Abstract
Background
Diagnosing Cushing syndrome (CS) can be challenging. The 24-hour urine free cortisol (UFC) measurement is considered gold standard. This is a laborious test, dependent on correct urine collection. Late-night salivary cortisol is easier and is used as a screening test for CS in adults, but has not been validated for use in children.
Objective
To define liquid chromatography tandem mass spectrometry (LC-MS/MS)-based cutoff values for bedtime and morning salivary cortisol and cortisone in children, and validate the results in children with and without CS.
Methods
Bedtime and morning salivary samples were collected from 320 healthy children aged 4 to 16 years. Fifty-four patients from the children’s outpatient obesity clinic and 3 children with pituitary CS were used for validation. Steroid hormones were assayed by LC-MS/MS. Cutoff levels for bedtime salivary cortisol and cortisone were defined by the 97.5% percentile in healthy subjects.
Results
Bedtime cutoff levels for cortisol and cortisone were 2.4 and 12.0 nmol/L, respectively. Applying these cutoff levels on the verification cohort, 1 child from the obesity clinic had bedtime salivary cortisol exceeding the defined cutoff level, but normal salivary cortisone. All 3 children with pituitary CS had salivary cortisol and cortisone far above the defined bedtime cutoff levels. Healthy subjects showed a significant decrease in salivary cortisol from early morning to bedtime.
Conclusions
We propose that bedtime salivary cortisol measured by LC-MS/MS with a diagnostic threshold above 2.4 nmol/L can be applied as a screening test for CS in children. Age- and gender-specific cutoff levels are not needed.
Keywords: salivary cortisol, Cushing syndrome, screening test, children
The overall incidence of Cushing syndrome (CS) is approximately 2 to 5 new cases per million people per year, with only 5% to 10% of all cases occurring in children [1], an incidence hampered by uncertainty as etiological classifications differ. In children, CS is most often diagnosed during infancy and in children above the age of 8 years. Delayed diagnosis may occur, with the condition first being diagnosed in adulthood [2].
In healthy individuals, cortisol is subject to overall regulation of the pituitary/hypothalamus via ACTH. Cortisol has a diurnal profile, with highest levels early in the morning and low levels in the evening [3]. In CS, patients are exposed to higher circulating levels of cortisol, and the diurnal profile is often disrupted or absent [4].
The most prominent symptom in children is growth stagnation and weight gain, unlike what is seen with alimentary obesity, in which there is increase in both height and body weight [5, 6]. The symptoms of hypercortisolism are influenced by cortisol levels, duration of disease, age, and gender [7-10].
The diagnosis of CS is largely based on laboratory testing and can be challenging to perform and interpret. Measurements of s-cortisol and p-ACTH in the morning and evening for evaluation of diurnal variation, the 1-mg dexamethasone suppression test, and 24-hour urine free cortisol (UFC) are the 3 most commonly used diagnostic tests in children [11]. The first 2 tests are not validated in children because age- and gender-specific reference limits or diagnostic cutoffs are lacking. For UFC, normal limits for children exist, and this test is considered the gold standard for the diagnosis of CS both in children and adults [11, 12].
Measurement of UFC is laborious. The caretaker must be trained in proper sampling or the child hospitalized for correct sampling. Even with appropriate preparation, the risk of sampling errors is substantial. Furthermore, fluid intake and renal function may affect cortisol excretion in the urine [11]. Thus, there is a need for a simple highly sensitive and specific screening test for CS in children.
In adults, late-night salivary cortisol is commonly used as a screening test for cortisol overproduction, and UFC obtained to confirm the diagnosis [11]. Data for diagnostic sensitivity and specificity in adults indicate that late-night salivary cortisol and UFC are equivalent [13]. Based on high activity of the enzyme 11β-HSD type II in salivary glands, which convert cortisol to cortisone, 1 study has suggested that salivary cortisone could be an even better biomarker for hypercortisolism [14]. There are a few studies that report on expected normal levels of salivary cortisol in children [15-17], but none of these use highly specific analytical methods such as liquid chromatography tandem mass spectometry (LC-MS/MS).
In the present study, we have established LC-MS/MS based age- and gender-specific reference levels for bedtime salivary cortisol and cortisone to potentially pave the way for salivary cortisol and cortisone as a screening test for CS in children.
