Abstract
Background & Purpose:
We investigated clinical and genetic factors associated with severe radiation-induced lymphopenia (RIL) in a randomized clinical trial of photon vs. proton radiation, with chemotherapy, for non-small cell lung cancer.
Methods:
XRCC1 rs25487 was genotyped in lymphocytes from serial peripheral blood samples. Severe RIL was defined as absolute lymphocyte count (ALC) <0.3×109 cells/L. Univariate and multivariate analyses were used to identify independent risk factors, which were then used to group patients according to risk of severe RIL.
Results:
Univariate analysis of the 178 patients in this analysis showed that older age, larger tumors, higher lung V5 and mean lung dose, and higher heart V5 and mean heart dose were associated with severe RIL during treatment (P<0.05). The XRCC1 rs25487 AA genotype was also associated with increased risk of severe RIL during treatment (AA vs. others: hazard ratio [HR] =1.065, 95% confidence interval [CI] 1.089–2.500, P = 0.018). Multivariate analyses showed that older age (HR=1.031, 95% CI 1.009–1.054, P=0.005), lung V5 (HR=1.039, 95% CI 1.023–1.055, P<0.0001), and AA genotype (AA vs. others, HR=1.768, 95% CI 1.165–2.684, P=0.007) were independently associated with higher incidence of severe RIL. These three risk factors (age ≥56 years, lung V5 ≥51% and XRCC1 rs25487 AA) distinguished patients at different risk of developing severe RIL (P<0.0001).
Conclusions:
Age, lung V5 and XRCC1 rs25487 AA were all linked with risk of severe RIL. Our predictive risk model may be helpful for identifying patients at high risk of severe RIL so that treatment can be modified.
Keywords: Radiation-induced Lymphopenia, non-small cell lung cancer, XRCC1 rs25487
INTRODUCTION
Low absolute lymphocyte counts (ALCs) during therapy have been linked not only with the risk of opportunistic infections (e.g. radiation-induced pneumonia) [1,2] but also with worse survival in patients receiving radiation therapy [3–9]. Moreover, the emergence of immunotherapy has greatly improved outcomes in some patients with lung cancer and has become a treatment option in major guidelines. Lymphocytes have crucial roles in cancer immunity, and severe lymphopenia resulting from current standard chemoradiation therapy regimens could undermine the antitumor effects of checkpoint inhibitors and other immune-modulating agents [10]. Therefore, it is important to identify factors that could predict the risk of severe radiation-induced lymphopenia (RIL).
Lymphocytes are known to be the most radiosensitive of the peripheral blood cells, with an LD50 as low as 2 Gy [11]. Lymphopenia is strongly associated with radiation dose and volume of organs at risk (e.g., lung, bone marrow, spleen) [12–14]. The entire body can be considered the organ at risk for lymphopenia, because peripheral lymphocytes circulate throughout the body and exist in all tissues, and lymphoblasts in the bone marrow can be exposed to radiation as well. Hence complete avoidance of treating lymphocytes during radiation therapy is not possible. Although dosimetric variables have been linked with lymphopenia [15], thus far they have not helped to identify patients who may be sensitive to RIL, which has been linked with poor overall survival (OS) in a variety of types of cancer [38]. A better understanding of the etiopathogenesis of homeostatic failure to restore lymphocyte counts may aid in formulating new therapeutic approaches to counter RIL.
Mechanistically, resting T and B lymphocytes show significant DNA fragmentation after exposure to 1–5 Gy, suggesting that the capacity for DNA repair is important in the development of lymphopenia [16]. The gene XRCC1, located on chromosome 19q13.2–13.3, encodes the XRCC1 protein, which acts as a scaffold for other proteins in the DNA repair complex [17]. Batar and others [18] observed a significant negative correlation between XRCC1 mRNA and protein expression and DNA damage level (micronucleus frequency) in lymphocytes exposed in vitro to 2 Gy of gamma rays. The single nucleotide polymorphism Arg399Gln (G >A, rs25487) is one of the most extensively studied polymorphisms in XRCC1 [19]. Alsbeih et al. [20] found that the wild-type XRCC1 399Arg (G) allele was associated with an increased risk of developing late reactions (subcutaneous and deep tissue fibrosis) to radiotherapy. On the other hand, Chang-Claude et al. [21] reported that XRCC1 399Gln (A) alleles were associated with decreased risk of acute skin reactions after radiotherapy (hazard ratio [HR]=0.51). Moreover, Yin et al. [22] found the XRCC1 399GlnGln (A/A) genotype to be associated with a reduced risk of radiation pneumonitis (adjusted HR for A/A vs. G/G=0.48; 95% confidence interval [CI] 0.24–0.97, P=0.041). However, we did not find any reports on associations between XRCC1 399 genotype and RIL in a PubMed search.
The importance of lymphocytes, T lymphocytes in particular, in antitumor immune effects underscores the need to identify risk factors and biomarkers for lymphopenia, particularly with the advent of checkpoint-inhibitor immunotherapy for many solid tumors. Here, we sought to identify genetic-, patient-, behavior-, and treatment-related factors that could predict the risk of severe RIL in patients with non-small cell lung cancer (NSCLC), as a first step in developing interventions to prevent or mitigate this form of radiation-induced toxicity.
