEAS-2B and HEK293 cells | Citrate-stabilized AuNPs | Exert higher toxicity | [141] |
| Hela cells | AuNPs | No indication of cytotoxicity | [142] |
| A549 and Vero cells | AuNPs conjugate | No toxicity | [143] |
| Caco-2 cells | AuNPs | Did not produce acute cytotoxicity | [144] |
| Vero cells | Porphyran-reduced AuNPs | No toxicological effects | [110] |
| Tumor ascites and normal peritoneal cells | Functionalized AuNPs | No morphological changes and cell death | [145] |
| HeLa cells | Silica-coated AuNRs and glucose-capped AuNPs | No toxicity effects | [146,147] |
| HEK293 cells | Phosphine-stabilized and thiol-stabilized AuNPs | Modified the gene expression and had no toxicity | [148] |
| Human carcinoma lung cell | Citrate-capped AuNPs | Induce toxicity | [133] |
| Human liver carcinoma cell | Citrate-capped AuNPs | No toxicity effects | [133] |
AuNPs have been associated to imbalances of oxidative status in vitro [171,121]. The current study showed that exposure of hepatocytes to AuNPs has yielded a time and dose-dependent increase in production of ROS in which the highest dose of AuNPs produced more damage. These were in line with the outcome of cell viability assay which indicated a rise in cell mortality. Thus, AuNPs were able to cause an initial oxidative damage which the cells tried to control the overproduction of ROS possibly because of the increase in the activity of antioxidant [[111], [112], [113]]. The mechanism of which has been presented in Fig. 5. Similarly, cell viability assays of human colorectal adenocarcinoma cells (HT29) exposed to AuNPs presented a significant reduction of viable cells. However, there were no genotoxic effects [115].
Fig. 5.
Toxicity mechanism of Gold nanoparticle (AuNPs) [34] (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Lopez-Chaves et al. [82] stated that HepG2 cells exposed to 10 nm AuNPs indicated tails moment similar to those from positive control in which cells were exposed to hydrogen peroxide. It is therefore possible that the 10 and 30 nm NPs were able to enter the nucleus which was confirmed by the NPs found inside the nucleus.
Though, treatments with AuNPs do not change the concentration of inflammatory markers when compared to the control [82]. This has also been reported by other authors [[172], [173], [174], [175]].
These could be attributed to the fact that the AuNPs was not sufficient enough to initiate an inflammatory response. They therefore concluded that the sizes of NPs determine their route of excretion. In addition, it has been proven that AuNPs induced oxidative status imbalances in the cells which were preceded by genetic, protein and lipid damage. An inflammatory response was seen in A549 cells in response to 20 nm AuNPs [137].
AuNPs caused a low long term toxicity effect on MG63 cells [116]. Guglielmo et al. (2012) determined the toxicity of coated and uncoated spherical AuNPs on BALB/c 3T3 fibroblast cells and revealed that DNA damage results via indirect oxidative stress. Several studies have stated that AuNPs have produced DNA damage, a cause of genotoxicity [125]. In vitro toxicological profile of AuNPs were evaluated in epithelial cells of airways and showed an elevation of lipid peoxidase as well as DNA damage and cytotoxicity in cells treated with AuNPs [117]. Predictive models involving the association of 12 various NPs and DNA indicated that AuNPs possess a great affinity to DNA and subsequently on their inhibition property in DNA replication are expected to be more vivid than for other NPs [135,136]. A significant increase in lipid peroxidation, as well as substantial DNA damage and cytotoxicity was observed in small airways of epithelial cells [117]. Several studies have given attention to size and time-dependent DNA damage caused by AuNPs but few studies talked about the dose-dependent DNA damage. Other researchers have observed DNA damage following exposure to 8 nm AuNPs [176] and 20 nm AuNPs [177].
The damage results due to the affinity of binding between AuNPs, thiol and amine groups is what cause the interaction with biomolecules leading to formation of free radicals [178,179,[180].
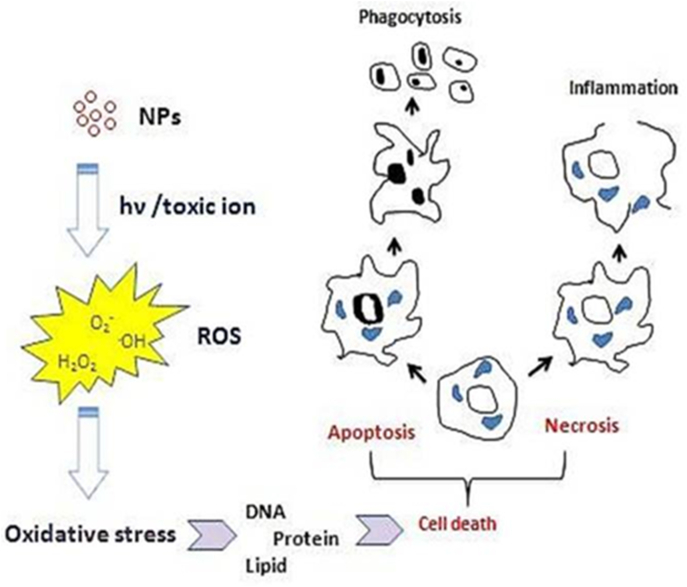
These very small particles are identified by their large surface areas which can lead to formation of reactive oxygen species (ROS). These ROS bring about cellular damage by destructing the proteins, DNA, membranes, and other organelles such as cytoplasm, mitochondria, and nucleus (Lewinski et al., 2012). Many other studies have indicated toxicities of AuNPs caused by production of ROS. Das et al. [119], observed AuNPs capped with trisodium citrate dihydrate (GNPC) and bovine serum albumin (GNPB) have slight hepatotoxic and nephrotoxic influences in MRC-5 cells. Studies on MRC-5 human lung fibroblasts demonstrated that AuNPs results in high lipid peroxidation, upegulation of antioxidants, expressions of protein and gene of stress response [[111], [112], [113]]. Khan et al. [120], also found out that AuNPs yield a great lipid peroxidation in liver of rats. Mateo et al. [121], confirmed the cytotoxicity effects of 3 AuNPs of variant sizes on human leukemia (HL-60) and hepatoma (HepG2) cell lines was associated with reduction of GSH following 72hrs incubation and simultaneous increase in production of ROS. GSH-capped AuNPs produce more reactive oxygen species than plain AuNPs in 3T3 cells [122].
Citrate-capped AuNPs were cytotoxic to HeLa and U937 cells and were cell culture media-dependent [123,124]. Residues of sodium citrate were seen to influence the cytotoxicity of AuNPs on human epithelial cells [181]. Martinez Paino et al. [125], reported elements of in vitro cytotoxicity and genotoxicity of AuNPs capped with either sodium citrate or polyamidoamine dendrimers at very low concentrations in HepG2 and PBMC cells. Oxidative stress was reflected in DNA damage in Balb/3T3 cells following treatment with AuNPs uncoated and coated with hyaluronic acid. However, their cell internalization and cytotoxicity were reduced [126]. Equally, large concentration of AuNPs caused oxidative stress by cell viability and deformations of actin and tubulin in C17.2 and PC12 cells [127]. Cytotoxicity in form of the disruption of actin cytoskeleton was observed after treatment of Balb/3T3 cells with citrate-stabilized AuNPs [25]. In comparison to gum-arabic and starch stabilized AuNPs, citrate coated showed grater cytotoxicity because of the citrate acidic property [160,161]. There were indications of autophagy and oxidative stress in MRC-5 cells exposed to 20 nm AuNPs [[111], [112], [113]].
Cell death caused by exposure to AuNPs largely depends on many factors which include NPs size. Previous studies showed that 1.4 nm AuNPs produced speedy cell death by necrosis whereas same AuNPs of 1.2 nm size produced cell death via apoptosis [182].
In addition, surface charge is yet another factor that plays a role in determining the type of cell death caused by NPs.
