To the Editor: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 501Y.V2 lineage (also known as B.1.351), first identified in South Africa in October 2020,1 has mutations that confer increased resistance to plasma from convalescent patients and vaccine recipients, as well as to some monoclonal antibodies.2-4 However, the immune response to 501Y.V2 is unknown. Similarly, the ability of antibodies elicited by 501Y.V2 infection to cross-react with other variants is unknown, but such cross-reactivity would have implications for the ability of second-generation vaccines based on the 501Y.V2 spike protein to protect against infection with the original and emerging SARS-CoV-2 lineages.5
We characterized the SARS-CoV-2 infections in a cohort of patients with coronavirus disease 2019 (Covid-19) who were hospitalized in the Groote Schuur Hospital, Cape Town (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org), after the emergence and dominance of 501Y.V2 in South Africa. Blood samples were obtained from 89 patients between December 31, 2020, and January 15, 2021; of these patients, 28 (31%) were randomly selected for SARS-CoV-2 sequencing, all of whom were shown by phylogenetic analysis to be infected with 501Y.V2 (Fig. S1A). Furthermore, at this time, the epidemic in Cape Town (Fig. S1B) and in South Africa as a whole was dominated by 501Y.V2, which accounted for more than 90% of infections. No patient in our study reported previous SARS-CoV-2 infection.
We first assessed the binding and neutralizing antibody responses of these patients to the 501Y.V2 spike protein. As with the original variant (D614G), 501Y.V2 elicited high-titer binding and neutralizing antibody responses (Fig. S2). Furthermore, titers of binding antibodies to the receptor-binding domain (including all of subdomain 1) and the full spike protein of the original variant were highly correlated with titers of binding antibodies to the corresponding proteins of the 501Y.V2 variant. Titration of a subset of 46 samples revealed that plasma samples had higher titers to the spike protein of 501Y.V2 than to the spike protein of the original variant (mean of 1.7 times as high), but high-level binding to the original variant remained (Fig. S3).
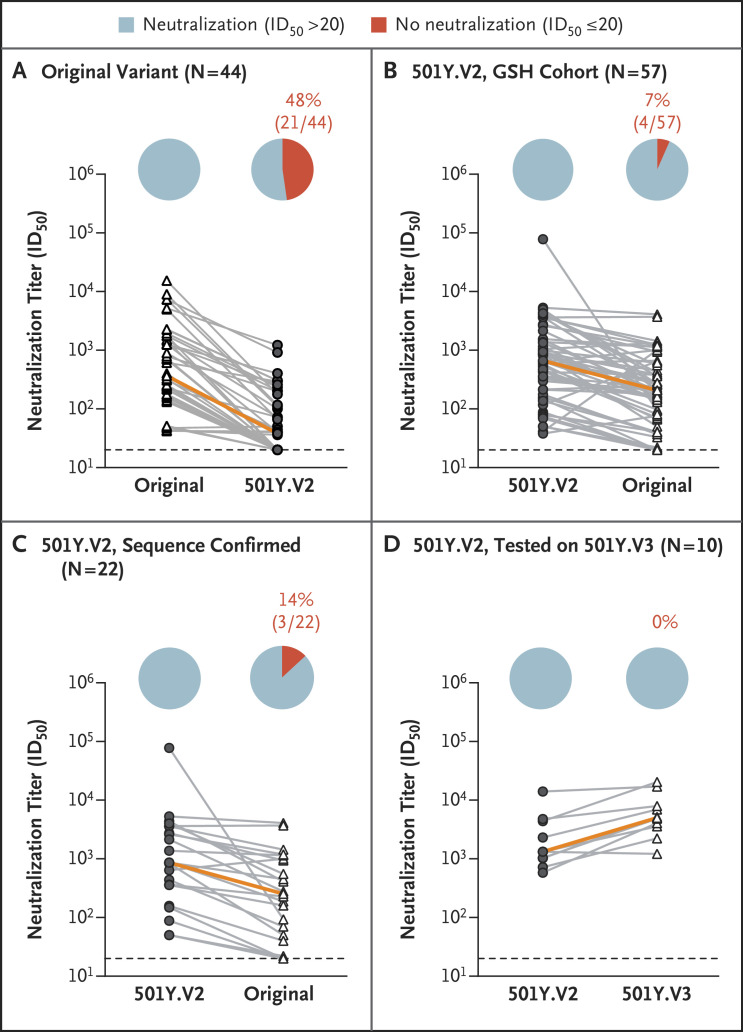
We previously reported that plasma from persons infected with the original variant showed substantially lower neutralization of the 501Y.V2 variant than of the original variant (Figures 1A and S4A).2 In the current study, we performed the reverse experiment by assessing the cross-reactivity of the plasma neutralizing responses in the Groote Schuur Hospital cohort of patients with 501Y.V2 infection against the original variant and against 501Y.V3 (P.1), the variant first described in Brazil. We first tested 57 plasma samples from patients at Groote Schuur Hospital against both 501Y.V2 and the original variant and found that 53 of 57 samples maintained neutralization activity against the original variant, with a geometric mean titer of 203 (95% confidence interval, 141 to 292), approximately one third of the titer against the 501Y.V2 variant (Figures 1B and S5A). When we limited the analysis to the 22 donors who had sequencing-confirmed infection with 501Y.V2 and had positive titers of binding antibodies, we observed the same pattern (Figure 1C). Finally, we tested a subset of 10 plasma samples against the 501Y.V3 (P.1) variant and found high levels of neutralization of this variant, with some samples showing higher potency against 501Y.V3 (P.1) than against 501Y.V2, a finding that may be due to the very different N-terminal domains of these variants (Figure 1D).