Patients and Methods
Study population and recruitment
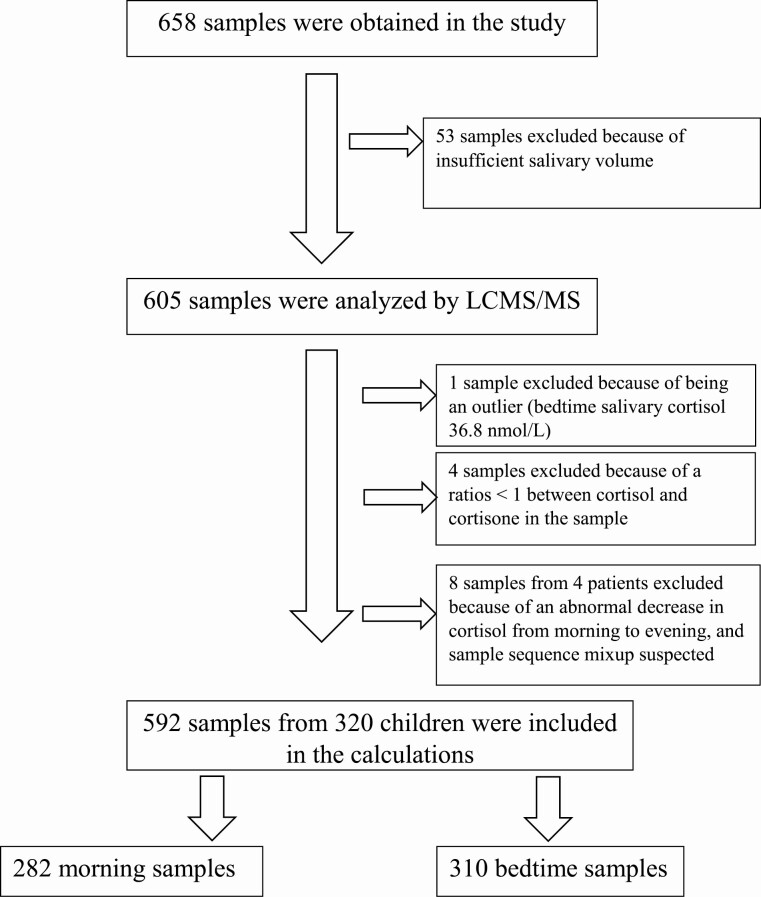
In the healthy cohort, 4- to 16-year-old children were recruited from 4 kindergartens and 4 schools in the city of Bergen, Norway. Of the 1270 children invited to participate, 320 were included in the study (Fig. 1). Children using systemic or local glucocorticoids were excluded from participation. A cohort to verify the findings (verification cohort) consisted of 54 consecutive patients aged 5 to 17 years from the Outpatient Obesity Clinic, Haukeland University Hospital, and 3 patients with recently diagnosed pituitary CS. Patients with CS had clinical features suggestive of hypercortisolism, and diagnostic tests were performed according to published pediatric and adult endocrinology protocols [18]. These included 09.00 p-ACTH, sleeping 00.00 s-cortisol, low-dose dexamethasone suppression test, corticotropin-releasing hormone testing, radiological investigations (pituitary magnetic resonance imaging scans), and bilateral synchronous inferior petrosal sinus sampling [18].
Figure 1.
Algorithm showing the inclusion and exclusion of samples obtained from healthy children.
Practical implementation
All children and their parents were given written information about the study and a consent form for the parents to sign along with sampling instructions and 2 salivary tubes for salivary samples marked morning and evening. Saliva was collected by an oral swab (Salivette, Sarstedt, Germany; blue cap specifically marked for cortisol) held in the mouth for about 2 minutes. Bedtime saliva was collected within 30 minutes of the child’s bedtime, and the morning sample immediately after awakening. Participants were instructed not to eat or brush their teeth at least 1 hour before the sampling. The tests were labelled by the parents for gender, age, date, and sampling time. The sampling had to be observed by an adult. The samples were brought along with the consent form to the school, where the project staff collected the saliva samples at specified dates. When the samples arrived at the laboratory, they were centrifuged and stored at -80°C until analysis. Patients from the obesity clinic and patients diagnosed with CS all performed 1 collection of bedtime salivary cortisol and 2 UFC collections at different days.
LC-MS/MS assay of steroids
The samples were analyzed by LC-MS/MS at the Hormone Laboratory, Department of Medical Biochemistry and Pharmacology, Haukeland University Hospital. The assay measures both endogenous and synthetic steroids. A detailed description of the method and performance metrics are provided in the data repository [19]. For the purpose of this study, corticosteroids (cortisol, cortisone, prednisolone, prednisone, dexamethasone) were analyzed. The limit of quantifications for cortisol, cortisone, prednisolone, prednisone, and dexamethasone were 45.7, 68.6, 68.6, 68.6, and 22.9 pM/L, respectively. The analytical precision (coefficient of variation %) ranged from 3.1% to 7.1%, and the accuracy ranged between 86% and 104% for all corticosteroids. Our laboratory is contributing to the external quality program SKML (Dutch Foundation for Quality Assessment in Medical Laboratories) for salivary cortisol.