METHODS
Patients
Patients had been enrolled in an institutional review board–approved, prospective randomized trial that compared outcomes after intensity-modulated (photon) radiation therapy (IMRT) or passive scattering proton therapy (PSPT) for locally advanced NSCLC [23]. All patients included in this study provided written informed consent for optional blood sample collection for subsequent biomarker analyses. Eligible patients were >18 years old; had a Karnofsky performance score of ≥70; and had stage IIA to IIIB disease, stage IV disease with a single brain metastasis, or recurrent tumor after surgical resection that could be treated definitively with concurrent chemoradiation. The median total radiation dose was 74 Gy (range 60.0–78.0 Gy) given in 1.8- to 2.4-Gy fractions. For this analysis, ALC values (number of cells ×109/L) of 178 eligible patients were obtained less than 30 days before treatment and weekly during concurrent chemoradiation, from which baseline and nadir ALC values were identified.
Clinical, dosimetric, and genetic data
All patient-related data (age, sex), behavior-related data (smoking history), disease-related data (disease stage, tumor histology, tumor volume), and treatment-related data were prospectively collected per protocol. The lung V5, mean lung dose (MLD), heart V5, and mean heart dose values were extracted from the delivered plans. Genotypes were determined from lymphocytes isolated from peripheral blood samples by real-time polymerase chain reaction (real-time PCR). The primer sequences, restriction enzymes, and PCR conditions used for the experiments are available upon request.
Statistical analysis
To visualize trends in peripheral blood lymphocyte numbers during treatment, we plotted ALC values over time during therapy (Suppl. Fig. S1). The nadir ALC value was defined as the minimum cell count during treatment for each patient. Optimal cutoff values for ALC nadir, age and lung V5 were determined by the methodoloty of Contal and O’Quigley [39,40]. The cutoff value to define severe RIL was ALC 0.3×109 cells/L, which was associated with OS with the best fit of Cox proportional hazards model. To analyze the cumulative incidence of severe RIL, we recorded the first time the ALC declined below 0.3×109 cells/L during treatment for each patient. A Cox proportional hazards regression model was used for univariate and multivariate analyses to assess potential associations of patient-, genetic-, behavior-, tumor-, and treatment-related factors with severe RIL; those factors were age, sex, Karnofsky performance status score, baseline ALC, gross tumor volume (GTV, in cm3), clinical disease stage, tumor location, smoking history, use of induction chemotherapy, lung and heart V5 (in %), and mean lung dose (MLD) and mean heart dose (in Gy). The criteria for including (or excluding) factors in the final multivariate Cox regression model for severe RIL were P<0.20 for inclusion and P>0.05 for removal in stepwise manner. The risk factors were assumed with equal weight to group patients for risk of severe RIL according to the number of risk factors. Kaplan-Meier curves were generated to visualize the cumulative incidence of severe RIL by risk group. All variables were analyzed as continuous when appropriate. All statistical tests were 2-sided, and analyses were performed using the SPSS ver. 24.0 statistical software package (IBM Corp., Armonk, NY) and SAS software (version 9.4; SAS Institute, Cary, NC).
RESULTS
Table 1 lists the characteristics of the 178 patients analyzed, of whom 95 were men and 83 were women, with a median age of 66 years (range 37–85 years). Roughly one-third of patients (n=64) received induction chemotherapy, and all patients received concurrent chemoradiation. Ninety percent of patients (n=160) had a baseline ALC of >1×109 cells/L, and the other 10% (n=18) had a baseline ALC of <1×109 cells/L. The ALC nadir value appeared from 1 to 8 weeks during treatment. According to the Common Terminology Criteria for Adverse Events v 5.0, the ALC nadir was grade 2 (i.e., <0.8–0.5×109 cells/L) in 16 patients (9%), grade 3 (i.e., <0.5–0.2×109 cells/L) in 103 patients (58%), and grade 4 (i.e., <0.2×109 cells/L) in 59 patients (33%). No differences in baseline ALCs or during-treatment nadir ALCs were found according to treatment modality (IMRT vs. PSPT) (Suppl. Table S1 and Suppl. Fig. S1). The median follow-up times were 24.6 months for all patients. In terms of OS, the optimal cutoff value for ALC nadir was 0.3×109 cells/L, which was also the median ALC value. ALC values below this threshold were associated with poorer OS after adjustment for other clinical factors (P=0.001, Suppl. Table S2; P=0.002, Suppl. Fig. S2). Thus we adopted ALC <0.3×109 cells/L as the definition of severe RIL for this study.
Table 1.
