Highlights
-
•
Ultrasound-assisted ILs pretreatment on the functional properties of soy protein hydrolysates was investigated.
-
•
The synergistic effects of ultrasound and ionic liquids not only depend on the type of ILs but also on its IL/protein ratio.
-
•
Structure of SPI pretreated with ultrasound-assisted ILs was changed.
Keywords: Soy protein, Ultrasound, Ionic liquids, Functional properties
Abstract
In this work, the effect of dual-frequency ultrasound-assisted ionic liquids (ILs) pretreatment on the functional properties of soy protein isolate (SPI) hydrolysates was investigated. The degree of hydrolysis (DH) of SPI pretreated by ultrasound and [BMIM][PF6] increased by 12.53% as compared to control (P < 0.05). More peptides with low molecular weight were obtained, providing support for the changes in DH. The trichloroacetic acid-nitrogen soluble index presented an increase, suggesting a better protein hydrolysate property. The increase in the calcium-binding activity showed the ultrasound-assisted ILs pretreatment could potentially improve bone health. The foaming capacity and stability of SPI hydrolysates pretreated by ultrasound-assisted [BMIM][PF6] always increased remarkably as compared to ultrasound-assisted [BDMIM][Cl] pretreatment. However, the synergistic effect of ultrasound-assisted [BMIM][PF6] on the emulsifying activity and antioxidant activities (DPPH and hydroxyl radical scavenging activity) was not as ideal as ultrasound-assisted [BDMIM][Cl] pretreatment, which may be affected by the structure of peptide. In conclusion, these results indicated the combination of dual-frequency ultrasound and ionic liquids would be a promising method to improve the functional properties of SPI hydrolysates and broaden the application scope of compound modification in proteolysis industry.
1. Introduction
Soy protein isolate (SPI), as a prime source of high-quality protein in the world, has been extensively used in beverage, baking and dairy alternatives due to its high nutritional value [1]. Compared with other plant proteins, SPI is rich in essential amino acids needed by human body, especially for the amount of lysine, which is normally lacking in other cereal [2]. Previous research demonstrated the essential amino acids in SPI could prevent cancer and reduce the levels of serum cholesterol [3]. Besides, SPI has a good supply of phytic acid, which is considered to be useful to inhibit the growth of cancer cells and reduce inflammation [4]. Due to the present of phytoestrogen, SPI could also prevent bone loss and promote a healthy heart in menopausal women. Therefore, the consumption of SPI containing at least 90% protein (dry basis) is increasing worldwidely. However, the natural SPI has compact globular structures, which leads to low molecular flexibility and limited functional properties, such as poor solubility, low emulsifying properties and antioxidant activities [5]. Therefore, a lot of efficient modification methods have been used to provoke expected property changes of SPI [6], [7].
Enzymatic modification is a safe and attractive method to improve the functional properties of SPI, as it can simplify the operational conditions, reduce by-products and be friendly to our environment [8]. Generally, the enzymatic hydrolysis process shows three important effects: smaller molecular weight, less secondary structure and more hydrophobic groups [9]. These changes can effectively modify the protein structure and conformation so as to enhance its properties. However, the enzymatic modification still has its drawbacks, such as longer reaction time, strict reaction temperature and pH, and low utilization rate of the enzyme [10]. This phenomenon indicates that single modification treatment does not have a satisfactory efficiency. So most work focuses on integrating various approaches to enhance the properties of protein.
In recent years, ultrasound technology has been commonly used to improve the efficiency of traditional enzymatic method. Due to its thermal and cavitation effects, ultrasound could disrupt the compact globular structures by affecting hydrogen bonds and hydrophobic interactions [11], increase the contact frequency between substrate and enzyme and break down protein aggregates [12]. These effects can increase the efficiency of protein enzymatic hydrolysis significantly. Therefore, many systematic types of research have been conducted on the ultrasound-assisted enzymolysis to improve the functional properties of protein [13], [14].
In general, ionic liquids (ILs) are organic salts and it was commonly formed by anions and cations [15]. ILs have strong potential applications in advanced biological reactions because of its unique characteristics, such as negligible vapour pressures, high chemical stability and enhanced reaction rates [16]. For example, ILs could be utilized as an attractive solvent for some insoluble components, including cellulose, chitin and collagen fibers [17], [18], [19]. Meng et al. [17] reported that the IL 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) could destroy the triple helical structure of collagen during the dissolution and regeneration, which indicated that [BMIM]Cl could become a green solvent for insoluble collagen fibers during the process of heating. It has also been reported that ILs can affect the protein refolding, aggregation and structural stability, which leads to the change in functional properties of the protein [20]. Apart from the application in protein, ILs can be also used to affect the enzyme performance, selectivity and stability [21]. Hu et al. [22] found [C10mim]Cl could bind and interact with the cell membrane, resulting in the changes of cell permeability and integrity. Therefore, it was necessary to remove ILs from product before its application. Further toxicological research should be carried out so as to determine the application scope of the product.
Recently, research on compound modification has attracted more and more attention, since it can combine the advantages of various modification methods [13], [14], [23]. The overall purpose of this research was to compare the synergistic effect of dual-frequency ultrasound and ionic liquid pretreatment on the hydrolysis of SPI. The functional properties (foaming and emulsifying properties, calcium-binding activity) and antioxidant activities were assessed. This research will provide ideas for the development of ultrasound-assisted ionic liquids in the proteolysis industry.
2. Materials and methods
2.1. Materials
Soybean protein isolate (SPI) was purchased from Shansong Biological Products Co. Ltd. (Shandong, China). 1-butyl-3-methyl imidazolium hexafluorate phosphate ([BMIM][PF6]) and 1-butyl-2,3-dimethyl imidazolium chloride ([BDMIM][Cl]) were obtained from Aladdin Industrial Corporation (Shanghai, China). 1, 1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) were bought from Sigma-Aldrich (St. Louis, MO, USA). Alcalase was supplied by Novozymes Biotechnology Co. Ltd. (Beijing, China). Other solvents were analytical grades.
2.2. Pretreatment of SPI by ultrasonic-assisted ionic liquids
SPI was initially dissolved in distilled water (1:50, w/v) and stirred at 25 °C for 30 min. Then ILs were added into the suspension, and the mixture was stirred at 25 °C for 2 h. The ratios of IL/SPI were 0.1 and 1 (by weight). The IL types and ratios were optimized by the previous research [24]. The pH of the mixture was kept at 7.0 using NaOH (0.25 M) during the whole stirring process and the condition of the magnetic stirring process was the same. Next, the SPI mixture was treated in an ultrasonic bath reactor equipped with frequency generators, which was manufactured by Jiangsu University as described by Wang et al [25]. The selected frequency was the combination of 20 and 28 kHz in a simultaneous working mode (pulsed on-time 2 s and off-time 2 s) with a temperature of 25 °C. The pretreatment time and power density were 40 min and 100 W/L, respectively. For control, the SPI without ultrasound and ionic liquids were treated by a magnetic stirring apparatus at the same conditions. The effect of dual-frequency ultrasound pretreatment (US) was used to compare the combined effect of dual-frequency ultrasound and ILs ([BMIM][PF6] and [BDMIM][Cl]).
2.3. Regeneration of SPI
SPI was regenerated based on a slightly modified procedure of Huang et al. [20]. Following ultrasound treatment, the mixture was condensed to 70–80 mL. Then the concentrated sample was mixed with ethanol (1:4, v/v) at 25 °C for 1 h and centrifuged (4500 g, 10 min). The resulting precipitate was washed by ethanol to remove the residual ionic liquids and the Folin-phenol reagent was used to check the existence of ILs according to Zhang et al [26]. Finally, the resulting residue was gathered and freeze-dried for 48 h to acquire the regenerated SPI.