The surface charge of 1.5 nm AuNPs was classified on how they caused cell death in human keratinocyte cell line HaCaT by apoptosis and necrosis [128]. It should be noted that AuNPs could cause cell death by various mechanisms taking into consideration distinctive cell lines. In the same vein, Chueh et al. [129], described reduction in growth of 3 mammalian cells (Vero, MRC-5, and NIH/3T3 cells) and was related with apoptosis and autophagy. Numerous studies that were published reaffirmed that NPs associate with membrane lipids resulting to deleterious effects on cells. A study by Leroueil et al. [183] stated that NH2-AuNPs produced physical damage to lipid membranes by atomic force microscopy. Camesano et al. [184] revealed that small AuNPs produced a loss of mass in lipid bilayers, indicating formation of pores and disruption of membrane. However, larger AuNPs of 20 nm did not disrupt the membrane.
A report of Chakraborty et al. [185] about the association of different AuNPs and bovine serum albumin (BSA) showed that BSA conserves its features with the exception of AuNRs.
The AuNRs results in significant loss of secondary and tertiary structures of protein.
Stelzer et al. [130] states that 10 nm AuNPs induced an elevation in estrogen accumulation in granulose cells of the ovary following incubation for 1–5 h.
Similarly, some internal cellular organelles involved in steroidogenesis were entered and altered because of the NPs. Elevation in concentration of blood testosterone were seen in studies involving mice following treatment with PEG-modified AuNPs [[90], [91], [92]].
The parameters of sperm validity in porcine gametes were not affected by AuNPs [186]. 50 nm AuNPs induced an effect on viability and motility on human spermatozoa [131].
Chuang et al. [138] demonstrated a complete toxicological study of AuNPs on mammalian cell lines. The outcome showed that AuNPs substantially modulate gene expression of 436 genes and protein function associated with apoptosis and progression of cell cycle. Similar report was put forward by Ng et al. [117], who state that AuNPs destabilize the expression of 19 genes in human fetal lung fibroblasts. AuNPs caused changes in gene expression following a single exposure of 0.01 mgkg−1 by Balasubramanian et al. [88,89].
However, from another perspective, AuNPs can be used to regulate gene expression thereby assisting in treatment of diseases by gene silencing [187].
Several studies have revealed that AuNPs results in changes to morphology of cells.
For instance, following exposure of A549 cells to AuNPs for 48 h, they assume circular shape because of the induced stress [133].
Vetten et al. [141] determined the effects of AuNPs on the reaction of ATP-based assay, involving the conversion of luciferin to luminescent oxyluciferin with the aid of ATP. They result in reduction of the luminescent signal which was the most observed effect at higher NPs levels. Several other studies have reported that AuNPs produce a decrease in levels of ATP of treated cells indicating a mitochondrial dysfunction [188,189].
More studies have confirmed the decrease in levels of GSH in cells following incubation with AuNPs [[90], [91], [92]].
For example, Gao et al. [134] evaluated the role of the association of 8 nm AuNPs with GSH on the series and progression of the events of apoptotic signaling in cell lines of human liver (HL7702 cells). There was an early decrease in cytosolic GSH of HL7702 cells, depolarization of mitochondrial transmembrane potential and subsequently apoptosis sets in.
Tsai et al. [116] used propidium iodide and annexin V double labelling in connection with flow cytometry to reveal the effects of AuNPs on the cell death of MG63 osteoblast-like cells.
A report by Patra et al. [133] demonstrated how substantial changes in nuclear morphology was observed after exposure of A549 cells to AuNPs which results in nuclear condensation thereby indicating cytotoxicity.
In another study by Liu et al. [[90], [91], [92]], hemolysis test showed hemocompatibility with human RBCs by polymer-modified AuNPs. Thus, it indicates a dose-dependent toxicological potential of SD-AuNPs in addition to significant decrease of cell viability and intracellular reactive oxygen species [190].
AuNPs produced a suppression of growth of cells in a dose-dependent manner in AGS, A549, NIH3T3, PK-15, and Vero cells. The mechanisms of suppression include delay of cell cycle and induction of apoptosis [138]. Chueh et al. [129], described evidence of reduction in cell growth and related to apoptosis in vero cells, autophagy in NIH3T3 cells, and DNA damage in MRC-5 cells after treatment with AuNPs. There was reduction in proliferation of breast cells (MDA-MB-231) by AuNPs [139].
Little or no immunotoxic, cytotoxic, and genotoxic effects was noticed on human cells following treatment with AuNPs [140]. Similarly, smaller citrate-stabilized AuNPs were non toxic to CHO, BEAS-2B and HEK293 cells while larger AuNPs exert higher toxicity [141]. No indication of cytotoxicity in hela cells post treatment with AuNPs [142]. There was no toxicity was observed in A549 and Vero cells treated with AuNPs conjugate [143]. Also, AuNPs did not produce acute cytotoxicity after treatment of Caco-2 cells to AuNPs [144]. No toxicological effects were noticed in vero cells exposed to porphyran-reduced AuNPs [110]. Functionalized AuNPs did not bring about morphological changes and cell death in tumor ascites and normal peritoneal cells [145]. In addition, silica-coated AuNRs and Glucose-capped AuNPs have produced no toxic effects in HeLa cells [146,147]. Phosphine-stabilized and thiol-stabilized AuNPs modified the gene expression and had no toxicity respectively in HEK293 cells [148].
Such incompatible results could arise from the inconsistencies in cell lines, toxicity assays, and physicochemical characters of NPs under study. For example, results from cytotoxicity could differ with the cell line used. AuNPs (diameter of 13 nm) capped with citrate was toxic in human carcinoma lung cell line but not in human liver carcinoma cell line at same concentration [133].
In view of this, it has become paramount to evaluate the toxicological effects of AuNPs and produce early markers that could indicate its health effects.
2. Conclusion
Presently, the conflict in data of AuNPs bioactivity in literature, portraying variations in laboratory protocols, makes it tough to determine and generalize vital issues of their effects. These do not give way for proper consensus and conclusion regarding the cytotoxicity of AuNPs. The outcome of these review showed small controversy but however, the primary toxicity and its extent is collectively determined by the characteristics, preparations and physicochemical properties of the AuNPs. Some studies have shown that AuNPs are not toxic, though many other studies contradict this statement. In order to have a holistic inference, more studies are required that will focus on characterization of the NPs and changes of physical properties before and after treatment with biological media.
2.1. Future directions
Subsequent studies should incorporate controlled experiment which includes supernatant control. Another vital perspective is to ascertain the realistic dose to use during toxicological evaluation of NPs which is the effective therapeutic dose and needs to be determined experimentally. Since most studies dwell on citrate or CTAB-capped AuNPs, there is the need to evaluate the toxicity and pharmacokinetics of functionalized AuNPs with their surface composition which in turn affects their toxicity. Functionalizing the NPs surface with more peculiar ligands would however help regulate and detoxify the uptake of these NPs. Further studies on use of AuNPs as medium or vehicle for drug delivery and treatment of chronic diseases such as diabetes and cancer should be encouraged.