Patient characteristics and univariate Cox regression analysis for lymphocyte nadir during radiotherapy
| Characteristic | All Patients (n=178) | Lymphocyte Nadir during Radiotherapy | Hazard | P Value | |
|---|---|---|---|---|---|
| >0.3×109/L (n=76) | <0.3×109/L (n=102) | Ratio (95% CI) | |||
| Age, years | |||||
| Median (range) | 66 (37–85) | 65 (37–81) | 66 (39–85) | 1.025 (1.004–1.046) | |
| 0.020 | |||||
| Mean (SD) | 64.7 (9.2) | 63.4 (9.7) | 65.8 (8.6) | ||
| Sex, no. (%) | |||||
| Female | 83 (46.6) | 34 (19.1) | 49 (27.5) | 1.00 | |
| Male | 95 (53.4) | 42 (23.6) | 53 (27.8) | 0.873 (0.592–1.2089) | |
| 0.495 | |||||
| Smoking pack-years | |||||
| Median (range) | 43 (0–244) | 37 (0–125) | 48 (0–244) | 1.003 (0.999–1.007) | |
| 0.150 | |||||
| Mean (SD) | 50.0 (39.8) | 43.9 (30.7) | 54.5 (44.9) | ||
| Tumor histology | |||||
| Squamous | 62 (34.8) | 25 (14.0) | 37 (20.8) | 1.00 | |
| Adeno | 92 (51.7) | 36 (20.2) | 56 (31.5) | 1.017 (0.671–1.541) | |
| 0.938 | |||||
| Other | 24 (13.5) | 15 (8.4) | 9 (5.1) | 0.542 (0.261–1.124) | |
| 0.100 | |||||
| GTV, cm3 | |||||
| Median (range) | 77.7 (1.9–686.6) | 55.5 (1.9–686.6) | 108.1 (5.7–673.7) | 1.001 (1.000–1.003) | |
| 0.030 | |||||
| Mean (SD) | 132.7 (136.4) | 118.8 (140.5) | 142.8 (133.1) | ||
| Disease stage, no. (%)* | |||||
| IIA-IIB | 12 (6.7) | 5 (2.8) | 7 (3.9) | 1.000 | |
| IIIA-IIIB | 152 (85.4) | 63 (35.4) | 89 (50) | 1.196 (0.553–2.584) | |
| 0.649 | |||||
| IV+recurrent | 14 (7.9) | 8 (4.5) | 6 (3.4) | 0.743 (0.249–2.214) | |
| 0.593 | |||||
| Tumor location | |||||
| LLL+RLL+RML | 53 (29.8) | 23 (12.9) | 30 (16.8) | 1.000 | |
| LUL+RUL | 116 (65.2) | 48 (27.0) | 68 (38.2) | 0.795 (0.280–2.260) | |
| 0.667 | |||||
| Mediastinum | 9 (5.1) | 5 (2.8) | 4 (2.2) | 1.052 (0.683–1.622) | |
| 0.818 | |||||
| KPS, no. (%) | |||||
| 90 | 56 (31.5) | 21 (11.8) | 35 (19.7) | 1.000 | |
| 80 | 109 (61.2) | 50 (28.1) | 59 (33.1) | 0.959 (0.444–2.072) | |
| 0.916 | |||||
| 70 | 13 (7.3) | 5 (2.8) | 8 (4.5) | 0.851 (0.406–1.873) | |
| 0.669 | |||||
| XRCC1 rs25487 | |||||
| AA | 42 (23.6) | 9 (5.1) | 33 (18.5) | 1.065 (1.089–2.500) | |
| 0.018 | |||||
| AG | 80 (44.9) | 43 (24.2) | 37 (20.8) | — | — |
| GG | 56 (31.5) | 24 (13.5) | 32 (18.0) | — | — |
| AG+GG | 136 (76.4) | 67 (37.6) | 69 (38.8) | 1.000 | |
| ALC at baseline (x109 cells/L) | |||||
| Median (range) | 1.63 (0.3–4.12) | 1.81 (0.3–3.62) | 1.48 (0.39–4.12) | 0.832 (0.624–1.109) | |
| 0.210 | |||||
| Mean (SD) | 1.77 (0.72) | 1.82 (0.69) | 1.72 (0.74) | ||
| Radiation modality | |||||
| Photons | 114 (64.0) | 47 (26.4) | 67 (37.6) | 1.000 | |
| Protons | 64 (36.0) | 29 (16.3) | 35 (19.7) | 0.792 (0.526–1.193) | |
| 0.265 | |||||
| Induction chemo, no. (%) | |||||
| No | 114 (64.0) | 50 (28.1) | 64 (36.0) | 1.000 | |
| Yes | 64 (36.0) | 26 (14.6) | 38 (21.3) | 1.128 (0.755–1.686) | |
| 0.557 | |||||
| Lung V5, % | <0.0001 | ||||
| Median (range) | 48.18 (12.3–89.4) | 43.84 (18.6–73.9) | 52.04 (12.3–89.4) | 1.031 (1.017–1.045) | |
| <0.001 | |||||
| Mean (SD) | 48.4 (14.1) | 45.3 (13.3) | 50.7 (14.3) | ||
| Mean lung dose, Gy | |||||
| Median (range) | 17.8 (1–22.8) | 16.0 (1–22.8) | 18.5 (1–22.7) | 1.073 (1.023–1.126) | |
| 0.004 | |||||
| Mean (SD) | 16.9 (4.3) | 16.3 (4.5) | 17.6 (3.8) | ||
| Heart V5, % | |||||
| Median (range) | 38.1 (0–100) | 29.5 (0–99.6) | 46 (0–100) | 1.013 (1.005–1.020) | |
| 0.001 | |||||
| Mean (SD) | 43.4 (27.6) | 37.8 (26.0) | 47.7 (28.2) | ||
| Mean heart dose, Gy | |||||
| Median (range) | 8.4 (0–37.0) | 5.8 (0–37.0) | 10.2 (0–34.6) | 1.028 (1.007–1.049) | |
| 0.009 | |||||
| Mean (SD) | 10.8 (9.0) | 9.3 (9.1) | 12.0 (8.8) | ||
Abbreviations: CI, confidence interval; SD, standard deviation; GTV, gross tumor volume; KPS, Karnofsky performance status score; LLL, left lower lobe; RLL, right lower lobe; RML, right middle lobe; LUL, left upper lobe; RUL, right upper lobe; ALC, absolute lymphocyte count.