Twenty-four-hour urine collection samples were analyzed using LC-MS/MS. The excreted cortisol during this period was calculated as the product of cortisol concentration and urine volume. A normal test was defined as a cortisol level below the age-specific cutoff levels given in Table 1. The analytical coefficient of variation was 10% at 140 nmol/L. The test for cortisol in urine is accredited according to ISO 15189:2012.
Table 1.
Age-specific reference levels for 24-h UFC in children, analyzed by LC-MS/MS
| Age, y | 24-h urine free cortisol, mcg/24 h | 24-h urine free cortisol, nmol/24 h |
|---|---|---|
| 3-8 | <20 | <55 |
| 9-12 | <37 | <102 |
| 13-17 | <56 | <155 |
Abbreviations: LC-MS/MS, liquid chromatography tandem mass spectometry; UFC, urine free cortisol
Quality assurance of all LC-MS/MS results was performed with the software SpecBase, which has been developed by the Hormone Laboratory, Department of Medical Biochemistry and Pharmacology, Haukeland University Hospital [19].
Statistics
Categorical data are reported as numbers (percent) and continuous data as median (range). The data were not normally distributed and therefore nonparametric statistics were applied. The Mann-Whitney U test was used to compare groups. The significance level was set to 0.05. Spearman correlation was used to evaluate the degree of correlation when appropriate. Dixon’s criteria were used to detect outliers [20]. The upper cutoff level for bedtime salivary cortisol and cortisone was defined as the 97.5th percentile (with 90% CI) in healthy children. The lower cutoff level for early morning salivary cortisol and cortisone was defined as the 2.5th percentile (with 90% CI) in healthy children. The need to divide reference data into subgroups (partitioning) was tested according to Harris and Boyd’s criteria [21].
Ethics
The study was approved by the local ethics committee (REK nr: 21745 and 2012/764). Written informed consent was obtained from a parent or legal guardian of each participant and also from participants 12 years and older.
Results
Patient characteristics
A total of 320 healthy children (174 girls) between 4 and 16 years of age were included in the study; 592 samples (310 evening samples and 282 morning samples) were included in the calculations (Tables 2 and 3). Fifty-three samples were excluded before analysis because of insufficient sample volume. Insufficient sample volume was most frequent in the youngest age group (14%, 10%, 9%, and 2% in age group 4-6 years, 7-9 years, 10-12 years, and 13-16 years, respectively).
Table 2.
Age and sex distribution of cortisol and cortisone levels for bedtime salivary samples, given in nmol/L
| N | Salivary cortisol median (range) | Salivary cortisol 97.5th percentile (90% CI) | Salivary cortisone median (range) | Salivary cortisone 97.5th percentile (90% CI) | |
|---|---|---|---|---|---|
| Total bedtime salivary samples | 310 | 0.34 (0.12-8.01) | 2.44 (1.64-3.81) | 2.88 (0.94-15.7) | 11.67 (8.99-13.42) |
| Boys | 137 | 0.35 (0.13-4.65) | 2.32 (1.12-4.57) | 2.88 (0.94-13.8) | 12.02 (8.42-13.7) |
| Girls | 173 | 0.35 (0.12-8.01) | 2.69 (1.69-6.47) | 2.91 (1.27-15.7) | 11.69 (7.59-14.4) |
| Age group 1 (4-6 y) | 40 | 0.35 (0.16-6.09) | 2.88 (1.60-15.7) | ||
| Boys | 14 | 0.34 (0.20-0.70) | 2.92 (1.60-5.55) | ||
| Girls | 26 | 0.38 (0.16-6.09) | 2.71 (1.61-15.7) | ||
| Age group 2 (7-9 y) | 76 | 0.34 (0.12-4.65) | 2.95 (1.46-4.65) | 2.94 (1.37-12.3) | 9.95 (6.8-12.3) |
| Boys | 39 | 0.32 (0.14-4.65) | 3.13 (1.65-12.3) | ||
| Girls | 37 | 0.35 (0.12-2.41) | 2.52 (1.37-9.28) | ||
| Age group 3 (10-12 y) | 103 | 0.29 (0.13-8.01) | 2.68 (0.93-8.01) | 2.63 (0.94-13.8) | 10.8 (6.16-13.8) |
| Boys | 42 | 0.30 (0.13-3.63) | 3.58 (0.82-3.63) | 2.56 (0.94-13.8) | 13.8 (5.1-13.8) |
| Girls | 61 | 0.30 (0.14-8.01) | 4.90 (0.97-8.01) | 2.79 (1.53-8.42) | 7.9 (6.01-8.42) |
| Age group 4 (13-16 y) | 91 | 0.35 (0.12-2.71) | 2.25 (1.36-2.71) | 3.34 (1.04-14.3) | 13.0 (9.89-14.3) |
| Boys | 42 | 0.31 (0.27-0.37) | 2.89 (2.41-3.71) | ||
| Girls | 49 | 0.39 (0.25-0.50) | 2.63 (1.39-2.71) | 3.34 (2.64-4.45) | 14.1 (9.83-14.3) |
When values are missing, there were too few patients in the subgroup to calculate the percentile.
