Abstract
Iran is one of the largest honey-producing countries worldwide and is considered as an important source of honey for international markets. However, since Iran is not registered for honey export to Europe, the quality of Iranian honey remains unknown to European traders. As the first step in filling this gap, we analyzed 225 honey samples using palynology, sensory, nuclear magnetic resonance (NMR) and conventional physicochemical analyses as outlined by the European Union coordinated control plan. The results show that while various types of genuine unifloral honey can be harvested in Iran, 85% of collected samples were adulterated. Performing principal component analysis on physicochemical parameters reveals that feeding tablet sugar and syrup of C4 origin to bees during the foraging season is a common mode of fraud. Replacement of natural nectar with sugar syrup together with presence of intensive aftertaste from Taraxacum and Eryngium affect the taste of unifloral honeys produced in Iran.
Keywords: Honey, Adulteration, NMR, Melissopalynology, Sensory, Physicochemical analyses
Honey, adulteration, NMR, melissopalynology, sensory, physicochemical analyses
1. Introduction
According to statistics from the Food and Agriculture Organization of the United Nations (FAOSTAT), Iran is ranked 9th worldwide in average annual production of honey between 1993 and 2018 (FAOSTAT, 2018). This fact implies that Iran can be a considerable supplier of honey for the international market. However, among the 10 largest honey-producing countries in the FAOSTAT report, Iran is the only one that cannot export honey to Europe. The reason is the lack of a monitoring program that ensures the rules and principles applied by Iranian certifying agents to provide guarantees equivalent to those laid down in Directives 96/93/EC and 2001/110/EC (Council of the European Union, 1996; 2001). Therefore, to the best of our knowledge, the widespread monitoring of Iranian honey has never been performed through random sampling and analysis by a European certified food quality control laboratory. Consequently, the source and quality of Iranian honey is unknown to the European market.
In former studies on the characterization of honey from different regions of Iran, sensory and detailed palynological analyses, as the core tools to identify botanical origin of honey, were not addressed sufficiently (Moloudian et al., 2018; Parviz et al., 2015; Zahedi Namini et al., 2018) or the methodology was poorly explained (Moloudian et al., 2018). However, before the botanical origin of honey samples is determined according to international conventions, any comparison of their physicochemical properties can be misleading. Another common issue in these studies is focusing on honey types that are not well defined. For instance, the concept of milkvetch or Gavan (common names for Astragalus spp.) honey exists only in Iran and China. However, in European melissopalynology laboratories the pollen of Astragalus is excluded from counting if the aim is verification of a unifloral honey. The reason, is the absence of any trace of Astragalus nectar in the taste of honey even if the sample contains a high percentage of Astragalus pollen. It is shown that Astragalus spp. is not a highly prolific source of nectar (Knuth et al., 1906). In addition, if the Astragalus honey exists, the standards for its organoleptic and palynological characteristics must be set up first so that its botanical origin can be approved prior to description of physicochemical properties. Another example of unusual honey types reported in the literature is pear honeydew (Moloudian et al., 2018). Honeydew is a liquid secreted by aphids and collected and processed by bees. The result is a dark honey with an electrical conductivity greater than 0.8 mS/cm Although some of blossom honey types such as Castanea, Calluna, Arbutus, Erica, and Tilia can also have electrical conductivity near or higher than 0.8 mS/cm (Oddo and Piro, 2004)., honey from pear and other fruit trees of Rosaceae has light color and electrical conductivity of less than 0.4 mS/cm (D.I.B., 2014). Therefore, due to the lack of compliance with international conventions, the questions about the quality of Iranian honey cannot be answered based on the former studies.
This study aims at utilizing the standard and state of the art methods used by commercial food control labs to introduce the source and quality of honey from one of the world's largest honey producers. In a collaborative project between the University of Goettingen, Bayer Bee Care center and Quality Service International (QSI GmbH), we tested Iranian honey samples using palynology, sensory, and physicochemical analyses as outlined by the European Union coordinated control plan (Council of the European Union, 2015).
2. Materials and methods
2.1. Sampling
For the present study, the Iranian provinces West Azerbaijan, East Azerbaijan and Ardabil were chosen because they are respectively ranked as the 1st to the 3rd for honey production in Iran (Agricultural Bank of Iran, 2011). Isfahan province, ranked nationally as the 6th, was also included because the honey from Khansar region has a good reputation nationwide. These four provinces account for approximately 58% of honey production in Iran, and their flora is representative of Irano-Turanian and Euxine-Hyrcanian floristic regions and the Zagros zone as the main floristic regions of Iran (Sagheb-Talebi et al., 2014). Moreover, during the sampling, we realized that many beekeepers spent spring and early summer in southern (Saharo-Sindian phytochorion) and northern (Hyrcanian phytochorion) areas of Iran. Therefore, the samples examined in this study could represent the main floral sources of honey from different regions of Iran. The beekeepers we met during the fieldworks believe that the main floral source of their honeys are Astragalus spp. and Eryngium spp.
In total, 225 honey samples were collected during two fieldworks in 2015 (29 samples) and 2017 (196 samples). The number of collected samples for each province ranges from 53 to 61. In both fieldworks, plants with flowers (bud and blossom) were sampled for the reference slide preparation. All honey samples were examined by palynology and sensory. However, since the samples taken from neighboring apiaries showed closely similar pollen spectra and thus had more likely similar physicochemical properties, 85 samples were excluded from physicochemical analyses.
2.2. Melissopalynology
Slide preparation was performed according to the method suggested by the International Honey Commission (IHC); 10 g of honey were mixed with 20 ml of water in a 50-ml conical tube. Then the solution was centrifuged for 10 min at 1000 g, the supernatant was decanted and a second wash and centrifugation for 5 min at 1000 g was carried out (Von Der Ohe et al., 2004). The concentrate was mixed with one droplet of glycerol-gelatin and mounted on a glass slide. After covering with a cover slip, the slides were left for 10 min to let the gelatin harden. To retain important particles, such as yeast and starch, on the background of slides, we avoided acetolysis.
To make reference slides, using the parental plants, which were taxonomically identified at the Tehran and Urmia Universities, the stamen anthers were dissected, washed with water and sieved to remove the large particles. After centrifuging for 5 min at 1000 g, the sediment was mounted on the slide as described above.
In each slide, at least 300 pollen grains were counted and identified under light microscopy with 400x magnification. The reference slides made in this study, the reference collections at QSI and the University of Goettingen were used for identification. The slides also were checked for the amount of starch, spores and yeast. The number of starch particles is reported as <10%, 10%–30% and >30% of counted pollen that are scored as low, medium and high levels, respectively. Spores were counted in the same way as starch and yeast with a hemocytometer only if it was very abundant in the background.