AJCC 6th edition.
In univariate analysis, we found that older age (HR=1.025, 95% CI 1.004–1.046, P=0.020), larger GTV (HR=1.001, 95% CI 1.000–1.003, P=0.030), higher MLD (HR=1.073, 95% CI 1.023–1.126, P=0.004), larger lung V5 (HR=1.031, 95% CI 1.017–1.045, P<0.0001), higher mean heart dose (HR=1.028, 95% CI 1.007–1.049, P=0.009), larger heart V5 (HR=1.013, 95% CI 1.005–1.020, P=0.001) and the XRCC1 rs25487 AA genotype (vs. AG/GG: HR=1.065, 95% CI 1.089–2.500, P=0.018) were all associated with severe RIL (ALC <0.3×109 cells/L) during treatment (Table 1).
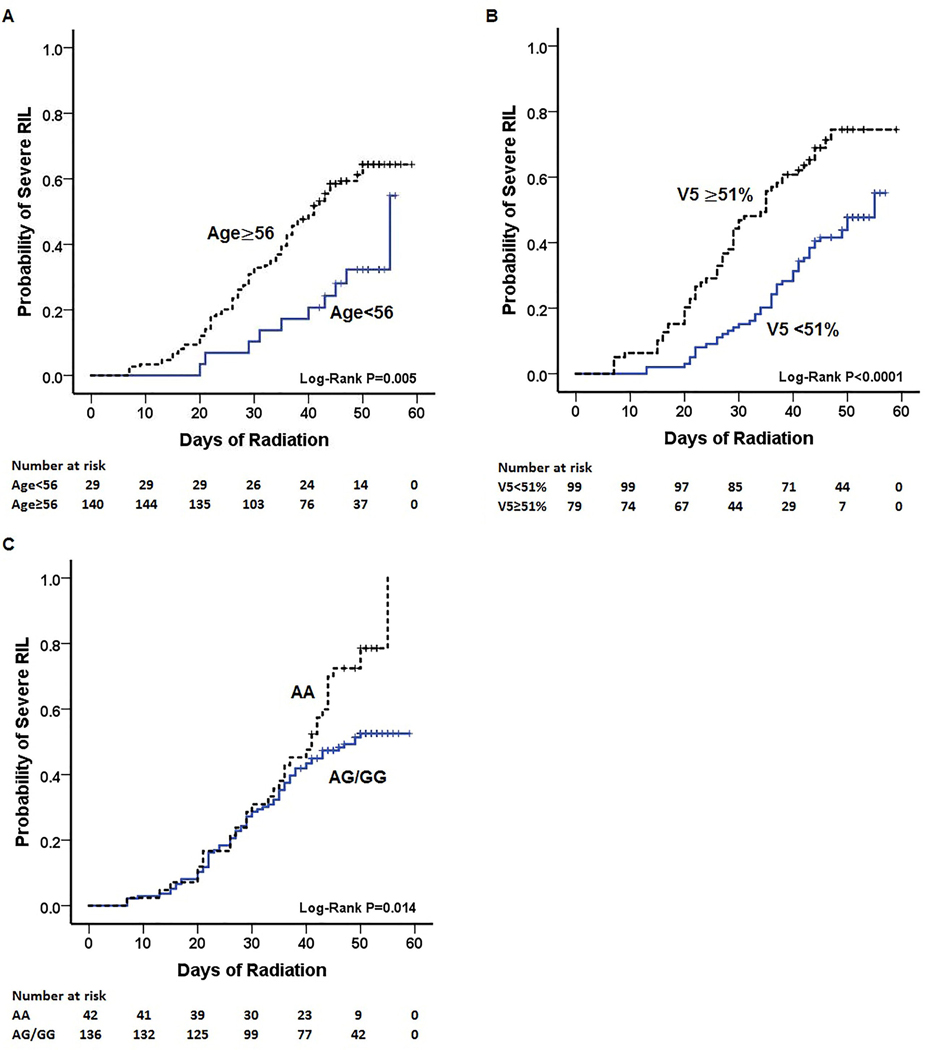
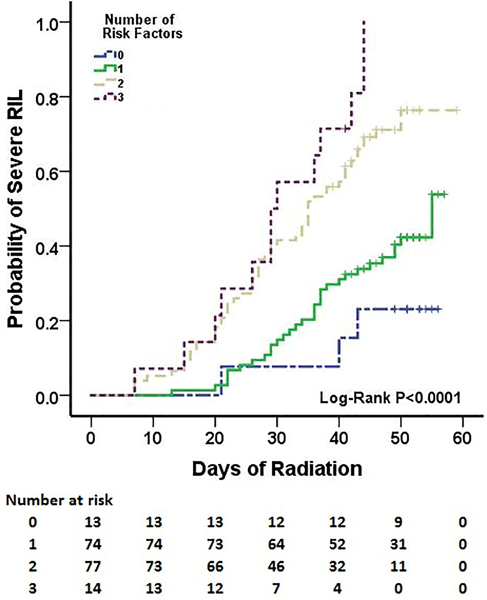
In multivariate analysis, older age (HR=1.031, 95% CI 1.009–1.054, P=0.005), higher lung V5 (HR=1.039, 95% CI 1.023–1.055, P<0.001), and the XRCC1 rs25487 AA genotype (vs. AG/GG: HR=1.768, 95% CI 1.165–2.684, P=0.007) were all independently associated with severe RIL (ALC <0.3×109/L) during treatment. Sex, smoking history, baseline ALC level, MLD, heart V5, mean heart dose, GTV, and radiation modality were not significantly associated with RIL in the multivariate analysis and thus were excluded from the final model (Table 2). Optimal cutoff values for age (56 years) and lung V5 (51%) were identified from the best fit of the Cox proportional hazards model for sever RIL. The cumulative incidence of severe RIL (ALC <0.3×109/L) during treatment stratified by age (56 years, P=0.005), lung V5 (51%, P<0.0001), and the XRCC1 rs25487 genotype (AA vs. AG/GG, P=0.014) are shown in Figure 1. Finally, we stratified patients for risk of severe RIL (ALC <0.3×109 cells/L) based on number of risk factors among the three identified in the multivariate analysis: age ≥56 years, lung V5 ≥51%, and XRCC1 rs25487 AA genotype (Table 3). Patients with 2–3 risk factors were at higher risk of severe RIL than were patients with 0–1 risk factor (HR=3.111, 95% CI 