Abstract
Robust physical activity after liver transplantation is an important determinant of longterm health, similar in its importance to the value of pretransplant activity for withstanding the immediate stress of transplantation. Although transplantation normally enables rapid recovery of liver synthetic and metabolic functions, the recovery of physical capacity and performance to normal levels is delayed and often incomplete. Anatomic measurements of sarcopenia and the physical performance indicators of frailty both tend to improve slowly, and they may, in fact, decrease further in the posttransplant period, especially when the common extrahepatic drivers of muscle loss, such as the elements of the metabolic syndrome, persist or intensify after transplantation. Posttransplant exercise improves fitness, which is a conclusion based on 2 observational studies and 3 randomized trials that assessed endpoints of strength testing, energy expenditure in metabolic equivalents, and peak or maximal oxygen uptake. Importantly, 1 controlled trial found that exercise also improved quality of life (QOL) measured by the Short Form 36 survey, consistent with multiple reports of the value of social support and engagement in sports activity for improving posttransplant QOL. Developing evidence-based standards for post–liver transplant physical activity baseline testing and sustainment of intensity and quality is a key unmet need in transplant hepatology. At present, it is reasonable for transplant teams to assess fitness and design a tailored exercise program when a recipient is first discharged, to record and reinforce progress at all posttransplant visits, and to set realistic longterm performance goals that will often achieve recommended standards for the healthy general population.
Liver transplantation is an inflection point in a recipient’s life. Its effects range from impacts on molecular and physical events to changes in personal outlook, resilience, and relationships. Pretransplant care is focused on solving the immediate problems that persons who need a liver transplant must overcome, typically over a duration of a year or less. In contrast, however, the new issues that liver recipients face in recovering and sustaining full function and quality of life (QOL) extend for decades.
There is now ample evidence that liver transplant recipients have a high prevalence of the metabolic syndrome and its complications, especially cardiovascular morbidity. Increasing physical activity and improving physical function are the primary modifiable risk factors for this major problem, along with learning the factors that may result in a recipient adopting either a vigorous or sedentary posttransplant physical activity profile. Those who have accepted and lived within the limitations of end-stage liver disease for years may harbor an unrealistically low or a falsely high expectation of the extent of improvement they are likely to experience after transplantation. In this review, we will consider the key events that may impact the extent and rapidity of recovery over time of physical and metabolic functionality and activity-related QOL after liver transplantation.
Changes in Physical Frailty, Muscle Mass, and Physical Activity After Liver Transplantation
PHYSICAL FRAILTY
Cirrhosis leads to muscle wasting, malnutrition, and functional impairments that manifest as the clinical phenotype of physical frailty, with increased risk of transplant wait-list mortality.(1–3) In transplant hepatology, a consensus toolkit for the measurement of frailty using 4 easily administered tests (the Karnofsky Performance Index, the Activities of Daily Living scale, the Liver Frailty Index [LFI], and the 6-minute walk test distance) was suggested by a consensus statement endorsed by the American Society of Transplantation.(4) The panel recommended that a single frailty assessment or cutoff should not be the sole criterion to define futility of liver transplantation but that it should be incorporated into the clinician’s overall assessment of a candidate’s global health status as a potential recipient, including their potential for posttransplant physical recovery. Although transplantation restores hepatic synthetic function and normalizes portal hypertension almost immediately for most recipients, physical frailty often persists, at least for the short and intermediate terms, if not indefinitely. In 1 study of over 500 liver transplant candidates, frailty was measured using the LFI, which is a composite performance test including grip strength, timed chair stands, and balance testing that measures physical frailty on a continuous scale, with a strong predictive value in persons with cirrhosis.(5,6) Among ~200 patients who underwent liver transplantation, median LFI scores worsened at 3 months, returned to pretransplant baseline at 6 months, and improved only modestly by 12 months after transplantation.(7) Only 40% of recipients achieved robust physical function by 1 year after transplant. Importantly, the only predictor of robustness after liver transplantation was high physical function before transplant, highlighting the importance of pretransplant efforts to preserve, if not improve, physical function prior to transplantation. Frailty is a cardinal manifestation of aging, and the concepts and metrics of frailty in cirrhosis are derived from the same constructs in geriatrics(1) As is the case with pretransplant frailty, persistent posttransplant frailty exhibits a similar phenotype to that of geriatric frailty. The intuitive concept from geriatrics that frailty adds to the impact of advanced age on physical performance is consistent with all reported information on this topic.
