Highlights
-
•
AAV is characterized by necrotizing small vessel vasculitis with positive serum ANCA.
-
•
MPO/PR3-ANCA and neutrophils play central roles in AAV pathogenicity.
-
•
Dysregulated complement system primes neutrophils.
-
•
MPO-ANCA directly activates neutrophils to induce NETosis followed by releasing NETs.
-
•
B cells, T cells, and dendritic cells also contribute to the pathogenicity of AAV.
Keywords: ANCA-Associated vasculitis, Anti-neutrophil cytoplasmic antibody, Neutrophil, NETs
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare disease, which is characterized by necrotizing small vessel vasculitis with positive serum ANCA (MPO/PR3-ANCA against myeloperoxidase/proteinase 3) and consists of three diseases: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis (EGPA). The AAV’s etiology involves genetic (single nucleotide polymorphism: SNP) and environmental factors (infection, harmful microparticles, and drugs) that affect phenotypic differences between regions and AAV subsets. ANCAs, especially MPO-ANCA, have pathogenicity and relevant to disease activities, playing a central role together with neutrophils. The overactivated neutrophils by ANCAs, as the effector cells, finally release reactive oxygen species, lytic enzymes, matrix metalloproteinases, and neutrophil extracellular traps (NETs) to cause vascular damage. The pathological process has been understood by the mechanism of ANCA production and small vessel vasculitis with the ANCA-cytokine-sequence-theory and the complementary peptide theory. These have been attributed to the various dysregulation of not only neutrophils and B cells but also complementary system, antigen presentation, and T cells. In particular, the complementary system has been elucidated that C5a is a crucial factor to activate neutrophils in vivo and in vitro. Subsequently, avacopan, a C5a receptor antagonist, was shown to be promising in the clinical trials. However, many issues are left unsolved; more personalized therapy based on phenotypes, the roles of SNPs from genome-wide association studies, and the characteristic of ANCA-producing B cells or treatment-refractory patients. Here, we overviewed the pathogenesis and pathology of AAV, focusing on the MPA and GPA subtypes.
1. Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) consists of disorders characterized by necrotizing small blood vessels and the presence of circulating ANCA. The most widely used classification of AAV is based on the 2012 International Chapel Hill Consensus Conference (CHCC). AAV is classified as a primary small vessel vasculitis, which consists of three diseases: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA) [1]. Each of these diseases shows characteristic clinical presentations, but shares similar pathological findings, including the necrotizing, pauci-immune vasculitis of small-sized vessels. Typically, MPA presents with glomerulonephritis and pulmonary capillaritis, including interstitial pneumonitis and lung hemorrhage, which often worsens rapidly. Additionally, involvement of the skin, nerves, and gastrointestinal tract is not uncommon. GPA presents with symptoms in the upper respiratory tract (ear, nose, and paranasal sinus inflammation), pulmonary nodule/hemorrhage, and glomerulonephritis. The histopathology of GPA is characterized by the presence of granulomatosis in addition to necrotizing vasculitis. In turn, EGPA is characterized by a preceding medical history of bronchial asthma or allergic rhinitis, followed by pulmonary infiltration, skin rash, peripheral neuropathy, and marked eosinophilia. The pathology of EGPA shows eosinophilic infiltration with granulomatosis and extravascular granuloma. In contrast, the histopathology of MPA and GPA presents with large amounts of neutrophils at the sites of vasculitis or necrosis.
ANCA and neutrophils are key components in MPA and GPA. We will discuss the role of AAV in pathogenicity and pathology.
1.1. Epidemiology
AAV is a rare disease. The incidence and prevalence are estimated at 20 per million per year and 46–184 per million people, respectively [2,3]. The peak age of onset of AAV is between 65 and 74 years [4] and is significantly higher in MPA [5]. Regional differences exist, and AAV is more common in white and Asian populations [2]. Regarding the AAV subsets, Northern Europe and Australia/New Zealand have a higher prevalence of GPA, whereas in Southern Europe and Asia, MPA has a higher prevalence [2]. A Japanese retrospective study showed that MPA is more frequent (MPA 50.0%, GPA 21.2%,EGPA 9.0%)and MPO-ANCA is predominant [6]. The phenotype may also be attributed to regional differences, as a case report of pulmonary fibrosis showed an MPO(+)ANCA predominance in Japan [7]. The reason for this difference has not been fully understood; however, it is thought to be related to genetic and environmental factors.
1.2. Etiology
It has been suggested that the onset of AAV is associated with both genetic and environmental factors.
1.2.1. Genetic factors
Although very few cases of familial AAV have been reported, there have been various genetic studies including genome-wide association studies (GWASs) that have identified single nucleotide polymorphisms (SNPs) related to susceptibility. GWASs have also suggested some differences between the three AAV subsets or in geography [8,9]. A GWAS on United Kingdom patients revealed obvious genetic differences in GPA and MPA, and the genetic association was stronger in classification by ANCAs compared to classification by clinical presentation [10]. In a summary of some studies, HLA-DR polymorphisms were shown to be related to susceptibility to MPA and MPO-ANCA(+) AAV, and HLA-DQ polymorphisms were shown to be related to resistance, whereas susceptibility to GPA and PR3-ANCA(+) AAV was shown to be associated to HLA-DP polymorphisms [[10], [11], [12]]. Furthermore, a GWAS from North American further identified HLA-DPB1∗04 as a risk allele for GPA [12]. In a regional differences study, Kawasaki et al. showed that HLA- DRB1∗09:01 is common in East Asia, but rare in Europe, and it is associated with MPA in Japan [11]. A study reported that the HLADRB1∗15 allele is highly frequent in African American GPA and reacts against sense/complementary PR3 138-169 peptide, which agrees with the theory of complementary peptide, as discussed below [13,14].
In the non-MHC gene, the single nucleotide polymorphism (SNP) of PTPN22 is associated with susceptibility to PR3-ANCA(+) AAV because the mutation suppresses IL-10 by upregulating the activity of PTPN22 [15]. Moreover, SNPs of SERPINA22 (encoding α1-antitrypsin, which is a main inhibitor of PR3), PRTN3 (encoding proteinase 3), and SEMA6A have been associated with resistance to GPA or PR3-ANCA(+) AAV [10,12].
However, the mechanisms by which these polymorphisms contribute to the pathogenesis of AAV remain unclear.
1.2.2. Environmental factors
There are several environmental factors that may cause AAV, including infection, harmful microparticles, and medical substances.
-
1)
Infection
Some of the infectious diseases were suggested to be involved in the pathogenesis in AAV. For instance, firstly reported AAV cases in 1982 were related to Ross River virus because of the geographical co-clustering of both diseases, similarity of the clinical presentations, and the positive serology of the virus [15]. Staphylococcus aureus, which is also a representative infectious factor, produces toxic shock syndrome toxin 1 that exacerbates GPA and supplies complementary PR3 or mimicry PR3 peptide, which leads to PR3-ANCA (we will discuss the theory of complementary peptide below) [13,14,16].
As a caveat, various infection (such as tuberculosis, HIV and infectious endocarditis) yields ANCA in the serum which does not cause AAV [17,18].
-
2)
Harmful microparticles
It has been reported that several harmful microparticles such as silica, asbestos, and metal cause AAV [19,20]. Silica is present in glass, cement, and soil. The increased incidence of MPO-ANCA(+) AAV in Japan may be related to the two massive earthquakes in the country (Hanshin-Awaji earthquake in 1995, the Great East Japan earthquake in 2011) [[21], [22], [23]]. Besides, the bronchoalveolar lavage fluid from patients presenting with diffuse alveolar hemorrhage after the Great East Japan earthquake shows a tendency toward higher silica concentrations, although there is a possibility that other microparticles cause AAV [24]. The frequency of AAV also increased after the 2014 Zhaotong earthquake in China [25]. On the other hand, this was not the case after the 2011 Christchurch Earthquake in New Zealand [26]. These differences may be caused by the amount of silica released into the air and the genetic factors that Asians tend to have AAV with positive MPO-ANCA, which is also a characteristic of silica-induced AAV.