2.046–4.729, P<0.0001z). The cumulative incidence of severe RIL during treatment stratified by number of risk factors is shown in Figure 2. The cumulative incidence of severe RIL was 75% in the high-risk groups (2–3 risk factors) and 39% in low risk groups (0–1 risk factor). In other words, lung V5 needs to be kept at <51% in patients aged ≥56 years or those with XRCC1 rs25487 AA genotype to reduce the risk of severe RIL.
Table 2.
Multivariate Cox regression analysis for severe radiation-induced lymphopenia (absolute lymphocyte count <0.3×109cells/L) during treatment
| Characteristics | HR (95% CI) | P Value |
|---|---|---|
| Age | 1.031 (1.009–1.054) | 0.005 |
| Lung V5, % | 1.039 (1.023–1.055) | <0.0001 |
| XRCC1 rs25487 | ||
| AA vs. AG | 2.088 (1.250–3.226) | 0.004 |
| AA vs. GG | 1.499 (0.921–2.439) | 0.104 |
| AA vs. AG+GG | 1.768 (1.165–2.684) | 0.007 |
Note: The thresholds for factors to be included in the final multivariate Cox regression model were P<0.2 for inclusion and P>0.05 for removal.
Fig 1.
Cumulative incidence of severe radiation-induced lymphopenia (RIL; absolute lymphocyte count <0.3×109 cells/L) during treatment for non-small cell lung cancer according to age (A), lung V5 (B), and XRCC1 rs25487 genotype (C).
Table 3.
Cox regression analyses of cumulative incidence of severe radiation-induced lymphopenia (absolute lymphocyte count < 0.3 × 109cells/L) during treatment stratified by the number of risk factors.
| Number of Risk Factors | HR | 95% CI | P Value |
|---|---|---|---|
| 1 vs. 0 | 2.155 | 0.659–7.042 | 0.204 |
| 2 vs. 0 | 5.682 | 1.767–18.182 | 0.004 |
| 3 vs. 0 | 9.346 | 2.625–33.333 | 0.0006 |
| 2 vs. 1 | 2.632 | 1.686–4.098 | <0.0001 |
| 3 vs. 1 | 4.329 | 2.232–8.333 | <0.0001 |
| 3 vs. 2 | 1.642 | 0.984–3.012 | 0.110 |
| 0–1 vs. 2–3 | 3.111 | 2.046–4.729 | <0.0001 |
Note: Risk factors were age ≥ 56 years, Lung V5 ≥ 51%, and XRCC1 rs25487 AA genotype.
Fig. 2.
