To the Editor: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant B.1.429 (also called CAL.20C or 452R.V1), first identified in California,1 is spreading rapidly in the United States and has been found in at least 25 other countries (see updates at https://www.gisaid.org/hcov19-variants/). This variant contains three spike mutations that are major targets for neutralizing antibodies; one mutation (L452R) is located in the receptor-binding motif, and another (W152C) in the N-terminal domain supersite. This has aroused concern about possible immune escape, which could compromise vaccine efficacy and increase the risk of reinfection. We measured the neutralizing activity of serum specimens obtained from 14 convalescent persons and from 49 recipients of one of two different vaccines based on the ancestral spike: an mRNA vaccine (mRNA-1273 [Moderna]; 26 recipients)2 and a protein nanoparticle vaccine (NVX-CoV2373 [Novavax]; 23 recipients).3 We selected mRNA-1273 samples that represented high, medium, and low neutralization titers. NVX-CoV2373 samples were randomly selected and were not preselected on the basis of antibody titers.
The neutralizing activity of all serum samples was tested against the B.1.429 variant and a variant of concern that first emerged in South Africa (B.1.351, also called 20H/501Y.V2). We compared this neutralizing activity to the activity the serum samples exhibited against the prototypical D614G variant. As compared with the D614G variant, we found that B.1.429 was approximately 2 to 3 times less sensitive to neutralization by convalescent serum and by serum samples obtained from vaccinated persons, whereas B.1.351 was approximately 9 to 14 times less sensitive to neutralization.
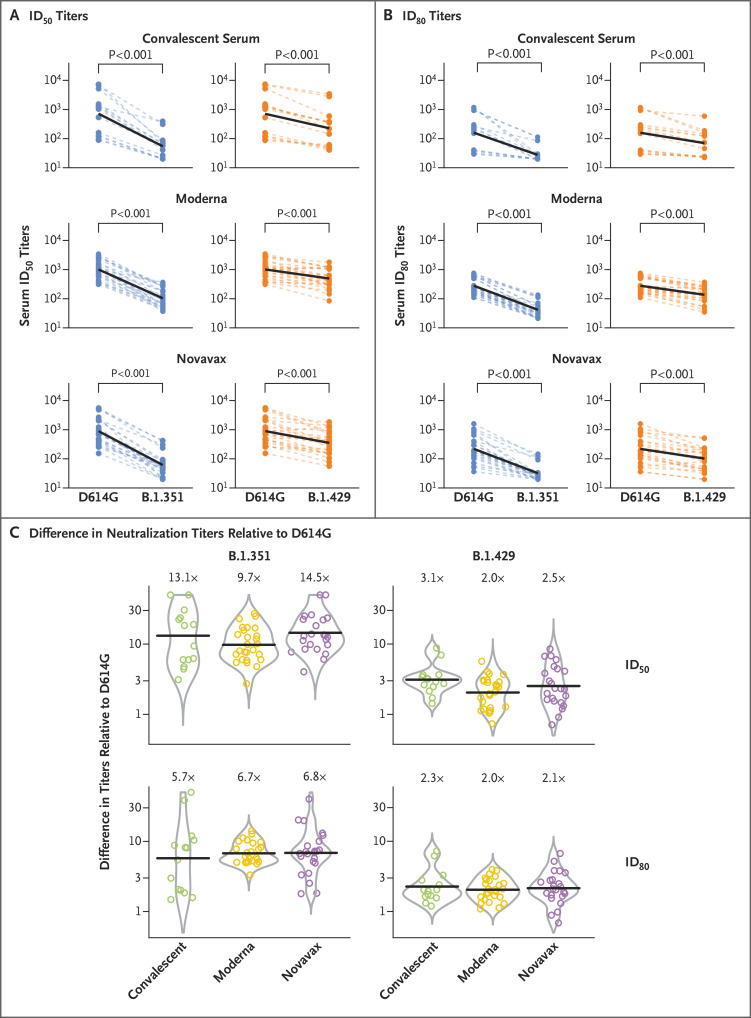
We constructed pseudoviruses with the D614G spike mutation alone (as the comparator variant) or combined with the additional mutations found in B.1.429 (S13I, W152C, and L452R) and B.1.351 (L18F, D80A, D215G, Δ242–244, R246I, K417N, E484K, N501Y, and A701V). Neutralization assays were performed with the use of a validated lentivirus-based spike-pseudotyped virus assay in 293T cells that were stably transduced to overexpress angiotensin-converting enzyme 2.4 The variant B.1.429 was neutralized by convalescent serum and by serum obtained from vaccinated persons, resulting in 50% inhibitory dilution (ID50) geometric mean titers of 225 to 495 (Figure 1A, and Table S1 in Supplementary Appendix 1, available with the full text of this letter at NEJM.org). The ID50 and ID80 titers against the B.1.429 variant for convalescent serum and for serum from persons who had received one of the vaccines were significantly lower than those against D614G (P<0.001) (Figure 1A and 1B, and Table S2 in Supplementary Appendix 2). The geometric mean ID50 titers against B.1.429 were 3.1 times (range, 1.4 to 8.8) lower than those against D614G for convalescent serum and were 2.0 and 2.5 times (range, 0.7 and 8.6) lower than against D614G for serum from persons who had received the mRNA-1273 and NVX-CoV2373 vaccines, respectively (Figure 1C and Table S1). The geometric mean ID50 titer against B.1.351 was 13.1 times lower than against D614G for convalescent serum and 9.7 times and 14.5 times lower than against D614G for serum from persons who had received the mRNA-1273 and NVX-CoV2373 vaccines, respectively (Figure 1C). Our findings regarding neutralization of the B.1.351 variant by serum obtained from recipients of the mRNA-1273 vaccine are consistent with those reported previously.5