2.3. Organoleptic (sensory) analysis
The taste and smell of the samples were described according to the IHC odor and aroma wheel (Piana et al., 2004) by the sensory team of QSI composed of 3 men and 4 women led by 3 sensory experts. The team leaders have between 10 and 25 years of experience in daily analysis of honey from different botanical and geographical origins. Informed consent was obtained from all participants in sensory experiments. The sensory room and questionnaire were in accordance with the method described by sensory group of international honey commission (Marcazzan et al., 2018). The questionnaire is provided as Table 1. The color of samples at the viscose state was compared with the Pfund color chart (USDA, 1985). The consistency of samples was recorded 3, 6 and 12 months after sampling to explore the rate of crystallization. Fluid, viscose, partly crystallized and crystallized are the four categories of consistency used in the QSI honey laboratory (Standard For Honey Codex Stan 12-1981, 2001). If the sensory (in particular taste and smell) matches any of the unifloral honey types, the sensory of the sample was judged as source specific.
Table 1.
The sensory questionnaire used in the study as described by Marcazzan et al. (2018).
| Sample code | Assessor | Date |
|---|---|---|
| Intensity of sensory descriptors: | ||
| Odour/Aroma (Global olfactory intensity) | ||
| Floral | ||
| Soft | ||
| Heady | ||
| Fruity | ||
| Fresh fruit | ||
| Tropical fruit | ||
| Sugary | ||
| Processed food | ||
| Like wine (winish) | ||
| Warm | ||
| Slight | ||
| Lactic | ||
| Caramelized | ||
| Toasty | ||
| Malty (Molasses) | ||
| Burned | ||
| Aromatic | ||
| Spicy | ||
| Resinous | ||
| Woody | ||
| Camphorated (Mentholated) | ||
| Esperidato (Citrus fruit) | ||
| Bitter almond | ||
| Chemical | ||
| Phenolic | ||
| Soap | ||
| Smocked | ||
| Vinegar (Pungent) | ||
| Ammoniacal | ||
| Vegetal | ||
| Green | ||
| Mouldy | ||
| Dry | ||
| Animal | ||
| Sulphuric | ||
| Proteic | ||
| Perspiration | ||
| Cat's urine | ||
| Taste Intensity | ||
| Sweetness | ||
| Sourness | ||
| Bitterness | ||
| Saltiness | ||
2.4. Physicochemical analyses
Besides assisting the palynology and sensory to approve the botanical origin of honey, physicochemical analyses were performed to detect adulteration. In the framework of the NMR project, QSI together with Bruker are developing a database for chemical profiles of honey samples collected worldwide to combat fraud in the honey business. To create the NMR database, conventional physicochemical parameters, including electrical conductivity, moisture content, hydroxymethylfurfural (HMF), diastase activity, sugars, pH and acidity, were also measured as described below. All measurements were carried out in duplicate and all methods were validated by Deutsche Akkreditierungsstelle GmbH.
2.4.1. Electrical conductivity
Electrical conductivity is routinely used together with palynology and sensory as a reliable method to determine the botanical origin of the honey. In general, blossom honey and honeydew have electrical conductivity of <0.5 and >0.8 mS/cm, respectively. There are some exceptions among blossom honey types which have electrical conductivity near or higher than 0.8 mS/cm (Oddo and Piro, 2004) for example Castanea, Calluna, Arbutus, Erica, and Tilia Between these two ranges, the honey is classified as blossom-honeydew. In addition, each unifloral honey type has a specific standard range of electrical conductivity (Beckh and Camps, 2009).
Electrical conductivity was measured as described by the harmonized methods of the European honey commission (Bogdanov et al., 1997) in 10 g of honey dissolved in 75 ml of demineralized water using the EC Electrode model LF413T3MIDS installed on the Schott instrument Titroline 7800 from SI analytics operating via software Titrisoft 3.1.5. .
2.4.2. Moisture content
The water content is an important factor to determine the shelf life of the honey. The higher the moisture, the faster the fermentation due to the yeast activity (Bogdanov et al., 1997). According to honey directive 2001/110/EC (Council of the European Union, 2001), the moisture content should not exceed 20 g/100 g.
As suggested by the harmonized methods of the European honey commission (Bogdanov et al., 1997), approximately 5 g of honey was melted at 60 °C, and any foam and large impurities were removed prior to measurement. The remaining sample was homogenized once again without severe stirring to avoid air bubble formation in the mixture. After cooling, the sample was measured on a Schmidt and Haensch automatic table refractometermodel ATR BR, operating via the software 3.2.
2.4.3. Hydroxymethylfurfural
The value of HMF is used to assess the freshness of the sample, the amount of heat the honey received and the potential adulteration (Pasias et al., 2017). The HMF content should not exceed 40 mg/kg after processing and/or blending. However, this value may reach 80 mg/kg if the honey comes from a tropical origin (Council of the European Union, 2001).
The base of the technique used for hydroxymethylfurfural evaluation was method number 10751 proposed by the German Institute for Standardization (DIN - Deutsches Institut für Normung, 2010). The measurement was performed on a Thermo Fisher ARENA auto-analyzer using 5 g of honey dissolved in demineralized water and reagents HMF R1 Amin (containing aminoacetophenone and glycerol 85%) and HMF R2 Barbi (containing barbituric acid).
2.4.4. pH and acidity
In general, honey is acidic with a pH ranging between 3.5 and 4.5 (Beckh and Camps, 2009). The duration of storage, adulteration with sugar (Yadata, 2014) and botanical origin (Sousa et al., 2016) can affect these values. The free acidity of honey may not be greater than 50 milliequivalents of acid per 1000 g (Council of the European Union, 2001).
Acidity and pH were measured as suggested by the harmonized methods of the European honey commission (Bogdanov et al., 1997). In a specimen cup, 10 g of honey was dissolved in 75 ml of demineralized water, and pH and acidity were then measured using the pH-Electrode model ScienceLine A 162 2 M-DIN-ID installed on the Schott instrument Titroline 7800 from SI analytics operating via the software Titrisoft 3.1.5.
2.4.5. Diastase activity
Diastase activity reflects the freshness of the honey and can also be used as an indicator for adulteration (Pasias et al., 2017). Diastase activity, which is measured after processing and/or blending, in general should not be less than 8 Schade units and in the case of honeys with low natural enzyme content not less than 3 Schade units (Council of the European Union, 2001).
The method used here was adopted based on the harmonized methods of the European Honey Commission (Bogdanov et al., 1997) and DIN method number 10750. First, 1 g of honey was dissolved in 4.5 ml of acetate buffer, and then the diastase was measured on a Thermo Fisher ARENA auto analyzer by applying the following reagents: i) reagent 1 corresponding to the DIN starch solution, ii) reagent 2 corresponding to the DIN dilute iodine solution, and iii) buffer containing sodium acetate and common salt dissolved in demineralized water.