2.4. Enzymatic hydrolysis method of SPI
The regenerated SPI was dissolved in distilled water (1: 50, w/v) and stirred for 1 h at 25 °C. Next, the SPI suspension was put into the water bath with a temperature of 50 °C for 10 min and 0.25 M NaOH was used to adjust the pH to 8.0. Then the hydrolysis reaction was started by the addition of Alcalase (E/S, 4000 U/g). The mixtures’ pH was kept stable during hydrolysis by adding 0.25 M NaOH. After 1 h, the mixture was heated to deactivate the enzyme in boiling water for 10 min, and centrifuged (4500 g, 10 min) to get the supernatant. All the supernatant was gathered and stored (4 °C) for further study.
2.5. Degree of hydrolysis (DH)
Memon et al. [7] procedure was used to determine the DH of SPI by the formula below:
| (1) |
where b stands for the NaOH consumed to maintain constant pH, Nb is NaOH concentration, α is the average degree of dissociation of the α-NH2 groups, which is 0.93 at 50 °C and pH (8.0), Mp is the proteins’ weight (g), htot is the total number of peptide bonds, which is 7.8 mmol/g for SPI.
2.6. Molecular weight distribution (MWD)
The MWD of SPI hydrolysates was analyzed on HPLC system (Waters 2695 E-2487, USA), using a TSK gel G2000 SWXL column (7.8 × 300 mm, Tosoh, Japan) with a UV detector at a wavelength of 220 nm. Acetonitrile with trifluoroacetic acid and water (the ratio is 40:0.1:60, v/v) in Milli-Q water was used as a mobile phase. An amount of 10 mg/mL samples were first treated by ultrasound for 5 min and filtered (0.45 µm filter) to remove the contaminants before injection. The flow rate was 0.5 mL/min and the column temperature was 30 °C. Five protein products, cytochrome c (12.38 kDa), bacitracin (1.42 kDa), aprotinin (6.5 kDa), Glycine-glycine-tyrosine-arginine (0.451 kDa), Glycine-glycine-glycine (0.189 kDa) were taken to make the molecular weight standard curve. Finally, peptide molecular distribution was calculated by the standard curve.
2.7. Functional properties
2.7.1. Trichloroacetic acid-nitrogen soluble index (TCA-NSI)
The TCA-NSI of SPI hydrolysates was calculated based on a slightly modified procedure of Wu et al. [27]. 2.5 mL of trichloroacetic acid (10%, w/v) was mixed with hydrolyzed SPI (2.5 mL), then centrifuged (4500 g, 15 min) to collect the supernatant. The protein supernatant was mixed with deionized water to obtain a suitable concentration. The soluble nitrogen content was analyzed by Lowry et al. [28], using bovine serum albumin (BSA) as standard. The solubility was expressed as the percentage of soluble nitrogen in the supernatant compared with the total amount of nitrogen in the samples.
2.7.1.1. Foaming capacity (FC) and stability (FS)
Hydrolysates of SPI and deionized water were mixed to obtain a concentration of 10 mg/mL. Then the hydrolysate dilution was homogenized using a mechanical homogenizer (HG-15A, Daihan Scientific Co., Ltd, Korea) at 6,500 rpm for 1 min. Finally, FC was calculated as follows:
| (2) |
where Va represents the volume (mL) before whipping; Vb represents the volume (mL) after whipping.
Forming stability (FS) was expressed as the percentage of the foam that remained after 30 min of whipping at 25 °C.
2.7.1.2. Emulsifying properties
Emulsifying properties of SPI hydrolysate was evaluated by the method of Pearce and Kinsella [29] with some modifications. The emulsions were prepared with 20 mL of proteins hydrolysate and soybean oil (5 g) in a 50 mL polypropylene tube. Then the mixture was homogenized (10,000 rpm, 1 min). 50 μL portions of pipetted emulsion from the bottom of the container were instantly mixed with 5 mL 0.1% SDS. The absorbance was read at a wavelength of 500 nm using spectrophotometer (Unic 7200, Unocal Corporation, China). The emulsifying activity (EA) and emulsion stability index (ESI) of SPI hydrolysate were calculated using the equations below.
| (3) |
| (4) |
where D2 represents the dilution ratio, C2 represents the initial SPI hydrolysates concentration (g/mL), φ represents the fraction of the oil phase (φ = 0.2), I represents the optical path (I = 1 cm), A0 and A60 are the absorbances at 0 min and 60 min, correspondingly.
2.7.1.3. Creaming index (CI) of emulsion
The emulsion (10 mL) was placed in a cleaned sample bottle (15 cm height and 1.4 cm internal diameter) and stored (4 °C). The serum phase (H0) and the height of emulsion (Ht) were read for 7 continuous days. Then, the CI was calculated as follows:
| (5) |
2.7.1.4. Calcium-binding activity
A slight modified procedure of Wang et al. [30] was used for the calcium-binding activity. 10 mL of hydrolysates were put into a 50 mL polypropylene tube and the pH was adjusted to 7.8 using NaOH or HCl (0.25 M). 1 mL CaCl2 (0.2 M) was then added and the mixture was placed in a thermostatic oscillator with the temperature of 40 °C for 1 h. After that, the solution was centrifuged (4500 g, 15 min). The supernatant was collected, placed in a beaker and mixed with ethanol (ethanol: supernatant = 7:1, v/v) for 1 h at 25 °C. Next, the mixture was centrifuged (4500 g, 10 min) to obtain the sediment. The sediment was dried at 40 °C. Finally, atomic absorption spectrometry was used to determine the content of calcium.
2.8. Determination of antioxidant activity
2.8.1. DPPH radical scavenging activity (DPPH-RSA)
A slight modified procedure of Liang et al. [31] was used to determine the DPPH-RSA. Briefly, DPPH was dissolved in 95% ethanol to obtain 0.1 mM solution. The SPI hydrolysates were diluted with deionized water to obtain different concentrations. 2 mL of diluted sample was added to 2 mL of 0.1 mM DPPH and kept in the darkness (30 min, 25 °C). At a wavelength of 517 nm, the absorbance was measured using the spectrophotometer. The DPPH-RSA was calculated as follows:
| (6) |
where Ai is the absorbance of the sample in DPPH at 517 nm, Aj is the absorbance of the sample in 95% ethanol at 517 nm, A0 is the absorbance of DPPH with 95% ethanol at 517 nm.
2.8.2. ABTS radical scavenging activity (ABTS-RSA)
Chen et al. [32] method with a minor adjustment was used to assess the ABTS-RSA. Potassium persulfate (2.6 mM) was mixed with ABTS (7.4 mM) at the ratio of 1:1 (v/v) and held in darkness (16 h) to obtain the ABTS radical cation. Prior to its usage, the radical cation solution was diluted in 0.2 mM phosphate buffer (pH = 7.4) to achieve an absorbance value of 0.70 ± 0.02 at 734 nm. 60 µL of sample was added to ABTS radical cation solution (4 mL) and kept in the dark for 6 min to react. At a wavelength of 734 nm, the absorbance value of the mixture was recorded using the spectrophotometer. The ABTS-RSA was calculated as follows:
| (7) |
where Ai represents the absorbance value of the sample in ABTS radical cation solution at 734 nm, Aj represents the absorbance value of the sample in 0.2 mM phosphate buffer (pH = 7.4) at 734 nm, A0 represents the absorbance value of phosphate buffer with ABTS radical cation solution at 734 nm.
2.8.3. Hydroxyl radical scavenging activity (OH-RSA)
The OH-RSA was performed based on the slightly adjusted procedure of Zhao et al. [6]. 1 mL of sample was mixed with 1 mL 3 mM FeSO4 and 1.0 mL 3 mM H2O2 and the mixture was kept at 25 °C (10 min) to react. Then 1.0 mL salicylic acid (6 mM) was added and allowed to react (15 min) at the same temperature. Finally, the absorbance values were recorded at wavelength of 510 nm using the spectrophotometer. The OH-RSA was calculated as follows:
| (8) |
where Ai is the absorbance of the sample in a mixture of FeSO4, H2O2 and salicylic acid at 510 nm, Aj is the absorbance of the sample in a mixture of FeSO4, H2O2 and deionized water at 510 nm, A0 is the absorbance of water with the mixture of FeSO4, H2O2 and salicylic acid at 510 nm.