-
3)
Drugs
It has been reported that the use of hydralazine, minocycline, propylthiouracil, and levamisole-contaminated cocaine is associated with the onset of AAV [[27], [28], [29], [30]]. The mechanism of propylthiouracil-induced AAV has been well elucidated. Propylthiouracil induces abnormal neutrophil extracellular traps (NETs) to product ANCA (see below in the section of Pathology) [[31], [32], [33]].
1.3. Anti-neutrophil cytoplasmic antibody
ANCAs are autoantibodies against cytoplasmic antigens expressed in the primary granules or the lysosome in neutrophils, especially MPO and PR3, which are detected using indirect immunofluorescence (IIF) microscopy on ethanol-fixed neutrophils. The staining patterns divide the ANCAs into perinuclear ANCA (p-ANCA) and cytoplasmic pattern (c-ANCA). Davies et al. first reported that eight patients had similar clinical symptoms of histopathology of the pauci-immune segmental necrotizing glomerulonephritis and the neutrophilic cytoplasm stained with IIF in 1982 [15]. Subsequently, Wounde et al. found that c-ANCA is specific to patients with GPA in 1985[34]. Falk and Jennette described p-ANCA in MPA in 1988[35]. P-ANCA mainly recognizes cationic myeloperoxidase (MPO), which leaks from the primary granules during ethanol fixation and attaches to the negatively charged nuclear membrane, whereas C-ANCA binds to proteinase 3 (PR3) in the cytoplasm [2]. Subsequently, the quantitative measurement of ANCAs for MPO and PR3 using enzyme-linked immunosorbent assay (ELISA) helps in the identification of MPO-ANCA and PR3-ANCA. Thus, ANCAs are useful not only for diagnosis, but also for disease activity parameters in active AAV [36].
However, it has been reported that the titer of ANCA is not always associated with disease activity, in particular for recurrence [37]. For one, it has been suggested that there is a difference in epitope or affinity [[38], [39], [40], [41]]. It is also possible that ceruloplasmin degradation products bind to ANCA, leading to false-negative results in ANCA(−) AAV patients [41]. Furthermore, there are primary granules and lysosomes that contain various other proteins, except for MPO or PR3 (ex. bactericidal permeability-increasing protein, elastase, cathepsin G, and lactoferrin). ANCAs can bind to those minor antigens, resulting in false positives (i.e., ANCA is negative using ELISA but positive using IIF) [42]. In general, although the detection of minor antigens for ANCA may be correlated with disease activity, whether these have a role in pathogenicity is not fully understood [43].
1.4. Structure, function, and epitope of MPO/PR-3
1.4.1. Myeloperoxidase
Myeloperoxidase is a glycosyl protein, mainly expressed in neutrophils, composed of two light chains (14 kDa) and two heavy chains (59 kDa) with a heme-containing enzyme that catalyzes a chemical reaction of chloride ion and hydrogen peroxide producing hypochlorous acid, which acts as a sterilizer [44]. The MPO gene is located on chromosome 17q22-q23. Gene expression is detected in granulocyte precursors; however, low or non-detectable in normal neutrophils [45]. Neutrophils in AAV increase the expression of MPO/PR-3, which correlates with disease activity [46,47]. Regarding intracellular distribution, MPO is not expressed on the cell membrane in stable conditions, but is expressed on the membrane in active AAV [48]. A few epitope analyses have also been reported. Most ANCAs in MPO-ANCA(+) AAV recognize an epitope at the C and N amino terminus of the MPO heavy chain, and this binding site has been associated with severe disease activity [38,49]. Another study reported that a linear epitope, human MPO 447-459, was detected not only in active AAV, but also in ANCA-negative AAV [41]. Regarding T cell response to MPO, several reports has identified immunodominant MPO T-cell epitopes, which are pathogenic to cause AAV in an animal model (e.g., mouse MPO 409–428, human MPO 431-439, and mouse MPO 447-461) [[50], [51], [52]].
1.5. Proteinase 3
Proteinase 3, encoded by PRTN3 located on chromosome 19, is a serine protease enzyme (29 kD glycoprotein) expressed in primary granules of neutrophils [53,54]. Its function is to digest proteins to remodel tissue, inactivate azurocidin (known as cationic antimicrobial protein), and activate inflammatory cytokines [[55], [56], [57]]. PR3 is usually expressed on the neutrophil cell surface with increasing neutrophil activation [56]. Epitope mapping studies have identified a complementary PR3 (cPR3) that is responsive to T cells (the cPR3 peptide model will be later discussed) [13,58].
1.6. Pathogenicity of ANCA
Several clinical reports have suggested that MPO-ANCA is pathogenic, whereas this remains controversial for PR3-ANCA [59]. 1) Transferring maternal anti-MPO-ANCA to neonates causes pulmonary hemorrhage and glomerulonephritis after birth [60,61]. 2) Rituximab (anti-CD20 monoclonal antibody), which depletes B cells but not plasma cells, is effective both as an induction and maintenance treatment of AAV by decreasing the serum level of ANCA [62,63].
In addition, some basic research has also shown that ANCAs cause AAV-like disease in animal models. Two landmark studies provided essential insights into ANCA pathogenesis [64]. Purified anti-MPO IgG or spleen cells from MPO-deficient mice immunized with murine MPO lead wild-type mouse and recombinase activating gene 2 (RAG2) deficient mice, which have deficits of B and T cells, to necrotizing pauci-immune glomerulonephritis and pulmonary capillaritis such as MPA [65]. Likewise, WKY (Wistar-Kyoto) rats immunized with human MPO produced anti-human MPO antibodies, which also cross-reacted with rat MPO, resulting in pauci-immune crescent glomerulonephritis and pulmonary hemorrhage [66]. In vitro studies have reported that ANCAs activate neutrophils by binding to Fcγ receptors (FcγRIIa and FcγRIIIb) after the Fab of ANCAs attach to the cell surface MPO or PR3, and, in turn, neutrophils produce reactive oxygen species (ROS) and release primary granules and NETs [[67], [68], [69], [70]]. Another report showed that ANCAs change CD11b into its active form on neutrophils to promote transmigration [71].
In contrast, the pathogenicity of PR3-ANCA in vivo is not well understood, partly because developing well-designed PR3-ANCA-induced AAV animal models has been unsuccessful [66]. For example, mice and rats immunized with chimeric human/mouse PR3 could produce anti-PR3 antibodies, but did not develop abnormalities in the kidney or lung [72]. Non-obese diabetic (NOD) mice immunized with recombinant mouse PR3 produced anti-PR3 antibodies without vasculitis. However, when mice bred with NOD and severe combined immunodeficiency (SCID) mice were transferred with splenocytes derived from NOD mice immunized with recombinant mouse PR3 in complete Freund’s adjuvant, they developed severe segmental and necrotizing glomerulonephritis, but still lacked granuloma [73]. The lower homology of mouse and human PR3 may contribute to the difficulty in developing GPA model mice [74].