2.4.6. Sugars
A large variety of sugars are measured by means of both NMR and HPLC (as a control for NMR). In the present study, however, we used only the values obtained from NMR except for erlose, which is not measured by NMR. This sugar can act as an indicator for botanical origin, particularly to distinguish between honeydew and blossom honey. The values of erlose oscillate between 0.1 and 0.9 g/100 g in blossom honey, while this range is between 0 and 3.4 g/100 g in honeydew. Detectable amounts of erlose do not exist in many types of unifloral honey (Horn & Lüllmann, 2017).
Two standards used for HPLC were 958do internal standard (31.25 g of xylose +1 ml of 1% methylene blue B 524 and 100 ml of 25% methanol) and standard measurement solution for the sugar spectrum (Spectrum chemical corp) containing Xylose (100.0), Fructose (40.1), Glucose (27.4), Saccharose (5.3), Turanose (3.1), Maltose (2.9), Trehalose (1.8), Isomaltose (2.0), Erlose (4.1), Melizitose (3.9), Maltotriose (2.9). The numbers in parenthesis shows concentration in g/100g.
For evaluation, a mixture of 1.25 g of honey, 250 μl of internal standard 958do and 23.8 ml of 25% methanol is passed through a membrane filter and measured using a refractive index (RI) detector on a Shimadzu HPLC system at 40 °C, with a dilution factor of 1, flow rate of 1.2 ml/min and injection volume of 4.0 μl for 12 min.
2.4.7. Isotopes
The C4-sugar content of honey is an indicator for adulteration by cheap sugar from corn and sugar cane. This value can be calculated from the difference in δ13C between the entire honey and its protein fraction (White and Winters, 1989).
All steps of measurement, including the preparation of honey and protein fraction, protein isolation and purification and determination of carbon isotopes, were performed based on the AOAC official method number 988.12 (Association of Official Analytical Chemists (AOAC), 2013). Both honey and protein fractions were measured on Picarro G2121-i Isotope and Gas Concentration Analyzer (operating via the software WinPro10 64 Bit Version 1903) together with a Thermo Fisher Delta V Advantage Isotope Ratio Mass Spectrometer (operating with the software Isolat 3.0).
2.4.8. NMR
NMR profiles were obtained of compounds belonging to different functional groups, such as saccharides, organic acids, amino acids, alkaloid, and alcohols, within honey. The final purpose of the NMR project is to establish a reference database of these profiles that can be used to determine the botanical and geographical origin as well as potential adulteration of an unknown sample. Such a database currently exists in QSI for many botanical origin and adulteration sources from various countries. The following compounds were detected in Iranian honey samples using NMR:
-
-
Saccharides (fructose, glucose, sucrose, turanose, maltose, melezitose, maltotriose, gentiobiose, and raffinose);
-
-
Organic acids (citric acid, malic acid, lactic acid, formic acid, fumaric acid, pyruvic acid, succinic acid, and acetic acid);
-
-
Amino acids and their derivatives (alanine, glutamine, proline, valine, tyrosine, phenylalanine, pyroglutamic acid)
-
-
Other organic chemicals, such as: shikimic acid; trigonelline; 2,3-butanediol; and ethanol.
All chemicals used in NMR were of analytical grade (>99% purity). The sample preparation method was adopted from BrukerBiospin (BrukerBiospin, Rheinstetten, Germany). The homogenized honey samples (5 g) were diluted in 17.5 ml of NMR-buffer (15.7 g of KH2PO4, 0.05 g of NaN3 in 1 L of deionized water) and adjusted to pH 3.1 with 1 M HCl and 1 M NaOH. A volume of 100 μl of standard solution (deuterium oxide containing 0.1% of 2,2,3,3-d(4)-3-(trimethylsilyl)propionic acid sodium salt (TSP)) was added to 900 μl of homogenized honey solution. The final solution was centrifuged at 14000 rpm, and 600 μl was transferred into an NMR-tube for direct measurement.
All measurements were performed on a Bruker AscendTM 400 MHz FoodScreener equipped with a 5-mm PA BBI 400SI H-BB-D-05 Z probe and Bruker SampleXpress (BrukerBiospin, Rheinstetten, Germany) for automatic sample change. The samples were measured without rotation.
1H-NMR-spectra were acquired at 300.1 K using the pulse programs noesygppr1d (1D spectra with water presaturation at 4.8 ppm) and jresgpprqf (2D J-resolved spectra, displaying chemical shift and spin-spin coupling information). For 1D spectra, 32 scans and 4 dummy scans of 64 k points were acquired with a spectral width of 20.6 ppm, a receiver gain of 16 and an acquisition time of 4.0 s. The 2D spectra were performed using 4 scans and 16 dummy scans of 8 k (F2-axis) and 40 k (F1-axis) points. The spectral widths were 16.7 ppm (F2) and 0.19 ppm (F1), receiver gain was 16 and acquisition times were 0.6 s (F2) and 0.3 s (F1). NOESY spectra were used for quantification, and JRES spectra were used for verification of compound identification. All spectra were automatically phased, baseline-corrected, and calibrated using TSP as a reference at 0.0 ppm. The compounds were quantified using the Honey-Profiling routine (release 1.0, BrukerBiospin, Rheinstetten, Germany) by automatic integration of the peak area calculated with an external standard (Spraul et al., 2009).
2.5. Statistics
Graphs for descriptive statistics were created in Excel. Principal component analysis (PCA) was performed in R by applying functions prcomp from package stats. Raw data were auto-scaled prior to PCA that means variables are shifted to be zero centered and then scaling is conducted by means of standard deviation.
3. Results and discussion
3.1. Melissopalynology
In the current study, palynological results are used to explore the main botanical origin of Iranian honey and examine whether any sample can be labeled as unifloral. Therefore, only the plant families, most frequent genera and plants that can produce unifloral honey are presented here.
