Abstract
Patient: Female, 17-year-old
Final Diagnosis: Drug toxicity
Symptoms: Seizure
Medication: —
Clinical Procedure: —
Specialty: Neurology
Objective:
Unusual clinical course
Background:
The electroencephalographic (EEG) findings associated with tetrahydrocannabinol (THC) use, particularly in concentrated form, are not well-described, despite the current widespread availability of these products. There is a lack of prior research describing the EEG findings in adolescent cannabis users, and the effects of THC on the seizure threshold have been variably reported.
Case Report:
A 17-year-old girl with no prior history of seizures or known seizure risk factors presented to an Emergency Department with acutely abnormal behavior in the setting of daily vaping of highly concentrated THC marijuana (“wax”). On admission, she had a witnessed generalized tonic-clonic seizure. Urine toxicology was positive for THC, and an extensive evaluation for other etiologies of her encephalopathy was unrevealing. Extended EEG on admission showed mild diffuse background slowing with occasional bifronto-centrally predominant sharp and spike wave discharges. Seven days later, without interim antiseizure medications, a repeat extended EEG showed resolution of the previously seen interictal findings.
Conclusions:
The clinical and EEG findings were temporally associated with the patient’s use of concentrated THC and may represent a constellation of symptoms of a THC wax toxidrome. In this case, THC was associated with lowering the seizure threshold and triggering a provoked seizure in an adolescent with no prior evidence of seizure tendency. This case also suggests the possibility of THC concentrate itself generating epileptiform discharges, as has previously been described with synthetic cannabinoid use.
Keywords: Cannabis, Electroencephalography, Marijuana Use, Seizures
Background
Interictal electroencephalographic (EEG) changes have not been previously described in the setting of the use of highly concentrated tetrahydrocannabinol (THC), despite these products being widely available in recent years. Although marijuana is the second most frequently used drug (after alcohol) in adolescents, with nearly 7% of 12th grade students in the United States reporting daily marijuana use in 2020 per the National Institute on Drug Abuse’s Monitoring the Future study, there is a paucity of research describing EEG findings among adolescent cannabis users, and the described effect of THC on seizure threshold has varied widely [1–3]. Limited data from animal models and human studies of chronic cannabis use describe a disruption in gamma EEG oscillations and thus a potential modulation of underlying network rhythms [4–7]. Recently, a limited number of case series have described seizure provocation and interictal EEG abnormalities following the use of synthetic cannabinoids (eg, “spice”). The mechanism of these outcomes is unknown but is thought to be potentially related to effects at the cannabinoid type 1 (CB1) receptor [8,9]. However, to our knowledge, this phenomenon has not been previously described with cannabis “wax,” which typically refers to a concentrated preparation with far higher amounts of THC (on the order of 50 times greater) than in other formulations [10].
We report a case of an adolescent with no prior seizure history or known epilepsy risk factors who experienced a generalized tonic-clonic seizure after use of cannabis wax. The patient was found to have interictal abnormalities on EEG, which normalized after 7 days of inpatient monitoring without further marijuana use.
Case Report
A 17-year-old girl with a history of depression and a prior psychiatric hospitalization, but no prior personal or family history of seizure, was brought in by her foster mother to the Emergency Department after 4 days of abnormal behavior, including emotional lability, paranoia, and irritability. Several hours before presentation, the patient’s foster mother found her unresponsive after a presumed unwitnessed fall, with a brief episode of “eye darting” and decreased responsiveness with no prodromal symptoms. The patient endorsed daily use of highly concentrated THC marijuana (wax) in the form of vaping cartridges, for at least the past 5 days. In the Emergency Department, she had a witnessed 1-min seizure with generalized tonic-clonic semiology and urinary incontinence. She was subsequently admitted for further evaluation.
The patient had no recent infectious symptoms, fevers, or sick contacts (including exposure to COVID-19). There was no prior history of seizure, syncope, or prior head trauma. She was previously diagnosed with depression and had an inpatient psychiatric hospitalization 6 months prior (for unspecified psychosis), and was started on olanzapine, which was self-discontinued 2 months before presentation. There was a family history of schizophrenia in the patient’s father and bipolar disorder in the patient’s mother, but no known family history of seizures or neurological disease (although available history was limited by the patient’s infrequent contact with her biological family).
On admission, a complete metabolic panel (obtained in the postictal period) was notable for a bicarbonate of 10 mmol/L (attributed to postictal metabolic acidosis), which resolved on repeat testing; urine pregnancy test and urinalysis were negative. Magnetic resonance imaging of the brain with and without contrast showed an incidentally enlarged adenohypophysis (felt to be clinically noncontributory in consultation with endocrinologists); results were otherwise unremarkable. An expanded urine toxicology screen was positive for THC. Broad-serum analyses to evaluate the patient’s encephalopathy were obtained and unremarkable. These analyses included SARS-CoV2 IgG and IgM, serum B12, HIV, syphilis rapid plasma reagin, thyroid studies, autoimmune encephalopathy panel, thyroglobulin and thyroperoxidase antibodies, antinu-clear antibodies, double-stranded DNA antibody, complement studies, and Sjogren’s antibodies. Multiple cerebrospinal fluid (CSF) studies were obtained and were also unremarkable, including basic CSF profile, bacterial culture, herpes simplex virus polymerase chain reaction, IgG index, oligoclonal bands, and autoimmune encephalopathy panel.