1.7. Pathology (mechanism of ANCA production and small vessel vasculitis)
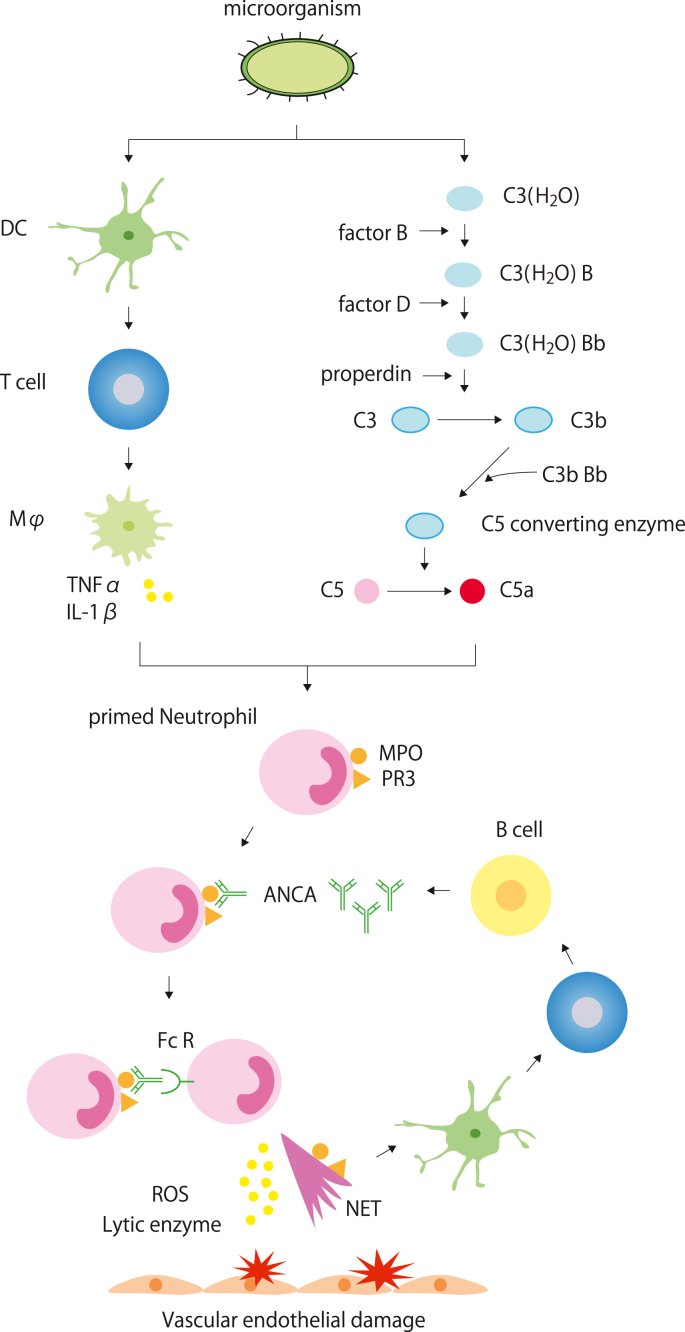
Neutrophils play a central role in AAV and their depletion ameliorates anti-MPO IgG-induced pauci-immune necrotizing and crescentic glomerulonephritis [75]. We will describe the mechanism of ANCA production and small vessel vasculitis with the ANCA-cytokine-sequence-theory [76] (Fig. 1). First, immunogen invades the body under infection and dendritic cells capture the antigen to present to T cells, which results in macrophage activation and production of inflammatory cytokines (e.g., TNFα, IL-1β) to prime neutrophils. Complement activation also contributes to this process. Following the priming of neutrophils, cytoplasmic MPO or PR3 is expressed on the membrane to bind to the Fab of ANCA, and the Fc region of ANCA-attached Fc receptors on neutrophils [[77], [78], [79]]. Neutrophils overactivated by ANCA release ROS, lytic enzymes, matrix metalloproteinases, and NETs to cause vascular damage [70]. The MPO or PR3 on the membrane or NETs, which are captured by antigen-presenting cells, are presented to CD4+ T cells or B cells that produce ANCAs. Several mechanisms maintain and promote this pathophysiology.
-
1)
Complement system
Fig. 1.
The mechanism of ANCA production and small-vessel vasculitis [76].
Microorganisms are either captured by dendritic cells or activate the alternative complement pathway. Dendritic cells lead macrophages to release inflammatory cytokines through antigen presentation to T cells. The alternative pathway produces C5a, which stabilizes the C3(H2O)Bb complex. Inflammatory cytokines or C5a stimulate neutrophils to express MPO and PR3 on their membrane [[77], [78], [79]]. Fab of ANCA binds them, while Fc of ANCA binds with the Fc receptor on neutrophils, which results in the release of ROS, lytic enzymes, and NETs that eventually cause vascular endothelial damage [70].
The histopathology of glomerulonephritis in AAV patients has been considered as pauci-immune; however, some reports have shown the deposition of complement in the kidney [80,81]. In addition, it has been reported that a low serum C3 level predicts a poor renal outcome in AAV and that the urinary level of complement is higher in active AAV than in remission [82,83].
Recent evidence from the MPO-ANCA-associated vasculitis model mouse has shown a role of the complement system, more specifically, the alternate pathway. Xiao et al. reported that crescentic glomerulonephritis caused by the transfer of anti-MPO IgG or anti-MPO splenocytes is blocked by C3 depletion with an injection of Cobra venom factor, in C5- or factor-B-deficient mice, but not in C4 deficient mice [84]. Furthermore, a C5-inhibiting monoclonal antibody (BB5.1) and a human C5aR antagonist (CCX168: avacopan) have been shown to improve MPO-ANCA-induced glomerulonephritis in mice. The importance of this pathway is also identified in C5aR/CD88 deficient mice [85,86]. An in vitro study also revealed evidence of complement participation in AAV. TNFα- and MPO-ANCA- or PR3-ANCA- primed human neutrophils in healthy persons produce substances that cause complement activation, including C5a [84,87]. Furthermore, Wang et al. suggested that the alternative pathway can be activated by proteins in NETs, including complement factor Bb and properdin, which are known secondary granules of neutrophils [88].
One phase II study (CLEAR trial) reported that avacopan could safely reduce the dose of steroids in induction treatment for relapsing active AAV [89]. The phase III study (ADVOCATE trial) indicated that avacopan was non-inferior to the active comparator prednisone at week 26 and superior to prednisone in sustained remission at week 52 in AAV patients receiving rituximab or cyclophosphamide/azathioprine [90,91].
-
2)
NETosis and NETs
NETosis was first described in 2004 as one of the programmed cell death mechanisms in which neutrophils trap and kill microorganisms by releasing cytoplasmic components (NETs) that contain DNA and various proteins such as histones, high-mobility group box 1 (HMGB1), LL37, neutrophil elastase, calprotectin, MPO, and PR3 [[92], [93], [94]]. Although NETs generally act as a biological defense against infection, their overproduction leads to damage of endothelial cells or activation of alternative complement pathways. NETs also provide MPO and PR3 to activate the adaptive immune system, producing ANCA [88,95]. The immunogenicity of NETs was previously shown, as its injection leads to ANCA production and AAV-like disease development. Conversely, the injection of myeloid dendritic cells loaded with DNase-treated NETs did not [96]. In addition, it has been reported that anti-histone antibodies suppress anti-GBM nephritis in mice, which often present rapidly progressive glomerulonephritis, serum MPO-ANCA, and ANCA-associated nephritis [97].
Several mechanisms can contribute to persistent NETs in vivo, including 1) overproduction by neutrophil hyperactivation, 2) suppression of NETs degradation, and 3) impairment in the degradation of NETs.
1) One study showed that neutrophils from AAV patients have a tendency to spontaneously release NETs, and the blood level of NETs components was higher in active AAV patients [98]. 2) DNase I degrades NETs. The rate of NET degradation and DNase I activity in MPA patients is lower than that in healthy controls [70,99]. 3) The abnormal forms of NETs induced by phorbol myristate acetate and propylthiouracil are difficult to digest by DNase I [31]. Nakazawa et al. provided evidence that propylthiouracil causes a conformational change, which leads to the impaired degradation of NETs for the pathogenesis of AAV induction [[31], [32], [33]].