2.8.4. Reducing power (RP)
Escudero et al. [33] procedure with a minor modification was applied for reducing power assay. 1 mL of sample was mixed with 2.5 mL phosphate buffer (0.2 mM, pH 6.6) and 2.5 mL 1% (w/v) potassium ferric cyanide (K3Fe(CN)6) in a 15 mL polypropylene tube. Then the mixture was placed in the water bath with a temperature of 50 °C to react. After 20 min, 2.5 mL trichloroacetic acid (10%, w/v) were added to stop the reaction and centrifuged (3000 g, 10 min). Next, 2.5 mL of supernatant was mixed with 2.5 mL deionized water and 0.5 mL ferric chloride solution (0.1% w/v). Finally, the absorbance value was recorded at wavelength of 700 nm using the spectrophotometer.
2.9. Statistical analysis
All experiments were conducted at least in triplicate. The data were evaluated by analysis of variance (ANOVA) under the significance level of P < 0.05 using Tukeys’ test and presented as mean ± SD using Minitab V.18. The figures and graphs were drawn using Origin Pro 8.0 software (Origin Lab Corporation, USA).
3. Results and discussion
3.1. Effect of ultrasonic-assisted ionic liquid pretreatments on DH of SPI
The degree of hydrolysis (DH) value was used to evaluate the extent of enzymatic proteolysis, as it is closely related to functional properties of protein hydrolysates. It was found high DH of protein hydrolysates could have the potential to enhance the antioxidant activities and surface hydrophobicity [6], [14], [34]. Fig. 1 shows the DH of SPI with different pretreatments. In the first 40 min, the DH increased rapidly, then slowed down thereafter, suggesting that the optimum SPI cleavage happened within 40 min of hydrolysis. The reason for the slow hydrolysis speed is mainly attributed to the reduction in the available substrate, product inhibition and enzyme auto-digestion [34].
Fig. 1.
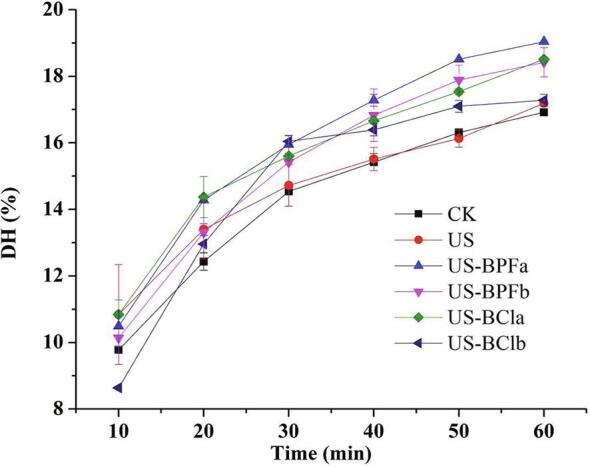
Effects of ultrasound-assisted ILs on the degree of hydrolysis of SPI. (CK, control; US, ultrasound pretreatment; US-BPFa and US-BPFb were ultrasound-assisted [BMIM][PF6] at IL/SPI ratio of 0.1 and 1, respectively; US-BCla and US-BClb were ultrasound-assisted [BDMIM][Cl] at IL/SPI ratio of 0.1 and 1, respectively).
Compared to the control, the DH of SPI increased after single ultrasonic pretreatment (P < 0.05), which was in line with previous study on other proteins [6], [35]. This result may be due that the ultrasound treatment destroyed the compact globular structures of SPI, thus, exposing more protein fractions to react with the enzyme [36].
When SPI was pretreated by ultrasound and ionic liquids, the DH of SPI increased significantly compared with the control and single ultrasound pretreatment (P < 0.05). This was mainly because the ILs could modify the protein conformation and caused change in the refolding state of protein, which resulted in loosened structure of SPI. In return, the binding rate between enzyme and protein got increase. When SPI was pretreated with ultrasound-assisted [BMIM][PF6], the highest DH was found at an IL/protein ratio of 0.1. However, the DH reduced slightly by adding more ILs, showing the ratio of IL/protein does play a significant role in affecting the protein hydrolytic process. A similar result was also observed in [BDMIM][Cl] pretreatment. This could be that high ILs concentration caused protein aggregation, thus, reducing the exposure of protein fractions. The higher DH was found in [BMIM][PF6] rather than that in [BDMIM][Cl] pretreatment, suggesting the type of ILs has a significant effect on the protein hydrolytic process. This can be explained by the fact that it is related to the ILs hydrophobicity [37]. In summary, the synergistic effects between ultrasound and ionic liquids were heavily dependent on the ILs type and its IL/protein ratio.
3.2. Molecular weight distribution (MWD) of SPI
Changes in MWD of SPI hydrolysates pretreated with ultrasound-assisted ILs are shown in Table 1. Compared to the control, the single ultrasound pretreatment caused an increase for 180–500 Da peptide fractions and the fractions below 180 Da peptide. It was found ultrasound treatment could produce high temperature, pressure and shear forces at the same time, which might help to break the cross-linkage between protein molecules. Thus, more protein molecules were easily broken down to release more peptide wih low molecular weight during the enzymatic process [38]. Moreover, the mechanical effects of the ultrasound may also help to expose more hydrophobic cores buried within the protein. Alcalase prefers to react with protein containing more hydrophobic residues [39].
Table 1.
Effects of ultrasound-assisted ILs on the molecular weight distribution of SPI hydrolysates.
| Samples | Percentage of SPI hydrolysates fractions (%) |
|||||
|---|---|---|---|---|---|---|
| >5KDa | 3-5KDa | 1-3KDa | 500–1000 Da | 180–500 Da | ≤180 Da | |
| CK | 2.62 | 3.13 | 16.72 | 26.19 | 45.67 | 5.67 |
| US | 2.6 | 3.19 | 16.21 | 25.55 | 46.02 | 6.43 |
| US-BPFa | 1.8 | 2.65 | 14.67 | 25.51 | 48.61 | 6.75 |
| US-BPFb | 2.34 | 2.97 | 16.1 | 25.59 | 46.95 | 6.05 |
| US-BCla | 2.17 | 2.92 | 15.79 | 25.82 | 47.17 | 6.12 |
| US-BClb | 3.43 | 3.45 | 16.48 | 23.86 | 46.73 | 6.04 |
When SPI was pretreated by ultrasound and [BMIM][PF6] at different concentrations, the percentage of low molecular weight peptide (sum of 180–500 Da and < 180 Da) was 55.36% and 53% for US-BPFa and US-BPFb, respectively. Furthermore, among all the pretreatments, US-BPFa had the highest percentage of low molecular weight peptide. This result indicates that the US-BPFa significantly resulted in the structural changes of protein molecules compared to the other pretreatments. This reason also explained the lower fractions with > 5 kDa, 3–5 kDa, and 1–3 kDa for US-BPFa. The relative percentage of the peptides with molecular weight (MW) over 3 kDa significantly increased in the pretreatment of US-BClb. The differences in the molecular weight distribution of SPI hydrolysates may indicate totally differences in their functional properties. In short, the changes in percentage of low molecular weight peptide are in consistent with the changes in DH.
