According to statistics from the World Health Organization, approximately 36.7 million people are currently living with HIV and 1.8 million become infected every year. Antiretroviral therapy has largely transformed HIV infection into a chronic disease. As a consequence, it is estimated that by the year 2030, 73% of HIV-infected individuals will be aged 50 and older and that 78% of individuals living with HIV will have cardiovascular disease (CVD).1 HIV-infected individuals have a significantly increased risk for a variety of CV complications including acute MI,2 heart failure with both reduced and preserved ejection fraction,3 sudden cardiac death,4 peripheral arterial disease,5 and stroke.6 In the US, CVD has become a key contributor to mortality among individuals living with HIV.7
The systematic review of longitudinal studies of CVD in HIV by Shah and colleagues in this issue,8 includes 80 studies with 793,635 individuals living with HIV and a total follow-up of 3.5 million person-years. A random-effects meta-analysis was performed to derive a pooled rate and risk of CVD among people living with HIV and to estimate the burden of CVD and HIV at the national, regional and global level. The authors report that the relative risk of CVD in persons living with HIV is 2.16 (95% CI 1.68–2.77) as compared to uninfected individuals. Over the past 26 years, the global population attributable fraction from CVD in the setting of HIV increased from 0.36% (95% CI 0.21–0.56%) to 0.92% (95% CI 0.55–1.41%) and disability adjusted life years increased from 0.74 (95% CI 0.44–1.16) to 2.57 (95% CI 1.53–3.92) million. Most of these increases took place in sub-Saharan Africa and the Asia Pacific regions.
The authors deserve high praise for the vast scope of their study and the large volume of work involved. Some of their findings confirm previous studies but on a broader scale; for example, the risk of incident CVD being two-fold higher in HIV and similar to other high risk groups such as diabetics.2 Most of the limitations of the study are inherent in this type of research and are unavoidable. First, MI and stroke are defined differently across the aggregated studies and the majority of cases were not clinically adjudicated but were categorized using coding. This lack of standardization of MI definition may be particularly problematic in HIV, where approximately half of all MIs are type 2;9 that is, demand related, for example in the setting of sepsis or illicit drug use.
Second, in a meta-analysis such as this without patient level data, the contributions of traditional risk factors (high levels of smoking and the metabolic syndrome) and HIV-specific risk factors (HIV medication, degree of HIV control, level of inflammation) cannot be adjusted for or even ascertained. Additionally, the CV risk of HIV-infected patients is likely distinctly different before and after the introduction of antiretroviral therapy. While the authors report that the impact of HIV and CVD was highest among individuals in sub-Saharan Africa, this finding is based on only a small number of studies from this region; most of the data comes from the U.S. and Europe. All of these issues likely contributed to the substantial heterogeneity for pooled risk ratios, as noted by the authors.
However, despite these limitations, the study by Shah et al demands our attention because of its global scope and sobering conclusions, a doubling of CV risk in people living with HIV, coupled with a tripling of the global burden of CVD in HIV. What are the implications of these findings?
Among the general population relative impact of traditional risk factors has been shifting over the past 2 to 3 decades. Hypercholesterolemia can now be well controlled with drug therapy in most patients, and control of hypertension has improved, at least in wealthier countries. The incidence and prevalence of type 2 diabetes has increased dramatically across most of the globe due to a higher prevalence of overweight and obesity, but outcomes in well treated patients with established diabetes has improved.10 The impact of smoking on CV risk has diminished as smoking rates have decreased in many countries and many at-risk groups, while in other places, little progress has been made.
Although HIV accounts for a relatively small proportion of CVD compared to these major risk factors, the relative risk of a CV event in a person living with HIV is in the range of traditional risk factors. Figure 1 compares the relative risk and population attributable risk of HIV from the study of Shah et al8 and odds ratios and traditional risk factors from the INTERHEART study.11 Because odds ratios can give inflated estimates of risk when the outcome is common,12 it is likely that the relative risks of traditional risk factors in INTERHEART are smaller, and thus closer to that of HIV. While the relative risk is unlikely to change much, the impact of HIV on CVD can only worsen as the HIV population ages. Has the time come to recognize HIV infection as a major CV risk factor, alongside diabetes, hypertension, hyperlipidemia, and smoking?
Figure 1.
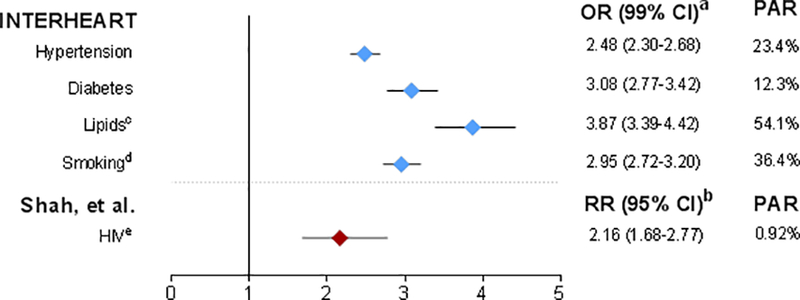
aRisk of acute MI; adjusted for age, sex, and smoking. Because ORs can give inflated estimates of risk when the outcome is common,12 it is likely that the RRs of traditional risk factors in INTERHEART are smaller, and thus closer to that of HIV.
bRisk of ASCVD (CVD, stroke, or MI); adjustment for confounders was limited to those available in the primary studies.
cHigh ApoB/ApoA1 ratio.
dOR (99% CI) for current smoking; PAR for any smoking.
ePAR for 2015 using prevalence data for HIV for the 15–49 year age group.