-
3)
Misfolded MPO/PR3 on the cell membrane with MHC class II
Several GWASs have detected SNPs related to MHC class II in AAV; however, the mechanism by which MHC class II works in disease development remains unclear. Arase and Tanimura et al. reported that MHC class II can transport misfolded ER proteins to the cell membrane and, as a result, autoantibodies bind the protein in autoimmunity [100,101]. They also reported that the MPO/HLA–DR complex on the neutrophilic cell membrane in MPA shows higher expression levels when compared to that in healthy controls, and the autoantibody binding to the MPO/HLA–DR complex correlates with an MPA-susceptibility allele [102]. Thus, it can be hypothesized that AAV patients are prone to express MPO/PR3 on the cell surface due to the presence of the MHC class II SNP.
-
4)
B lymphocytes
B cells play an essential role in AAV. Activated B cells are essential for ANCA production and increase in active GPA patients [103]. B cell depletion with rituximab is effective in both induction and maintenance treatment of AAV [63,104]. IL-10-producing regulatory B cells that are characterized by positive CD5 suppress inflammation [105]. The percentage of CD5+ B cells in the peripheral blood of patients with active AAV was shown to be lower than that in remission after rituximab treatment or than that of healthy controls [106]. B cells are activated by cytokines released from neutrophils, including B cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS). Overproduction of these cytokines may increase autoreactive B cells. Indeed, the levels of circulating BAFF in active AAV are higher than those in inactive AAV or healthy controls [107,108].
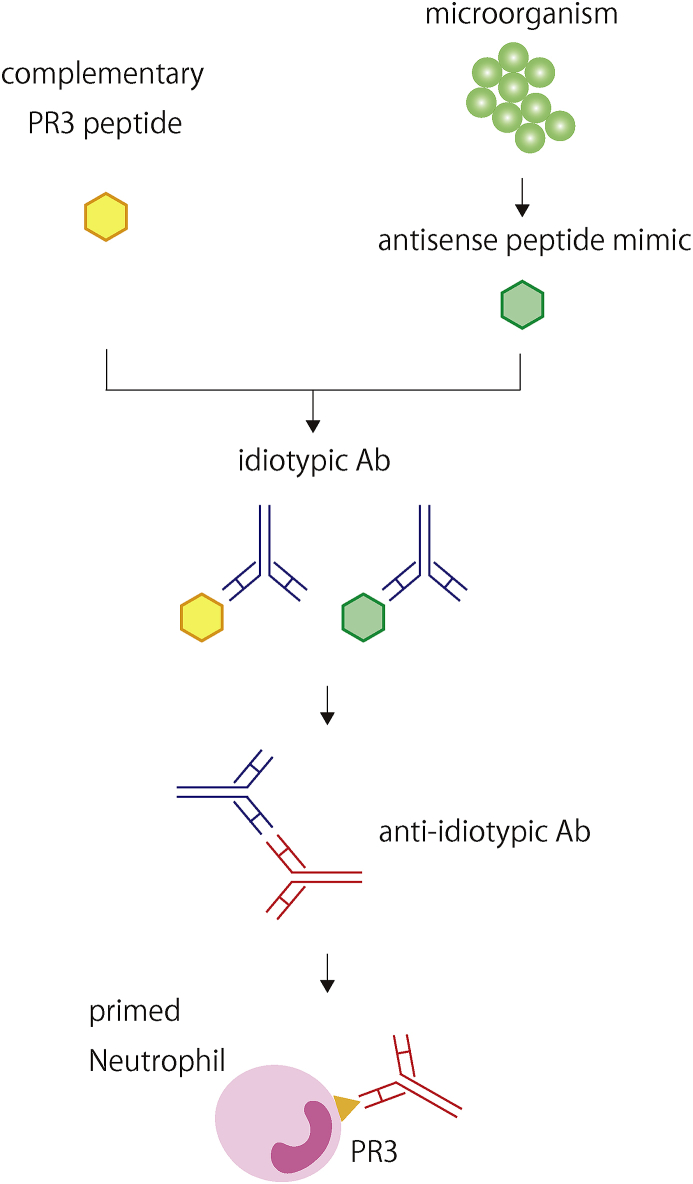
In addition, Jennette and Falk et al. proposed the theory of autoantigen complementarity, which is dependent on B cell adaptive immune response [13,14] (Fig. 2). First, a complimentary peptide, antisense of autoantigen, or its mimic is produced by the host or by the microorganism invading the body. Following this, the immune system produces an idiotypic antibody for the complementary peptide or the mimic. Finally, an anti-idiotypic antibody that cross-reacts with the autoantigen epitopes emerges. Indeed, mice immunized with a part of human complimentary PR3 (cPR3) peptide produce antibodies against not only the cPR3 peptide, but also the PR3 peptide. In addition, serum from GPA patients contains antibodies against both PR3 and cPR3 [13]. Moreover, cPR3 can lead to T lymphocyte activation [58]. Bioinformatics analysis identified a mimicry homolog of the cPR3 peptide from S. aureus [13]. Several clinical findings agree with these speculations. S. aureus colonization in the upper respiratory tract is associated with the GPA relapse rate [109,110]. Furthermore, a randomized placebo-controlled study reported that trimethoprim-sulfamethoxazole reduces relapsing events in GPA patients receiving maintenance therapy [111].
-
5)
T lymphocytes
Fig. 2.
Theory of autoantigen complementarity [13,14].
When a complementary peptide, which is antisense to the autoantigen, is produced, or an antisense peptide mimic invades the body, the immune system produces an idiotypic antibody for the complementary peptide or mimic. Following this, an anti-idiotypic antibody is produced and cross-reacts with the autoantigen epitopes.
T cells participate in AAV pathology by activating neutrophils or B cells by releasing cytokines. CD8+ T cells, which were primed by phytohemagglutinin, produce IFNγ that leads neutrophils to express MHC class II in vitro, and the population of CD8+CD28+CD11b + cells, which produces IFNγ in active AAV peripheral blood cells, was detected, but not in patients in remission [112]. It was reported that anti CD8+ antibody suppresses murine glomerulonephritis upon administration of murine MPO and anti-mouse GBM globulin [52]. Regarding CD4+ T cells, Th1 cells express IFN or CD40L to contribute to classical macrophage activation, which results in the production of TNFα and IL-1β. Th2 cells release IL-4 and IL-13 to induce anti-inflammatory macrophages. In AAV, several studies have reported that Th1 is predominant in active AAV, and Th2 increases in remission [113,114].
However, the evidence of the efficacy of T cell-specific treatments is still limited, although the effectiveness of cyclophosphamide or corticosteroids targeting various cell types, including T cells, has been established in AAV. For example, abatacept binds CD80/86 on antigen-presenting cells through its CTLA-4 to prevent T cell activation. This treatment has been effective with a high frequency of disease remission and prednisone discontinuation, but only in patients with non-severe relapsing GPA [115]. It is not known whether abatacept may have a role in the treatment of the severe form of AAV.
1.8. Future perspective
The future of AAV research lends considerable optimism for better management of the disease once considered untreatable. Based on recent advances in the understanding of its pathogenesis, new treatments, in addition to avacopan, have been in the process of evaluation. A phase 3 clinical trial with belimumab, an anti-BLyS monoclonal antibody for the maintenance treatment of MPA and GPA (NCT01663623) is being conducted, as is a clinical trial with hydroxychloroquine (NCT04316494). Hydroxychloroquine is known to suppress flares in SLE [109], and one of the mechanisms reported is that its accumulation on lysosomes suppresses the presence of autoantigens with MHC class II [116]. It could reduce the expression of the MPO/HLA–DR complex on the neutrophilic surface.