MUSCLE AND BONE MASS
Loss of muscle mass, sarcopenia, is the anatomic correlate of frailty in cirrhosis. A North American transplant hepatology expert panel reviewed the evidence connecting sarcopenia with adverse outcomes in liver transplantation and methods to measure muscle mass. They proposed standardized adoption of the skeletal muscle index (SMI), which is the cross-sectional area on imaging of muscle at vertebral level L3 normalized to the square of patient height, with sex-specific cutoffs of 50 and 39 cm2/m2 for North American men and women, respectively.(8) The slow and incomplete recovery of posttransplant frailty compared with rapid normalization of hepatic synthetic function is mirrored by delayed improvement in measured muscle and bone mass after transplantation. Before transplantation, excess ammonia delivered to skeletal muscle is the central metabolic driver of muscle loss in end-stage liver disease, mainly by inhibition of the mammalian target of rapamycin pathway that supports muscle protein synthesis(9,10) Because ammonia excess is no longer present after transplantation, recovery of muscle mass and function would be expected. However, immunosuppression with calcineurin inhibitors that inhibit the mammalian target of rapamycin pathway,(9) along with other abnormalities, may account for prolonged recovery. In a similar manner, posttransplant medical regimens are thought to contribute to delayed recovery of diminished bone mineral density, with persistently high fracture risk.(11) Future studies will help to better define the impact of common posttransplant medical regimens and exercise on the recovery of muscle and bone mass and function.
Anatomic sarcopenia frequently persists after liver transplantation, and de novo sarcopenia may develop even in those with normal pretransplant muscle mass. Sarcopenia, measured as SMI on computed tomography imaging, was present in 66% of 53 recipients before transplant and increased to 86% by 13 months after transplant, with only 2 sarcopenic recipients improving to normal SMI,(12) findings that have been confirmed in other studies in both adults(13,14) and children.(15) In contrast, when recipients with potential alternative causes of muscle loss or with a high risk of allograft liver disease recurrence were excluded, a highly selected cohort of 40 recipients showed improvement in sarcopenia, which decreased from 55% to 30% 1 year after transplant, suggesting that factors other than the transplant itself might be the key culprits in posttransplant sarcopenia.(16) In another study of 382 adult liver transplant recipients, higher muscle mass, measured by creatinine excretion at 1 year after transplant, was associated with longer recipient and allograft survival.(17)
MEASURED PHYSICAL ACTIVITY AND EXERCISE CAPACITY
Adult transplant candidates are among the most sedentary of all populations with chronic disease when activity is objectively measured with personal monitoring, similar to that of persons with chronic lung disease, advanced heart failure, and renal failure on dialysis. Daily step counts among liver transplant candidates, 3164 ± 2842 steps, and percentage of waking time spent in moderate-to-vigorous activity, 4.9% ± 6.9%, both averaged about one-third of that of healthy adults, and the latter measurement was a highly significant predictor of wait-list mortality.(18)
After liver transplantation, physical activity levels generally improve. Recipients’ expectations and intensity of motivation are important determinants of their achieved level of physical performance, which may reach that of most nontransplanted individuals.(19) On the other hand, those who have been highly sedentary for an extended time before transplant may lack the capacity or the readiness and inclination to make physical recovery a priority. After transplantation for nonalcoholic steatohepatitis cirrhosis, recipients’ physical activity and resting energy expenditure were reported to be lower than that of nontransplanted patients with nonalcoholic fatty liver disease.(20) In 17 recipients transplanted mainly for hepatitis C virus (HCV) and alcohol cirrhosis, resting energy expenditure increased transiently at 1 month after transplantation and returned to a level no different from pretransplant baseline by 1 year.(21) In another study of 20 liver transplant recipients, aerobic fitness was measured by peak oxygen consumption (VO2peak) before and a mean of 16 months after transplantation. It improved only modestly by 8%, an increase that was largely attributed to increased hemoglobin and cessation of beta blockade.(22)
A relationship of physical inactivity with the posttransplant metabolic syndrome (PTMS) was suggested by a study of 204 liver transplant recipients that showed a significant association between sedentary activity and PTMS, with its commonly occurring components of posttransplant obesity, hepatic steatosis, diabetes, hypertension, and hyperlipidemia. PTMS had a prevalence of 58.8% in the study cohort.(23) Half of recipients reported performing no formal exercise. Those who did exercise reported an average of 90 ± 142 minutes/week at a mean metabolic equivalent (MET) score of 3.6 ± 1.5, both well below the recommended levels for the general population. The findings raise a key question of whether and to what extent vigorous posttransplant exercise will prove to have value for prevention and management of PTMS and its cardiovascular complications.