Table 3.
Age and gender distribution of cortisol and cortisone levels for the early morning salivary samples given in nmol/L
| N | Salivary cortisol median (range) | Salivary cortisol 2.5th percentile (90% CI) | Salivary cortisone median (range) | Salivary cortisone 2.5th percentile (90% CI) | |
|---|---|---|---|---|---|
| Total morning salivary samples | 282 | 8.57 (0.88-52.4) | 0.49 (0.15-2.55) | 28.1 (0.36-56.1) | 4.24 (1.04-12.95) |
| Boys | 125 | 7.99 (0.11-22.66) | 1.0 (0.11-2.60) | 27.6 (0.36-51.2) | 5.06 (0.36-12.7) |
| Girls | 157 | 9.05 (0.08-52.4) | 0.45 (0.98-16.7) | 28.46 (0.4-56.1) | 3.57 (0.98-16.7) |
| Age group 1 (4-6 y) | 35 | 8.32 (5.84-10.56) | 0.14 (0.14-31.1) | 29.6 (22.8-32.5) | 0.65 (0.65-18.8) |
| Boys | 15 | 8.34 (0.144-16.6) | 0.14 (0.14-5.72) | 31.3 (0.65-37.3) | 0.65 (0.65-21.7) |
| Girls | 20 | 7.32 (2.53-28.8) | 2.54 (2.54-4.77) | 25.3 (16.9-55.1) | 16.8 (16.8-21.2) |
| Age group 2 (7-9 y) | 63 | 7.82 (3.68-45.7) | 1.68 (0.40-3.05) | 27.7 (3.68-45.7) | 9.24 (3.7-16.9) |
| Boys | 30 | 7.72 (2.5-20.9) | 2.53 (2.53-3.62) | 27.6 (12.9-45.7) | 12.9 (12.9-19.1) |
| Girls | 33 | 8.80 (0.4-17.5) | 0.40 (0.40-3.16) | 27.6 (3.6-45.5) | 3.68 (3.68-17.4) |
| Age group 3 (10-12 y) | 95 | 9.17 (0.40-56.1) | 1.16 (0.40-19.43) | 29.1 (0.40-56.1) | 6.05 (0.40-19.43) |
| Boys | 37 | 8.47 (2.6-22.4) | 2.61 (2.61-4.39) | 27.0 (12.8-38.6) | 12.8 (12.8-19.9) |
| Girls | 58 | 9.48 (0.08-24.6) | 0.13 (0.08-4.34) | 32.5 (0.40-56.1) | 0.95 (0.39-21.6) |
| Age group 4 (13-16 y) | 89 | 8.56 (0.11-5.24) | 0.45 (0.11-2.57) | 27.7 (0.36-55.2) | 2.14 (0.36-11.53) |
| Boys | 43 | 6.47 (0.11-22.7) | 0.21 (0.11-3.0) | 25.7 (0.36-51.2) | 0.83 (0.37-11.52) |
| Girls | 46 | 9.29 (0.45-52.4) | 0.45 (0.45-3.38) | 27.8 (1.47-55.2) | 1.94 (1.47-17.2) |
Fifty-four consecutive patients, 22 boys and 32 girls, attending the outpatient obesity clinic, Haukeland University Hospital, were enrolled in the study. Their median age was 13 [5-17] years (Table 4). The 3 patients with CS were 2 boys ages 15 and 16 years, respectively, and 1 girl age 16 years.
Table 4.