In contrast, many questions remain. Regarding the treatment, available evidence suggests that AAV therapy might be optimized using a personalized approach guided by the ANCA type of the patient. Nevertheless, it is far from precision medicine. Second, although GWASs have revealed several SNPs correlated with AAV, their role has been mostly unclear. Further studies that combine GWAS with phenotype, transcriptome, and proteomics may help answer these questions. Third, the characteristics of ANCA-producing B cells have not been fully identified. Rituximab is shown to be effective in AAV by depleting all of the B cells, not plasma cells [117]; however, the safety profile of rituximab is not better than conventional therapy using cyclophosphamide [63]. Also, some AAV patients are refractory to the treatment. The ineffectiveness maybe because autoreactive B cells can be rescued from the deletion in the presence of high concentrations of BAFF [118]. More specific or complete suppression therapy for ANCA-producing B cells is warranted. Fourth, although the C5a antagonist is effective, the histopathologies show pauci-immune glomerulonephritis. This suggests that activation of neutrophils rather than immune complex formation plays a central role in the pathogenesis of AAV. More investigation is needed to examine how the complement system is activated in AAV. Besides, the detailed analysis of C5a-activated neutrophils in AAV is lacking.
The understanding of AAV and its treatments have advanced; however, more basic and clinical research studies are needed to answer the questions on its pathogenesis, pathology, and more specific treatments.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This article was supported by the Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Research Committee of Intractable Vasculitis Syndrome of the Ministry of Health, Labour and Welfare of Japan.
Contributor Information
Daisuke Tsukui, Email: tsukui@med.teikyo-u.ac.jp.
Yoshitaka Kimura, Email: yo.kimura.july@med.teikyo-u.ac.jp.
Hajime Kono, Email: kono@med.teikyo-u.ac.jp.
References
- 1.Jennette J.C., Falk R.J., Bacon P.A., Basu N., Cid M.C., Ferrario F. Revised international Chapel Hill Consensus conference nomenclature of vasculitides. Arthritis Rheum. 2012;65(1):1–11. doi: 10.1002/art.37715. 2013. [DOI] [PubMed] [Google Scholar]
- 2.Geetha D., Jefferson J.A. ANCA-associated vasculitis: core curriculum 2020. Am. J. Kidney Dis. 2020;75(1):124–137. doi: 10.1053/j.ajkd.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Watts R.A., Mahr A., Mohammad A.J., Gatenby P., Basu N., Flores-Suarez L.F. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol. Dial. Transplant. 2015;30(Suppl 1):i14–22. doi: 10.1093/ndt/gfv022. [DOI] [PubMed] [Google Scholar]
- 4.Ntatsaki E., Watts R.A., Scott D.G. Epidemiology of ANCA-associated vasculitis. Rheum. Dis. Clin. N. Am. 2010;36(3):447–461. doi: 10.1016/j.rdc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Solans-Laque R., Fraile G., Rodriguez-Carballeira M., Caminal L., Castillo M.J., Martinez-Valle F. Clinical characteristics and outcome of Spanish patients with ANCA-associated vasculitides: impact of the vasculitis type, ANCA specificity, and treatment on mortality and morbidity. Medicine (Baltim.) 2017;96(8) doi: 10.1097/MD.0000000000006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sada K.E., Yamamura M., Harigai M., Fujii T., Dobashi H., Takasaki Y. Classification and characteristics of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis in a nationwide, prospective, inception cohort study. Arthritis Res. Ther. 2014;16(2):R101. doi: 10.1186/ar4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homma S., Matsushita H., Nakata K. Pulmonary fibrosis in myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitides. Respirology. 2004;9(2):190–196. doi: 10.1111/j.1440-1843.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 8.Gomes A.M., Nery F., Ventura A., Almeida C., Seabra J. Familial clusters of ANCA small-vessel vasculitis. NDT Plus. 2009;2(1):34–35. doi: 10.1093/ndtplus/sfn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prendecki M., Cairns T., Pusey C.D. Familial vasculitides: granulomatosis with polyangitis and microscopic polyangitis in two brothers with differing anti-neutrophil cytoplasm antibody specificity. Clin Kidney J. 2016;9(3):429–431. doi: 10.1093/ckj/sfw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons P.A., Rayner T.F., Trivedi S., Ju Holle, Watts R.A., Jayne D.R. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 2012;367(3):214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki A., Hasebe N., Hidaka M., Hirano F., Sada K.E., Kobayashi S. Protective role of HLA-DRB1∗13:02 against microscopic polyangiitis and MPO-ANCA-positive vasculitides in a Japanese population: a case-control study. PloS One. 2016;11(5) doi: 10.1371/journal.pone.0154393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie G., Roshandel D., Sherva R., Monach P.A., Lu E.Y., Kung T. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1∗04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheum. 2013;65(9):2457–2468. doi: 10.1002/art.38036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendergraft W.F., 3rd, Preston G.A., Shah R.R., Tropsha A., Carter C.W., Jr., Jennette J.C. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat. Med. 2004;10(1):72–79. doi: 10.1038/nm968. [DOI] [PubMed] [Google Scholar]
- 14.Jennette J.C., Falk R.J. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat. Rev. Rheumatol. 2014;10(8):463–473. doi: 10.1038/nrrheum.2014.103. [DOI] [PubMed] [Google Scholar]
- 15.Davies D.J., Moran J.E., Niall J.F., Ryan G.B. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br. Med. J. 1982;285(6342):606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popa E.R., Stegeman C.A., Abdulahad W.H., van der Meer B., Arends J., Manson W.M. Staphylococcal toxic-shock-syndrome-toxin-1 as a risk factor for disease relapse in Wegener’s granulomatosis. Rheumatology. 2007;46(6):1029–1033. doi: 10.1093/rheumatology/kem022. [DOI] [PubMed] [Google Scholar]
- 17.Csernok E., Moosig F. Current and emerging techniques for ANCA detection in vasculitis. Nat. Rev. Rheumatol. 2014;10(8):494–501. doi: 10.1038/nrrheum.2014.78. [DOI] [PubMed] [Google Scholar]
- 18.Fukasawa H., Hayashi M., Kinoshita N., Ishigaki S., Isobe S., Sakao Y. Rapidly progressive glomerulonephritis associated with PR3-ANCA positive subacute bacterial endocarditis. Intern. Med. 2012;51(18):2587–2590. doi: 10.2169/internalmedicine.51.8081. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Puerta J.A., Gedmintas L., Costenbader K.H. The association between silica exposure and development of ANCA-associated vasculitis: systematic review and meta-analysis. Autoimmun. Rev. 2013;12(12):1129–1135. doi: 10.1016/j.autrev.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane S.E., Watts R.A., Bentham G., Innes N.J., Scott D.G. Are environmental factors important in primary systemic vasculitis? A case-control study. Arthritis Rheum. 2003;48(3):814–823. doi: 10.1002/art.10830. [DOI] [PubMed] [Google Scholar]