3.3. TCA-NSI
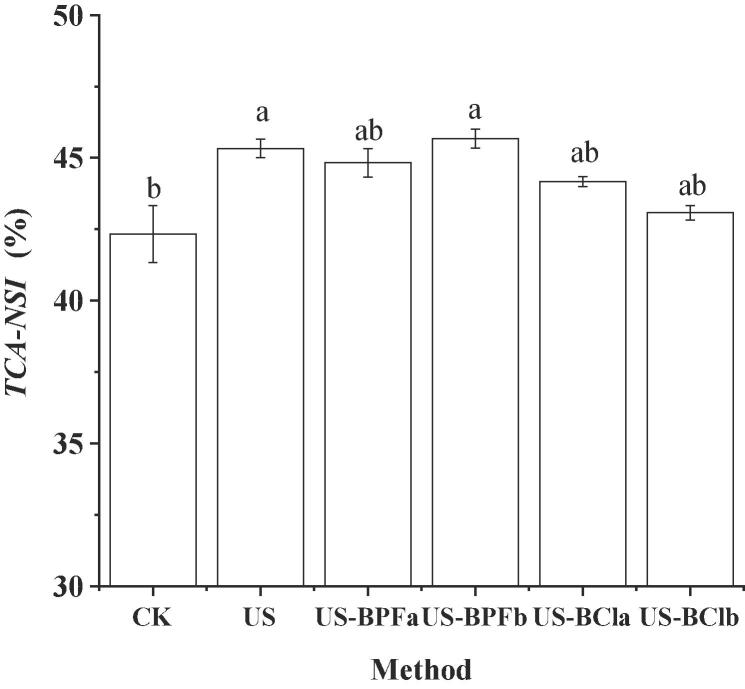
TCA-NSI is a very important value that can reflect the degree of DH, hence becoming the most practical and convenient means to measure the hydrolysis process [40]. The TCA-NSI value of SPI hydrolysates is shown in Fig. 2. The highest TCA-NSI value of 45.67% was obtained during the combination of ultrasound and [BMIM][PF6] (ratio of 1) pretreatment, followed by the single ultrasound pretreatment (45.30%), whereas the lowest value was observed in the control pretreatment (42.33%). This data trend was consistent with the results of [BMIM][PF6] in DH.
Fig. 2.
Effects of ultrasound-assisted ILs on the TCA-NSI. (CK, control; US, ultrasound pretreatment; US-BPFa and US-BPFb were ultrasound-assisted [BMIM][PF6] at IL/SPI ratio of 0.1 and 1, respectively; US-BCla and US-BClb were ultrasound-assisted [BDMIM][Cl] at IL/SPI ratio of 0.1 and 1, respectively). Note: the different letter reveals significant differences (P < 0.05).
However, the combined effect between ultrasound and [BDMIM][Cl] is not as ideal as single ultrasound treatment. This result could be due to the interaction between ionic liquids and proteins, which resulted in the different aggregation state of protein molecules and a decrease in the effectiveness of ultrasound, thus influencing the TCA-NSI value.
3.4. Foaming capacity (FC) and stability (FS)
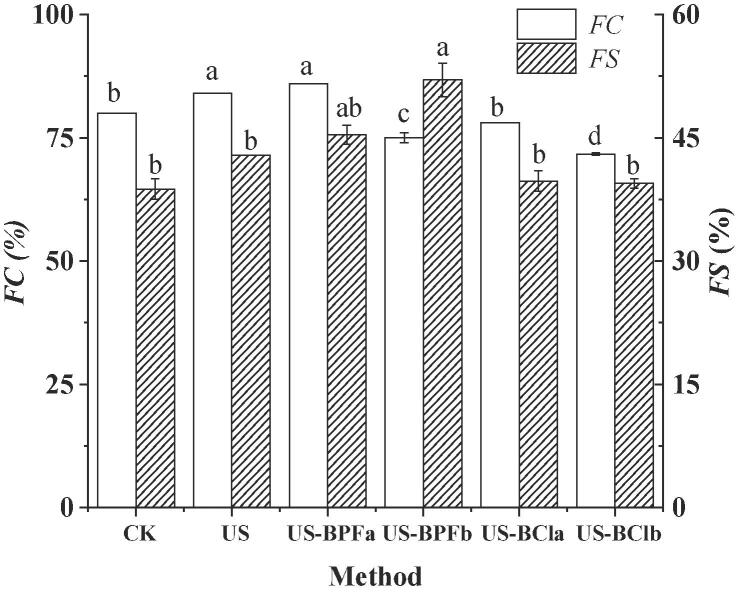
The FC and FS generally depend on the surface activity of protein and the interfacial activity in the fluid-air interface, which keeps air bubbles in the suspension. Fig. 3 displays the impact of ultrasound-assisted ionic liquids on the foam formation and stability of SPI hydrolysates.
Fig. 3.
Effects of ultrasound-assisted ILs on the foam capacity and stability. (CK, control; US, ultrasound pretreatment; US-BPFa and US-BPFb were ultrasound-assisted [BMIM][PF6] at IL/SPI ratio of 0.1 and 1, respectively; US-BCla and US-BClb were ultrasound-assisted [BDMIM][Cl] at IL/SPI ratio of 0.1 and 1, respectively). Note: the different letter reveals significant differences (P < 0.05).
With single ultrasound pretreatment, the FC and FS of SPI hydrolysates increased by 5% and 9.5% as compared to the control, respectively. The ultrasound and sonochemistry effects could destroy the partial tighten structure or loosen the protein structure by damaging chemical bonds [41]. Thereby, the loosened structure would lead to the changes for SPI hydrolysates properties and improve the interfacial adsorption capacity of protein hydrolysates and the foaming properties. The maximum FC value observed was 86% for ultrasound-assisted [BMIM][PF6] pretreatment at an IL/protein ratio of 0.1. However, compared with control and single ultrasound pretreatment (P < 0.05), the FC value reduced significantly with adding high concentration ionic liquids. This can be explained as the high level of salts could change the structure of SPI, resulting in the increase of surface tension of hydrolysates and the screening of the electrostatic double-layer forces [42]. Another reason is mainly due to protein aggregation. A similar result was found in ultrasound combined [BDMIM][Cl] pretreatment.
Interestingly, the FS was the highest for the ultrasound-assisted [BMIM][PF6] pretreatment at an IL/protein ratio of 1, proving that the effect of ionic liquids is complex. For the combination of ultrasound and [BDMIM][Cl] pretreatment, the FS result is not as good as the ultrasound-assisted [BMIM][PF6] pretreatment (P < 0.05). A possible reason is that the different hydrogen bond-forming capability of ionic liquids, which caused changes in protein structure and refolding state and further affected the FS of SPI hydrolysates.
3.5. Emulsion properties (EA and ESI)
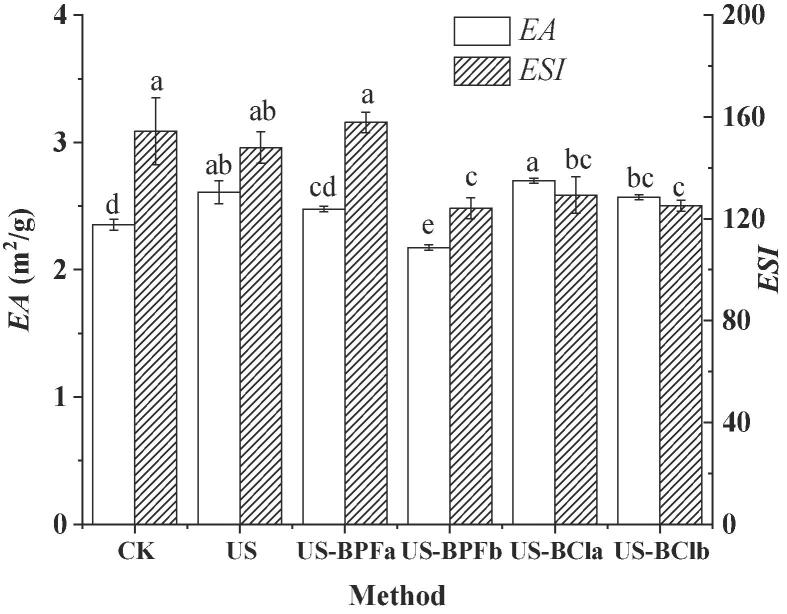
Emulsion activity (EA) is an important index since it not only indicates the effectiveness of emulsifiers but also represents the ability of polypeptides for being adsorbed at the oil–water interface [29]. The EA of SPI hydrolysates pretreated by different methods was shown in Fig. 4.
Fig. 4.