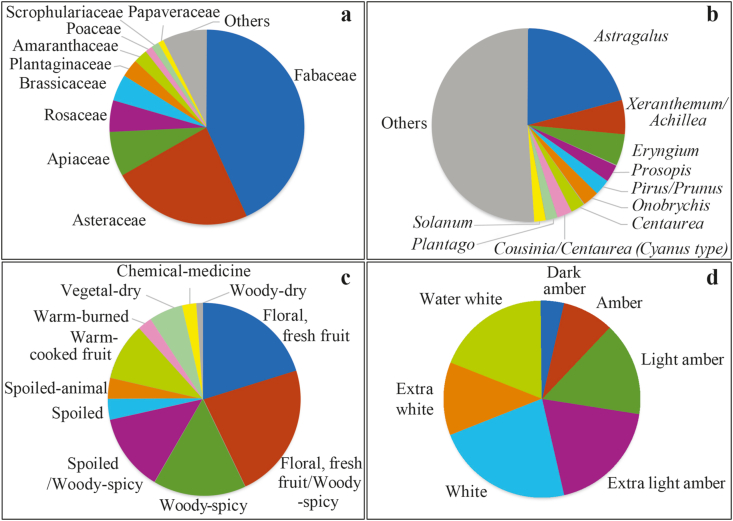
In total, pollen types from 101 taxa were identified. From a palynological point of view, the 10 most frequently visited plant families by bees in landscapes of Iran are Fabaceae, Asteraceae, Apiaceae, Rosaceae, Brassicaceae, Plantaginaceae, Amaranthaceae, Poaceae, Scrophulariaceae and Papaveraceae (family concept follows APG IV (Chase et al., 2016)). The relative abundance of these families is shown in Figure 1.a where “others” includes Lamiaceae, Malvaceae, Rutaceae, Salicaceae, Thymelaeaceae, Boraginaceae, Polygonaceae, Caryophyllaceae, Cannabaceae, Cyperaceae, Rhamnaceae, Myrtaceae, Solanaceae, Lythraceae, Rubiaceae, Convolvulaceae, Euphorbiaceae, Acanthaceae, Campanulaceae, Hypericaceae, Juglandaceae, Urticaceae, Caprifoliaceae, Ranunculaceae, Aquifoliaceae, Betulaceae, Oleaceae, Cucurbitaceae, Elaeagnaceae, Smilacaceae, and Xanthorrhoeaceae, in descending order of abundance.
Figure 1.
Pie charts demonstrating the relative abundance of most important plant families (a) and genera (b), honey smells and tastes (c) and honey colors (d). N = 225.
The pollen types belonging to the genera Astragalus, Xeranthemum/Achillea, Eryngium, Prosopis, Pyrus/Prunus, Onobrychis/Alhagi, Centaurea, Cousinia/Centaurea (C. cyanus type), Plantago and Solanum were the most frequently seen pollen types at the genus level (Figure 1.b). The slash sign is used between genera names with very similar pollen grains that cannot be separated under a light microscope.
Among the taxa that are internationally accepted as a source of unifloral honey, pollen of Brassica napus (rapeseed honey), Centaurea cyanus (cornflower honey), Citrus spp. (orange honey), Helianthus annuus (sunflower honey), Pyrus/Prunus spp. (fruit blossom honey), Taraxacum spp. (dandelion honey), Tilia spp. (lime honey), Trifolium spp. (clover honey), and Ziziphus jujuba (jujube honey) were detected. The pollen of Prosopis was also found in many samples but in Iran this plant grows as a small shrub that is locally called Jeqjeqeh (which means rattle) and differs from the tropical Prosopis that is the source of mesquite honey. Samples containing sufficient percentages of pollen from Centaurea cyanus, Citrus, Helianthus annuus, Taraxacum, Thymus and Tilia to be considered as unifloral honey are discussed in section 3.4.
The relative abundance of starch grains did not exceed 10% in any sample. The spores and yeast were too scattered in the background to be considered for counting.
3.2. Organoleptic (sensory) analysis
Using the honey wheel, the taste and smell of samples were classified into families and when possible into subfamilies. The taste and smell of all samples fell into the same classes. The pie chart in Figure 1.c shows the relative abundance of these classes. In this paper, a slash symbol is used to separate different tastes when more than one taste is detected within a sample (e.g., woody/spoiled), a comma symbol is used if the name of the taste family has two parts (e.g., floral, fresh fruit) and a dash symbol is used between family and subfamily names (e.g., woody-spicy).
The woody-spicy class, individually or together with the spoiled and floral, fresh fruit classes, was the most frequently perceived flavor (51%), followed by floral, fresh fruit (20% alone and 23% together with woody-spicy), spoiled (4% spoiled, 4% spoiled-animal and 13% spoiled/woody-spicy), warm (warm-cooked fruit 10% and warm-burned 2%), vegetal-dry (6%), chemical-medicine (2%) and woody-dry (1%). The few samples with organoleptic properties of unifloral honey are discussed in section 3.4.
The color of samples covers all seven Pfund color categories (USDA, 1985) from water white to dark amber. Figure 1.d illustrates the percentage of samples falling into each category. The number of samples in white tones is slightly higher than the number of those in amber tones.
All samples, including those collected during the fieldwork and those collected by the partners, were viscose when they arrived in Germany. However, after one year, the majority of samples were crystalized. Table 2 displays the change in consistency of samples in one year.
Table 2.
Changes in crystallization of samples in one year. Values are calculated as the percentage of the total number of samples.