Figure 1 illustrates possible trajectories of physical performance over time for liver transplant recipients. The vertical axis represents level of performance expressed quantitatively using any objective metric, such as the 6-minute walk distance, components of the LFI (grip strength, chair stand performance, and postural stability), maximum oxygen consumption (VO2max), usual gait speed, or the Karnofsky Performance Index, and the horizontal axis indicates time. From points A to C, it is common for transplant candidates to experience gradual physical decline over time, perhaps with a reversible deficit attributed to an acute illness as shown at point B. After recovery from the acute transplant episode at point C, occurrence of major complications such as advanced renal failure, ischemic cholangiopathy, intra-abdominal infection, or severe recurrent allograft liver disease could negatively impact performance, shown at point D. More frequently, recipients may slowly improve as shown at point E, or those with strong motivation and an optimal course as shown at point F may achieve a level of performance equivalent to that of patients without chronic liver disease(19)
FIG. 1.
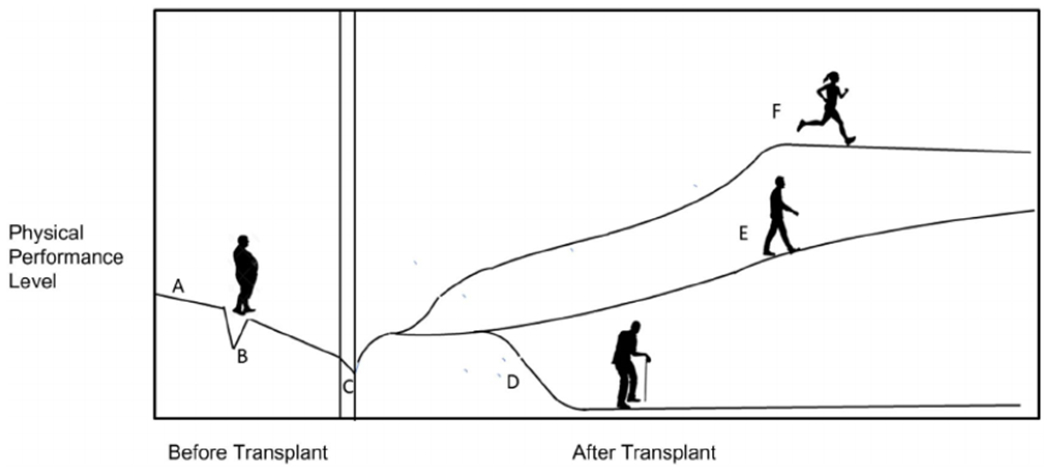
Potential physical activity trajectories of liver transplant recipients: physical performance level at transplant listing (A); loss of performance from acute illness with partial improvement (B); acute effect of transplant episode (C); effect of a major complication, eg, rejection, acute kidney injury, or infection (D); incomplete/delayed functional recovery (E); and robust functional recovery toward normal (F). The physical decline from wait-listing (A) through an acute illness (B) continues through transplantation (C). The posttransplant recovery of robust activity may be impaired by major complications (D), may follow a slow recovery pathway (E), or, with strong motivation, may recover to a normal or nearly normal level of fitness (F).