2.2. Regulatory considerations
Regulation for the use of particles in food and other consumables should be enforced especially the smaller-sized that pose the greatest risk. Safety guidelines associating their properties to toxicities should be put in place. Nanowastes generally should be neutralized before disposal to eliminate its hazardous nature or reactivity. Government and other stakeholders should proactively develop a wide strategy for management including recycling of nanowastes to inhibit unintended consequences. Handling of these NPs should be done with care and caution as well as improvement of the NPs toxicity assays. Strict regulations should be ensured for laws and policies of managing all aspects of NPs development in this fast growing field of nanotechnology.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.100991.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jennings T., Strouse G. Past, present, and future of gold nanoparticles. Adv. Exp. Med. Biol. 2007;620:34. doi: 10.1007/978-0-387-76713-0_3. [DOI] [PubMed] [Google Scholar]
- 2.Bracamonte M.V., Bollo S., Labbe P., Rivas G.A., ferreyra N.F. Quaternized chitosan as support for the assembly of gold nanoparticles and glucose oxidase. Physiochemical characterization of the platform and evaluation of its biocatalytic activity. Electrochmica Acta. 2011;56:1316–1322. [Google Scholar]
- 3.Aillon K.L., Xie Y., El-Gendy N., Berkland C.J., Forrest M.L. Nanomaterials physiochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009;61(8):457–466. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsich E., Travan A., Donati I., Luca A.D., Benincasa M., Crosera M., Paoletti Biological response of hydrogels embedding gold nanoparticles. Collods and SurfaceB: Biointerfaces. 2011;83:331–339. doi: 10.1016/j.colsurfb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins J.T., Halaney D.L., Sokolov K.V., Ma L.L., Shipley H.J., Mahajan S., Louden C.L., Asmis R., Milner T.E., Johnsons K.P., Feldman M.D. Excretion and toxicity of gold–iron nanoparticles. Mater. Sci. Eng. C. 2013;33(1):550–556. doi: 10.1016/j.nano.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Dykman L., Khelbstov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem. Soc. Rev. 2012;41:2256–2282. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 7.Pissuwan D., Niidome T., Cortie M.B. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Contr. Release. 2011;149:65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Pissuwan D., Valenzuela S.M., Cortie M.B. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006;24:62–67. doi: 10.1016/j.tibtech.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Frohlich E., Roblegg E. Models for oral uptake of nanoparticles in consumer products. Toxicology. 2012;291:10–17. doi: 10.1016/j.tox.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra P., Das D., Wahab A.A.A. Gold nanoparticles in molecular diagnostics and therapeutics. Digest Journal of nanomedicine and biostructures. 2010;5(2):363–367. [Google Scholar]
- 11.Surendra N., Nidhi G., Ramesh C. Cationic polymer based nanocarriers for delivery of therapeutic nucleic acids. J. Biomed. Nanotechnol. 2011;7(4):504–520. doi: 10.1166/jbn.2011.1313. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X.D., Wu H.Y., Wu D., Wang Y.Y., Chang J.H., Zhai Z.B., Meng A.M., Liu P.X., Zhang L.A., Fan F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010;5:771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., Zhou H., Yang L., Du G., Pai-Panandiker A.S., Huang X., Yan B. Enhancement of cell recognition in vitro by dual-ligand cancer targeting gold nanoparticles. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber A., Bundschuh M., Klingelhofer D. Gold nanoparticles: recent aspects for human toxicology. J. Occup. Med. Toxicol. 2013;8:32. doi: 10.1186/1745-6673-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne H.J., Lynch I., de Jong W.H., Kreyling W.G., Loft S., Park M.V.D.Z., Riediker M., Warheit D. The European Network on the Health and Environmental Impact of Nanomaterials; 2010. Protocols for assessment of biological hazards of engineered nanomaterials; pp. 1–30. [Google Scholar]
- 16.De Jong W.H. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29 doi: 10.1016/j.biomaterials.2007.12.037. 1912–19. [DOI] [PubMed] [Google Scholar]
- 17.Van Doren E.A.F., Temmerman P.R.H.D., Francisco A.D., Mast J. Determination of the volume specific surface area by using transmission electron tomography for characterization and definition of nanomaterials. Journal of Nanobiiotechnology. 2011;9:17. doi: 10.1186/1477-3155-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patra C.R., Bhattacharya R., Mukhopadhyay D., Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv. Drug Deliv. Rev. 2010;62:346–361. doi: 10.1016/j.addr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prime D., Paul S., Joseph-Franks P.W. Gold nanoparticle charge trapping and relation to organic polymer memory devices. Philos transact A Math Phys Eng Sci. 2009;367(1905):4215–4225. doi: 10.1098/rsta.2009.0141. [DOI] [PubMed] [Google Scholar]
- 20.Tsoukalas D. From silicon to organic nanoparticle memory devices. Philos transact A Math Phys Eng Sci. 2009;367(1905):4169–4179. doi: 10.1098/rsta.2008.0280. [DOI] [PubMed] [Google Scholar]
- 21.Hashmi A.S.K., Hutchings G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006;45:7896–7936. doi: 10.1002/anie.200602454. [DOI] [PubMed] [Google Scholar]
- 22.Heaven M.W., Dass A., White P.S., Holt K.M., Murray R.W.J. Crystal structure of the gold nanoparticle [N(C8H17)4][Au25(SCH2CH2Ph)18] Am. Chem. Soc. 2008;130:3754. doi: 10.1021/ja800561b. 3755. [DOI] [PubMed] [Google Scholar]
- 23.McPherson J.S., Thompson D.T. Selectivity of gold catalysis for application of commercial interest. Tropics in Catalysis. 2009;52:743–750. [Google Scholar]
- 24.Sardar R., Funston A.M., Mulvaney P., Murray R.W. Gold nanoparticles: past, present, and future. Langmuir. 2009;25(24):13840–13851. doi: 10.1021/la9019475. [DOI] [PubMed] [Google Scholar]
- 25.Coradeghini R., Gioria S., Garcia C.P., Nativo P., Franchini F., Gilliland D., Ponti J., Rossi F. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol. Lett. 2013;217:205–216. doi: 10.1016/j.toxlet.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Jo M.R., Bae S.H., Go M.R., Kim H.J., Hwang Y.G., Choi S.J. Toxicity and biokinetics of colloidal gold nanoparticles. Nanomaterials. 2015;5:835–850. doi: 10.3390/nano5020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung J.H., Ji J.H., Park J.D., Song M.Y., Song K.S., Ryu H.R., Yoon J.U., Jeon K.S., Jeong J., Han B.H., Chung Y.H., Chang H.K., Lee J.H., Kim D.W., Kelman B.J., Yu I.J. Subchronic inhalation toxicity of gold nanoparticles. Part. Fibre Toxicol. 2011;8:16. doi: 10.1186/1743-8977-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravensthorpe M. IOP Publishihing Natural News Web. 2013. Colloidal gold: the great rejuvenator of mind and body.https://thezeitgeistindia.blogspot.com/2013/12/new-postthree-beneficial-colloids-and.html Accessed. [Google Scholar]