Such recognition might have salutary consequences. First, elevating HIV infection to the status of a major CV risk factor might stimulate research in this area. Treatment of CVD among individuals living with HIV relies on clinical trial data from non-HIV populations, even though HIV-associated atherosclerosis has distinct features (such as more non-calcified plaques) and mechanisms, including chronic inflammation/immune activation and the impact of antiretroviral therapy. Clinical trial data specific to HIV would be helpful; however it is not feasible in an era of limited resources to do outcome-driven clinical trials for all types of interventions and patient populations. As such, smaller proof-of-concept mechanistic studies that target HIV-specific factors will be critical to move the field forward. Precision medicine approaches, including “omics” methodologies, studies of healthcare disparities, digital monitoring, and implementation science aimed at CV risk in HIV will also be invaluable.
It is important to note that elevating HIV to the level of the other major risk factors could improve awareness, and thus the prevention, detection, and treatment of CVD among individuals living with HIV, along with better control of their traditional risk factors. All of these things could theoretically reduce CVD and CVD mortality in HIV. Often neither the HIV specialist nor the cardiologist has the requisite knowledge to treat these individuals optimally, and awareness among all caregivers of the excess cardiovascular risk remains crucial. Traditional risk calculators underestimate cardiovascular risk in HIV,13 but recognition of HIV as a major risk factor would reduce the need for risk calculation. The European Society of Cardiology guidelines recommend lipid-lowering therapy to reduce low-density lipoprotein cholesterol to <70 mg/dL in individuals infected with HIV.14
In 2004 our group reported rapid carotid artery intima-media progression in HIV and concluded that this finding would presage a high rate of CV events.15 Fourteen years later, the study by Shah et al confirms this high rate of CV events on a global scale. The rate continues to increase even in the setting of treated and suppressed HIV disease. The key challenges for cardiologists and HIV clinicians continue to be unraveling the mechanisms underlying HIV-associated atherosclerosis, accurately predicting individuals at risk, and providing interventions to reduce this risk. The time to consider HIV as a CV risk factor is now.
Acknowledgments
Funding and Support:
This was funded in part by the National Institute of Allergy and Infectious Diseases (K24AI112393 to PYH).
Abbreviations:
- Apo
apolipoprotein
- ASCVD
atherosclerotic cardiovascular disease
- CI
confidence interval
- CVD
cardiovascular disease
- HIV
human immunodeficiency virus
- MI
myocardial infarction
- OR
odds ratio
- PAR
population attributable risk
- RR
risk ratio
Footnotes
Conflict of Interest Disclosures:
Dr. Hsue has received honoraria from Gilead and Merck, outside of the submitted work. Dr. Waters reports no conflict of interest.
References
- 1.Smit M, Brinkman K, Geerlings S, Smit C, Thyadarajan K, Sighem Av, de Wolf F, Hallett TB; ATHENA observational cohort. Future challenges for clinical care of an ageing population infected with HIV: a modeling study. Lancet Infect Dis 2015;15:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez-Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol 2017;2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012;59:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman JA, Duncan MS, Alcorn CW, So-Armah K, Butt AA, Goetz MB, Tindle HA, Sico J, Tracy RP, Justice AC, Freiberg MS. Association of HIV infection and risk of peripheral artery disease. Circulation 2018; March 13. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012;60:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, Freiberg MS, Lloyd-Jones DM Patterns of cardiovascular mortality in HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol 2016;117:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah A, Stelzle D, Lee KK, Beck Eduard J., Alam Shirjel, Clifford Sarah, Longenecker Chris T., Strachan Fiona E., Bagchi Shashwatee, Whiteley William, Rajagopalan Sanjay, Kottilil Shyamasundaran, Nair Harish, Newby David E., McAllister David A., Mills Nicholas L.. Global burden of atherosclerotic cardiovascular disease in people living with the human immunodeficiency virus: a systematic review and meta-analysis. Circulation (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane HM, Paramsothy P, Drozd DR, Nance RM, Delaney JA, Heckbert SR, Budoff MJ, Burkholder GA, Willig JH, Mugavero MJ, Mathews WC, Crane PK, Moore RD, Eron JJ, Napravnik S, Hunt PW, Geng E, Hsue P, Rodriguez C, Peter I, Barnes GS, McReynolds J, Lober WB, Crothers K, Feinstein MJ, Grunfeld C, Saag MS, Kitahata MM; Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Cohort. Types of myocardial infarction among human immunodeficiency virus-infected individuals in the United States. JAMA Cardiol. 2017;2:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A, Gudbjörnsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 13.Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, D’Agostino RB Sr. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation 2018;137:2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catapano A, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS guidelines for the management of dyslipidemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 15.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004;109:1603–1608. [DOI] [PubMed] [Google Scholar]