- 21.Yashiro M., Muso E., Itoh-Ihara T., Oyama A., Hashimoto K., Kawamura T. Significantly high regional morbidity of MPO-ANCA-related angitis and/or nephritis with respiratory tract involvement after the 1995 great earthquake in Kobe (Japan) Am. J. Kidney Dis. 2000;35(5):889–895. doi: 10.1016/s0272-6386(00)70260-5. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi Y., Saito A., Ojima Y., Kagaya S., Fukami H., Sato H. The influence of the Great East Japan earthquake on microscopic polyangiitis: a retrospective observational study. PloS One. 2017;12(5) doi: 10.1371/journal.pone.0177482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuhara N., Tanino Y., Sato S., Nikaido T., Misa K., Fukuhara A. High incidence of ANCA-positive interstitial pneumonia after the 2011 fukushima disaster. Allergol. Int. 2016;65(1):117–119. doi: 10.1016/j.alit.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Ebisawa K., Yamada N., Kobayashi M., Katahira M., Konno H., Okada S. Cluster of diffuse alveolar hemorrhage cases after the 2011 tohoku region pacific coast earthquake. Respir Investig. 2013;51(1):2–8. doi: 10.1016/j.resinv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Cui Z., Long J.Y., Huang W., Wang J.W., Wang H. The frequency of ANCA-associated vasculitis in a national database of hospitalized patients in China. Arthritis Res. Ther. 2018;20(1):226. doi: 10.1186/s13075-018-1708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farquhar H.J., McGettigan B., Chapman P.T., O’Donnell J.L., Frampton C., Stamp L.K. Incidence of anti-neutrophil cytoplasmic antibody-associated vasculitis before and after the February 2011 Christchurch Earthquake. Intern. Med. J. 2017;47(1):57–61. doi: 10.1111/imj.13246. [DOI] [PubMed] [Google Scholar]
- 27.Dobre M., Wish J., Negrea L. Hydralazine-induced ANCA-positive pauci-immune glomerulonephritis: a case report and literature review. Ren. Fail. 2009;31(8):745–748. doi: 10.3109/08860220903118590. [DOI] [PubMed] [Google Scholar]
- 28.Sethi S., Sahani M., Oei L.S. ANCA-positive crescentic glomerulonephritis associated with minocycline therapy. Am. J. Kidney Dis. 2003;42(2):E27–E31. doi: 10.1016/s0272-6386(03)00671-1. [DOI] [PubMed] [Google Scholar]
- 29.Panamonta O., Sumethkul V., Radinahmed P., Laopaiboon M., Kirdpon W. Propylthiouracil associated antineutrophil cytoplasmic antibodies (ANCA) in patients with childhood onset Graves’ disease. J. Pediatr. Endocrinol. Metab. 2008;21(6):539–543. [PubMed] [Google Scholar]
- 30.Jin Q., Kant S., Alhariri J., Geetha D. Levamisole adulterated cocaine associated ANCA vasculitis: review of literature and update on pathogenesis. J. Community Hosp. Intern. Med. Perspect. 2018;8(6):339–344. doi: 10.1080/20009666.2018.1536242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakazawa D., Tomaru U., Suzuki A., Masuda S., Hasegawa R., Kobayashi T. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64(11):3779–3787. doi: 10.1002/art.34619. [DOI] [PubMed] [Google Scholar]
- 32.Waldhauser L., Uetrecht J. Oxidation of propylthiouracil to reactive metabolites by activated neutrophils. Implications for agranulocytosis. Drug Metab. Dispos. 1991;19(2):354–359. [PubMed] [Google Scholar]
- 33.Waldhauser L., Uetrecht J. Antibodies to myeloperoxidase in propylthiouracil-induced autoimmune disease in the cat. Toxicology. 1996;114(2):155–162. doi: 10.1016/s0300-483x(96)03476-2. [DOI] [PubMed] [Google Scholar]
- 34.van der Woude F.J., Rasmussen N., Lobatto S., Wiik A., Permin H., van Es L.A. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1(8426):425–429. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 35.Falk R.J., Jennette J.C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N. Engl. J. Med. 1988;318(25):1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 36.De’Oliviera J., Gaskin G., Dash A., Rees A.J., Pusey C.D. Relationship between disease activity and anti-neutrophil cytoplasmic antibody concentration in long-term management of systemic vasculitis. Am. J. Kidney Dis. 1995;25(3):380–389. doi: 10.1016/0272-6386(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 37.Birck R., Schmitt W.H., Kaelsch I.A., van der Woude F.J. Serial ANCA determinations for monitoring disease activity in patients with ANCA-associated vasculitis: systematic review. Am. J. Kidney Dis. 2006;47(1):15–23. doi: 10.1053/j.ajkd.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki K., Kobayashi S., Yamazaki K., Gondo M., Tomizawa K., Arimura Y. Analysis of risk epitopes of anti-neutrophil antibody MPO-ANCA in vasculitis in Japanese population. Microbiol. Immunol. 2007;51(12):1215–1220. doi: 10.1111/j.1348-0421.2007.tb04017.x. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M., Sasaki M., Nakabayashi I., Akashi M., Tomiyasu T., Yoshikawa N. Two types of myeloperoxidase-antineutrophil cytoplasmic autoantibodies with a high affinity and a low affinity in small vessel vasculitis. Clin. Exp. Rheumatol. 2009;27(1 Suppl 52):S28–S32. [PubMed] [Google Scholar]
- 40.Yoshida M., Yamada M., Sudo Y., Kojima T., Tomiyasu T., Yoshikawa N. Myeloperoxidase anti-neutrophil cytoplasmic antibody affinity is associated with the formation of neutrophil extracellular traps in the kidney and vasculitis activity in myeloperoxidase anti-neutrophil cytoplasmic antibody-associated microscopic polyangiitis. Nephrology. 2016;21(7):624–629. doi: 10.1111/nep.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth A.J., Ooi J.D., Hess J.J., van Timmeren M.M., Berg E.A., Poulton C.E. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J. Clin. Invest. 2013;123(4):1773–1783. doi: 10.1172/JCI65292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Hussain T., Hussein M.H., Conca W., Al Mana H., Akhtar M. Pathophysiology of ANCA-associated vasculitis. Adv. Anat. Pathol. 2017;24(4):226–234. doi: 10.1097/PAP.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 43.Talor M.V., Stone J.H., Stebbing J., Barin J., Rose N.R., Burek C.L. Antibodies to selected minor target antigens in patients with anti-neutrophil cytoplasmic antibodies (ANCA) Clin. Exp. Immunol. 2007;150(1):42–48. doi: 10.1111/j.1365-2249.2007.03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klebanoff S.J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- 45.McInnis E.A., Badhwar A.K., Muthigi A., Lardinois O.M., Allred S.C., Yang J. Dysregulation of autoantigen genes in ANCA-associated vasculitis involves alternative transcripts and new protein synthesis. J. Am. Soc. Nephrol. 2015;26(2):390–399. doi: 10.1681/ASN.2013101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohlsson S., Hellmark T., Pieters K., Sturfelt G., Wieslander J., Segelmark M. Increased monocyte transcription of the proteinase 3 gene in small vessel vasculitis. Clin. Exp. Immunol. 2005;141(1):174–182. doi: 10.1111/j.1365-2249.2005.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J.J., Pendergraft W.F., Alcorta D.A., Nachman P.H., Hogan S.L., Thomas R.P. Circumvention of normal constraints on granule protein gene expression in peripheral blood neutrophils and monocytes of patients with antineutrophil cytoplasmic autoantibody-associated glomerulonephritis. J. Am. Soc. Nephrol. 2004;15(8):2103–2114. doi: 10.1097/01.ASN.0000135058.46193.72. [DOI] [PubMed] [Google Scholar]
- 48.Hess C., Sadallah S., Schifferli J.A. Induction of neutrophil responsiveness to myeloperoxidase antibodies by their exposure to supernatant of degranulated autologous neutrophils. Blood. 2000;96(8):2822–2827. [PubMed] [Google Scholar]