- 29.Yah C.S. The toxicity of Gold Nanoparticles in relation to their physiochemical properties. Biomed. Res. 2013;24(3):400–413. 2013. [Google Scholar]
- 30.Lewinski N., Colvin V., Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 31.Yah C.S., Iyuke S.E., Simate G.S. Nanoparticles toxicity and their routes of exposures. PJPS. 2012;25(2):477–491. [PubMed] [Google Scholar]
- 32.Uboldi C., Bonacchi D., Lorenzi G., Hermanns M.I., Pohl C., Baldi G., Unger R.E., Kirkpatrick C.J. Gold nanoparticles induce cytotoxicity in the alveolar type-II cell lines A549 and NCIH441. Part. Fibre Toxicol. 2009;6:18. doi: 10.1186/1743-8977-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg H., Galyean A., Leopold M. Evaluating engineered nanoparticles in natural waters. Trac. Trends Anal. Chem. 2011;30(1):72–83. [Google Scholar]
- 34.Khalili J.F., Jafari S., Ali M.E. A review of molecular mechanisms involved in toxicity of nanoparticles. Adv. Pharmaceut. Bull. 2015 Nov;5(4):447–454. doi: 10.15171/apb.2015.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renault S., Baudrimont M., Mesmer-DudonsN, Gonzalez P., Mornet S., Brisson A. Impacts of gold nanoparticle exposure on two freshwater species: a phytoplanktonic alga (Scenedesmus subspicatus) and a benthic bivalve (Corbicula fluminea) Gold Bull. 2008;41(2):116–126. [Google Scholar]
- 36.Chithrani B.D., Ghazani A.A., Chan W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 37.Khan J.A., Pillai B., Das T.K., Singh Y., Maiti S. Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem. 2007;8:1237. doi: 10.1002/cbic.200700165. [DOI] [PubMed] [Google Scholar]
- 38.Fanord F., Fairbairn K., Kim H., Garces A., Bhethanabotla V., Gupta V.K. Bisphosphonate modified gold nanoparticles: a useful vehicle to study the treatmentNanotechnology. 2011;22 doi: 10.1088/0957-4484/22/3/035102. [DOI] [PubMed] [Google Scholar]
- 39.Donaldson K., Borm P.J.A., Castranova V., Gulumian M. The limits of testing particle-mediated oxidative stress in vitro in predicting diverse pathologies; relevance for testing of nanoparticles. Part. Fibre Toxicol. 2009;6:13. doi: 10.1186/1743-8977-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slepička P., Slepičková Kasálková N., Siegel J., Kolská Z., Švorčík V. Methods of gold and silver nanoparticles preparation. Materials. 2020;13:1. doi: 10.3390/ma13010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro H.P.S., Wender H., Alencar M.A.R.C., Teixeira S.R., Dupont J., Hickmann J.M. Third-order nonlinear optical response of colloidal gold nanoparticles prepared by sputtering deposition. J. Appl. Phys. 2013;114:183104. [Google Scholar]
- 42.PradeepaVidya S.M., Mutalik S., Udaya K., BhatHuilgol P. Avadhan. Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life sci. 2016;153:171–179. doi: 10.1016/j.lfs.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 43.Kalita S., Kandimalla R., Sharma K.K., Kataki A.C., Deka M., katoky J. Amoxicillin functionalized gold nanoparticles reverts MRSA resistance. Mater. Sci. Eng. C. 2016;61:720–727. doi: 10.1016/j.msec.2015.12.078. [DOI] [PubMed] [Google Scholar]
- 44.Amini A., Kamali M., Amini B., Najafi A. enhanced antibacterial activity of imipenem-immobilized on surface of spherical and rod gold nanoparticles. J. Phys. D. 2019;52(6) [Google Scholar]
- 45.Singh R., Patil S., Singh N., gupta S. Dual functionality nanobioconjugates targeting intracellular bacteria in cancer cells with enhanced antimicrobial activity. Sci. Rep. 2017;7(2):5792. doi: 10.1038/s41598-017-06014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shittu K.O., Bankole M.T., Abdulkareem A.S., Abubakre O.K. Application of gold nanoparticles for improved drug efficiency. Adv. Nat. Sci: Nanosci. Nanotechnol. 2017;8(3) [Google Scholar]
- 47.Dhamecha, D., Jalalpure, S., Jadhav, K. & Sajjan, D. (2016) Green synthesis of gold nanoparticles using Pterocarpus marsupium: Characterization and biocompatibility studies, Part. Science and Technology, 34:2, 156-164.
- 48.Ahmed A., Khan A.K., Anwar A, Ali S.A., Shah M.R. Biofilm inhibitory effect of chlorhexidine conjugated gold nanoparticles against Klebsiella pneumonia. Microb. Pathog. 2016;98:50–56. doi: 10.1016/j.micpath.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 49.Shilo M., Berenstein P., Dreifuss T., Nash T., Goldsmith G., Kazimirsky G., Motiei G., Frenkel D., Brodie C., Popovtzer R. Insulin-coated gold nanoparticles as a new concept for personalized and adjustable glucose regulation. Nanoscale. 2015;7(48):20489–20496. doi: 10.1039/c5nr04881h. [DOI] [PubMed] [Google Scholar]
- 50.Lan M.-Y., Hsu Y.-B., Hsu C-H, Ho C.-Y., Lin J.-C., Lee S.W. Induction of apoptosis by high-dose gold nanoparticles in nasopharyngeal carcinoma cells. Auris Nasus Larynx. 2013;40:563–568. doi: 10.1016/j.anl.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Rattanata N., Klaynongsruang S., Leelayuwat C., Limpaiboon T., Lulitanond A., Boonsiri P., Chio-Srichan S., Soontaranon S., Rugmai S., Daduang J. Gallic acid conjugated with gold nanoparticles antibacterial activity and mechanism of action on food borne pathogens. Int. J. Nanomed. 2016;11(11):3347–3356. doi: 10.2147/IJN.S109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnaby A.S.N., Lee A., Mirkin C.A. Probing the inherent stability of RNA immobilized on nanoparticle constructs. Proc. Natl. Acad. Sci. U.S.A. 2014;111(27):9739–9744. doi: 10.1073/pnas.1409431111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Artiga A., Serrano-Sevilla I., De Matteis L., Mitchell S.G., de la Fuente J.M. Current status and future perspectives of gold nanoparticle vectors for siRNA delivery. J. Mater. Chem. B Mater. Biol. Med. / 2019;7(6):876–896. doi: 10.1039/c8tb02484g. [DOI] [PubMed] [Google Scholar]
- 54.Griffith L.G., Swartz M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 55.Morgan E., Wupperfcld D., Morales D., Reich N. ShapeOmalters. Gold nanoparlicle shape impacts the biological activity of siRNA delivery. Bioconjugate Chem. 2019;30(3):853–860. doi: 10.1021/acs.bioconjchem.9b00004. [DOI] [PubMed] [Google Scholar]
- 56.Calderon-Gonzalez R., Teran-Navarro H., Garcia I., Marradi M., Salcines-Cuevas D., Yanez-Diaz S., Solis- Angulo A., Frande-Cabanes E., Farinas M.C., Garcia-Castano A., Gomez-Roman J., Penades S., Rivera F., Freire J., Alvarez-Dominguez C. Gold glyconanoparticles coupled to listeriolysin O 91-99 peptide serve as adjuvant therapy against melanoma. Nanoscale. 2017;9(30):10721–10732. doi: 10.1039/c7nr02494k. [DOI] [PubMed] [Google Scholar]
- 57.Lyu Z., Zhou F., Liu Q., Xue H., Yu Q., Chen H. A universal platform for macromolecular deliveryinto cells using gold nanoparticle layers via the . nhotoporation effect. Adv. Funct. Mater. 2016;26(32) 5787-S795. [Google Scholar]
- 58.Hiilin J., Carrillo-Carrion C., Soliman M.G., Pfeiffer C., Valdeperez D., Masood A., Chakraborly I., Zhu L., Gallego M., Yue Z., Carril M., Feliu N., Escudero A., Alkilany A.M., Pelaz B., del Pino P., Parak W.J. Selected standard protocols for the • synthesis, phase transfer, and characterization of inorganic colloidal nanoparticles. Chem. Mater. 2017;29(1):399–461. [Google Scholar]