| Consistency | month 3 | month 6 | month 12 |
|---|---|---|---|
| Viscose | 73% | 33% | 28% |
| Partly crystalized | 16% | 20% | 5% |
| Crystalized | 11% | 47% | 67% |
3.3. Authenticity
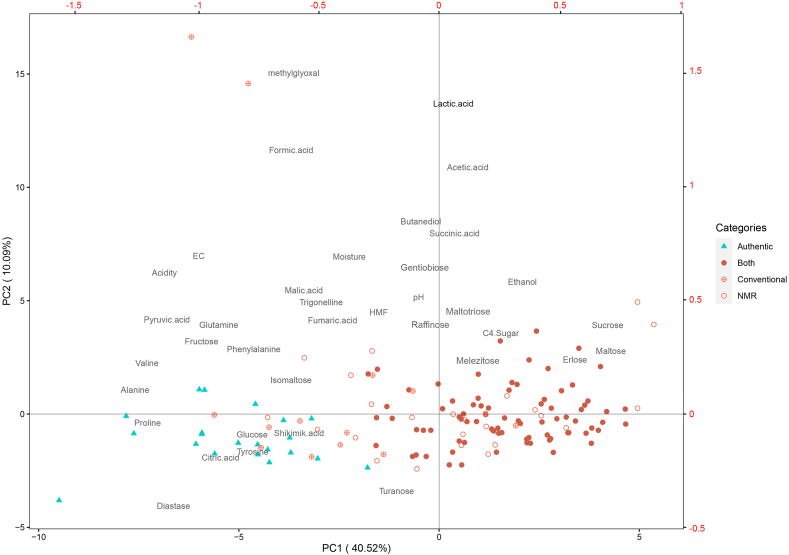
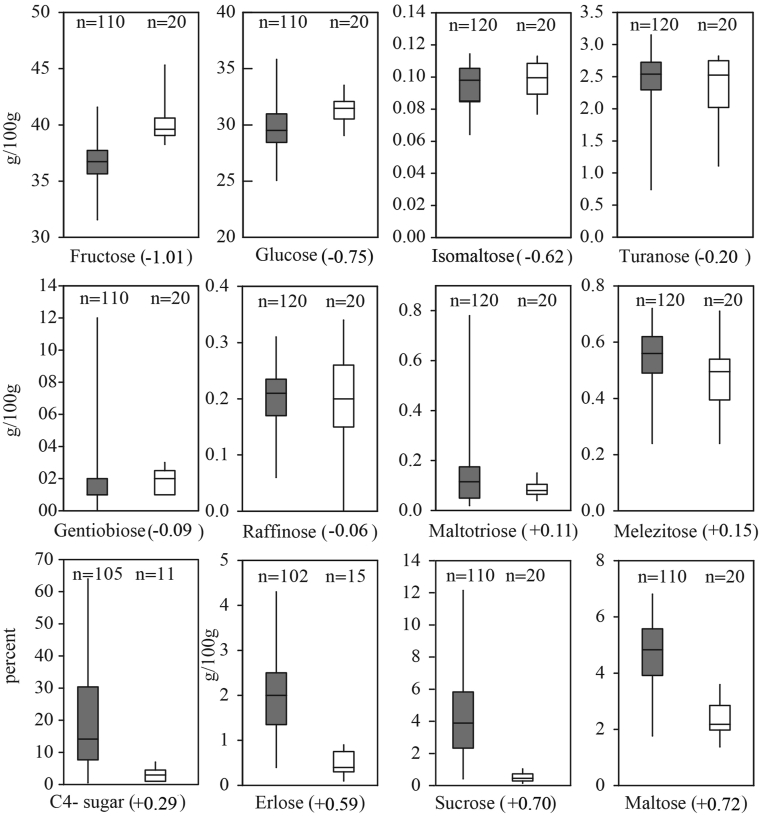
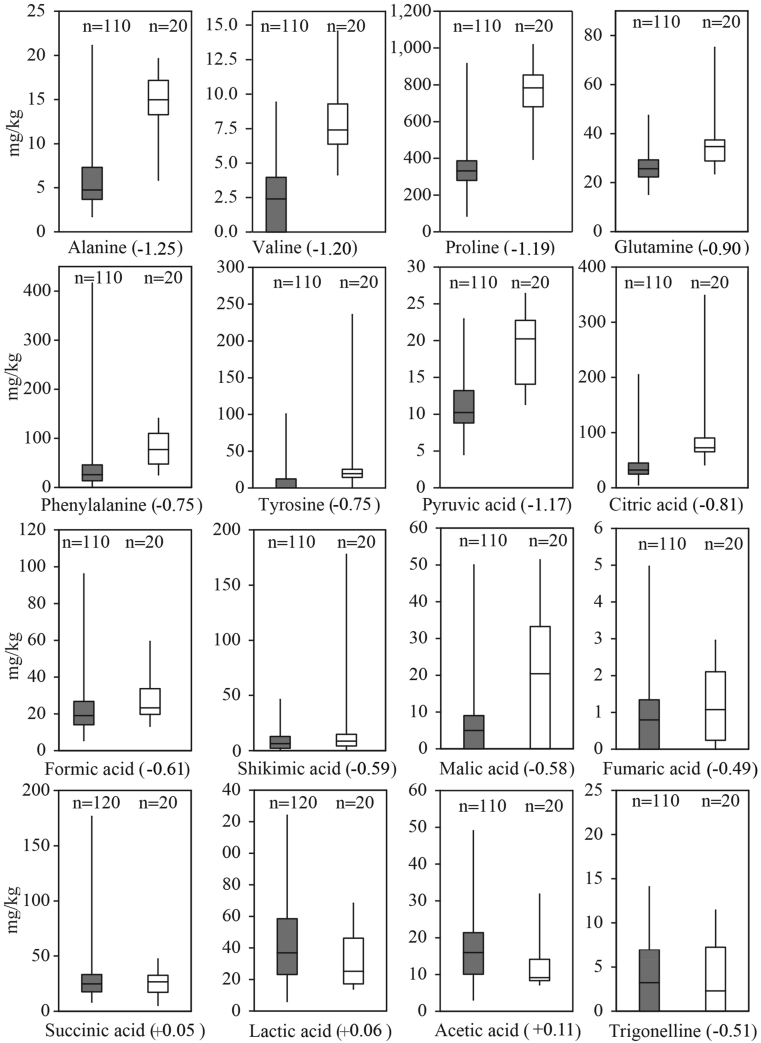
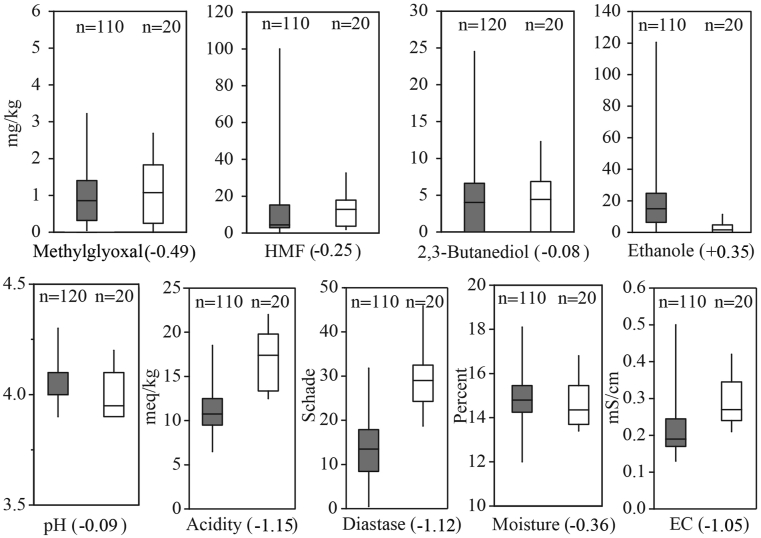
Authentication is the key result of physicochemical analyses that must be addressed prior to other honey properties; otherwise, exploring the botanical origin of an adulterated honey is pointless. The authenticity of Iranian samples was tested by comparing the physicochemical results with the honey directive (Council of the European Union, 2001) and the NMR reference database comprising the data from over 20,000 samples collected worldwide. The outcome shows that only 15% of samples were authentic. The remaining samples were assessed as adulterated: 57% by both NMR and conventional methods, 18% only by NMR and 10% exclusively by conventional methods. In many cases, there were several parameters indicating adulteration of one sample; thus, it was not possible to quantify the contribution from each parameter to overall assessment. However, PCA was applied to identify parameters that play essential roles in authentication. Before performing statistical tests, the notion that the unequal numbers of adulterated and authentic samples may affect the reliability of the tests must be considered. The PCA in Figure 2 shows that the adulterated and authentic samples can be mainly separated through axis PC1. Disaccharides (maltose and sucrose) and C4 sugars are placed to the far right of PC1 that is dominantly populated by adulterated samples. Conversely, monosaccharides (glucose and fructose), Amino acids (e.g. proline, valine, alanine, and glutamine) and diastase activity are located to the left of PC1, close to authentic samples. Samples that are labeled as adulterated only based on one method (either NMR or conventional) appear mainly on transition between adulterated and authentic samples implying their borderline quality. The boxplots in Figures 3, 4, and 5 show the range of values for different parameters in authentic and adulterated samples. The number written in round brackets next to the parameter's name shows the loading on PC1.