Posttransplant Rehabilitation and Exercise Training
EXERCISE, OXYGEN UPTAKE, AND PHYSICAL PERFORMANCE AFTER TRANSPLANTATION
Observational nonrandomized studies of exercise after liver transplant were reported for 2 recipient cohorts that included a total of 58 patients who participated in supervised exercise.(24,25) One cohort of 38 recipients began individually tailored exercise at 3 weeks after transplant, which continued through month 6 after transplant; 18 of the 38 patients completed the study with attrition due mainly to posttransplant complications, employment constraints, and travel issues.24) The other cohort of 20 recipients, all of whom reported fatigue, began a 12-week exercise program 1 year or longer after transplant; 18 of the 20 recruited patients completed the intervention(25) Both studies showed improvement in exercise capacity measured by VO2max or VO2peak, and physical function measured by the 6-minute walk distance and knee muscle strength. Recipients in 1 study also reported improvement in subjective fatigue.(25)
Three randomized trials of posttransplant exercise have now been published. In a study of 151 recipients randomized at 2 months after transplant to a 10-month, graded, home-based exercise and diet intervention compared with usual care, participants randomized to the intervention arm showed significantly greater improvements in exercise capacity measured by VO2peak compared with that of control patients(26) Both the intervention and usual care arms showed a 30% improvement in quadriceps strength over the study period. Notably, only 119 (79%) of the patients completed the trial, and the reasons why 32 patients did not complete the trial were not reported.
In a smaller trial of 15 liver transplant recipients randomized to either a program of 24 exercise treadmill sessions over 12 weeks versus usual care, 9 participants in the exercise group significantly increased their 6-minute walk distance by 19% and resting energy expenditure by 30%, compared with no change in 6 control patients.(27)
A third randomized trial involving 54 recipients compared the impact on aerobic fitness of a 24-week site-based exercise intervention beginning 6 months after transplant. The intervention involved two 75-minute sessions per week of moderate-to-high intensity resistance and aerobic exercise versus usual care.(28) Baseline aerobic fitness levels measured by VO2peak for the intervention and control arms were similar, 16.4 and 17.4 mL/kg/minutes, respectively. This low level of aerobic fitness matched that reported for other patient groups with advanced cirrhosis.(29) Of the 54 patients, 50 completed the trial. VO2peak increased by 15% among exercisers and only 7% among controls, a significant difference favoring the intervention arm. Global muscle strength increased 31% in the intervention arm, again significantly greater than a 9% improvement in the control arm.
The reports provide consistent support for the concept that structured exercise training initiated early after liver transplantation improves recipients’ exercise capacity and physical function. Whether the reported improvements are sustainable over time after completing an exercise program is not known. Although an optimal program has not been defined, it appears that recipients who exercise regularly will benefit in terms of measurable performance, along with improved QOL as discussed below.
Physical Activity and QOL After Liver Transplantation
TRAJECTORY AND DETERMINANTS OF POSTTRANSPLANT QOL
Two systematic reviews of studies of QOL in liver transplantation reinforce the consensus that QOL improves after transplant, but whether it usually recovers to general population levels is not as clear.(30,31) Although early improvements in psychological distress and personal function are usually sustained, indicators of social and role function, physical distress, and general health perception often worsen over the long term (>12 years after transplant).(32) Predictors of worse posttransplant QOL include hepatocellular carcinoma and receiving a deceased circulatory death donor organ.(33) A cross-sectional survey study of 61 liver transplant recipients found significant associations of negative self-perceived body image with self-reported sedentary physical activity and low QOL.(34) With respect to liver disease etiology, QOL was lowest for recipients transplanted for HCV and alcoholic liver disease(32)
PHYSICAL ACTIVITY AS A KEY DETERMINANT OF QOL