- 59.Guo Q., Guo Q., Yuan J., Zeng J. Biosynthesis of gold nanoparticles using a kind of flavonol: Dihydromyricetin. Colloid. Surface. Physicochem. Eng. Aspect. 2014;441:127–132. [Google Scholar]
- 60.Khoobchandani M., Katti K., Maxwell A., Fay W.P., Katti K.V. Laminin receptor-avid nanotherapeutic GCg-AuNP as a potential alternative therapeutic approach to prevent yfestenosis. Int. J. Mol. Sci. 2016;17(3):316. doi: 10.3390/ijms17030316. (104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bibikova O., Singh P., Popov A., Akchurin G., Skaptsov A., Skovorodkin I., Khanadeev V., Mikhalevich D., Kinnunen M., Akchurin G., Bogatyrev V., Khlebtsov N., Vainio S.J., Meglinski I., Tuchin V. Shape dependent interaction of gold nanoparticles with cultured cells at laser exposure. Laser Phys. Lett. 2017;14(5) [Google Scholar]
- 62.Lee S.M., Park H., Yoo K.H. Synergistic cancer therapeutic effects of locally delivered drug and heat/using multifunctional nanoparticles. Adv. Mater. 2010;36:4049–4053. doi: 10.1002/adma.201001040. [DOI] [PubMed] [Google Scholar]
- 63.Dong S., Tang C., Zhou H., Zhao H. Photochemical synthesis of gold nanoparticles by the sunlight radiation using a seeding approach. Gold Bull. 2004;(37):3–4. [Google Scholar]
- 64.Giljohann D.A., Seferos D.S., Daniel W.L., Massich M.D., Patel P.C., Mirkin C.A. Gold nanoparticles for biology and medicine. Angew. Chem. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gurunathan S., Kim J.H. Biocompatible gold nanoparticles ameliorate retinoic acid-induced cell death and induce differentiation in F9 teratocarcinormi stem cells. Nanomaterials. 2018;8(6):396. doi: 10.3390/nano8060396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Payne J.N., Waghwani H.K., Connor M.G., Hamilton W., Tockstein S., Moolani H., Chavda F., Badwaik V., Lawrenz M.B., Dakshinamurthy R. Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front. Microbiol. 2016;7:607. doi: 10.3389/fmicb.2016.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai H.Z., Chen W.Y., Wu C.Y., Chen Y.C. Potent antibacterial nanoparticles for pathogenic bacteria. ACS Appl. Mater. Interfaces. 2015;7(3):2046–2054. doi: 10.1021/am507919m. [DOI] [PubMed] [Google Scholar]
- 68.You Q., Zhang X., Wu F.G., Chen Y. Colorimelric and test stripe-based assay of bacteria by using vancomycin-modified gold nanoparticles. Sensor. Actuator. B Chem. 2019;281:408–414. /3. [Google Scholar]
- 69.Silvero C M.J., Rocca D.M., de la Villarmois E.A., Fournier K., Lanterna A.E., Perez M.£., Becerra M.C., Scaiano J.C. Selective photoinduced antibacterial activity of amoxicillin-coated gold nanoparticles: from one-step synthesis to in vivo cytocompatibility. ACS Omega. 2018;3(1):1220–1230. doi: 10.1021/acsomega.7b01779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haddada M.B., Jeannot K., Spadavecchia J. Novel synthesis and characterization of doxycycline- loaded gold nanoparticles: the golden doxycycline for antibacterial applications. Part. Part. Sysl. Charact. 2019;736(2):1800395. [Google Scholar]
- 71.Anwar A., Siddiqui R., Raza Shah M., Ahmed Khan N. Gold nanoparticles conjugation enhances antiacanthamoebic properties of nystatin, fluconazole and amphotericin B. J. Microbiol. Biotechnol. 2019;29(1):71–177. doi: 10.4014/jmb.1805.05028. [DOI] [PubMed] [Google Scholar]
- 72.Qiu H., Min Y., Rodgers Z., Zhang L., Wang A.Z. Nanomedicine approaches to improve cancer immunotherapy. Wiley Interdiscip. Rev. Nanomcd. /Nanobiotechnol. 2017;9(5) doi: 10.1002/wnan.1456. , e!456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grimaldi A.M., Incoronato M., Salvatore M., Soricelli A. Nanoparticle-based strategies for cancer immunotherapy and immunodiagnostics. Nanomedicine. 2017;12(19):2349–2365. doi: 10.2217/nnm-2017-0208. [DOI] [PubMed] [Google Scholar]
- 74.Hu X., Wu T., Bao Y., Zhang Z. Nanotechnology based therapeutic modality to boost anti-tumor immunity and collapse tumor defense. J. Contr. Release. 2017;256:26–45. doi: 10.1016/j.jconrel.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 75.Mahjub, R., Jatana, S., Lee, S. E., Qin, Z., Pauli, G., Soleimani, M., Madadi, S. and Li, S. D. Recent advances in applying nanolechnologies for cancer immunotherapy. J. Contr. Release 288, 239-263 A201&). [DOI] [PubMed]
- 76.Etame A.B., Smith C.A., Chan W.C., Rutka J.T. Design and potential application of PEGylated gold, nanoparticles with size-dependent permeation through brain microvasculature. Nanomed: XNanotechnol Biol Med. 2011;7:992–1000. doi: 10.1016/j.nano.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Jia J., Zhang Y., Xin Y., Jiang C., Yan B., Zhai S. Interactions between nanoparticles and dendritic cells: from the perspective of cancer immunotherapy. Front. Oncol. 2018;8:404. doi: 10.3389/fonc.2018.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Englinger B., Pirker C., Heffeter P., Terenzi A., Kowol C.R., Keppler B.K., Berger W. Metal drugs/and the anticancer immune response. Cheni. Rev. 2019;119(2):1519–1624. doi: 10.1021/acs.chemrev.8b00396. [DOI] [PubMed] [Google Scholar]
- 79.Jeff M.M.J.M., Bulte W.M. Springer; New York, NY, USA: 2008. Nanoparticles in Biomedical Imaging, Emerging Technologies and Applications. Fundamental Biomedical Technologies; p. 102. [Google Scholar]
- 80.Lopez-Campos F., Candini D., Carrasco E., Berenguer Frances M.A. Nanoparticles applied to cancer immunoregulation. Rep. Practical Oncol. Radiother. 2019;24(1):47–55. doi: 10.1016/j.rpor.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fischer H.C., Chan W.C.W. Nanotoxicity: the growing need for in vivo study. Curr. Opin. Biotechnol. 2007;18:565–571. doi: 10.1016/j.copbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 82.Lopez-Chaves C., Soto-Alvaredo J., Montes-Bayon M., Bettmer J., Llopis J., Sanchez-Gonzalez C. Gold nanoparticles: distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2018;14:1–12. doi: 10.1016/j.nano.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 83.Rattanapinyopituk K., Shimada A., Morita T., Sakurai M., Asano A., Hasegawa T. Demonstration of the clathrin-and caveolinmediated endocytosis at the maternal–fetal barrier in mouse placenta after intravenous administration of gold nanoparticles. J. Vet. Med. Sci. 2014;76:377–387. doi: 10.1292/jvms.13-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sadauskas E., Wallin H., Stoltenberg M., Vogel U., Doering P., Larsen A. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 2007;4:10. doi: 10.1186/1743-8977-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen J., Wang H., Long W., Shen X., Wu Di, Song S.S., Sun Y.M., Liu P.X., Fan S., Fan F. Int. J. Nanomed. 2012;8:2409e2419. doi: 10.2147/IJN.S46376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y.S., Hung Y.C., Hong M.Y., Onischuk A.A., Chiou J.C., Sorokina I.V., Tolstikova T., Huang G.S. J. Nanomater. 2012;2012 Article ID 746960, 1e11. [Google Scholar]
