To the Editor: The emergence of two variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) — B.1.1.7 in the United Kingdom and B.1.351 in South Africa — has aroused concern that these variants may escape immunity resulting from either previous infection or vaccination. In an attempt to measure the resistance of these variants to neutralization elicited by infection or vaccination, we generated recombinant vesicular stomatitis virus–based SARS-CoV-2 pseudoviruses containing the spike protein of the Wuhan-1 reference strain (wild-type), the D614G mutation, and the B.1.1.7 and B.1.351 variants. (Details regarding the recombination process are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.)
We next evaluated pseudovirus resistance to neutralization using convalescent serum obtained from 34 patients 5 months after infection with coronavirus disease 2019 (Covid-19) and serum from 50 participants obtained 2 to 3 weeks after receipt of the second dose of inactivated-virus vaccines — BBIBP-CorV (Sinopharm)1 or CoronaVac (Sinovac)2 — which were developed in China (Table S1 in the Supplementary Appendix). We first determined the serum neutralizing-antibody titer against wild-type pseudovirus and observed similar geometric mean titers (GMTs) in serum obtained from convalescent patients and from vaccinees (Figure 1A), which suggested a low antibody response after two-dose inoculation induced by BBIBP-CorV or CoronaVac.1,2 Notably, undetectable neutralization titers were seen in 4 of 34 convalescent serum samples, in 6 of 25 BBIBP-CorV serum samples, and in 4 of 25 CoronaVac serum samples.
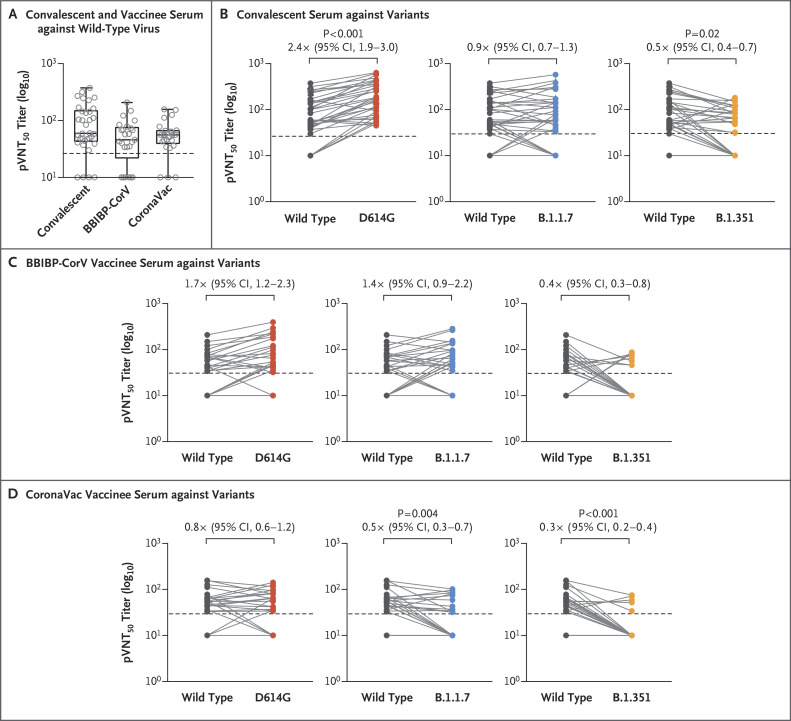
Figure 1. Neutralization of SARS-CoV-2 Pseudoviruses in Convalescent and Vaccinee Serum Samples.