Effects of ultrasound-assisted ILs on the emulsion properties of SPI hydrolysates. (CK, control; US, ultrasound pretreatment; US-BPFa and US-BPFb were ultrasound-assisted [BMIM][PF6] at IL/SPI ratio of 0.1 and 1, respectively; US-BCla and US-BClb were ultrasound-assisted [BDMIM][Cl] at IL/SPI ratio of 0.1 and 1, respectively). Note: the different letter reveals significant differences (P < 0.05).
Compared to control, the EA of SPI hydrolysates increased remarkably after single ultrasound pretreatment (P < 0.05), indicating a smaller number of dispersed fat droplets were obtained. One possible reason may be attributed that the high levels of hydrodynamic shear associated with ultrasonic cavitations could reduce the particle size of protein and increase the uniformity of protein distribution [43]. Thus, it will further influence the size of SPI hydrolysates. The highest EA value was observed in the sample treated by ultrasound-assisted [BDMIM][Cl] at an IL/protein ratio of 0.1. One possible reason is that ultrasound-assisted [BDMIM][Cl] treatment could affect the structure of SPI by improving molecular flexibility and surface hydrophobicity, which further induced the production of peptide that could be adsorbed at the oil–water interface.
Emulsion stability index (ESI) reflects the emulsion ability to resist breakdown. As shown in Fig. 4, the ESI value of SPI hydrolysates decreased significantly after US-BPFb, US-BCla and US-BClb pretreatments in comparison with that of US (P < 0.05). The decrease in ESI value could be attributed to the low molecular weight peptides (below 180 Da) present in the SPI hydrolysates (confirmed in Table 1). The small peptide cannot unfold and re-orient at the interface as the large peptides do, causing less efficiency in plummeting the interfacial tension and stabilizing the emulsions. Similarly, Zou et al. [35] and Mintah et al. [44] also found that emulsion stability reduced significantly with decreasing size of peptides. However, the lowest percentage of low molecular weight peptide and high ESI in control is not consisitent with this conclusion. The structure of peptide may also influence the ESI significantly.
3.6. Creaming index
Creaming index (CI) is a critical indicator to evaluate the creaming stability. Fig. 5. shows the changes in CI for emulsions stabilized by SPI hydrolysates during 1 week storage.
Fig. 5.
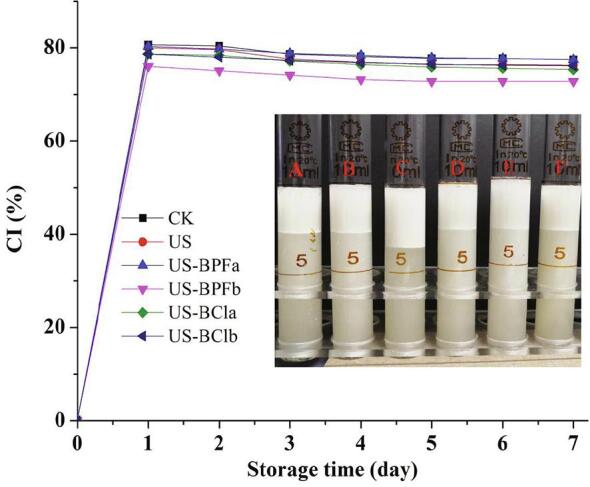
Effects of ultrasound and ILs on the creaming index of SPI hydrolysates. (CK, control; US, ultrasound pretreatment; US-BPFa and US-BPFb were ultrasound-assisted [BMIM][PF6] at IL/SPI ratio of 0.1 and 1, respectively; US-BCla and US-BClb were ultrasound-assisted [BDMIM][Cl] at IL/SPI ratio of 0.1 and 1, respectively; A: CK, B:US-BPFa, C: US-BPFb, D: US-BCla, E: US-BClb, F: US).
The emulsions formed rapidly into two layers within 1 day, as shown in Fig. 5. And then during the following days, the creaming rate slowed down. The lowest CI was obtained in a sample pretreated by combined ultrasound and [BMIM][PF6] at an IL/protein ratio of 1. The result of DH indicates that the highest DH was found after the combination ultrasound and [BMIM][PF6] pretreatment. Therefore, it can be inferred that the DH was closely related to the CI. However, there is no obvious change among the other different pretreatments, indicating that the creaming stability was on the same level.
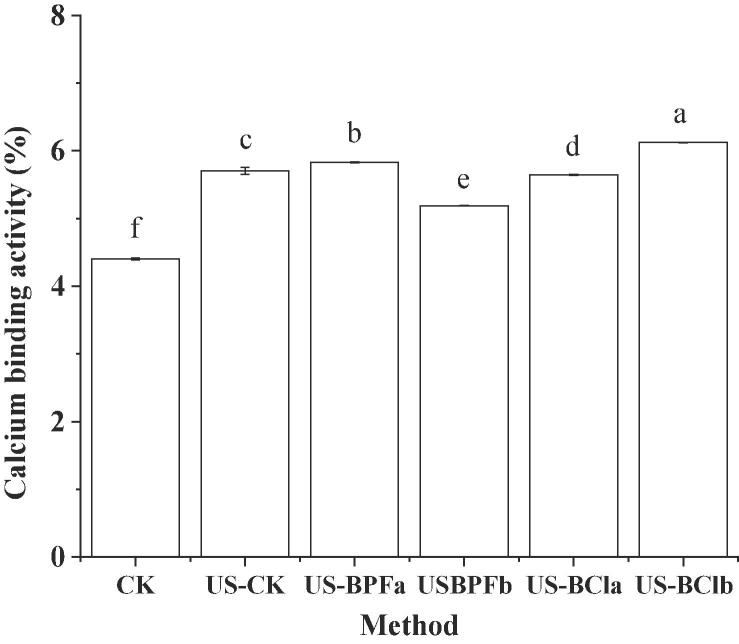
3.7. Calcium-binding abilitiy
The effect of ultrasound and ionic quids pretreatment on the calcium-binding ability of SPI hydrolysates was shown in Fig. 6. After ultrasound pretreatment, the calcium-binding ability of SPI hydrolysates significantly increased when compared to control (P < 0.05). It was reported that hydrophobic amino acids play a significant role in the process of binding calcium ions [45]. The ultrasonic cavitation was beneficial to change the secondary structure of protein and expose more hydrophobic groups and regions, which could provide more contact chance between enzyme and protein. Then the number of hydrophobic amino acids in SPI hydrolysates will be increased and it will subsequently bind with the calcium ions. Therefore, the calcium-binding ability of SPI hydrolysates could be improved significantly.
Fig. 6.
Effects of ultrasound-assisted ILs on the calcium binding ability of SPI hydrolysates. (CK, control; US, ultrasound pretreatment; US-BPFa and US-BPFb were ultrasound-assisted [BMIM][PF6] at IL/SPI ratio of 0.1 and 1, respectively; US-BCla and US-BClb were ultrasound-assisted [BDMIM][Cl] at IL/SPI ratio of 0.1 and 1, respectively). Note: the different letter reveals significant differences (P < 0.05).
For ultrasound-assisted [BMIM][PF6] pretreatment, a slight increase in calcium-binding ability was observed at an IL/protein ratio of 0.1. While at an IL/protein ratio of 1, a significant decrease in the calcium-binding ability was seen. This was possibly influenced by the difference in the degree of hydrolysis. Chen et al. [46] reported that the proper hydrolysis might result in the exposure of hydrophobic groups, therefore promoted calcium-binding ability. However, there was a total opposite trend for [BDMIM][Cl] pretreatment, further indicating the complex effects of ionic liquids. The highest calcium-binding ability with US-BClb pretreatment may be due to the highest relative percentage of the peptides with molecular weight (MW) over 3 kDa. The molecular weight of the peptides from the protein hydrolysates is always one of the most important factors concerning desired functional properties [47].