- 87.Kim K.T., Zaikova T., Hutchison J.E., Tanguay R.L. Toxicol. Sci. 2013;133:275e288. doi: 10.1093/toxsci/kft081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balasubramanian S.K., Jittiwat J., Manikandan J., Ong C.N., Yu L.E., Ong W.Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials. 2010;31:2034–2042. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 89.Balasubramanian S.K. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rat. Biomaterials. 2010;31:2034–2042. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 90.Liu H., Liu T., Wang H., Li L., Tan L., Fu C., Nie G., Chen D., Tang F. Biomaterials. 2013;34:6967e6975. doi: 10.1016/j.biomaterials.2013.05.059. [DOI] [PubMed] [Google Scholar]
- 91.Liu M., Gu X., Zhang K., Ding Y., Wei X., Zhang X., Zhao Y. J. Nano Res. 2013;15:1745e1759. [Google Scholar]
- 92.Liu X., Huang H., Liu G., Zhou W., Chen Y., Ji J. Nanoscale. 2013;5:3982e3991. doi: 10.1039/c3nr00284e. [DOI] [PubMed] [Google Scholar]
- 93.Cho E.C., Xie J.W., Wurm P.A., Xia Y.N. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009;9:1080–1084. doi: 10.1021/nl803487r. [DOI] [PubMed] [Google Scholar]
- 94.Cho W.S., Cho M.J., Jeong J. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009;236:16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X.D., Wu D., Shen X., Liu P.X., Yang N., Zhao B., Zhang H., Sun Y.M., Zhang L.A., Fan F.Y. Int. J. Nanomed. 2011;6:2071e2081. doi: 10.2147/IJN.S21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sadauskas E., Jacobsen N.R., Danscher G., Stoltenberg M., Vogel U., Larsen A., Kreyling W., Wallin H. Biodistribution of gold nanoparticles in mouse lung following intratracheal instillation. Chem. Cent. J. 2009;3:16. doi: 10.1186/1752-153X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sonavane G., Tomoda K., Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloid. Surface. B. 2008;66:274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 98.Lasagna-Reeves C., Gonzalez-Romero D., Barria M.A., Olmedo I., Clos A., Ramanujam V.M.S., Urayama A., Vergara L., Kogan M.J., Soto C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010;393:649–655. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 99.Lipka J., Semmler-BehnkeM, Sperling R.A., Wenk A., Takenaka S., Schleh C., Kissel T., Parak W.J., Kreyling W.G. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials. 2010;31:6574–6581. doi: 10.1016/j.biomaterials.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 100.Cho W.S., Cho M., Jeong J., Choi M., Cho H.Y., Han B.S., Kim S.H., Kim H.O., Lim Y.T., Chung B.H., Jeong J. Toxicol. Appl. Pharmacol. 2009;236:16e24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 101.Xiaomin Li, Zhenpeng Hu, Jinlong Ma, Xinyu Wang, Yapei Zhang, Wei Wang, Zhi Yuan, The Systematic Evaluation of Size-dependent Toxicity and Multi-Time Biodistribution of Gold Nanoparticles, Colloids and Surfaces B: Biointerfaces 10.1016/j.colsurfb.2018.04.005. [DOI] [PubMed]
- 102.Yahyaei B., Nouri M., Bakherad S., Hassani M., Pourali P. Effects of biologically produced gold nanoparticles: toxicity assessment in different rat organs after intraperitoneal injection. Amb. Express. 2019;9:38. doi: 10.1186/s13568-019-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X.D., Wu D., Shen X., Liu P.X., Fan F.Y., Fan S. J. Biomaterials. 2012;33:4628e4638. doi: 10.1016/j.biomaterials.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 104.Hassanen E.I., Morsy E.A., Hussien A.M., Ibrahim M.A., Farroh K.Y. The effect of different concentrations of gold nanoparticles on growth performance, toxicopathological and immunological parameters of broiler chickens. Biosci. Rep. 2020 doi: 10.1042/BSR20194296. 40 BSR20194296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sousa F., Mandal S., Garrovo C., Astolfo A., Bonifacio A., Latawiec D., Menk R.H., Arfelli F., Huewel S., Legname G., Galla H.J., Krol S. Functionalized gold nanoparticles: a detailed in vivo multimodal microscopic brain distribution study. Nanoscale. 2010;2:2826–2834. doi: 10.1039/c0nr00345j. [DOI] [PubMed] [Google Scholar]
- 106.Vecchio G., Galeone A., Brunetti V., Maiorano G., Rizzello L., Sabella S., Cingolani R., Pompa P.P. Nanomedicine. 2012;8:1e7. doi: 10.1016/j.nano.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Pompa P.P., Vecchio G., Galeone A., Brunetti V., Sabella S., Maiorano G., Falqui A., Bertoni G., Congolani R. In vivo toxicity assessment of gold nanoparticles in Drosophila melanogaster. Nano Res. 2011;4:405–413. [Google Scholar]
- 108.Nghiem T.H.L., Nguyen T.T., Fort E., Nguyen T.P., Hoang T.M.N., Nguyen T.Q., Tran H.N. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012;3 015002/1e015002/5. [Google Scholar]
- 109.Chen Y.S., Hung Y.C., Liau I., Huang G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4:858–864. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Venkatpurwar V., Mali V., Bodhankar S., Pokharkar V. Toxicol. Environ. Chem. 2012;94:1357e1367. [Google Scholar]
- 111.Li J.J., Hartono D., Ong C.-N., Bay B.-H., Yung L.-Y.L. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials. 2010;31:5996–6003. doi: 10.1016/j.biomaterials.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 112.Li J.J., Hartono D., Ong C.N., Bay B.H., Yung L.Y. Biomaterials. 2010;31:5996e6003. doi: 10.1016/j.biomaterials.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 113.Li T., Albee B., Alemayehu M. Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna. Anal. Bioanal. Chem. 2010;398(2):689–700. doi: 10.1007/s00216-010-3915-1. [DOI] [PubMed] [Google Scholar]
- 114.Nam S.H., Lee W.M., Shin Y.J. Derivation of guideline values for gold (III) ion toxicity limits to protect aquatic ecosystems. Water Res. 2014;48(1):126–136. doi: 10.1016/j.watres.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 115.Schneider, T., Westermann, M. & Glei, M. In vitro uptake and toxicity studies of metal nanoparticles and metal oxide nanoparticles in human HT29 cells. Arch. Toxicol.. DOI 10.1007/s00204-017-1976-z. [DOI] [PubMed]
- 116.Tsai S.W., Liaw J.W., Kao Y.C., Huang M.Y., Lee C.Y., Rau L.R., Huang C.Y., Wei K.C., Ye T.C. PloS One. 2013;8:1e12. doi: 10.1371/journal.pone.0076545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ng C.T., Li J.J., Gurung R.L., Hande M.P., Ong C.N., Bay B.H., Yung L.Y.L. Exp. Biol. Med. 2013;238:1355e1361. doi: 10.1177/1535370213505964. [DOI] [PubMed] [Google Scholar]
- 118.Kang S.J., Yum Y.N., Kim J.H., Song H., Jeong J., Lim Y.T. Induction of DNA damage in L5178Y cells treated with gold nanoparticle. Biomol.Ther. 2009;17:6. doi: 10.4062/biomolther.2009.17.1.92. [DOI] [Google Scholar]
- 119.Das S., Debnath N., Mitra S., Datta A., Goswami A. Biometals. 2012;25:1009e1022. doi: 10.1007/s10534-012-9567-1. [DOI] [PubMed] [Google Scholar]
- 120.Khan H.A., Abdelhalim M.A.K., Al-Ayed M.S., Alhomida A.S. Saudi J. Biol. Sci. 2012;19:461e464. doi: 10.1016/j.sjbs.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mateo D., Morales P., Ávalos A., Haza A.I. Oxidative stress contributes to gold nanoparticle-induced cytotoxicity in human tumor cells. Toxicol. Mech. Methods. 2014;24:161–172. doi: 10.3109/15376516.2013.869783. [DOI] [PubMed] [Google Scholar]