Characteristics of patients included from the obesity clinic and the 3 patients with CS: 24-h UFC
| Patients from obesity clinic (n = 54) | CS (n = 3) | |
|---|---|---|
| Girls, n (%) |
31 (57.4) |
1 (33.3) |
| Age, median y (range) |
13 (5-17) |
15 (15, 16) |
| BMI, median kg/m2 (range) |
33 (24-118) |
29.4 (27.4-32.1) |
| 24-h UFC nmol/24-h median (range) |
41 (8-190) |
803 (265-1286) |
| Bedtime salivary cortisol median nmol/L (range) |
0.4 (0.12-15.4) |
18.0 (6.34-21.6) |
| Bedtime salivary cortisone median nmol/L (range) |
2.6 (1.02-8.54) |
32.4 |
Abbreviations: BMI, body mass index; CS, Cushing syndrome; UFC, urine free cortisol.
Bedtime salivary cortisol and cortisone
In total, 310 bedtime salivary samples (173 from girls) were collected between 18:00 and 23:57. One outlier was identified for bedtime salivary cortisol (salivary cortisol 36.9 nmol/L), based on Dixon’s criteria, and excluded from all calculations. The overall median cortisol level was 0.34 (0.12-8.01) nmol/L (Table 2). There was no significant difference between sexes for the median bedtime salivary cortisol levels (P = 0.40), or between the age categories (P = 0.15). The 97.5th percentile for bedtime salivary cortisol in the total cohort was 2.4 (90% CI, 1.97-3.87 nmol/L). The need for further partitioning into subgroups was tested and not found necessary [21].
The median level for bedtime cortisone was 2.91 (90% CI, 0.94-15.7) nmol/L (Table 2). There were no differences between sex (P = 0.89) or age categories (P = 0.33). The 97.5th percentile for bedtime salivary cortisone was 12.0 (90% CI, 9.63-13.21) nmol/L.
The median ratio of cortisone to cortisol in bedtime salivary samples was 8.72 (90% CI, 1.61-20.34) nmol/L. According to our clinical experience, a ratio below 1 is strongly suspicious of sample contamination by hydrocortisol-containing content (eg, creams). Four samples had a ratio below 1 and were excluded (Fig. 1). There was no correlation between age and bedtime salivary cortisol or cortisone, and no correlation between the collection time of the evening samples and the cortisol or cortisone levels (not shown).
Morning salivary cortisol and cortisone
In total, 282 morning salivary samples (157 from girls) were collected between 05:27 and 09:55 AM (Table 3). No outliers were identified for morning salivary cortisol or cortisone. The overall median morning salivary cortisol level was 8.56 (90% CI, 0.75-28.8) nmol/L. Median levels for each age categories are shown in Table 3. There were no significant sex (P = 0.14), or age (P = 0.23) difference. The 2.5th percentile for early morning salivary cortisol in the whole cohort was 0.61 (90% CI, 0.19-2.55) nmol/L. Further partitioning into subgroups was tested and not found necessary [21].
The median morning salivary cortisone was 28.1 (90% CI, 0.36-56.1) nmol/L, for girls 28.5 (90% CI, 0.4-56.1) nmol/L, and 27.6 (90% CI, 0.36-51.2) nmol/L for boys (Table 3). Girls had significantly higher morning salivary cortisone than boys (P = 0.01), but there were no significant differences between age groups (P = 0.23). The 2.5th percentile for morning salivary cortisone was 4.24 (90% CI, 1.04-13.0) nmol/L for girls and boys combined, 3.70 (90% CI, 1.47-16.7) nmol/L for girls, and 5.06 (90% CI, 0.46-12.6) nmol/L for boys. Partitioning into sex-specific cutoff levels was not warranted according to the Harris and Boyd criteria [21].
The median ratio of cortisone to cortisol in early morning salivary samples was 3.46 (90% CI, 1.52-11.3) nmol/L. There was no correlation between age and early morning salivary cortisol or cortisone, or between the collection time of the morning samples and the cortisol or cortisone levels (not shown).
Diurnal variation from morning to evening
The median decrease in salivary cortisol from early morning to late evening was 8.20 (90% CI, 0.10-25.7) nmol/L, representing a 95.5% (90% CI, 22.8-99.2) decrease. The 2.5th percentile for the percent decrease in salivary cortisol was 74% (90% CI, 63-80). The median decrease in salivary cortisone from early morning to late evening was 25.5 (90% CI, 5.41-53.0) nmol/L, representing an 89.7% (90% CI, 32.7-99.9) decrease. The 2.5th percentile for the percent decrease in salivary cortisone was 59.7% (90% CI, 55-66). Four patients had an increase in the salivary cortisol and cortisone from morning to evening. The 8 samples from these 4 patients were excluded from the calculations because the increase suggested that the reported sample times were mixed up.