Cumulative incidence of severe radiation-induced lymphopenia (RIL; absolute lymphocyte count <0.3×109 cells/L) during treatment by number of the risk factors (age ≥56 years; lung V5 ≥51%; and XRCC1 rs25487 AA genotype). The low-risk groups had 0–1 factor, the high-risk groups had 2–3 factors.
DISCUSSION
We hypothesized that genetic variations in the DNA repair gene XRCC1 could, with other clinical and dosimetric factors, predict RIL (defined in this study as ALC <0.3×109 cells/L). Indeed, we confirmed in the present study that the XRCC1 rs25487 AA genotype was associated with severe RIL during treatment for NSCLC. The protein product of the DNA repair gene XRCC1 is crucial in DNA repair, both in base excision repair [24, 25] and non-homologous end-joining [26–28]. The functional effect, if any, of the single nucleotide polymorphism rs25487, also known as Gln399Arg, is not clear, although some studies suggest that amino acid substitutions in evolutionary conserved regions can affect protein function [29]. In one study, the (A) allele was associated with reduced repair of genetic damage from the nitrosamine NNK in cultured human lymphocytes, leading the authors to propose that the amino-acid change in the XRCC1 protein could have led to deficiencies in DNA repair [30]. These findings lead us in turn to propose that the reduced DNA repair capacity of XRCC1 rs25487 AA may explain the RIL observed in the current study, perhaps through compromises in immunity that lead to decreased inflammatory responses of normal tissues (e.g., lungs and skin) and worse OS. Specifically, the (A) allele has been linked with decreased radiation-associated toxicity in normal tissues, and correspondingly in less pneumonitis, less acute skin reactions, and less subcutaneous and deep tissue fibrosis [20–22]. A meta-analysis suggested that both the XRCC1 rs25487 AG and AA genotypes could predict poor OS among patients with lung cancer (G/A vs. G/G: HR 1.23; 95% CI 1.06–1.44; A/A vs. G/G: HR 2.03; 95% CI 1.20–3.45) [31]. However, direct evidence that XRCC1 399Gln/Arg determines lymphocyte radiosensitivity and affects radiotherapy outcomes remains limited. Therefore, the above assumptions need to be further verified through basic research.
The current study further showed increasing age to be associated with severe RIL during treatment for NSCLC. This phenomenon could be explained by the telomere theory, that is, telomeres become shorter with age (i.e., over time) [32–34] and suggests that telomeres may be a marker of cellular senescence. Age-related decreases in mean telomere restriction fragment length have been linked with chromosomal radiosensitivity and apoptotic response in breast cancer [35]. This association between radiosensitivity and telomere shortening was also observed in peripheral blood lymphocytes, which could result from defects in homologous recombination repair of double-strand breaks and telomere uncapping [36]. Therefore aging could possibly induce severe RIL via age-related telomere shortening and increased radiosensitivity.
Finally, we found that lung V5 was independently associated with lymphocyte nadir during radiotherapy. Although MLD, mean heart dose, heart V5, and GTV were linked with RIL in univariate analyses, these factors were excluded from the final multivariate model because of their correlation with lung V5. This result was consistent with findings from another study in which 711 patients with NSCLC were treated with definitive radiotherapy; lung V5 was the factor most strongly associated with lymphocyte nadir, and higher lymphocyte counts during treatment were associated with better OS and disease control [15]. In a mathematical modeling approach, Yovino et al. [37] found that the radiation dose to circulating lymphocytes in patients receiving fractionated radiation for high-grade glioma could be as high as 2.2 Gy, with 99% of circulating lymphocytes receiving at least 0.5 Gy, after a typical regimen of 30 daily treatments with 2-Gy fractions. The incidental doses received when lymphocytes are within the radiation portal during fractionated radiation therapy could be sufficient to result in lymphopenia [38]. We realize that the most accurate dosimetric variable for RIL would be the dose to the lymphocytes when lymphocytes are considered an organ at risk. However, no reliable way of calculating dose to lymphocytes has been found to date. Therefore, in the current analysis, we included dose variables for the lung and heart. (Although we acknowledge that dose to the great vessels may have added valuable information, that dose was not measured and thus that information was not available to us). Finally, we used lung V5 as a surrogate for low-dose exposure in multivariate model to clarify the effects of radiation dose and volume on RIL because it was the strongest predictor of severe RIL. Exposure of more circulating lymphocytes to low-dose irradiation associated with the use of larger radiation fields could increase lymphocyte destruction, as suggested by our findings regarding greater reductions of circulating lymphocyte numbers being associated with lung V5.
Therefore, we built a predictive model consisting of the XRCC1 rs25487 genotype, lung V5, and age for predicting the occurrence of severe lymphopenia during radiation therapy (Figure 2). Notably, the only factor that is modifiable in the proposed model is the lung V5, suggesting that every effort should be made to meet the dose constraint for lung V5, especially for older patients or those with the XRCC1 rs25487 AA genotype. We also propose that this model could be used to aid in the choice of radiation modality (protons vs. photons) that would allow the greatest reduction in lung V5.