- 122.Pongsuchart M., Danladkaew C., Khomvarn T., Sereemaspun A. Int. Proc. Chem. Biol. Environ. Eng. 2012;27:98e102. [Google Scholar]
- 123.Maiorano G., Sabella S., Sorce B., Brunetti V., Malvindi M.A., Cingolani R., Pompa P.P. ACS Nano. 2010;4:7481e7491. doi: 10.1021/nn101557e. [DOI] [PubMed] [Google Scholar]
- 124.Sabella S., Brunetti V., Vecchio G., Galeone A., Maiorano G., Cingolani R., Pompa P.P. J. Nano Res. 2011;13:6821e6835. doi: 10.1039/c1nr10233h. [DOI] [PubMed] [Google Scholar]
- 125.Martinez Paino I.M., Spolon Marangoni V., de Cassia Silva de Oliveira R., Greggi Antunes L.M., Zucolotto V. Toxicol. Lett. 2012;215:119e125. doi: 10.1016/j.toxlet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 126.Di Guglielmo C., De Lapuente J., Porredon C., Ramos-Lopez D., Sendra J., Borras M. J. Nanosci. Nanotechnol. 2012;12:6185e6191. doi: 10.1166/jnn.2012.6430. [DOI] [PubMed] [Google Scholar]
- 127.Soenen S.J., Manshian B., Montenegro J.M., Amin F., Meermann B., Thiron T., Cornelissen M., Vanhaecke F., Doak S., Parak W.J. ACS Nano. 2012;6:5767e5783. doi: 10.1021/nn301714n. [DOI] [PubMed] [Google Scholar]
- 128.Schaeublin N.M., Braydich-Stolle L.K., Schrand A.M., Miller J.M., Hutchison J., Schlager J.J., Hussain S.M. Nanoscale. 2011;3:410e420. doi: 10.1039/c0nr00478b. [DOI] [PubMed] [Google Scholar]
- 129.Chueh P.J., Liang R.Y., Lee Y.H., Zheng Z.M., Chuang S.M. J. Hazard Mater. 2014;264:303e312. doi: 10.1016/j.jhazmat.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 130.Stelzer R., Hutz V. J. Reprod. Develop. 2009;55:685e690. doi: 10.1262/jrd.20241. [DOI] [PubMed] [Google Scholar]
- 131.Moretti E., Terzuoli G., Renieri T., Iacoponi F., Castellini C., Giordano C., Collodel G. Andrologia. 2013;45:392e396. doi: 10.1111/and.12028. [DOI] [PubMed] [Google Scholar]
- 132.Choi S.Y., Jeong S., Jang S.H., Park J., Park J.H., Ock K.S., Lee S.Y., Joo S.W. Toxicol. Vitro. 2012;26:229e237. doi: 10.1016/j.tiv.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 133.Patra H.K., Banerjee S., Chaudhuri U., Lahiri P., Dasgupta A.K. Cell selective response to gold nanoparticles. Nanomedicine. 2007;3:111–119. doi: 10.1016/j.nano.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 134.Gao W., Xu K., Ji L., Tang B. Toxicol. Lett. 2011;205:86e95. doi: 10.1016/j.toxlet.2011.05.1018. [DOI] [PubMed] [Google Scholar]
- 135.Li K., Zhao X., Hammer B.K., Du S., Chen Y. ACS Nano. 2013;7:9664e9674. doi: 10.1021/nn402472k. [DOI] [PubMed] [Google Scholar]
- 136.Li W.Q., Wang F., Liu Z.M., Wang Y.C., Wang J., Sun F. Small. 2013;9:1708e1714. doi: 10.1002/smll.201201079. [DOI] [PubMed] [Google Scholar]
- 137.Bachand D., Allen A., Bachand M., Achyuthan K.E., Seagrave J.C., Brozik S.M. J. Nano Res. 2012;14:1212e1222. [Google Scholar]
- 138.Chuang S.M., Lee Y.H., Liang R.Y., Roam G.D., Zeng Z.M., Tu H.F., Wang S.K., Chueh P.J. Biochim. Biophys. Acta. 2013;1830:4960e4973. doi: 10.1016/j.bbagen.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 139.Jain S., Coulter J.S., Butterworth K.T., Hounsell A.R., McMahon S.J., Hyland W.B., Muir M.F., Dickson G.R., Prise K.M., Currell F.J. Radiother. Oncol. 2014;110:342e347. doi: 10.1016/j.radonc.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 140.Pfaller T., Colognato R., Nelissen I., Favilli F., Casals E., Ooms D., Leppens H., Ponti J., Stritzinger R., Puntes V. Nanotoxicology. 2010;4:52e72. doi: 10.3109/17435390903374001. [DOI] [PubMed] [Google Scholar]
- 141.Vetten M.A., Tloleng N., Rascher D.T., Skepu A., Keter F.K., Boodhia K., Koekemoer L.A., Andraos C., Tshikhudo R., Gulumian M. Part. Fibre Toxicol. 2013;10:50e65. doi: 10.1186/1743-8977-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salado J., Insausti M., Lezama L., Gil de Muro I., Moros M., Pelaz B., Grazu V., de la Fuente J.M., Rojo T. Nanotechnology. 2012;23 doi: 10.1088/0957-4484/23/31/315102. 315102/1e315102/10. [DOI] [PubMed] [Google Scholar]
- 143.Cho T.J., MacCuspie R.I., Gigault J., Gorham J.M., Elliott J.T., Hackley V.A. Langmuir. 2014;30:3883e3893. doi: 10.1021/la5002013. [DOI] [PubMed] [Google Scholar]
- 144.Aueviriyavit S., Phummiratch D., Maniratanachote R. Toxicol. Lett. 2014;224:73e83. doi: 10.1016/j.toxlet.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 145.Raji V., Kumar J., Rejiya C.S., Vibin M., John A., Abraham A. J. Exp. Nanosci. 2012;7:174e188. [Google Scholar]
- 146.Kaur H., Pujari G., Semwal M.K., Sarma A., Avasthi D.K. Nucl. Instrum. Methods Phys. Res., Sect. B. 2013;301:7e11. [Google Scholar]
- 147.Mallick S., Sun I.C., Kim K., Yi D.K. J. Nanosci. Nanotechnol. 2013;13:3223e3229. doi: 10.1166/jnn.2013.7149. [DOI] [PubMed] [Google Scholar]
- 148.Leifert A., Pan Y., Kinkeldey A., Schiefer F., Setzler J., Scheel O., Lichtenbeld H., Schmid G., Wenzel W., Jahnen-Dechent W. Proc. Natl. Acad. Sci. U.S.A. 2013;110:8004e8009. doi: 10.1073/pnas.1220143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meng J., Li Y., Liu J., Cheng X., Guo H., Zhang W., Wu X., Xu H. Nanotoxicology. 2014;8:686e696. doi: 10.3109/17435390.2013.822593. [DOI] [PubMed] [Google Scholar]
- 150.Schmid G., Kreyling W.G., Simon U. Toxic effects and biodistribution of ultrasmall gold nanoparticles. Arch. Toxicol. 2017;91:3011–3037. doi: 10.1007/s00204-017-2016-8. [DOI] [PubMed] [Google Scholar]
- 151.Fraga S. Short-and long-term distribution and toxicity of gold nanoparticles in the rat after a single-dose intravenous administration. Nanomed.-Nanotechnol. 2014;10:1757–1766. doi: 10.1016/j.nano.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 152.Hirn S. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011;77:407–416. doi: 10.1016/j.ejpb.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abdelhalim M.A.K., Moussa S.A.A. Saudi J. Biol. Sci. 2013;20:177e181. doi: 10.1016/j.sjbs.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Daraee H., Eatemadi A., Abbasi E., Fekri Aval S., Kouhi M., Akbarzadeh A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016;44:410–422. doi: 10.3109/21691401.2014.955107. [DOI] [PubMed] [Google Scholar]
- 155.Lin Z., Monteiro-Riviere N.A., Kannan R., Riviere J.E. A computational framework for interspecies pharmacokinetics, exposure and toxicity assessment of gold nanoparticles. Nanomedicine. 2016;11:107–119. doi: 10.2217/nnm.15.177. [DOI] [PubMed] [Google Scholar]
- 156.Ambwani S, Kandpal D, Arora S, Ambwani TK. 2ND INTERNATIONAL CONFERENCE ON EMERGING TECHNOLOGIES: MICRO TO NANO 2015 (ETMN-2015): Contributory papers presented in 2nd International Conference on Emerging Technologies: Micro to Nano 2015. AIP Publishing; 2016. Cytotoxic effects of gold nanoparticles exposure employing in vitro animal cell culture system as part of nanobiosafety; p. 020091. [Google Scholar]