3.8. Antioxidant activity
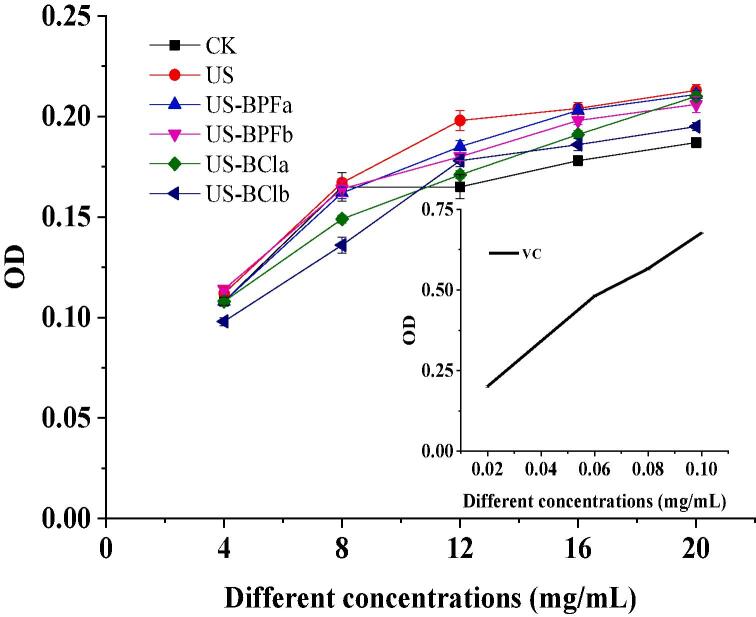
3.8.1. DPPH-RSA
The DPPH-RSA is one of the in vitro assessments of the free RSA of various antioxidants [48]. The effect of ultrasound-assisted ionic quids pretreatment on the DPPH-RSA of SPI hydrolysates was shown in Table 2. IC50 (50% inhibitory concentration) value (mg/mL) is the concentration at which DPPH radicals were scavenged by 50%. The final result was expressed by the IC50 value. The lower IC50 value indicates the higher effect of free RSA.
Table 2.
Effects of ultrasound-assisted ILs on the antioxidant activity of SPI hydrolysates.
| Samples | DPPH-IC50 (mg/mL) | ABTS-IC50(mg/mL) | Hydroxyl-IC50 (mg/mL) |
|---|---|---|---|
| VC | 0.0068 ± 0.001f | 0.145 ± 0.003c | 0.10 ± 0.02f |
| CK | 12.04 ± 0.04c | 0.960 ± 0.065a | 1.45 ± 0.03 cd |
| US | 11.21 ± 0.03d | 0.827 ± 0.017b | 1.34 ± 0.05d |
| US-BPFa | 14.26 ± 0.21a | 0.955 ± 0.015ab | 1.51 ± 0.04bc |
| US-BPFb | 13.55 ± 0.04b | 0.945 ± 0.035ab | 1.64 ± 0.03a |
| US-BCla | 13.28 ± 0.32b | 1.055 ± 0.005a | 1.23 ± 0.02e |
| US-BClb | 9.59 ± 0.23e | 0.935 ± 0.035ab | 1.62 ± 0.03ab |
Note: Mean value ± standard deviation from three separate samples. Different letters in the same column indicate significant differences at P < 0.05.
The lowest IC50 value was obtained in the sample pretreated by ultrasound and [BDMIM][Cl] pretreatments at an IL/protein ratio of 1. This result indicates that the synergistic effect is beneficial to improve the DPPH-RSA of SPI hydrolysates. After a single ultrasound pretreatment, the IC50 value of SPI hydrolysates decreased compared to the control (P < 0.05). The increase in free RSA may be attributed to the difference in the small peptide or amino acids. Several researchers also reported that smaller molecular weight of the peptides could have higher DPPH-RSA [35], [49]. However, the IC50 value increased significantly for the other three pretreatments (US-BPFa, US-BPFb and US-BCla). Compared to the ultrasound-assisted [BDMIM][Cl] pretreatment, combined ultrasound and [BMIM][PF6] pretreatment always showed a negative effect on the DPPH-RSA of SPI hydrolysates. This phenomenon could be attributed to the different compound about anions and cations, thus leading to the different hydrogen bond forming capability and aggregation state of protein. The more aggregates of SPI, the less enzyme’s sensitive sites of protein fractions, thus bring the lower antioxidant activity of hydrolysates. In conclusion, all the IC50 values were higher than the that of Vc, suggesting that the DPPH radical scavenging ability of SPI hydrolysates was lower than Vc.
3.8.2. ABTS-RSA
ABTS-RSA is an outstanding tool for measuring the hydrophilic and hydrophobic antioxidants. Table 2. displays the impact of ultrasound and ionic liquids pretreatment on the ABTS-RSA of SPI hydrolysates. The final result was also expressed by IC50.
Compared with the control, the IC50 value reduced significantly after single ultrasonic pretreatment (P < 0.05), indicating the improved ABTS-RSA. This could be linked to the fact that ultrasound treatment could produce high turbulence and shear energy in shaped cavitations, which could emerge smaller molecules and expose more hydrophobic ends [31]. It was reported that peptide with low molecule weight and high hydrophobic ends could have high ABTS radical scavenging ability [35]. With the addition of ionic liquids, the value of IC50 started to increase. These results showed the synergistic effect is not as effective as single ultrasound pretreatment.
3.8.3. OH-RSA
Among the oxygen free radicals, OH-RSA is the most reactive free radical, since it reacts with everything in living organisms, such as DNA, lipids and proteins. This special nature could cause harmful effects on our body, including the ageing, cancer and other diseases. The effect of ultrasound and ionic liquids pretreatments on the hydroxyl radical scavenging activity of SPI hydrolysates was shown in Table 2.
Compared to the control, the IC50 value of SPI pretreated with single ultrasound and ultrasound-assisted [BDMIM][Cl] treatment at an IL/protein ratio of 0.1 decreased by 7.6% and 15.2%, respectively. The decrease in the IC50 value shows the hydroxyl radical scavenging activity get improved. One possible reason is that ultrasound treatment destroyed the protein’s compact structure and exposed more hydrophobic amino acids. The hydrophobic amino acids could play an important role on radical scavenging [50]. However, the IC50 value of SPI increased after the other three (US-BPFa, US-BPFb and US-BClb) pretreatments, especially for the ILs pretreatment at an IL/protein ratio of 1. This may be due that ILs type and concentration have different influences on the structure of SPI, which leads to different properities of hydrolysates.
3.8.4. RP
The RP could be used to evaluate the ability of an antioxidant in reducing ferric (Fe3+) to ferrous (Fe2+) ion by transferring electrons. The effect of ultrasound and ionic liquids pretreatments on the reducing power assay of SPI hydrolysates was shown in Fig. 7.
Fig. 7.
Effects of ultrasound-assisted ILs on the reducing power of SPI hydrolysates. (CK, control; US, ultrasound pretreatment; US-BPFa and US-BPFb were ultrasound-assisted [BMIM][PF6] at IL/SPI ratio of 0.1 and 1, respectively; US-BCla and US-BClb were ultrasound-assisted [BDMIM][Cl] at IL/SPI ratio of 0.1 and 1, respectively).
When the concentration of hydrolysates is 4 mg/mL, there was no significant difference between control and the pretreatments. However, with the increase of concentration, the reducing power of SPI hydrolysates prepared with different pretreatments increased remarkably, compared with the control (P < 0.05). This suggests the reducing power of SPI hydrolysates increased significantly after ultrasound and ILs pretreatments. The difference can be attributed to the specific amino acid or peptide. Moreover, Zhao et al. [48] also found that the reducing power of peptide mainly depends on the concentration of hydrolysates, thus, the higher the hydrosylate concentration, the higher the reducing power.