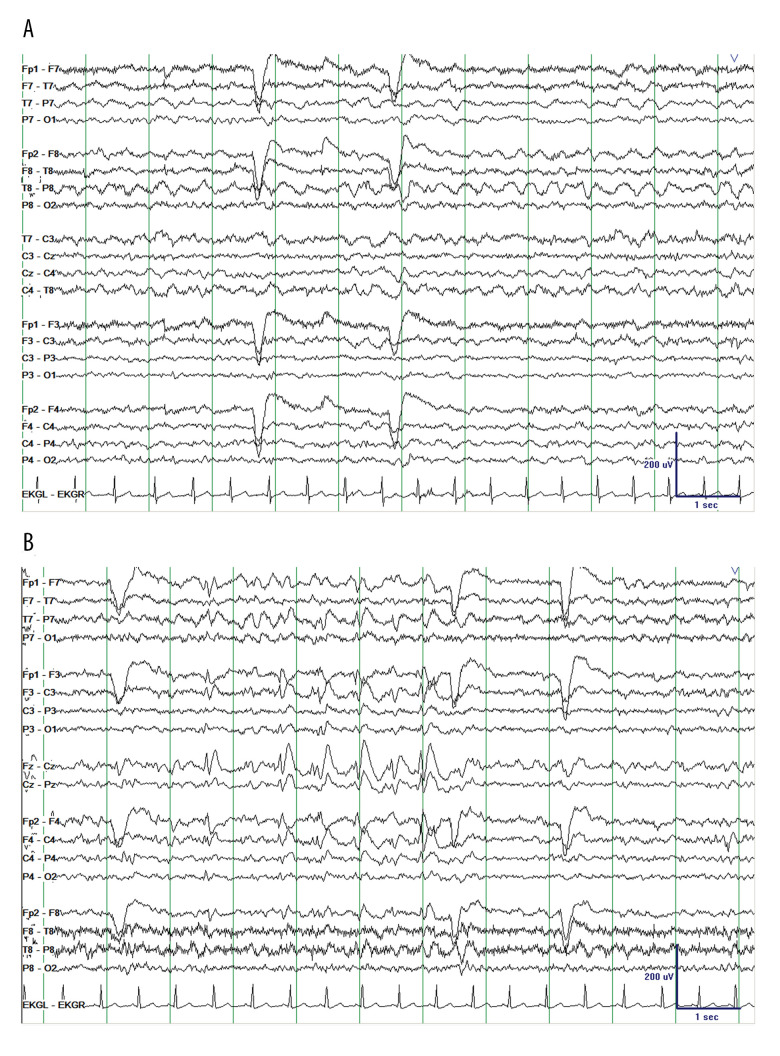
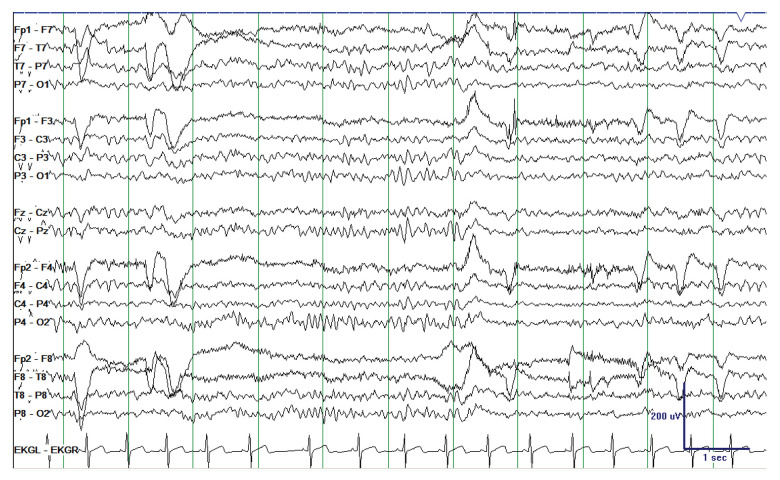
Extended EEG was performed on admission, and it showed mild diffuse background slowing with occasional bifronto-centrally predominant sharp and spike wave discharges, frequently occurring in brief runs without ictal evolution (Figure 1). The patient was not given antiseizure medications and had no further seizures during the admission. Psychiatry evaluated the patient and felt her presentation was most likely related to substance-induced exacerbation of an underlying thought disorder. She was restarted on olanzapine with some improvement of her mental status, although with ongoing paranoid thought content and disorganized thought process. An extended EEG was repeated 7 days after admission and demonstrated complete normalization of the background (Figure 2). The patient was ultimately discharged to an inpatient psychiatric hospital.
Figure 1.
Electroencephalography (longitudinal bipolar montage, sensitivity 10 μV/mm) on presentation demonstrating (A) mild diffuse background slowing with (B) occasional bifronto-centrally predominant sharp and spike wave discharges, frequently occurring in (very) brief runs without ictal evolution and primarily observed in wakefulness and N1 sleep.