Figure 2.
PCA displaying the position of adulterated and authentic samples relative to different parameters. The numbers inside the round brackets on each axis show the contribution from that axis to total variation in dataset. Hollow, crossed and solid circles show adulterated samples determined by NMR, conventional and both NMR and conventional methods, respectively. Bottom, left, top and right axes show PC1 score, PC2 score, loadings on PC1, and loadings on PC2.
Figure 3.
Boxplots showing the range of values for sugars in adulterated (grey boxes) and authentic (white boxes) samples.
Figure 4.
Boxplots showing the range of values for amino acids, organic acids and alkaloids in adulterated (grey boxes) and authentic (white boxes) samples.
Figure 5.
Boxplots showing the range of values for other parameters in adulterated (grey boxes) and authentic (white boxes) samples.
The positive scores and the higher contents of sucrose and maltose for adulterated samples compared to authentic samples suggests that the adulteration might be related to the feeding of tablet sugars to bee colonies even when they have access to natural nectar. The source of tablet sugar could be partly sugarcane that is also used to produce white sugar in Iran. That can explain the higher content of C4 sugars in adulterated samples. Replacing natural nectar with sugar might be the reason for the lower values of the compounds and parameters of natural honey such as monosaccharides, amino acids and enzyme in adulterated samples.
During the fieldwork, we frequently saw storage of white sugar close to apiaries. Beekeepers told us that they receive sugar subsidy from the government. They solve the white sugar in a bowl of water and locates it next to beehives.
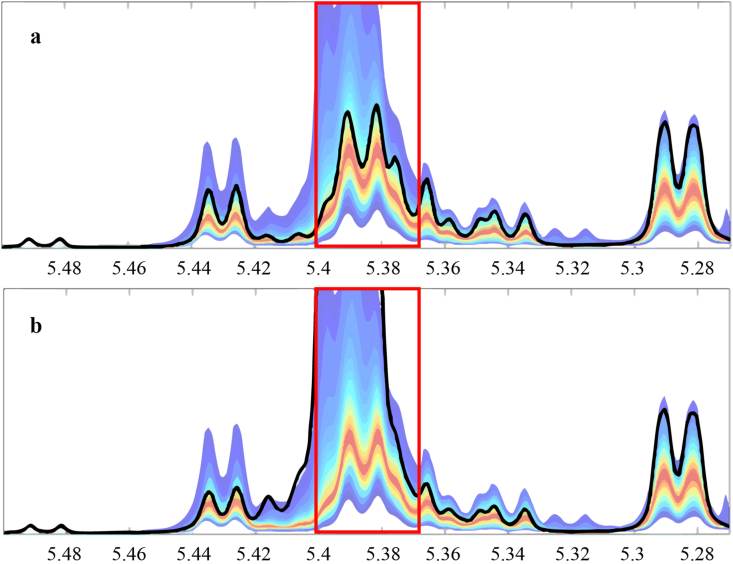
To check whether higher values of saccharides, especially sucrose and maltose, are associated with a specific botanical origin, such as Astragalus, the NMR quantile plots were checked. Figure 6 displays plots for one authentic sample (top) and one adulterated sample (bottom) with 81% and 70% Astragalus spp. pollen, respectively. The peaks inside the rectangles show maltose and sucrose. The black line that displays values for the current sample is much higher inside the rectangles for the adulterated sample, although it has a lower content of Astragalus pollen. Therefore, the high values of sugars are not related to floral source.
Figure 6.
Quantile plots for one authentic (a) and one adulterated (b) sample. The thick black line shows the values for current sample and the background shades display values for samples in reference database.
Since there is a common misconception in Iran that crystallization of honey is due to adulteration, we calculated the percentage of adulterated samples for viscose and crystallized (plus partly crystallized) honeys at their consistency state after one year. This value is higher for crystalized (90%) compared to viscose (74%) samples; however, this small difference can be due to the larger number of individuals in the crystalized group (72% of samples). In addition, two-thirds of the viscose samples were fake, suggesting that consistency is not a reliable measure for authentication.
3.4. Botanical origin
According to Codex Alimentarius, “honey may be designated according to floral source if it comes wholly or mainly from that particular source and has the organoleptic, microscopic and physicochemical properties corresponding with that origin” (Standard For Honey Codex Stan 12-1981, 2001). By this definition, identification of floral source can be obtained by putting together all the results previously presented in this study. Among the parameters used for identification, palynology is given priority in this study. Thus, if the percentage of a pollen type belonging to a source plant met the standard ranges found in the literature (bmbl, 2011; D.I.B., 2014; Oddo and Piro, 2004), the respective sample was included in Table 3 as potential unifloral honey. Further assessment is performed based on adulteration (adult.), sensory (sens.), electrical conductivity and fructose/glucose (F/G) ratio, as these are the routinely used parameters in honey evaluation. Unifloral source of a sample is approved if all parameters are in accordance with the standards (stand.).
Table 3.
The possible unifloral honeys found in this study. Approved unifloral honeys are marked with an asterisk. The names of some plants are repeated twice when both authentic and adulterated (adult.) samples are present with accepted pollen contents. NA is used when the standard (stand.) is not available. Yes/No in the sensory (sens.) column indicates whether or not the sample(s) has typical sensory features. The lower 4 rows display properties of potential samples with locally acknowledged unifloral origin. The column “NO.” indicates the number of samples found with the respective properties.