Our study did have several limitations. First, we did not have information on lymphocyte subtypes (e.g. CD4+ and CD8+ T cells), and thus we could not evaluate associations of lymphocyte subtypes with genetic, tumor, or patient characteristics. Second, we did not perform studies to verify the mechanism of XRCC1 399Gln/Arg genotype and lymphocyte radiosensitivity. Finally, our data were obtained exclusively from one treatment center, and thus findings from the current study and the model developed should be validated with independent data sets from multicenter studies. We are planning future collaborative studies with other institutions to enroll larger numbers of patients, or perhaps patients with different diseases, to validate our findings.
In conclusion, older age, higher lung V5, and the presence of the XRCC1 rs25487 AA genotype were found to be independently associated with higher risk of severe RIL. Our risk stratification analysis further showed that the risk of severe RIL (ALC <0.3×109/L) during treatment for individual patients was increased by the number of risk factors present for that patient. With validation, our predictive model can help to guide personalized treatment for patients with NSCLC receiving definitive radiotherapy and immunotherapy.
Supplementary Material
Highlights.
Retrospective analysis of prospective clinical trial to identify factors predicting radiation-induced lymphopenia
Age, lung V5 and XRCC1 rs25487 AA genotype were all independently linked with lymphopenia during chemoradiation for locally advanced non-small cell lung cancer
Combinations of these risk factors distinguished patients at different risk for lymphopenia
ACKNOWLEDGEMENTS
The authors are extremely grateful for the expert editorial work performed by Ms. Christine Wogan.
Funding: Supported in part by National Cancer Institute Grants P01 CA021230 and P30 CA016672.
Footnotes
Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Terrones-Campos C, Ledergerber B, Vogelius IR, Specht L, Helleberg M, Lundgren J. Lymphocyte count kinetics, factors associated with the end-of-radiation-therapy lymphocyte count, and risk of infection in patients with solid malignant tumors treated with curative-intent radiation therapy. Int J Radiat Oncol Biol Phys. 2019. November 15;105(4):812–823. doi: 10.1016/j.ijrobp.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Reckzeh B, Merte H, Pflüger KH, Pfab R, Wolf M, Havemann K. Severe lymphocytopenia and interstitial pneumonia in patients treated with paclitaxel and simultaneous radiotherapy for non-small-cell lung cancer. J Clin Oncol. 1996. April;14(4):1071–6. PubMed PMID: 8648359. [DOI] [PubMed] [Google Scholar]
- 3.Mendez JS, Govindan A, Leong J, Gao F, Huang J, Campian JL. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol. 2016. April;127(2):329–35. doi: 10.1007/s1106-0015-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015. June;38(3):259–65. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015. October;13(10):1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014. December;36(12):1747–53. doi: 10.1002/hed.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang Y, Yuan BY, Chen GW, Zhao XM, Hu Y, Zhu WC, et al. Association between circulating lymphocyte populations and outcome after stereotactic body radiation therapy in patients with hepatocellular carcinoma. Front Oncol. 2019. September 10; 9:896. doi: 10.3389/fonc.2019.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho O, Chun M, Kim SW, Jung YS, Yim H. Lymphopenia as a potential predictor of ipsilateral breast tumor recurrence in early breast cancer. Anticancer Res. 2019. August;39(8):4467–4474. doi: 10.21873/anticanres.13620. [DOI] [PubMed] [Google Scholar]
- 9.Deng W, Xu C, Liu A, van Rossum PSN, Deng W, Liao Z, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol. 2019. April;133:9–15. doi: 10.1016/j.radonc.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Pike LRG, Bang A, Mahal BA, Taylor A, Krishnan M, Spektor A, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. 2019. January 1;103(1):142–151. doi: 10.1016/j.ijrobp.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res 1990;123(2):224–227. [PubMed] [Google Scholar]
- 12.Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys 2017;99(1):128–135. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Chadha AS, Liu G, Chen HC, Das P, Minsky BD, Mahmood U, et al. Does unintentional splenic radiation predict outcomes after pancreatic cancer radiation therapy? Int J Radiat Oncol Biol Phys 2017;97(2):323–332. doi: 10.1016/j.ijrobp.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 14.Cho O, Chun M, Chang SJ, Oh YT, Noh OK. Prognostic value of severe lymphopenia during pelvic concurrent chemoradiotherapy in cervical cancer. Anticancer Res 2016;36(7):3541–3547. [PubMed] [Google Scholar]
- 15.Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014;89(5):1084–1091. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol 1987;139(10):3199–3206. [PubMed] [Google Scholar]
- 17.Hoeijmakers JH. DNA repair mechanisms. Maturitas 2001;38(1):17–23. [DOI] [PubMed] [Google Scholar]