- 49.Gou S.J., Xu P.C., Chen M., Zhao M.H. Epitope analysis of anti-myeloperoxidase antibodies in patients with ANCA-associated vasculitis. PloS One. 2013;8(4) doi: 10.1371/journal.pone.0060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooi J.D., Chang J., Hickey M.J., Borza D.B., Fugger L., Holdsworth S.R. The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc. Natl. Acad. Sci. U. S. A. 2012;109(39):E2615–E2624. doi: 10.1073/pnas.1210147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Free M.E., Stember K.G., Hess J.J., McInnis E.A., Lardinois O., Hogan S.L. Restricted myeloperoxidase epitopes drive the adaptive immune response in MPO-ANCA vasculitis. J. Autoimmun. 2020;106:102306. doi: 10.1016/j.jaut.2019.102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang J., Eggenhuizen P., O’Sullivan K.M., Alikhan M.A., Holdsworth S.R., Ooi J.D. CD8+ T cells effect glomerular injury in experimental anti-myeloperoxidase GN. J. Am. Soc. Nephrol. 2017;28(1):47–55. doi: 10.1681/ASN.2015121356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin K.R., Kantari-Mimoun C., Yin M., Pederzoli-Ribeil M., Angelot-Delettre F., Ceroi A. Proteinase 3 is a phosphatidylserine-binding protein that affects the production and function of microvesicles. J. Biol. Chem. 2016;291(20):10476–10489. doi: 10.1074/jbc.M115.698639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korkmaz B., Moreau T., Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90(2):227–242. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Guyot N., Wartelle J., Malleret L., Todorov A.A., Devouassoux G., Pacheco Y. Unopposed cathepsin G, neutrophil elastase, and proteinase 3 cause severe lung damage and emphysema. Am. J. Pathol. 2014;184(8):2197–2210. doi: 10.1016/j.ajpath.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Crisford H., Sapey E., Stockley R.A. Proteinase 3; a potential target in chronic obstructive pulmonary disease and other chronic inflammatory diseases. Respir. Res. 2018;19(1):180. doi: 10.1186/s12931-018-0883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kessenbrock K., Frohlich L., Sixt M., Lammermann T., Pfister H., Bateman A. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J. Clin. Invest. 2008;118(7):2438–2447. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J., Bautz D.J., Lionaki S., Hogan S.L., Chin H., Tisch R.M. ANCA patients have T cells responsive to complementary PR-3 antigen. Kidney Int. 2008;74(9):1159–1169. doi: 10.1038/ki.2008.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kallenberg C.G. Pathogenesis of PR3-ANCA associated vasculitis. J. Autoimmun. 2008;30(1–2):29–36. doi: 10.1016/j.jaut.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Bansal P.J., Tobin M.C. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann. Allergy Asthma Immunol. 2004;93(4):398–401. doi: 10.1016/S1081-1206(10)61400-7. [DOI] [PubMed] [Google Scholar]
- 61.Schlieben D.J., Korbet S.M., Kimura R.E., Schwartz M.M., Lewis E.J. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. Am. J. Kidney Dis. 2005;45(4):758–761. doi: 10.1053/j.ajkd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Guillevin L., Pagnoux C., Karras A., Khouatra C., Aumaitre O., Cohen P. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N. Engl. J. Med. 2014;371(19):1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 63.Stone J.H., Merkel P.A., Spiera R., Seo P., Langford C.A., Hoffman G.S. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salama A.D., Little M.A. Animal models of antineutrophil cytoplasm antibody-associated vasculitis. Curr. Opin. Rheumatol. 2012;24(1):1–7. doi: 10.1097/BOR.0b013e32834d2d52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao H., Heeringa P., Hu P., Liu Z., Zhao M., Aratani Y. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Invest. 2002;110(7):955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Little M.A., Smyth L., Salama A.D., Mukherjee S., Smith J., Haskard D. Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am. J. Pathol. 2009;174(4):1212–1220. doi: 10.2353/ajpath.2009.080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Falk R.J., Terrell R.S., Charles L.A., Jennette J.C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl. Acad. Sci. U. S. A. 1990;87(11):4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porges A.J., Redecha P.B., Kimberly W.T., Csernok E., Gross W.L., Kimberly R.P. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J. Immunol. 1994;153(3):1271–1280. [PubMed] [Google Scholar]
- 69.Rarok A.A., Limburg P.C., Kallenberg C.G. Neutrophil-activating potential of antineutrophil cytoplasm autoantibodies. J. Leukoc. Biol. 2003;74(1):3–15. doi: 10.1189/jlb.1202611. [DOI] [PubMed] [Google Scholar]
- 70.Nakazawa D., Shida H., Tomaru U., Yoshida M., Nishio S., Atsumi T. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J. Am. Soc. Nephrol. 2014;25(5):990–997. doi: 10.1681/ASN.2013060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radford D.J., Savage C.O., Nash G.B. Treatment of rolling neutrophils with antineutrophil cytoplasmic antibodies causes conversion to firm integrin-mediated adhesion. Arthritis Rheum. 2000;43(6):1337–1345. doi: 10.1002/1529-0131(200006)43:6<1337::AID-ANR16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 72.van der Geld Y.M., Hellmark T., Selga D., Heeringa P., Huitema M.G., Limburg P.C. Rats and mice immunised with chimeric human/mouse proteinase 3 produce autoantibodies to mouse Pr3 and rat granulocytes. Ann. Rheum. Dis. 2007;66(12):1679–1682. doi: 10.1136/ard.2006.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Primo V.C., Marusic S., Franklin C.C., Goldmann W.H., Achaval C.G., Smith R.N. Anti-PR3 immune responses induce segmental and necrotizing glomerulonephritis. Clin. Exp. Immunol. 2010;159(3):327–337. doi: 10.1111/j.1365-2249.2009.04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfister H., Ollert M., Frohlich L.F., Quintanilla-Martinez L., Colby T.V., Specks U. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood. 2004;104(5):1411–1418. doi: 10.1182/blood-2004-01-0267. [DOI] [PubMed] [Google Scholar]
- 75.Xiao H., Heeringa P., Liu Z., Huugen D., Hu P., Maeda N. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am. J. Pathol. 2005;167(1):39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Csernok E. Anti-neutrophil cytoplasmic antibodies and pathogenesis of small vessel vasculitides. Autoimmun. Rev. 2003;2(3):158–164. doi: 10.1016/s1568-9972(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 77.Witko-Sarsat V., Lesavre P., Lopez S., Bessou G., Hieblot C., Prum B. A large subset of neutrophils expressing membrane proteinase 3 is a risk factor for vasculitis and rheumatoid arthritis. J. Am. Soc. Nephrol. 1999;10(6):1224–1233. doi: 10.1681/ASN.V1061224. [DOI] [PubMed] [Google Scholar]
- 78.Halbwachs-Mecarelli L., Bessou G., Lesavre P., Lopez S., Witko-Sarsat V. Bimodal distribution of proteinase 3 (PR3) surface expression reflects a constitutive heterogeneity in the polymorphonuclear neutrophil pool. FEBS Lett. 1995;374(1):29–33. doi: 10.1016/0014-5793(95)01073-n. [DOI] [PubMed] [Google Scholar]
- 79.Brachemi S., Mambole A., Fakhouri F., Mouthon L., Guillevin L., Lesavre P. Increased membrane expression of proteinase 3 during neutrophil adhesion in the presence of anti proteinase 3 antibodies. J. Am. Soc. Nephrol. 2007;18(8):2330–2339. doi: 10.1681/ASN.2006121309. [DOI] [PubMed] [Google Scholar]
- 80.Xing G.Q., Chen M., Liu G., Heeringa P., Zhang J.J., Zheng X. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J. Clin. Immunol. 2009;29(3):282–291. doi: 10.1007/s10875-008-9268-2. [DOI] [PubMed] [Google Scholar]
- 81.Chen M., Xing G.Q., Yu F., Liu G., Zhao M.H. Complement deposition in renal histopathology of patients with ANCA-associated pauci-immune glomerulonephritis. Nephrol. Dial. Transplant. 2009;24(4):1247–1252. doi: 10.1093/ndt/gfn586. [DOI] [PubMed] [Google Scholar]
- 82.Gou S.J., Yuan J., Wang C., Zhao M.H., Chen M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin. J. Am. Soc. Nephrol. 2013;8(11):1884–1891. doi: 10.2215/CJN.02790313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manenti L., Vaglio A., Gnappi E., Maggiore U., Allegri L., Allinovi M. Association of serum C3 concentration and histologic signs of thrombotic microangiopathy with outcomes among patients with ANCA-associated renal vasculitis. Clin. J. Am. Soc. Nephrol. 2015;10(12):2143–2151. doi: 10.2215/CJN.00120115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao H., Schreiber A., Heeringa P., Falk R.J., Jennette J.C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 2007;170(1):52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huugen D., van Esch A., Xiao H., Peutz-Kootstra C.J., Buurman W.A., Tervaert J.W. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71(7):646–654. doi: 10.1038/sj.ki.5002103. [DOI] [PubMed] [Google Scholar]