| Plant | No. | Pollen% |
Adult. | Sens. | EC |
F/G |
|||
|---|---|---|---|---|---|---|---|---|---|
| Stand. | study | Stand. | Study | Stand. | Study | ||||
| C. cyanus∗ | 1 | >10 | 28 | No | Yes | >30 | 0.39 | >1.10 | 1.25 |
| Citrus spp. | 1 | >20 | 20 | No | No | 0.10–0.30 | 0.23 | >1.10 | 1.20–1.23 |
| Citrus spp. | 1 | >20 | 23 | Yes | No | 0.10–0.30 | 0.15 | >1.10 | 1.23 |
| Helianthus spp. | 3 | >50 | 50–53 | Yes | No | 0.20–0.40 | 0.43–0.50 | >1.10 | 1.12–1.14 |
| Taraxacum spp.∗ | 4 | >05 | 6–17 | No | Yes | 0.37–0.65 | 0.23–0.30 | >0.85 | 1.20–1.35 |
| Taraxacum spp. | 10 | >05 | 5–10 | Yes | Yes | 0.37–0.65 | 0.13–0.33 | >0.85 | 1.12–1.29 |
| Thymus spp. | 1 | >13 | 17 | No | No | 0.35–0.75 | 0.34 | >1.17 | 1.25 |
| Tilia spp. | 1 | >20 | 73 | Yes | No | 0.37–0.97 | 0.16 | >0.94 | 1.31 |
| Astragalus spp. | 8 | NA | 62–81 | No | - | 0.24–0.47 | 0.23–0.42 | NA | 1.20–1.36 |
| Astragalus spp. | 12 | NA | 60–86 | Yes | - | 0.24–0.47 | 0.17–0.27 | NA | 1.21–1.28 |
| Eryngium spp. | 1 | >45 | 50 | No | - | NA | 0.34 | NA | 1.25 |
| Eryngium spp. | 2 | >45 | 50–53 | Yes | - | NA | 0.17–0.26 | NA | 1.22–1.28 |
The results approved only one unifloral honey to be assigned to C. cyanus with certainty. The 4 authentic dandelion (Taraxacum spp.) samples had the typical sensory features, but their electrical conductivity values were too low. However, as electrical conductivity is not a characterizing parameter for dandelion honey (Oddo and Piro, 2004), a unifloral source of these samples can be accepted. The other 10 dandelion samples also showed typical sensory features, but they were assessed as adulterated.
Among the locally acknowledged floral sources, Astragalus spp. and Eryngium spp. were found with reasonable pollen percentages. However, no standard range is available for the properties of these honey types. For Eryngium spp., the minimum accepted pollen percentage is considered to be 45% in this study only because this is the suggested lower limit for two other genera in the family Apiaceae: Foeniculum vulgare (Parvanov et al., 2011) and Coriandrum sativum (Atanassova et al., 2012) that can produce unifloral honey. In the family Fabaceae, the acceptable lower limits for pollen percentage differ from 20% for Robinia spp. to 70% for Trifolium spp. and 80% for Lotus spp. (bmbl, 2011). As the predominance of Astragalus pollen in Iranian samples is more similar to ranges of Trifolium spp. and Lotus spp. than underrepresented Robinia spp., samples with greater than 60% Astragalus spp. pollen were selected for Table 3. The standard range of electrical conductivity for Astragalus honey is quoted from one study (Moloudian et al., 2018) with unclear palynology and sensory analyses. These uncertainties prevented approval of samples with Astragalus and Eryngium origins. Moreover, potential Astragalus and Eryngium honey types constitute only nine and one percent of the samples tested in this study, respectively. This finding is in contrast to the common belief that these two plants are the main source of honey in Iran.
Since the electrical conductivity of all samples is less than 0.50 mS/cm, each sample that is neither unifloral nor adulterated can be categorized as polyfloral blossom honey according to the honey directive. The spicy taste in almost half of the samples is due to the presence of Eryngium. Plants from the Apiaceae family typically have a persistent strong anise-like flavor (Dinkov & Ivanov, 2009; Parvanov et al., 2011). The spoiled odor perceived in approximately 20% of samples can be emitted from Taraxacum, which is found in trace amounts in many samples. The intensive odor of the nectar of Taraxacum can be easily realized particularly when another fragrant nectar is not present in the honey (Oddo and Piro, 2004).
Pollen of Tilia spp. is usually underrepresented in honey (Oddo and Piro, 2004); thus, a sample with 70% of Tilia pollen as reported in this study must have a strong flavor of lime honey. However, such pure sample and some of the others listed in Table 3 with accepted pollen contents (e.g. Citrus, Helianthus, and Thymus) did not have respective sensory features. According to the results of this study, the most probable reasons are replacement of natural nectar with sugar and dominance of nectar with intensive flavor from Taraxacum spp. and Eryngium spp.
4. Conclusion
This study indicates that the flora of Iran has the potential for production of genuine unifloral honeys with international marketability. However, apparently Iranian beekeepers are not informed about these honey types. They establish apiaries in rangelands where Astragalus and Eryngium are the dominant plants. Since the nectar yield of these plants is not enough beekeepers have to feed sugar to bees during the foraging season. Moreover, the Iranian beekeepers are not trained to avoid lands covered by flowers that secret nectar with intensive aftertaste. As a result, the main source of authentic Iranian honey is nectar of various wild flowers (multifloral), sometimes with unpleasant aftertaste. Also the quality of Iranian honey does not comply with international standards, and therefore, Iran cannot act as producer of some unifloral honey types for global market unless the beekeeping practices undergo fundamental changes.
Declarations
Author contribution statement
Elmira Khansaritoreh, Kamaleddin Alizadeh: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yasaman Salmaki, Elias Ramezani, Shahin Zarre: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tayebeh Akbari Azirani, Farnood Henareh: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Gudrun Beckh, Hermann Behling: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Bayer CropScience and Quality Service International GmbH.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Special thanks goes to all colleagues in QSI, in particular the experts in pollen department who thoroughly helped this project with their several years of experience in palynology and sensory. We would also like to show our gratitude to Dr. Sebastian Bachmann and Dr. Jane van der Meulen from NMR department in QSI for assisting us in physicochemical aspects of the project. We thanks Mr. Arne Duebecke for his revision of adulteration section. We thank also the state organizations of Iran for their great help in data acquisition.