- 18.Batar B, Guven G, Eroz S, Bese NS, Guven M. Decreased DNA repair gene XRCC1 expression is associated with radiotherapy-induced acute side effects in breast cancer patients. Gene 2016;582(1):33–37. doi: 10.1016/j.gene.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Matullo G, Palli D, Peluso M, Guarrera S, Carturan S, Celentano E, et al. XRCC1, XRCC3, XPD gene polymorphisms, smoking and (32)P-DNA adducts in a sample of healthy subjects. Carcinogenesis 2001;22(9):1437–1445. [DOI] [PubMed] [Google Scholar]
- 20.Alsbeih G, Al-Harbi N, Al-Hadyan K, El-Sebaie M, Al-Rajhi N. Association between normal tissue complications after radiotherapy and polymorphic variations in TGFB1 and XRCC1 genes. Radiat Res 2010;173(4):505–511. doi: 10.1667/RR1769.1. [DOI] [PubMed] [Google Scholar]
- 21.Chang-Claude J, Popanda O, Tan XL, Kropp S, Helmbold I, von Fournier D, et al. Association between polymorphisms in the DNA repair genes, XRCC1, APE1, and XPD and acute side effects of radiotherapy in breast cancer patients. Clin Cancer Res 2005;11(13):4802–4809. [DOI] [PubMed] [Google Scholar]
- 22.Yin M, Liao Z, Liu Z, Wang LE, Gomez D, Komaki R, et al. Functional polymorphisms of base excision repair genes XRCC1 and APEX1 predict risk of radiation pneumonitis in patients with non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2011;81(3):e67–73. doi: 10.1016/j.ijrobp.2010.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao Z, Lee JJ, Komaki R, Gomez DR, O’Reilly MS, Fossella FV, et al. Bayesian adaptive randomization trial of passively scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small cell lung cancer. J Clin Oncol 2018;36(18):1813–1822. doi: 10.1200/JCO.2017.74.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sak A, Fegers I, Groneberg M, Stuschke M. Effect of separase depletion on ionizing radiation-induced cell cycle checkpoints and survival in human lung cancer cell lines. Cell Prolif 2008; 41(4):660–670. doi: 10.1111/j.1365-2184.2008.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caldecott KW. XRCC1 and DNA strand break repair. DNA Repair (Amst) 2003; 2(9):955–969. [DOI] [PubMed] [Google Scholar]
- 26.Bétermier M, Bertrand P, Lopez BS. Is non-homologous end-joining really an inherently error-prone process? PloS Genet 2014;10(1):e1004086. doi: 10.1371/journal.pgen.1004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, Javadekar SM, Pandey M, Srivastava M, Kumari R, Raghavan SC. Homology and enzymatic requirements of microhomology-dependent alternative end joining. Cell Death Dis 2015;6:e1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta A, Eckelmann B, Adhikari S, Srivastava M, Kumari R, Raghavan SC. Microhomology-mediated end joining is activated in irradiated human cells due to phosphorylation-dependent formation of the XRCC1 repair complex. Nucleic Acids Res 2017;45(5):2585–2599. doi: 10.1038/cddis.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savas S, Kim DY, Ahmad MF, Shariff M, Ozcelik H. Identifying functional genetic variants in DNA repair pathway using protein conservation analysis. Cancer Epidemiol Biomarkers Prev 2004;13(5):801–807. [PubMed] [Google Scholar]
- 30.Abdel-Rahman SZ, El-Zein RA. The 399Gln polymorphism in the DNA repair gene XRCC1 modulates the genotoxic response induced in human lymphocytes by the tobacco-specific nitrosamine NNK. Cancer Lett 2000;159(1):63–71. [DOI] [PubMed] [Google Scholar]
- 31.Cui Z, Yin Z, Li X, Wu W, Guan P, Zhou B. Association between polymorphisms in XRCC1 gene and clinical outcomes of patients with lung cancer: a meta-analysis. BMC Cancer 2012; 12:71. doi: 10.1186/1471-2407-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telomeres Olovnikov A., telomerase, and aging: origin of the theory. Exp Gerontol 1996; 31: 443–448. [DOI] [PubMed] [Google Scholar]
- 33.Olovnikov A. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 1973;41:181–190. [DOI] [PubMed] [Google Scholar]
- 34.Greider CW, Blackburn EH. The telomere terminal transferase of tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 1987; 51:887–898. [DOI] [PubMed] [Google Scholar]
- 35.Barwell J, Pangon L, Georgiou A, Docherty Z, Kesterton I, Ball J, et al. Is telomere length in peripheral blood lymphocytes correlated with cancer susceptibility or radiosensitivity? Br J Cancer 2007;97(12):1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong KK, Chang S, Weiler SR, Docherty Z, Kesterton I, Ball J, et al. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat Genet 2000;26(1):85–88 [DOI] [PubMed] [Google Scholar]
- 37.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 2013;31(2):140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018;123:42–51. doi: 10.1016/j.critrevonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Contal Cecile, O’Quigley John. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comp Stat Data Analys 1999;30(3):253–70. 10.1016/S0167-9473(98)00096-6. In this issue. [DOI] [Google Scholar]
- 40.Mandrekar JN, Mandrekar SJ, Cha SS. Cutpoint determination methods in survival analysis using SAS. Proceedings of the 28th SAS Users Group International Conference (SUGI 28) 2003. Paper 261–28. In this issue. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.