- 157.Simpson C.A., Salleng K.J., Cliffel D.E., Feldheim D.L. Nanomed. Nanotechnol. Biol. Med. 2013;9:257e263. doi: 10.1016/j.nano.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 158.Bogdanov A.A., Gupta S., Koshkina N. Gold nanoparticles stabilized with mpeg-grafted poly(L-Lysine): in vitro and in vivo evaluation of a potential theranostic agent. Bioconjugate Chem. 2015;26(1):39–50. doi: 10.1021/bc5005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tiwari P.M., Vig K., Dennis V.A., Singh S.R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 2011;1(1):31–63. doi: 10.3390/nano1010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vijayakumar S., Ganesan S. J. Nanomater. 2012;2012 Article ID 734398, 1e9. [Google Scholar]
- 161.Vijayakumar S., Ganesan S. Vitro cytotoxicity assay on gold nanoparticles with different stabilizing agents. J. Nanomater. 2012;2012:9. [Google Scholar]
- 162.Bozich J.S., Lohse S.E., Torelli M.D. Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to daphnia magna. Environ-Sci Nano. 2014;1(3):260–270. [Google Scholar]
- 163.Alkilany A.M., Nagaria P.K., Hexel C.R., Shaw T.J., Murphy C.J., Wyatt M.D. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small. 2009;5(6):701–708. doi: 10.1002/smll.200801546. [DOI] [PubMed] [Google Scholar]
- 164.Zhang G., Yang Z., Lu W. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30(10):1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhai J., Hinton T.M., Waddington L.J. Lipid-Peg conjugates sterically stabilize and reduce the toxicity of phytantriol-based lyotropic liquid crystalline nanoparticles. Langmuir. 2015;31(39):10871–10880. doi: 10.1021/acs.langmuir.5b02797. [DOI] [PubMed] [Google Scholar]
- 166.Simpson C.A., Salleng K.J., Cliffel D.E., Feldheim D.L. In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles. Nanomedicine. 2013;9(2):257–263. doi: 10.1016/j.nano.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 167.Pan Y., Leifert A., Ruau D. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009;5(18):2067–2076. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 168.Dragoni S., Franco G., Regoli M., Bracciali M., Morandi V., Sgaragli G., Bertelli E., Valoti M. Gold nanoparticles uptake and cytotoxicity assessed on rat liver precision-cut slices. Toxicol. Sci. 2012;128(1):186–197. doi: 10.1093/toxsci/kfs150. [DOI] [PubMed] [Google Scholar]
- 169.Botha T.L., James T.E., Wepener V. Comparative aquatic toxicity of gold nanoparticles and ionic gold using a species sensitivity distribution approach. J. Nanomater. 2015 doi: 10.1155/2015/986902. Article ID 986902. [DOI] [Google Scholar]
- 170.Griffith L.G., Swartz M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 171.Piryazev A.P., Azizova O.A., Aseichev A.V., Dudnik L.B., Sergienko V.I. Effect of gold nanoparticles on production of reactive oxygen species by human peripheral blood leukocytes stimulated with opsonized zymosan. Bull. Exp. Biol. Med. 2013;156:101–103. doi: 10.1007/s10517-013-2288-9. [DOI] [PubMed] [Google Scholar]
- 172.Downs T.R., Crosby M.E., Hu T., Kumar S., Sullivan A., Sarlo K. Silica nanoparticles administered at the maximum tolerated dose induce genotoxic effects through an inflammatory reaction while gold nanoparticles do not. Mutat Res Toxicol Environ Mutagen. 2012;745:38–50. doi: 10.1016/j.mrgentox.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 173.Khan H.A., Abdelhalim M.A.K., Alhomida A.S., Al-Ayed M.S. Effects of naked gold nanoparticles on proinflammatory cytokinesmRNAexpression in rat liver and kidney. BioMed Res. Int. 2013;2013:590730. doi: 10.1155/2013/590730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang C., Yang H., Wu J., Meng Z., Xing R., Tian A. No overt structural or functional changes associated with PEG-coated gold nanoparticles accumulation with acute exposure in the mouse heart. Toxicol. Lett. 2013;222:197–203. doi: 10.1016/j.toxlet.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 175.Sousa A.A., Hassan S.A., Knittel L.L., Balbo A., Aronova M.A., Brown P.H. Biointeractions of ultrasmall glutathione-coated gold nanoparticles: effect of small size variations. Nanoscale. 2016;8:6577–6588. doi: 10.1039/c5nr07642k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li J.J., Zou L., Hartono D., Ong C.N., Bay B.H., Lanry Yung L.Y. Gold nanoparticles induce oxidative damage in lung fibroblasts in vitro. Adv. Mater. 2008;20:138–142. doi: 10.1002/adma.200701853. [DOI] [Google Scholar]
- 177.Auffan M., Rose J., Orsiere T., De Meo M., Thill A., Zeyons O. CeO2 nanoparticles induce DNA damage towards human dermal fibroblasts in vitro. Nanotoxicology. 2009;3:161–171. doi: 10.1080/17435390902788086. [DOI] [Google Scholar]
- 178.Siddiqi N.J., Abdelhalim M.A., El-Ansary A.K., Alhomida A.S., Ong W.Y. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J. Neuroinflammation. 2012;9:123. doi: 10.1186/1742-2094-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ojea-Jimenez I., Puntes V. Instability of cationic gold nanoparticle bioconjugates: the role of citrate ions. J. Am. Chem. Soc. 2009;131:13320–13327. doi: 10.1021/ja902894s. [DOI] [PubMed] [Google Scholar]
- 180.Siddiqi N.J., Abdelhalim M.A., El-Ansary A.K., Alhomida A.S., Ong W.Y. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J. Neuroinflammation. 2012;9:123. doi: 10.1186/1742-2094-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Freese C., Uboldi C., Gibson M.I., Unger R.E., Weksler B.B., Romero I.A., Couraud P.O., Kirkpatrick C.J. Part. Fibre Toxicol. 2012;9:1e11. doi: 10.1186/1743-8977-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pan Y., Neuss S., Leifert A., Fischler M., Wen F., Simon U., Schmid G., Brandau W., Jahnen-Dechent W. Small. 2007;3:1941e1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 183.Leroueil P.R., Berry S.A., Duthie K., Han G., Rotello V.M., McNerny D.Q., Baker J.R., Orr B.G., Banaszak H., Mark M. Nano Lett. 2008;8:420e424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 184.Camesano T.A., D'Angelo S.M. Canada; Montreal, QC: 2011. 85th ACS Colloid and Surface Science Symposium. [Google Scholar]
- 185.Chakraborty S., Joshi P., Shanker V., Ansari Z.A., Singh S.P., Chakrabarti P. Langmuir. 2011;27:7722e7731. doi: 10.1021/la200787t. [DOI] [PubMed] [Google Scholar]
- 186.Tiedemann D., Taylor U., Rehbock C., Jakobi J., Klein S., Kues W.A., Barcikowski S., Rath D. Analyst. 2014;139:931e942. doi: 10.1039/c3an01463k. [DOI] [PubMed] [Google Scholar]
- 187.Young K.L., Scott A.W., Hao L., Mirkin S.E., Liu G., Mirkin C.A. Nano Lett. 2012;12:3867e3871. doi: 10.1021/nl3020846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Suh K.S., Lee Y.S., Seo S.H., Kim Y.S. Biol. Trace Elem. Res. 2013;153:428e436. doi: 10.1007/s12011-013-9679-7. [DOI] [PubMed] [Google Scholar]
- 189.Jebali A., Kazemi B. Toxicol. Vitro. 2013;27:1847e1854. doi: 10.1016/j.tiv.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 190.Chaicherd S., Killingsworth M.C., Pissuwan D. Toxicity of gold nanoparticles in a commercial dietary supplement drink on connective tissue fibroblast cells. SN Applied Sciences. 2019;1:336. doi: 10.1007/s42452-019-0354-2. 2019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.