Figure 2.
Repeat electroencephalography 7 days after presentation demonstrating normalization of the background.
Discussion
This case report highlights the presence of interictal epileptiform abnormalities on EEG following a first lifetime seizure (and another possible seizure within the prior 24 hours), likely provoked by use of high-dose THC wax concentrate, in an adolescent. In this case, we suspect the patient’s interictal discharges may have reflected a transient state of increased epileptogenicity following THC concentrate use, and that her diffuse background slowing was a related nonspecific finding reflective of her initial encephalopathy after frequent THC use and during the postictal period. Notably, these findings completely resolved on a repeat study with no interim antiseizure medication administration. The patient had no further seizures during her hospitalization, further supporting the theory that the initial clinical seizure(s) and EEG abnormalities were provoked by THC use. As with spice use, the mechanism of this epileptogenicity is not fully understood, although we surmise it could be related to THC’s partial agonism of the CB1 receptor. Further, high-dose THC may lower the seizure threshold by decreasing GABA transmission, as has been previously proposed [8]. It is also possible that the patient may have had an underlying seizure tendency, although no historical risk factors suggested this. If the tendency existed, it may still imply that use of the THC concentrate lowered the patient’s seizure threshold.
Limitations of this case include unknown details about the specific THC (and possible CBD) concentrations in the wax product used by the patient, as well as an inability to obtain the compound for analysis. Other potential confounding factors in this patient’s presentation include her suspected underlying psychiatric diagnosis, although this would not be expected to contribute to the epileptiform findings noted on EEG. Finally, while the patient did not receive antiseizure medications in the interim between the 2 EEG studies, it is possible that the olanzapine started during this time may have affected the background rhythm; however, olanzapine is typically associated with lowering the seizure threshold, and therefore would not be expected to reduce epileptiform discharges [11].
Conclusions
Amid conflicting evidence surrounding the effect of THC on the seizure threshold, this case supports a previously undescribed effect of high-dose THC concentrate in triggering a provoked seizure in an adolescent [2]. Moreover, as the patient had no prior evidence of an underlying seizure tendency, this case additionally suggests the possibility of THC concentrate itself generating epileptiform discharges, as has been previously described in several cases of synthetic marijuana use [8,9]. As the use of THC concentrate, particularly in inhaled or vaped form, has a widely increasing prevalence among adolescents, it should therefore be considered in the differential diagnosis for otherwise unexplained presentations of first lifetime seizure in patients of this age group.
Footnotes
Department and Institution Where Work Was Done
This work was performed at the University of California San Francisco (UCSF), Department of Neurology, San Francisco, CA.
Conflicts of Interest
None.
References:
- 1.Monitoring the Future Study . Trends in prevalence of various drugs. National Institute on Drug Abuse; 2020. [cited 2021 Feb 18]. https://www.drugabuse.gov/drug-topics/trends-statistics/monitoring-future/monitoring-future-study-trends-in-prevalence-various-drugs. [Google Scholar]
- 2.Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Curr Pharm Des. 2014;20(13):2186–93. doi: 10.2174/13816128113199990426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon E, Devinsky O. Alcohol and marijuana: Effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266–72. doi: 10.1046/j.1528-1157.2001.19301.x. [DOI] [PubMed] [Google Scholar]
- 4.Soltesz I, Alger BE, Kano M, et al. Weeding out bad waves: Towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16(5):264–77. doi: 10.1038/nrn3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards CR, Skosnik PD, Steinmetz AB, et al. Sensory gating impairments in heavy cannabis users are associated with altered neural oscillations. Behav Neurosci. 2009;123(4):894–904. doi: 10.1037/a0016328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raver SM, Haughwout SP, Keller A. Adolescent cannabinoid exposure permanently suppresses cortical oscillations in adult mice. Neuropsychopharmacology. 2013;38(12):2338–47. doi: 10.1038/npp.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skosnik PD, Cortes-Briones JA, Hajós M. It’s all in the rhythm: The role of cannabinoids in neural oscillations and psychosis. Biol Psychiatry. 2016;79(7):568–77. doi: 10.1016/j.biopsych.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Gounder K, Dunuwille J, Dunne J, et al. The other side of the leaf: Seizures associated with synthetic cannabinoid use. Epilepsy Behav. 2020;104(Pt A):106901. doi: 10.1016/j.yebeh.2020.106901. [DOI] [PubMed] [Google Scholar]
- 9.de Havenon A, Chin B, Thomas KC, et al. The secret “spice”: An undetectable toxic cause of seizure. Neurohospitalist. 2011;1(4):182–86. doi: 10.1177/1941874411417977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akutsu M. Current status of drug problems and drug analysis. Yakugaku Zasshi. 2019;139(5):693–97. doi: 10.1248/yakushi.18-00166-2. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 11.Hedges D, Jeppson K, Whitehead P. Antipsychotic medication and seizures: A review. Drugs Today. 2003;39(7):551–57. doi: 10.1358/dot.2003.39.7.799445. [DOI] [PubMed] [Google Scholar]