Presence of exogenous steroids in the salivary samples
No evidence was found for the presence of the synthetic steroids prednisone, prednisolone, and dexamethasone in the salivary samples.
Verification cohort from the outpatient obesity clinic and patients with CS
The median bedtime salivary cortisol level in the group of patients from the obesity clinic was 0.4 (90% CI, 0.12-15.4) nmol/L, and for cortisone 2.6 (90% CI, 1.02-8.54) nmol/L. One patient had a bedtime salivary cortisol above the cutoff level as defined by our study (15 nmol/L), but had a normal salivary cortisone concentration in the same sample. None of these patients had a bedtime salivary cortisone above the cutoff level defined in this study.
The patients with obesity had a median UFC level of 41 (90% CI, 8-190) nmol/24 hours. One child had an excretion of urinary cortisol above the upper cutoff level for the test (Table 1). The 3 patients diagnosed with CS showed clearly elevated UFC at 1093, 265, and 1286 nmol/24 hours, respectively. Their bedtime salivary cortisol levels were 6.34, 21.6, and 18.0 nmol/L, respectively, with all 3 clearly elevated.
Discussion
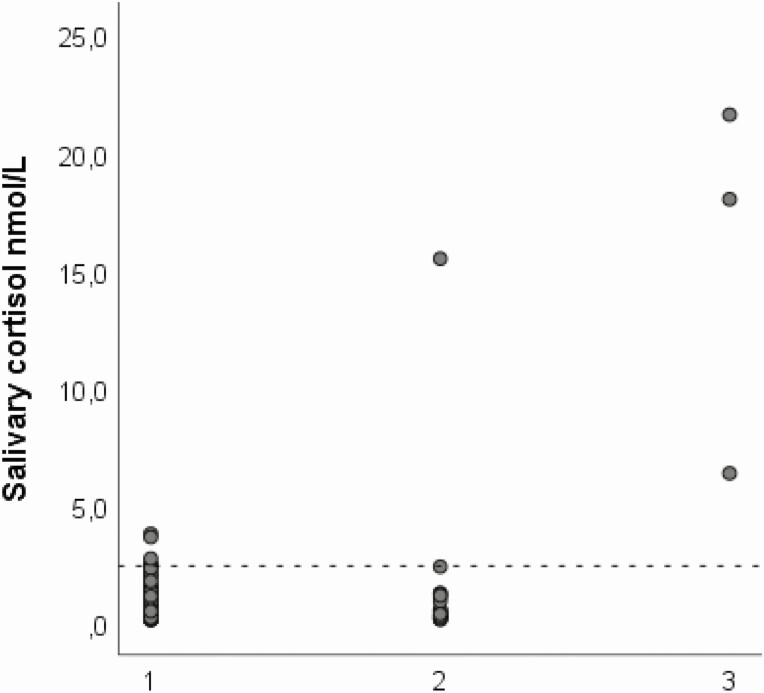
The use of salivary samples to diagnose abnormal cortisol levels is attractive because it is easy to perform, and glucocorticoids in saliva are thought to reflect the free and biological active levels [13, 22]. Late-night salivary cortisol measurement is established as a valid screening test for CS in adults [11]. We present here LC-MS/MS-based cutoff levels for bedtime and morning salivary cortisol and cortisone in a large cohort of 4- to 16-year-old children. The cutoff level for bedtime salivary cortisol was 2.4 nmol/L, slightly lower than that of adults using the same method (2.8 nmol/L) [23]. The cutoff level was validated in an independently collected cohort of children with obesity, and in - children with verified CS. The patients with overt CS had salivary cortisol and cortisone far above the defined cutoff values (Fig. 2).
Figure 2.
Dot plot showing the distribution of bedtime salivary cortisol (nmol/L) for (1) healthy children, (2) children from the validation cohort, and (3) patients with Cushing syndrome. Dotted line indicating the cutoff level for bedtime salivary cortisol found in this study.
Our data support that age and gender-specific cutoff levels are not required for bedtime salivary cortisol or cortisone. We also found no association between the cortisol levels and the time point for collection of the evening sample, which underpins that the sample could be pragmatically collected at bedtime without the need for a strict fixed sampling time point.