Multiple reports identify physical activity as a highly significant determinant of QOL. Posttransplant physical activity, self-care, and mobility were all associated with improved QOL.(35) This is supported by data demonstrating that total energy expenditure in METs has also been strongly associated with QOL in transplant recipients.(36) Participation in group sports activities was associated with improved physical function and QOL, independent of comorbidities, for up to 5 years after transplant.(37,38)
EXERCISE AND QOL
Exercise and QOL are closely linked in general populations. Exercise not only improves physical wellbeing, but it also strongly impacts the mental health components of QOL. Exercise is regularly included in behavioral support for management of depression and anxiety. Although it stands to reason that exercise would enhance QOL for transplant recipients, high-quality randomized trial evidence supporting this inference is limited.(38) Because transplant recipients with better QOL are more likely to engage in exercise, it is difficult to disentangle the causality of whether exercise improves QOL. At present, the best support for this statement is the randomized exercise trial of 151 liver transplant recipients cited earlier that found that patients randomized to its exercise arm had significantly better QOL Short Form 36 scores than the patients in its usual care group.(26)
SOCIAL SUPPORT, GAMING, AND THE TRANSPLANT GAMES
Transplant recipients, caregivers, and providers intuitively associate physical and emotional health with social support in important activities, including posttransplant physical performance.(19) Making a sustained effort to gain the health benefits of a guideline-based physical activity program can represent a watershed change from the sedentary pretransplant state experienced by many patients.(18) Group physical activities that hold appeal for recipients may provide the positive incentive needed for that change. For example, the organized sports of the Transplant Games, started in 1978 by Maurice Slapak, an English transplant surgeon, now engage the interest and energy of recipients worldwide with a positive impact on posttransplant health.(39,40) In general, most liver transplant recipients rightly view their overall experience as lifesaving, transformative, and highly beneficial.(30,31) The social interaction and teamwork involved in group-based transplant-related physical activities can deliver significant intangible benefits in terms of self-esteem and confidence.
A Personalized Exercise Program for Liver Transplant Recipients
Current society practice guidelines generally recommend exercise for liver transplant recipients without providing specific detail. In our current practice, we begin planning for the recovery of a recipient’s physical activity at a candidate’s pretransplant evaluation. Setting a realistic pace and expected goals for posttransplant recovery will benefit from personalized appreciation of a candidate’s physical performance status; their duration and extent of inactivity, muscle loss, and bone health at the time of listing; and importantly, by their observed activity and level of desire for improvement while wait-listed.
We use pretransplant data to help engage recipients and their caregivers with physical therapists, nutritionists, and physicians in beginning a tailored physical recovery program as soon as possible, normally in a meeting at posttransplant hospital discharge or on the first transplant return visit. On subsequent visits, we reassess progress, primarily relying on the LFI. We take advantage of the confidence that recipients place in their transplant team by asking that the physician or other lead provider at each visit make a point of encouraging activity, especially group or recreational sporting activity whenever circumstances permit.(38–40)
At present we lack specific consensus physical activity target levels for liver transplant recipients. However, because of the importance of physical activity to mitigate risk of the cardiovascular complications of the PTMS,(21) it seems reasonable at present to encourage recipients who can do so to work toward meeting the United States population guidelines of 150 minutes/week of moderate-to-vigorous activity, along with resistance exercise training for 15-20 minutes twice weekly(41) We encourage recipients to track their activity intensity and duration using wearable accelerometers as described for pretransplant exercise programs.(42)
Conclusion
This review describes current evidence supporting the significance and value of recognizing and overcoming posttransplant frailty, sarcopenia, and inactivity in order to improve physical function and QOL and to mitigate the risk of PTMS. Post–liver transplant care offers an opportunity for transformational change to enable recipients to pursue the proven benefits of physical activity, mitigation of longterm metabolic risk, and improved QOL.