4. Conclusion
In this study, a combination of dual-frequency ultrasound and ILs with different types or concentrations was found to change the functional properties of SPI hydrolysates differently. The effect of ultrasound-assisted [BMIM][PF6] at a IL/protein ratio of 0.1 had the most significant influences on most of the functional properties of SPI hydrolysates. However, the synergistic effect for the ultrasound and [BDMIM][Cl] is not as good as the [BMIM][PF6] or single ultrasound pretreatment. Especially, the ionic liquids with high concentration possibly led to the protein aggregation, thus presenting the negative effect sometimes. Compared to the control, the degree of hydrolysis (DH) of SPI treated by ultrasound and ILs increased significantly and more peptides with low molecular weight were obtained. The result showed these important two indexes (DH, MWD) were closely related to its functional and antioxidant properties. In a word, application of ultrasound combined with suitable ionic liquid could affect the structure of the protein and change its refolding state, resulting in an improved antioxidant and functional properties.
Funding
The authors are grateful for the support provided by the National Natural Science Foundation of China (31701540) and Natural Science Foundation of Jiangsu Province (BK20170539) and A project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
CRediT authorship contribution statement
Wenxue Zhang: Conceptualization, Investigation, Methodology, Formal analysis, Software, Writing - original draft, Writing - review & editing. Liurong Huang: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing - review & editing. Wenwen Chen: Investigation. Jiale Wang: Investigation. Shiheng Wang: Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cui C., Zhao M., Yuan B., Zhang Y., Ren J. Effect of pH and pepsin limited hydrolysis on the structure and functional properties of soybean protein hydrolysates. J. Food Sci. 2013;78(12):C1871–C1877. doi: 10.1111/1750-3841.12309. [DOI] [PubMed] [Google Scholar]
- 2.Astawan M., Prayudani A.P.G. The overview of food technology to process soy protein isolate and its application toward food industry. World Nutrit. J. 2020;4(1):12–17. [Google Scholar]
- 3.Henkel J. Soy: health claims for soy protein, question about other components. Fda Consumer. 2000;34(3):18–20. [PubMed] [Google Scholar]
- 4.Vucenik, I., Shamsuddin, A.M. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J. Nutrit., 133, 3778–3784, 2003. [DOI] [PubMed]
- 5.Yuan B., Ren J., Zhao M., Luo D., Gu L. Effects of limited enzymatic hydrolysis with pepsin and high-pressure homogenization on the functional properties of soybean protein isolate. LWT Food Sci. Technol. 2012;46(2):453–459. [Google Scholar]
- 6.Zhao F., Liu X., Ding X., Dong H., Wang W. Effects of high-intensity ultrasound pretreatment on structure, properties, and enzymolysis of soy protein isolate. Molecules. 2019;24(20):3637. doi: 10.3390/molecules24203637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Memon A.H., Ding R., Yuan Q., Wei Y., Liang H. Facile synthesis of alcalase-inorganic hybrid nanoflowers used for soy protein isolate hydrolysis to improve its functional properties. Food Chem. 2019;289:568–574. doi: 10.1016/j.foodchem.2019.03.096. [DOI] [PubMed] [Google Scholar]
- 8.Xue P., Sun N.a., Li Y., Cheng S., Lin S. Targeted regulation of hygroscopicity of soybean antioxidant pentapeptide powder by zinc ions binding to the moisture absorption sites. Food Chem. 2018;242:83–90. doi: 10.1016/j.foodchem.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay K., Ghosh S. Preparation and characterization of papain-modified sesame (Sesamum indicum L.) protein isolates. J. Agric. Food. Chem. 2002;50(23):6854–6857. doi: 10.1021/jf020320x. [DOI] [PubMed] [Google Scholar]
- 10.Ren X., Zhang X.i., Liang Q., Hou T., Zhou H. Effects of different working modes of ultrasound on structural characteristics of zein and ace inhibitory activity of hydrolysates. J. Food Qual. 2017;2017:1–8. [Google Scholar]
- 11.Ayim I., Ma H., Alenyorege E.A., Ali Z., Donkor P.O. Influence of ultrasound pretreatment on enzymolysis kinetics and thermodynamics of sodium hydroxide extracted proteins from tea residue. J. Food Sci. Technol. 2018;55(3):1037–1046. doi: 10.1007/s13197-017-3017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L., Ding X., Li Y., Ma H. The aggregation, structures and emulsifying properties of soybean protein isolate induced by ultrasound and acid. Food Chem. 2019;279:114–119. doi: 10.1016/j.foodchem.2018.11.147. [DOI] [PubMed] [Google Scholar]
- 13.Galante M., De Flaviis R., Boeris V., Spelzini D. Effects of the enzymatic hydrolysis treatment on functional and antioxidant properties of quinoa protein acid-induced gels. LWT–Food. Sci. Technol. 2020;118:108845. doi: 10.1016/j.lwt.2019.108845. [DOI] [Google Scholar]
- 14.Dabbour M., He R., Mintah B., Tang Y., Ma H. Ultrasound assisted enzymolysis of sunflower meal protein: kinetics and thermodynamics modeling. J. Food Process Eng. 2018;41(7):e12865. doi: 10.1111/jfpe.2018.41.issue-710.1111/jfpe.12865. [DOI] [Google Scholar]
- 15.Tang D.Y.Y., Yew G.Y., Koyande A.K., Chew K.W., Vo D.-V., Show P.L. Green technology for the industrial production of biofuels and bioproducts from microalgae: a review. Environ. Chem. Lett. 2020;18(6):1967–1985. [Google Scholar]
- 16.Harada L.K., Pereira J.F.B., Campos W.F., Silva E.C., Moutinho C.G., Vila M.M.G.C., Oliveira J.M., Teixeira J.A., Balcão V.M., Tubino M. Insights into protein-ionic liquid interactions aiming at macromolecule delivery systems. J. Braz. Chem. Soc. 2018;29(10):1983–1998. [Google Scholar]
- 17.Meng Z., Zheng X., Tang K., Liu J., Ma Z., Zhao Q. Dissolution and regeneration of collagen fibers using ionic liquid. Int. J. Biol. Macromol. 2012;51(4):440–448. doi: 10.1016/j.ijbiomac.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Swatloski R.P., Spear S.K., Holbrey J.D., Rogers A.D. Dissolution of cellulose [correction of cellose] with ionic liquids. J. Am. Chem. Soc. 2002;124(18):4974–4975. doi: 10.1021/ja025790m. [DOI] [PubMed] [Google Scholar]
- 19.Jaworska M.M., Gorak A. Modification of chitin particles with chloride ionic liquids. Mater. Lett. 2016;164:341–343. [Google Scholar]
- 20.Huang L., Jia S., Zhang W., Ma L., Ding X. Aggregation and emulsifying properties of soybean protein isolate pretreated by combination of dual-frequency ultrasound and ionic liquids. J. Mol. Liq. 2020;301:112394. doi: 10.1016/j.molliq.2019.112394. [DOI] [Google Scholar]
- 21.Hernandez F.F.J. Keys for the use of ionic liquids as reaction media in enzyme-catalyzed processes. J. Bioprocess. Biotechn. 2018;8(2):1–3. [Google Scholar]
- 22.Hu L.-X., Xiong Q., Shi W.-J., Huang G.-Y., Liu Y.-S., Ying G.-G. New insight into the negative impact of imidazolium-based ionic liquid [C10mim]Cl on Hela cells: From membrane damage to biochemical alterations. Ecotoxicol. Environ. Saf. 2021;208:111629. doi: 10.1016/j.ecoenv.2020.111629. [DOI] [PubMed] [Google Scholar]
- 23.Khoo K.S., Chew K.W., Yew G.Y., Manickam S., Ooi C.W., Show P.L. Integrated ultrasound-assisted liquid biphasic flotation for efficient extraction of astaxanthin from Haematococcus pluvialis. Ultrason. Sonochem. 2020;67:105052. doi: 10.1016/j.ultsonch.2020.105052. [DOI] [PubMed] [Google Scholar]
- 24.Huang L., Zhang W., Yan D., Ma L., Ma H. Solubility and aggregation of soy protein isolate induced by different ionic liquids with the assistance of ultrasound. Int. J. Biol. Macromol. 2020;164:2277–2283. doi: 10.1016/j.ijbiomac.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y.-Y., Tayyab Rashid M., Yan J.-K., Ma H. Effect of multi-frequency ultrasound thawing on the structure and rheological properties of myofibrillar proteins from small yellow croaker. Ultras. Sonochem. 2021;70:105352. doi: 10.1016/j.ultsonch.2020.105352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.X. Zhang, L. Xu, X. Wang, H. Wang, A method for determination of ionic liquid residues in regenerated cellulose membrane, CN 103257140 A (In Chinese), 2013.