The screening for CS in children is a major clinical challenge. It involves collection of at least 2 UFC, which is time consuming and vulnerable to sampling errors. Collection of salivary is considerably easier, and it could reduce the frequency of delayed diagnosis by enabling wider screening by salivary cortisol measurements. Delayed diagnosis is a recognized problem [24], and it is suspected that several children with CS actually have had this condition from childhood, but remain undiagnosed until adulthood. In perspective of the recent appreciation of low-grade cortisol overproduction as a condition that predispose for metabolic complications, an easy screening test is certainly desirable. Whether such mild autonomous cortisol production occurs in children is currently unknown, and salivary corticosteroid measurements have the potential to advance our understanding in this field.
The lack of decrease in serum cortisol from early morning to late evening is a diagnostic marker for CS in children. International literature has considered a night-time nadir of less than 50% of the morning concentration as normal [25]. Norwegian pediatric guidelines for diagnostics of CS consider a decrease of more than 75% from morning to evening as adequate in children older than 2 years of age [26]. Others have considered a night-time nadir of less than 50% of the morning concentration as normal [25]. A smaller drop could be abnormal. In saliva, we found a similar biorhythm with median salivary cortisol and cortisone levels decreasing 95.6% and 89.7%, respectively. The 2.5th percentile for corresponding reduction in salivary cortisol was 74%. Hence, a decrease of salivary cortisol of less 75% from morning to evening suggests a disturbed cortisol biorhythm that should be investigated further.
The data from the validation cohort support the cutoff levels identified. This cohort comprised 54 children with obesity, representing a group in which CS is sometimes suspected. All but 1 child had bedtime salivary cortisol below 2.4 nmol/L, indicating that this cutoff correctly classifies healthy children and those with CS. The patients with pituitary CS also had UFC far above the upper cutoff level. We acknowledge that the small number of patients with CS included in this study is a major limitation. Although this precludes estimation of the test’s diagnostic sensitivity, these initial results indeed show promising ability to discriminate healthy and disease—analogous to adults. One likely reason for the lack of validated tests is that CS is a very rare condition in children, and recruiting a sufficiently sized cohort would require a coordinated network of collaborating diagnostic centers that agree on standardized tests. In this study, we reached out to both national and international centers. Although few CS patients were included, we argue that the well-characterized salivary corticoid levels in healthy children contribute significantly to the current knowledge and is an important step toward improved diagnostic methods for CS in children.
Our data do not indicate that salivary cortisone has greater diagnostic value than salivary cortisol, as suggested by Perogramvos et al [14]. Rather, both can be used interchangeably. This fact, and also that girls showed significantly higher morning salivary cortisone than boys in our study, makes us speculate about whether there exists age and/or gender dependency of 11β-HSD type II activity in salivary glands. We have not been able to find support for that in the literature.
The ability of the highly sensitive LC-MS/MS method to detect even very low-level traces of exogenous glucocorticoids is very useful to identify false tests in patients exposed to local or systemic drugs. Hydrocortisone-contaminated samples can easily be identified because the cortisol levels are very high relative to cortisone. Measuring the cortisone/cortisol ratio enables the detection of sample contamination with various skin creams containing hydrocortisone. Such creams are frequently used, and hydrocortisone may easily be transferred onto the swabs used to collect saliva. In our study, 1 child in the validation cohort had elevated salivary cortisol but normal salivary cortisone, indicating contamination with hydrocortisone. Her UFC was normal. As such, the measurement of multiple glucocorticoids by LC-MS/MS serves to increase the robustness of our data and is an important strength of this study.
Samples with insufficient salivary volume were most frequently found, as expected, in the youngest age group. Still, 86% of samples from children aged 4 to 6 years were successful, indicating that it is a useful method also for the youngest children. One child had both UFC measurements above the upper reference limit for the assay. She had normal bedtime salivary cortisol and cortisone; subsequent clinical and biochemical reevaluation excluded CS.
In conclusion, bedtime salivary cortisol levels above 2.4 nmol/L in children are abnormal and should raise suspicion of CS. This finding is an important advance in the diagnostics of CS in children that lack validated screening tests. Further studies are needed to accurately assess the diagnostic performance for salivary cortisol, and we call for international collaboration to collect data in this rare disease.
Acknowledgments
The authors thank Dr. Nora Alicia Guldhaug for assistance with calculating the urine-free cortisol levels.
Financial Support: This research was financed by The Hormone Laboratory, Department of Medical Biochemistry and Pharmacology, Haukeland University Hospital, Bergen, Norway.