Abbreviations:
- HCV
hepatitis C virus
- LFI
Liver Frailty Index
- MET
metabolic equivalent
- PTMS
posttransplant metabolic syndrome
- QOL
quality of life
- SMI
skeletal muscle index
- VO2max
maximum oxygen consumption
- VO2peak
peak oxygen consumption
REFERENCES
- 1).Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Tandon P, Montano-Loza AJ eds. Frailty and Sarcopenia in Cirrhosis. The Basics, the Challenges and the Future. Cham, Switzerland: Springer Nature; 2019:1–279. [Google Scholar]
- 3).Ooi PH, Hager A, Mazurak VC, Dajani K, Bhargava R, Gilmour SM, Mager DR. Sarcopenia in chronic liver disease: impact on outcomes. Liver Transpl 2019;25:1422–1438. [DOI] [PubMed] [Google Scholar]
- 4).Lai JC, Sonnenday CJ, Tapper EB, Duarte-Rojo A, Dunn MA, Bernal W, et al. Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant 2019;19:1896–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, Feng S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Lai JC, Covinsky KE, McCulloch CE, Feng S. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol 2018;113:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Lai JC, Segev DL, McCulloch CE, Covinsky KE, Dodge JL, Feng S. Physical frailty after liver transplantation. Am J Transplant 2018;18:1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology 2019;70:1816–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Davuluri G, Krokowski D, Guan BJ, Kumar A, Thapaliya S, Singh D, et al. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J Hepatol 2016;65:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Krol CG, Dekkers OM, Kroon HM, Rabelink TJ, van Hoek B, Hamdy NAT. Longitudinal changes in BMD and fracture risk in orthotopic liver transplant recipients not using bone-modifying treatment. J Bone Mineral Res 2014;29:1763–1769. [DOI] [PubMed] [Google Scholar]
- 12).Tsien C, Garber A, Narayanan A, Shah SN, Barnes D, Eghtesad B, et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol 2014;29:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Jeon JY, Wang H-J, Ock SY, Xu W, Lee J-D, Lee JH, et al. Newly developed sarcopenia as a prognostic factor for survival in patients who underwent liver transplantation. PLoS One 2015;10:e0143966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Bhanji RA, Takahashi N, Moynagh MR, Narayanan P, Angirekula M, Mara KC, et al. The evolution and impact of sarcopenia pre- and post-liver transplantation. Aliment Pharmacol Ther 2019;49:807–813. [DOI] [PubMed] [Google Scholar]
- 15).Mager DR, Hager A, Ooi PH, Siminoski K, Gilmour SM, Yap JYK. Persistence of sarcopenia after pediatric liver transplantation is associated with poorer growth and recurrent hospital admissions. J Parenter Enteral Nutr 2019;43:271–280. [DOI] [PubMed] [Google Scholar]
- 16).Bergerson JT, Lee J-G, Furlan A, Sourianarayanane A, Fetzer DT, Tevar AD, et al. Liver transplantation arrests and reverses muscle wasting. Clin Transplant 2015;29:216–221. [DOI] [PubMed] [Google Scholar]
- 17).Stam SP, Oste MCJ, Eisenga MF, Blokzijl H, van den Berg AP, Bakker SJL, de Meijer VE. Posttransplant muscle mass measured by urinary creatinine excretion rate predicts long-term outcomes after liver transplantation. Am J Transplant 2019;19:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, et al. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl 2016;22:1324–1332. [DOI] [PubMed] [Google Scholar]
- 19).Beekman L, Berzigotti A, Banz V. Physical activity in liver transplantation: a patient’s and physicians’ experience. Adv Ther 2018;35:1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Singhvi A, Sadowsky S, Cohen A, Demzik A, VanWagner L, Rinella M, Levitsky J. Resting and exercise energy metabolism after liver transplantation for nonalcoholic steatohepatitis. Transplantation Direct 2017;3:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Ferreira L, Santos LF, Anastácio LR, Lima AS, Correia MITD. Resting energy expenditure, body composition, and dietary intake: a longitudinal study before and after liver transplantation. Transplantation 2013;96:579–585. [DOI] [PubMed] [Google Scholar]