Panel A shows the 50% pseudovirus neutralization titer (pVNT50) in convalescent serum collected from 34 recovered patients approximately 5 months after SARS-CoV-2 infection and in serum collected from 50 vaccinees who had received either the BBIBP-CorV or CoronaVac vaccine 2 to 3 weeks after the second dose against recombinant vesicular stomatitis virus–based SARS-CoV-2 pseudovirus bearing the Wuhan-1 (wild-type) spike protein. Box plots indicate the median and interquartile range (IQR); the whiskers represent 1.5 times the IQR. Panel B shows changes in the reciprocal serum pVNT50 titer in 34 convalescent serum samples against the D614G, B.1.1.7, and B.1.351 variants, as compared with wild-type virus. Panels C and D show changes in the reciprocal pVNT50 titer in serum samples obtained from the 25 recipients of the BBIBP-CorV vaccine and 25 recipients of the CoronaVac vaccine, respectively, against the D614G, B.1.1.7, and B.1.351 variants, as compared with wild-type virus. Factor changes in the geometric mean titer and 95% confidence interval (CI) in the pVNT50 titers, as compared with those for wild-type virus, are shown under the P values. Only P values of less than 0.05 (indicating significance) are shown. Each data point is the average of duplicate assay results. In each panel, the horizontal dashed line represents the lower limit of detection of the assay (titer, <30); this limit was assigned a value of 10 for geometric mean calculations and was considered to be seronegative. In all panels, calculations were performed with the use of the two-tailed Kruskal–Wallis test after adjustment for the false discovery rate.
We next assessed the neutralizing activity of convalescent serum and vaccinee serum against D614G, B.1.1.7, and B.1.351 variants as compared with wild-type pseudovirus. The convalescent serum was significantly more effective (by a factor of 2.4; 95% confidence interval [CI], 1.9 to 3.0) in neutralizing the D614G pseudovirus, had a similar effect to that of the wild-type virus in neutralizing the B.1.1.7 variant, and was significantly less effective (by a factor of 0.5; 95% CI, 0.4 to 0.7) in neutralizing the B.1.351 pseudovirus (Figure 1B). Moreover, 9 of 30 convalescent serum samples showed complete loss of neutralizing activity against B.1.351. For the BBIBP-CorV vaccinee serum samples, although the GMTs of neutralization against the variants were not significantly different from the GMTs against the wild-type virus, 20 serum samples showed complete or partial loss of neutralization against B.1.351 (Figure 1C). For the CoronaVac vaccinee serum samples, we observed a marked decrease in the GMTs in the serum neutralization of B.1.1.7 (by a factor of 0.5; 95% CI, 0.3 to 0.7) and B.1.351 (by a factor of 0.3; 95% CI, 0.2 to 0.4). In addition, most of the serum samples showed complete or partial loss of neutralization against B.1.351 (Figure 1D).
Our findings suggest that B.1.1.7 showed little resistance to the neutralizing activity of convalescent or vaccinee serum, whereas B.1.351 showed more resistance to the neutralization of both convalescent serum (by a factor of 2) and vaccinee serum (by a factor of 2.5 to 3.3) than the wild-type virus. Most of the vaccinee serum samples that were tested lost neutralizing activity, a finding that was consistent with the results of other recent studies of neutralization by convalescent serum or serum obtained from recipients of messenger RNA or BBIBP-CorV vaccines.3-5 Our findings also highlight the importance of sustained viral monitoring and evaluation of the protective efficacy of vaccines in areas where variants are circulating.
Supplementary Appendix
Disclosure Forms
This letter was published on April 6, 2021, at NEJM.org.
Footnotes
Supported by a grant (L202038) from the Beijing Natural Science Foundation and a grant (81773494) from the Natural Science Foundation of China, both to Dr. Ma.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2021;21:39-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021;21:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang B, Dai L, Wang H, et al. Neutralization of SARS-CoV-2 VOC 501Y.V2 by human antisera elicited by both inactivated BBIBP-CorV and recombinant dimeric RBD ZF2001 vaccines. February 2, 2021. (https://www.biorxiv.org/content/10.1101/2021.02.01.429069v1). preprint.
- 4.Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2-elicited serum — preliminary report. N Engl J Med. DOI: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021. March 8 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.