Figure 1. Cross-Reactivity of Neutralizing Antibody Responses.
Plasma samples from patients infected with the original variant (D614G) (Panel A) and from patients in the Groote Schuur Hospital (GSH) cohort who were infected with the 501Y.V2 variant (Panels B, C, and D) were compared for their neutralization cross-reactivity against other variants. One analysis (Panel C) was limited to samples from the 22 patients who had positive titers of binding antibodies and in whom sequencing had confirmed infection with 501Y.V2. A subset of 10 samples (Panel D) was assayed against 501Y.V3 pseudoviruses. Neutralizing antibody responses elicited by 501Y.V2 infection were more cross-reactive than those elicited by infection with the original variant. Plasma from patients infected with the original variant elicited titers against 501Y.V2 that were, on average, approximately one ninth of the titer elicited against the original variant (Panel A). In contrast, plasma from patients who had been infected with 501Y.V2 elicited responses against the original variant that were one third (Panel B) and one fourth (Panel C) of those elicited against the 501Y.V2 variant. Plasma from some of the patients infected with 501Y.V2 elicited even greater responses against 501Y.V3 than against 501Y.V2 (approximately three times as high) (Panel D). In each graph, the orange line indicates the slope between the median neutralization potencies of the samples tested. In the pie charts, blue indicates the percentage of samples with neutralization activity and red the percentage of samples with no detectable neutralization activity. The threshold of detection for the neutralization assay is a 50% inhibitory dilution (ID50) of 20. All experiments were performed in duplicate, and the mean values are shown. Data for the original variant plasma are from Wibmer et al.2
Overall, we found that 501Y.V2 elicits robust neutralizing antibody responses against both the original variant and 501Y.V3 (P.1), which indicates high levels of cross-reactivity. Our data indicate that vaccines built on the spike protein of 501Y.V2 may be promising candidates for the elicitation of cross-reactive neutralizing antibody responses to SARS-CoV-2.
Supplementary Appendix
Disclosure Forms
This letter was published on April 7, 2021, at NEJM.org.
Footnotes
Supported by the South African Medical Research Council (grants 96825, SHIPNCD 76756, and DST/CON 0250/2012), the Centers for Disease Control and Prevention (grant 5 U01IP001048-05-00), the ELMA South Africa Foundation (grant 20-ESA011) and the Wellcome Centre for Infectious Diseases Research in Africa, which is supported by core funding from the Wellcome Trust (grant 203135/Z/16/Z). Dr. Wibmer is supported by the Fogarty International Center of the National Institutes of Health (award number R21TW011454) and the FLAIR Fellowship program (award number FLR\R1\201782). Dr. Burgers is supported by the European and Developing Countries Clinical Trials Partnership 2 of the European Union Horizon 2020 program (grant TMA2016SF-1535-CaTCH-22). Dr. Moore is supported by the South African Research Chairs Initiative of the Department of Science and Innovation and the National Research Foundation (grant 98341).
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Tegally H, Wilkinson E, Giovanetti M, et al. Emergence of a SARS-CoV-2 variant of concern with mutations in spike glycoprotein. Nature 2021. March 9 (Epub ahead of print).33690265 [Google Scholar]
- 2.Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med 2021. March 2 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 3.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. February 12, 2021. (https://www.biorxiv.org/content/10.1101/2021.01.25.428137v3). preprint. [DOI] [PubMed]
- 4.Cele S, Gazy I, Jackson L, et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature 2021. March 29 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet 2021;397:952-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.