Figure 1. Neutralization of B.1.429 and B.1.351 Pseudoviruses in Serum Samples Obtained from Convalescent Persons and Vaccine Recipients.
Convalescent serum samples were obtained from infected persons 1 to 8 weeks after resolution of coronavirus disease 2019 infection or 2 to 10 weeks after the most recent positive SARS-CoV-2 test. Serum samples were obtained from recipients of the Moderna vaccine on day 57 (28 days after the second vaccine dose), and Novavax serum samples were obtained from vaccine recipients on day 35 (14 days after the second vaccine dose). Results are shown as the difference in neutralization titers of matched samples (Panels A and B) and the difference in titers relative to the D614G variant (the ratio of titers against the variant indicated) for each sample set (Panel C). Lower values indicate stronger cross-neutralization of the variant virus. Dashed thin lines in Panels A and B represent individual samples, and thick black lines represent the geometric means of each sample group, as indicated at the right. Thick black bars in Panel C represent the geometric mean titer differences for the sample sets, which are also labeled above each set. Circles in Panel C represent the differences in titers relative to D614G for individual samples. P values for the comparison of the reciprocal neutralization titers at 50% inhibitory dilution (ID50) and 80% inhibitory dilution (ID80) are pairwise comparisons of the data shown in Panels A and B, calculated with the use of the Wilcoxon signed-rank test. P values less than 0.001 correlate to Q (adjusted P) values less than 0.0019 (see Table S2 in Supplementary Appendix 2). Differences in the neutralization titers among the three sample sets shown in Panel C were not significant (P>0.05 by the Wilcoxon rank-sum test).
The modestly lower value in neutralization titers against the B.1.429 variant seen in this study is similar to that we saw previously when neutralization of the B.1.1.7 variant was tested with the same assay using serum samples obtained from recipients of the mRNA-1273 and NVX-CoV2373 vaccines.4 These results, and the high efficacy shown by these vaccines, suggest that vaccine-elicited neutralizing antibodies are likely to remain effective against the B.1.429 variant. The magnitude of resistance seen with the B.1.351 variant is of greater concern with respect to current vaccines.
Supplementary Appendix 1
Supplementary Appendix 2
Disclosure Forms
This letter was published on April 7, 2021, at NEJM.org.
Footnotes
Drs. Shen and Montefiori were supported by a grant (3UM1-AI068618-14S1) from the COVID-19 Prevention Network, and Dr. Korber was supported by a grant (XB3W00) from Los Alamos National Laboratory.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Zhang W, Davis BD, Chen SS, Martinez JMS, Plummer JT, Vail E. Emergence of a novel SARS-CoV-2 strain in southern California, USA. January 20, 2021. (https://www.medrxiv.org/content/10.1101/2021.01.18.21249786v1). preprint. [DOI] [PMC free article] [PubMed]
- 2.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020;383:2427-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 2020;383:2320-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen X, Tang H, McDanal C, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe 2021. March 05 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu K, Werner AP, Koch M, et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med. DOI: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.