- 22).Lemyze M, Dharancy S, Neviere F, Pruvot F-R, Declerck N, Wallaert B. Aerobic capacity in patients with chronic liver disease: very modest effect of liver transplantation. Presse Med 2010;39:e174–e181. [DOI] [PubMed] [Google Scholar]
- 23).Kallwitz ER, Loy V, Mettu P, Von Roenn N, Berkes J, Coder SJ. Physical activity and metabolic syndrome in liver transplant recipients. Liver Transpl 2013;19:1125–1131. [DOI] [PubMed] [Google Scholar]
- 24).Beyer N, Aadahl M, Strange B, Kirkegaard P, Hansen BA, Mohr T, Kjasr M. Improved physical performance after orthotopic liver transplantation. Liver Transpl Surg 1999;5:301–309. [DOI] [PubMed] [Google Scholar]
- 25).van den Berg-Emons RJ, van Ginneken BT, Nooijen CF, Metselaar HJ, Tilanus HW, Kazemier G, Stam HJ. Fatigue after liver transplantation: effects of a rehabilitation program including exercise training and physical activity counseling. Phys Ther 2014;94:857–865. [DOI] [PubMed] [Google Scholar]
- 26).Krasnoff JB, Vintro AQ Ascher NL, Bass NM, Paul SM, Dodd MJ, Painter PL. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant 2006;6:1896–1905. [DOI] [PubMed] [Google Scholar]
- 27).Garcia AM, Veneroso CE, Soares DD, Lima AS, Correia MI. Effect of a physical exercise program on the functional capacity of liver transplant patients. Transplant Proc 2014;46:1807–1808. [DOI] [PubMed] [Google Scholar]
- 28).Moya-Nájera D, Moya-Herraiz Á, Compte-Torrero L, Hervás D, Borreani S, Calatayud J, et al. Combined resistance and endurance training at a moderate-to-high intensity improves physical condition and quality of life in liver transplant patients. Liver Transpl 2017;23:1273–1281. [DOI] [PubMed] [Google Scholar]
- 29).Jones JC, Coombes JS, Macdonald GA. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl 2012;18:146–151. [DOI] [PubMed] [Google Scholar]
- 30).Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol 2008;48: 567–577. [DOI] [PubMed] [Google Scholar]
- 31).Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long-term quality of life. Liver Int 2014;34:1298–1313. [DOI] [PubMed] [Google Scholar]
- 32).Ruppert K, Kuo S, DiMartini A, Balan V. In a 12-year study, sustainability of quality of life benefits after liver transplantation varies with pretransplantation diagnosis. Gastroenterology 2010;139:1619–1629. [DOI] [PubMed] [Google Scholar]
- 33).McLean KA, Drake TM, Sgro A, Camilleri-Brennan J, Knight SR, Ots R, et al. The effect of liver transplantation on patient-centered outcomes: a propensity-score matched analysis. Transpl Int 2019;32:808–819. [DOI] [PubMed] [Google Scholar]
- 34).Zimbrean PC, Gan G, Deng Y, Emre S. Body image in liver transplantation recipients. Liver Transpl 2019;25:712–723. [DOI] [PubMed] [Google Scholar]
- 35).Painter P, Krasnoff J, Paul SM, Ascher NL. Physical activity and health-related quality of life in liver transplant recipients. Liver Transpl 2001;7:213–219. [DOI] [PubMed] [Google Scholar]
- 36).Masala D, Mannocci A, Unim B, Del Cimmuto A, Turchetta F, Gatto G, et al. Quality of life and physical activity in liver transplantation patients: results of a case-control study in Italy. Transplant Proc 2012;44:1346–1350. [DOI] [PubMed] [Google Scholar]
- 37).Cicognani E, Mazzoni D, Totti V, Roi GS, Mosconi G, Nanni Costa A. Health-related quality of life after solid organ transplantation: the role of sport activity. Psychol Health Med 2015;20:997–1004. [DOI] [PubMed] [Google Scholar]
- 38).Neale J, Smith AC, Bishop NC. Effects of exercise and sport in solid organ transplant recipients: a review. Am J Phys Med Rehabil 2017;96:273–288. [DOI] [PubMed] [Google Scholar]
- 39).Slapak M Sport and transplantation. Ann Transplant 2005;10:60–67. [PubMed] [Google Scholar]
- 40).Neuberger J, Armstrong MJ, Fisher J, Mark P, Schmidtke K, Sharif A, Vlaev I. Sport and exercise in improving outcomes after solid organ transplantation: overview from a UK meeting. Transplantation 2019;103(suppl 1):S1–S11. [DOI] [PubMed] [Google Scholar]
- 41).Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res 2015;117:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, Maícias-Rodríguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24:122–139. [DOI] [PubMed] [Google Scholar]