- 86.Xiao H., Dairaghi D.J., Powers J.P., Ertl L.S., Baumgart T., Wang Y. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J. Am. Soc. Nephrol. 2014;25(2):225–231. doi: 10.1681/ASN.2013020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schreiber A., Xiao H., Jennette J.C., Schneider W., Luft F.C., Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 2009;20(2):289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H., Wang C., Zhao M.H., Chen M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 2015;181(3):518–527. doi: 10.1111/cei.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jayne D.R.W., Bruchfeld A.N., Harper L., Schaier M., Venning M.C., Hamilton P. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 2017;28(9):2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Merkel P.A., Jayne D.R., Wang C., Hillson J., Bekker P. Evaluation of the safety and efficacy of avacopan, a C5a receptor inhibitor, in patients with antineutrophil cytoplasmic antibody-associated vasculitis treated concomitantly with rituximab or cyclophosphamide/azathioprine: protocol for a randomized, double-blind, active-controlled, phase 3 trial. JMIR Res Protoc. 2020;9(4) doi: 10.2196/16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jayne D.R., Merkel P.A., Schall T., Bekker P., ADVOCATE Study Group Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 2021;384(7):599–609. doi: 10.1056/NEJMoa2023386. [DOI] [PubMed] [Google Scholar]
- 92.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18(2):134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 93.Sorensen O.E., Borregaard N. Neutrophil extracellular traps - the dark side of neutrophils. J. Clin. Invest. 2016;126(5):1612–1620. doi: 10.1172/JCI84538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 95.Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PloS One. 2012;7(2) doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sangaletti S., Tripodo C., Chiodoni C., Guarnotta C., Cappetti B., Casalini P. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;120(15):3007–3018. doi: 10.1182/blood-2012-03-416156. [DOI] [PubMed] [Google Scholar]
- 97.Kumar S.V., Kulkarni O.P., Mulay S.R., Darisipudi M.N., Romoli S., Thomasova D. Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe GN. J. Am. Soc. Nephrol. 2015;26(10):2399–2413. doi: 10.1681/ASN.2014070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soderberg D., Kurz T., Motamedi A., Hellmark T., Eriksson P., Segelmark M. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology. 2015;54(11):2085–2094. doi: 10.1093/rheumatology/kev217. [DOI] [PubMed] [Google Scholar]
- 99.Hakkim A., Furnrohr B.G., Amann K., Laube B., Abed U.A., Brinkmann V. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. U. S. A. 2010;107(21):9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arase H. Rheumatoid rescue of misfolded cellular proteins by MHC class II molecules: a new hypothesis for autoimmune diseases. Adv. Immunol. 2016;129:1–23. doi: 10.1016/bs.ai.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Tanimura K., Jin H., Suenaga T., Morikami S., Arase N., Kishida K. beta2-Glycoprotein I/HLA class II complexes are novel autoantigens in antiphospholipid syndrome. Blood. 2015;125(18):2835–2844. doi: 10.1182/blood-2014-08-593624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hiwa R., Ohmura K., Arase N., Jin H., Hirayasu K., Kohyama M. Myeloperoxidase/HLA class II complexes recognized by autoantibodies in microscopic polyangiitis. Arthritis Rheum. 2017;69(10):2069–2080. doi: 10.1002/art.40170. [DOI] [PubMed] [Google Scholar]
- 103.Popa E.R., Stegeman C.A., Bos N.A., Kallenberg C.G., Tervaert J.W. Differential B- and T-cell activation in Wegener’s granulomatosis. J. Allergy Clin. Immunol. 1999;103(5 Pt 1):885–894. doi: 10.1016/s0091-6749(99)70434-3. [DOI] [PubMed] [Google Scholar]
- 104.Jones R.B., Tervaert J.W., Hauser T., Luqmani R., Morgan M.D., Peh C.A. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N. Engl. J. Med. 2010;363(3):211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 105.Mauri C. Regulation of immunity and autoimmunity by B cells. Curr. Opin. Immunol. 2010;22(6):761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 106.Bunch D.O., McGregor J.G., Khandoobhai N.B., Aybar L.T., Burkart M.E., Hu Y. Decreased CD5(+) B cells in active ANCA vasculitis and relapse after rituximab. Clin. J. Am. Soc. Nephrol. 2013;8(3):382–391. doi: 10.2215/CJN.03950412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krumbholz M., Specks U., Wick M., Kalled S.L., Jenne D., Meinl E. BAFF is elevated in serum of patients with Wegener’s granulomatosis. J. Autoimmun. 2005;25(4):298–302. doi: 10.1016/j.jaut.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 108.Nagai M., Hirayama K., Ebihara I., Shimohata H., Kobayashi M., Koyama A. Serum levels of BAFF and APRIL in myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis: association with disease activity. Nephron Clin. Pract. 2011;118(4):c339–c345. doi: 10.1159/000323393. [DOI] [PubMed] [Google Scholar]
- 109.Stegeman C.A., Tervaert J.W., Sluiter W.J., Manson W.L., de Jong P.E., Kallenberg C.G. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann. Intern. Med. 1994;120(1):12–17. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- 110.Zycinska K., Wardyn K.A., Zielonka T.M., Demkow U., Traburzynski M.S. Chronic crusting, nasal carriage of Staphylococcus aureus and relapse rate in pulmonary Wegener’s granulomatosis. J. Physiol. Pharmacol. 2008;59(Suppl 6):825–831. [PubMed] [Google Scholar]
- 111.Stegeman C.A., Tervaert J.W., de Jong P.E., Kallenberg C.G. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N. Engl. J. Med. 1996;335(1):16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- 112.Iking-Konert C., Vogl T., Prior B., Wagner C., Sander O., Bleck E. T lymphocytes in patients with primary vasculitis: expansion of CD8+ T cells with the propensity to activate polymorphonuclear neutrophils. Rheumatology. 2008;47(5):609–616. doi: 10.1093/rheumatology/ken028. [DOI] [PubMed] [Google Scholar]
- 113.Szczeklik W., Jakiela B., Wawrzycka-Adamczyk K., Sanak M., Hubalewska-Mazgaj M., Padjas A. Skewing toward Treg and Th2 responses is a characteristic feature of sustained remission in ANCA-positive granulomatosis with polyangiitis. Eur. J. Immunol. 2017;47(4):724–733. doi: 10.1002/eji.201646810. [DOI] [PubMed] [Google Scholar]
- 114.Abdulahad W.H., van der Geld Y.M., Stegeman C.A., Kallenberg C.G. Persistent expansion of CD4+ effector memory T cells in Wegener’s granulomatosis. Kidney Int. 2006;70(5):938–947. doi: 10.1038/sj.ki.5001670. [DOI] [PubMed] [Google Scholar]
- 115.Langford C.A., Monach P.A., Specks U., Seo P., Cuthbertson D., McAlear C.A. An open-label trial of abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener’s) Ann. Rheum. Dis. 2014;73(7):1376–1379. doi: 10.1136/annrheumdis-2013-204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schrezenmeier E., Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 117.von Borstel A., Sanders J.S., Rutgers A., Stegeman C.A., Heeringa P., Abdulahad W.H. Cellular immune regulation in the pathogenesis of ANCA-associated vasculitides. Autoimmun. Rev. 2018;17(4):413–421. doi: 10.1016/j.autrev.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 118.McClure M., Gopaluni S., Jayne D., Jones R. B cell therapy in ANCA-associated vasculitis: current and emerging treatment options. Nat. Rev. Rheumatol. 2018;14(10):580–591. doi: 10.1038/s41584-018-0065-x. [DOI] [PubMed] [Google Scholar]