References
- Agricultural Bank of Iran . 2011. Ranking of Provinces Based on the Quantity of Agricultural Products.https://www.bki.ir/Portals/0/SBank/ProvinceRating.pdf (in Farsi) [Google Scholar]
- Association of Official Analytical Chemists AOAC . 2013. AOAC Official Method 998.12 C-4 Plant Sugars in Honey. [Google Scholar]
- Atanassova J., Yurukova L., Lazarova M. Pollen and inorganic characteristics of Bulgarian unifloral honeys. Czech J. Food Sci. 2012;30(6):520–526. [Google Scholar]
- Beckh G., Camps G. Neue Spezifikationen für Trachthonige. Dtsch. Lebensm.-Rundsch. 2009:105–110. [Google Scholar]
- bmbl . 2011. Neufassung der Leitsätze für Honig.https://www.bmel.de/SharedDocs/Downloads/Ernaehrung/Lebensmittelbuch/LeitsaetzeHonig.pdf?__blob=publicationFile [Google Scholar]
- Bogdanov S., Martin P., Luellmann C. Harmonised methods of the European honey commission. Apidologie. 1997;28(Extra Isuue):1–59. [Google Scholar]
- Chase M.W., Christenhusz M.J.M., Fay M.F., Byng J.W., Judd W.S., Soltis D.E., Mabberley D.J., Sennikov A.N., Soltis P.S., Stevens P.F., Briggs B., Brockington S., Chautems A., Clark J.C., Conran J., Haston E., Möller M., Moore M., Olmstead R., Weber A. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016 [Google Scholar]
- Council of the European Union . 1996. COUNCIL DIRECTIVE 96/93/EC of 17 December 1996 on the Certification of Animals and Animal Products.https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31996L0093&from=EN [Google Scholar]
- Council of the European Union . 2001. COUNCIL DIRECTIVE 2001/110/EC of 20 December 2001 Relating to Honey.https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32001L0110&from=EN [Google Scholar]
- Council of the European Union . 2015. Coordinated Control Plan to Establish the Prevalence of Fraudulent Practices in the Marketing of Honey Outline.https://ec.europa.eu/food/sites/food/files/safety/docs/official-controls_food-fraud_honey_control-plan-outline_201512.pdf [Google Scholar]
- D.I.B. 2014. Honigsorten-Bezeichnungen (Issue 3.4)https://deutscherimkerbund.de/userfiles/downloads/satzung_richtlinien/Merkblatt_Sorten_3_4_neu.pdf?__blob=publicationFile [Google Scholar]
- DIN - Deutsches Institut für Normung . 2010. DIN 10751-1:2010-08 Analysis of Honey - Determination of Hydroxymethylfurfural - Part 1: Winkler Photometric Method. [Google Scholar]
- Dinkov D., Ivanov T. Proceedings of the 41st Apimondia Congress, February, 1–11. 2009. Sensorial characteristics and composition of Bulgarian’s coriander (Coriandrum sativum L.) honey. http://www.researchgate.net/profile/Dinko_Dinkov2/publication/267247448_SENSORIAL_CHARACTERISTICS_AND_COMPOSITION_OF_BULGARIAN’S_CORIANDER_(CORIANDRUM_SATIVUM_L.)_HONEY/links/54f07c0d0cf2432ba65b2bb4.pdf. [Google Scholar]
- FAOSTAT . 2018. Production of Natural Honey in World.http://www.fao.org/faostat/en/#data/QL/visualize [Google Scholar]
- Horn H., Lüllmann C. 2017. Der Honig : Imker, Analytik, Gesetz, Gesundheit.https://www.bienenundnatur.de/fachthemen/fuer-sie-gelesen/helmut-horn-cord-luellmann-der-honig/ [Google Scholar]
- Knuth P., Davis J.R.A., Müller H. Clarendon Press; 1906. Handbook of Flower Pollination Based upon Hermann Müller’s Work “The Fertilisation of Flowers by Insects”. [Google Scholar]
- Marcazzan G.L., Mucignat-Caretta C., Marina Marchese C., Piana M.L. A review of methods for honey sensory analysis. J. Apicult. Res. 2018;57(1) [Google Scholar]
- Moloudian H., Abbasian S., Nassiri-Koopaei N., Tahmasbi M.R., Afzal G.A., Ahosseini M.S., Yunesian M., Khoshayand M.R. Characterization and classification of Iranian honey based on physicochemical properties and antioxidant activities, with chemometrics approach. Iran. J. Pharm. Res. (IJPR) 2018;17(2):708–725. [PMC free article] [PubMed] [Google Scholar]
- Oddo L.P., Piro R. Main European unifloral honeys: descriptive sheets. Apidologie. 2004;35:38–81. [Google Scholar]
- Parvanov P., Dinkov D., Tananaki C., Mihaylova G. Sensorial characteristics and composition of Bulgarian’s fennel (Foeniculum vulgate Mill.), bee honey: I. Quality parameters. J. Mount. Agric. Balkans. 2011;14(1):1–22. [Google Scholar]
- Parviz M., Karimi F., Rezaei M., Javanmard M.R., Javadzadeh M., Allahdadi G. Assessment of the physicochemical quality of Iranian honey. Qual. Assur. Saf. Crop Foods. 2015;7(5):629–634. [Google Scholar]
- Pasias I.N., Kiriakou I.K., Proestos C. HMF and diastase activity in honeys: a fully validated approach and a chemometric analysis for identification of honey freshness and adulteration. Food Chem. 2017;229:425–431. doi: 10.1016/j.foodchem.2017.02.084. [DOI] [PubMed] [Google Scholar]
- Piana M.L., Oddo L.P., Bentabol A., Bruneau E., Bogdanov S., Declerck C.G. Sensory analysis applied to honey: state of the art. Apidologie. 2004;35:26–37. [Google Scholar]
- Sagheb-Talebi K., Sajedi T., Pourhashemi M. Springer; 2014. Forests of Iran : a Treasure from the Past, a hope for the Future. [Google Scholar]
- Sousa J.M.B., Souza E.L., Marques G., Benassi M. de T., Gullón B., Pintado M.M., Magnani M. Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT - Food Sci. Technol. 2016;65:645–651. [Google Scholar]
- Spraul M., Schütz B., Rinke P., Koswig S., Humpfer E., Schäfer H., Mörtter M., Fang F., Marx U.C., Minoja A. NMR-based multi parametric quality control of fruit juices: SGF profiling. Nutrients. 2009;1(2):148–155. doi: 10.3390/nu1020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standard For Honey Codex Stan 12-1981 . 2001. Codex Stan. [Google Scholar]
- USDA . Federal Register. 1985. United States standards for grades of extracted honey.https://www.ams.usda.gov/sites/default/files/media/Extracted_Honey_Standard%5B1%5D.pdf [Google Scholar]
- Von Der Ohe W., Oddo L.P., Piana M.L., Morlot M., Martin P. Harmonized methods of melissopalynology. Apidologie. 2004;35:18–25. [Google Scholar]
- White J.W., Winters K. Honey protein as internal standard for stable carbon isotope ratio detection of adulteration of honey. J. Assoc. Off. Anal. Chem. 1989;72(6):907–911. [PubMed] [Google Scholar]
- Yadata D. Detection of the electrical conductivity and acidity of honey from different areas of tepi. Food Sci. Technol. 2014;2(5):59–63. [Google Scholar]
- Zahedi Namini N., Mousavi M.H., Mahmoudi R., Hassanzadeh P. Hygienic quality of the honey samples produced in the Iran in comparison with international standards. Int. Food Res. J. 2018;25(3):982–988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.