- 27.Wu W., He L., Liang Y., Yue L., Peng W., Jin G., Ma M. Preparation process optimization of pig bone collagen peptide-calcium chelate using response surface methodology and its structural characterization and stability analysis. Food Chem. 2019;284:80–89. doi: 10.1016/j.foodchem.2019.01.103. [DOI] [PubMed] [Google Scholar]
- 28.Lowry OliverH., Rosebrough NiraJ., Farr A.L., Randall RoseJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 29.Pearce K.N., Kinsella J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food. Chem. 1978;26(3):716–723. [Google Scholar]
- 30.Wang L.i., Ding Y., Zhang X., Li Y., Wang R., Luo X., Li Y., Li J., Chen Z. Isolation of a novel calcium-binding peptide from wheat germ protein hydrolysates and the prediction for its mechanism of combination. Food Chem. 2018;239:416–426. doi: 10.1016/j.foodchem.2017.06.090. [DOI] [PubMed] [Google Scholar]
- 31.Liang Q., Ren X., Ma H., Li S., Xu K., Oladejo A.O. Effect of low-frequency ultrasonic-assisted enzymolysis on the physicochemical and antioxidant properties of corn protein hydrolysates. J. Food Qual. 2017;2017:1–10. [Google Scholar]
- 32.Chen X.-W., Wang J.-M., Yang X.-Q., Qi J.-R., Hou J.-J. Subcritical water induced complexation of Soy protein and Rutin: improved interfacial properties and emulsion stability. J. Food Sci. 2016;81(9):C2149–C2157. doi: 10.1111/1750-3841.13403. [DOI] [PubMed] [Google Scholar]
- 33.Escudero E., Mora L., Fraser P.D., Aristoy M.-C., Toldrá F. Identification of novel antioxidant peptides generated in spanish dry-cured ham. Food Chem. 2013;138(2-3):1282–1288. doi: 10.1016/j.foodchem.2012.10.133. [DOI] [PubMed] [Google Scholar]
- 34.S. Ketnawa, M.O. Alvarez, S. Benjakul, R.S. kuen, Gelatin hydrolysates from farmed Giant catfish skin using alkaline proteases and its antioxidative function of simulated gastro-intestinal digestion. Food Chem., 2016, 192, 34-42. [DOI] [PubMed]
- 35.Zou Ye, Wang Wei, Li Qian, Chen Yao, Zheng Daheng, Zou Yanmin, Zhang Min, Zhao Ting, Mao Guanghua, Feng Weiwei, Wu Xiangyang, Yang Liuqing. Physicochemical, functional properties and antioxidant activities of porcine cerebral hydrolysate peptides produced by ultrasound processing. Process Biochem. 2016;51(3):431–443. [Google Scholar]
- 36.Zhou Cunshan, Ma Haile, Ding Qingzhi, Lin Lin, Yu Xiaojie, Luo Lin, Dai Chunhua, Yagoub Abu El-Gasim A. Ultrasonic pretreatment of corn gluten meal proteins and neutrase: effect on protein conformation and preparation of ACE (angiotensin converting enzyme) inhibitory peptides. Food Bioprod. Process. 2013;91(4):665–671. [Google Scholar]
- 37.Zhao Hua. Protein stabilization and enzyme activation in ionic liquids: specific ion effects. J. Chem. Technol. Biotechnol. 2016;91(1):25–50. doi: 10.1002/jctb.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Qingzhi, Zhang Ting, Niu Shuai, Cao Feifan, Wu-Chen Ricardo Antonio, Luo Lin, Ma Haile. Impact of ultrasound pretreatment on hydrolysate and digestion products of grape seed protein. Ultrason. Sonochem. 2018;42:704–713. doi: 10.1016/j.ultsonch.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Jin Jian, Ma Haile, Wang Bei, Yagoub Abu El-Gasim A., Wang Kai, He Ronghai, Zhou Cunshan. Effects and mechanism of dual-frequency power ultrasound on the molecular weight distribution of corn gluten meal hydrolysates. Ultrason. Sonochem. 2016;30:44–51. doi: 10.1016/j.ultsonch.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Bah Clara S.F., Carne Alan, McConnell Michelle A., Mros Sonya, Bekhit Alaa El-Din A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016;202:458–466. doi: 10.1016/j.foodchem.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 41.G.Y., Tan, X., Chew, K. W., Chang, J. S., Tao, Y., Jiang, N., & P.L. Show, Thermal-Fenton mechanism with sonoprocessing for rapid non-catalytic transesterification of microalgal to biofuel production. Chem. Eng. J., 2020, 127264.
- 42.Samin Ali Mohamed, Manan Muhamad. A., Idris Ahmed Kamal, Yekeen Nurudeen, Said Mohamed, Alghol Ali. Protein foam application for enhanced oil recovery. J. Dispersion Sci. Technol. 2017;38(4):604–609. [Google Scholar]
- 43.Yang Xue, Li Yunliang, Li Songtao, Ren Xiaofeng, Olayemi Oladejo Ayobami, Lu Feng, Ma Haile. Effects and mechanism of ultrasound pretreatment of protein on the Maillard reaction of protein-hydrolysate from grass carp (Ctenopharyngodon idella) Ultrason. Sonochem. 2020;64:104964. doi: 10.1016/j.ultsonch.2020.104964. [DOI] [PubMed] [Google Scholar]
- 44.Mintah Benjamin Kumah, He Ronghai, Dabbour Mokhtar, Xiang Jiahui, Agyekum Akwasi Akomeah, Ma Haile. Techno-functional attribute and antioxidative capacity of edible insect protein preparations and hydrolysates thereof: effect of multiple mode sonochemical action. Ultrason. Sonochem. 2019;58:104676. doi: 10.1016/j.ultsonch.2019.104676. [DOI] [PubMed] [Google Scholar]
- 45.Liu B., Zhuang Y., Sun L. Identification and characterization of the peptides with calcium-binding capacity from tilapia (Oreochromis niloticus) skin gelatin enzymatic hydrolysates. J. Food Sci. 2020;85(1):114–122. doi: 10.1111/1750-3841.14975. [DOI] [PubMed] [Google Scholar]
- 46.Chen D., Mu X., Huang H., Nie R., Liu Z., Zeng M. Isolation of a calciumbinding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J. Funct. Foods. 2014;6(1):575–584. [Google Scholar]
- 47.Wang J., Su Y., Jia F., Jin H. Characterization of casein hydrolysates derived from enzymatic hydrolysis. Chem. Cent. J. 2013;7:62. doi: 10.1186/1752-153X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Qiang, Xiong Hua, Selomulya Cordelia, Chen Xiao Dong, Zhong Honglan, Wang Shenqi, Sun Wenjing, Zhou Qiang. Enzymatic hydrolysis of rice dreg protein: effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012;134(3):1360–1367. doi: 10.1016/j.foodchem.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 49.Wang Bin, Li Li, Chi Chang-Feng, Ma Jia-Hui, Luo Hong-Yu, Xu Yin-feng. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013;138(2-3):1713–1719. doi: 10.1016/j.foodchem.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Ajibola Comfort F., Fashakin Joseph B., Fagbemi Tayo N., Aluko Rotimi E. Renin and angiotensin converting enzyme inhibition with antioxidant properties of african yam bean protein hydrolysate and reverse-phase hplc-separated peptide fractions. Food Res. Int. 2013;52(2):437–444. [Google Scholar]