To the Editor: The BNT162b2 vaccine was shown to have 95% efficacy against coronavirus disease 2019 (Covid-19).1 To date, the two-dose vaccine protocol has not been approved in Israel for persons previously infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); however, administration of a single dose is now being considered.
In addition to the original virus first identified in Wuhan, China, SARS-CoV-2 variants first identified in the United Kingdom (B.1.1.7), South Africa (B.1.351), and Brazil (P.1) have been detected in recent months.2 Samples from persons who had been vaccinated or previously infected with the original virus or the B.1.1.7 variant were shown to have significantly less neutralizing activity against the B.1.351 variant than against the other variants.3,4 In this study, we investigated whether one dose of the BNT162b2 vaccine would increase neutralizing activity against the B.1.1.7, B.1.351, and P.1 variants in persons previously infected with SARS-CoV-2.
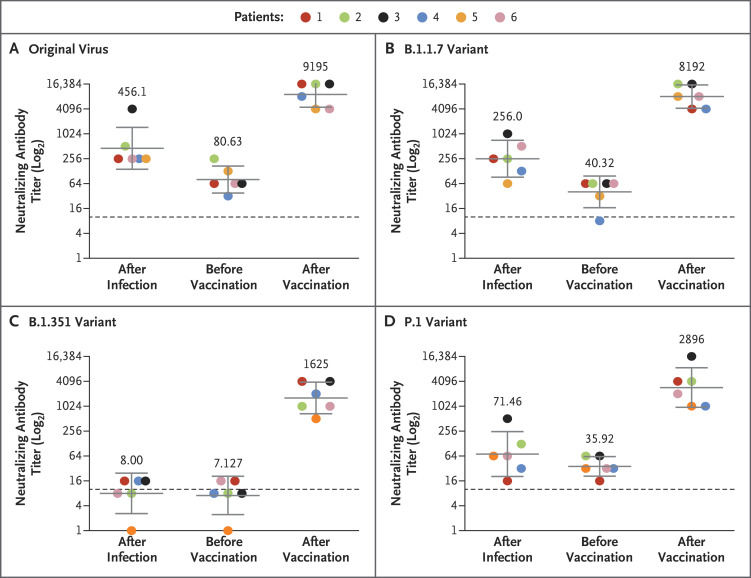
A microneutralization assay with isolates of the original virus (sublineage B.1) and the B.1.1.7, B.1.351, and P.1 variants was performed on 18 serum samples from six health care workers previously infected with SARS-CoV-2, with a sample obtained from each patient at three time points: 1 to 12 weeks after natural infection, immediately before vaccination, and 1 to 2 weeks after vaccination (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). All six health care workers were women (32 to 67 years of age) and had been infected with the original virus (sublineage B.1), as determined by sequencing of SARS-CoV-2 performed at the time of diagnosis. Samples obtained at the first time point had neutralizing activity against the original virus and the B.1.1.7 and P.1 variants, with geometric mean titers of 456, 256, and 71, respectively, but had little or no neutralizing activity against the B.1.351 variant, with a geometric mean titer of 8. At the second time point, geometric mean titers were 81, 40, 36, and 7 for the original virus and the B.1.1.7, P.1, and B.1.351 variants, respectively. Of note, at the third time point, geometric mean titers were 9195, 8192, 2896, and 1625 for the original virus and the B.1.1.7, P.1, and B.1.351 variants, respectively — that is, the titers after vaccination were 114, 203, 81, and 228 times as high as the titers immediately before vaccination (Figure 1 and Table S2).
Figure 1. Neutralizing Response against the Original Virus and Variants after SARS-CoV-2 Infection and One Dose of the BNT162b2 Vaccine.
Serum samples from six patients previously infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), obtained 1 to 12 weeks after natural infection, immediately before receiving one dose of the BNT162b2 vaccine, and 1 to 2 weeks after vaccination, were tested with a microneutralization assay for the neutralizing response against sublineage B.1 of the original virus (Panel A), the B.1.1.7 variant first identified in the United Kingdom (Panel B), the B.1.351 variant first identified in South Africa (Panel C), and the P.1 variant first identified in Brazil (Panel D). Dashed lines indicate the cutoff titer. Solid lines and numbers indicate the geometric mean titer, and 𝙸 bars show the 95% confidence interval.
This study showed that, in our small cohort, one vaccine dose substantially increased neutralizing activity against all variants tested, with similar titers detected across patients for each variant. This highlights the importance of vaccination even in previously infected patients, given the added benefit of an increased antibody response to the variants tested. Limitations of the study include the small cohort of only women and the lack of evaluation of T-cell response. However, we think the fact that all six patients responded similarly to vaccination supports our conclusions. Further studies could investigate the effects of a second vaccine dose on neutralizing activity against variants of concern in persons who have and persons who have not been previously infected.
Supplementary Appendix
Disclosure Forms
This letter was published on April 7, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet 2021;397:952-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2-elicited serum — preliminary report. N Engl J Med. 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. February 12, 2021. (https://www.biorxiv.org/content/10.1101/2021.01.25.428137v3). preprint. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.