Glossary
Abbreviations
- CS
Cushing syndrome
- LC-MS/MS
liquid chromatography tandem mass spectometry
- UFC
urine free cortisol
Additional Information
Disclosures: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Stratakis CA. Cushing syndrome in pediatrics. Endocrinol Metab Clin North Am. 2012;41(4):793-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan LF, Storr HL, Grossman AB, Savage MO. Pediatric Cushing’s syndrome: clinical features, diagnosis, and treatment. Arq Bras Endocrinol Metabol. 2007;51(8):1261-1271. [DOI] [PubMed] [Google Scholar]
- 3. Melmed S, Polonsky KS, Reed Larsen P, et al. Williams textbook of endocrinology. 10 ed. Amsterdam: Elsevier; 2003:451-551. [Google Scholar]
- 4. Tourniaire J, Chalendar D, Rebattu B, et al. The 24-h cortisol secretory pattern in Cushing’s syndrome. Acta Endocrinol (Copenh). 1986;112(2):230-237. [DOI] [PubMed] [Google Scholar]
- 5. Lodish MB, Gourgari E, Sinaii N, et al. Skeletal maturation in children with Cushing syndrome is not consistently delayed: the role of corticotropin, obesity, and steroid hormones, and the effect of surgical cure. J Pediatr. 2014;164(4):801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magiakou MA, Mastorakos G, Oldfield EH, et al. Cushing’s syndrome in children and adolescents. Presentation, diagnosis, and therapy. N Engl J Med. 1994;331(10):629-636. [DOI] [PubMed] [Google Scholar]
- 7. Afshari A, Ardeshirpour Y, Lodish MB, et al. Facial plethora: modern technology for quantifying an ancient clinical sign and its use in cushing syndrome. J Clin Endocrinol Metab. 2015;100(10):3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keil MF, Graf J, Gokarn N, Stratakis CA. Anthropometric measures and fasting insulin levels in children before and after cure of Cushing syndrome. Clin Nutr. 2012;31(3):359-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lodish MB, Hsiao HP, Serbis A, et al. Effects of Cushing disease on bone mineral density in a pediatric population. J Pediatr. 2010;156(6):1001-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stratakis CA, Mastorakos G, Mitsiades NS, Mitsiades CS, Chrousos GP. Skin manifestations of Cushing disease in children and adolescents before and after the resolution of hypercortisolemia. Pediatr Dermatol. 1998;15(4):253-258. [DOI] [PubMed] [Google Scholar]
- 11. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lodish MB, Keil MF, Stratakis CA. Cushing’s syndrome in pediatrics: an update. Endocrinol Metab Clin North Am. 2018;47(2):451-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manetti L, Rossi G, Grasso L, et al. Usefulness of salivary cortisol in the diagnosis of hypercortisolism: comparison with serum and urinary cortisol. Eur J Endocrinol. 2013;168(3):315-321. [DOI] [PubMed] [Google Scholar]
- 14. Perogamvros I, Owen LJ, Keevil BG, Brabant G, Trainer PJ. Measurement of salivary cortisol with liquid chromatography-tandem mass spectrometry in patients undergoing dynamic endocrine testing. Clin Endocrinol (Oxf). 2010;72(1):17-21. [DOI] [PubMed] [Google Scholar]
- 15. Törnhage CJ. Reference values for morning salivary cortisol concentrations in healthy school-aged children. J Pediatr Endocrinol Metab. 2002;15(2):197-204. [DOI] [PubMed] [Google Scholar]
- 16. Gröschl M, Rauh M, Dörr HG. Circadian rhythm of salivary cortisol, 17alpha-hydroxyprogesterone, and progesterone in healthy children. Clin Chem. 2003;49(10):1688-1691. [DOI] [PubMed] [Google Scholar]
- 17. Kiess W, Meidert A, Dressendörfer RA, et al. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37(4 Pt 1):502-506. [DOI] [PubMed] [Google Scholar]
- 18. Storr HL, Chan LF, Grossman AB, Savage MO. Paediatric Cushing’s syndrome: epidemiology, investigation and therapeutic advances. Trends Endocrinol Metab. 2007;18(4):167-174. [DOI] [PubMed] [Google Scholar]
- 19. Åstrøm UG. Supplement: Late night saliva cortisol as a screening test for Cuhsing’s syndrome in children [Supplemental file]. ProMED-mail website. https://zenodo.org/record/4399427#.YC-VKTKg9aR. Accessed December 29, 2020.
- 20. Reed AH, Henry RJ, Mason WB. Influence of statistical method used on the resulting estimate of normal range. Clin Chem. 1971;17(4):275-284. [PubMed] [Google Scholar]
- 21. Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference ranges. Clin Chem. 1990;36(2):265-270. [PubMed] [Google Scholar]
- 22. Perogamvros I, Owen LJ, Newell-Price J, Ray DW, Trainer PJ, Keevil BG. Simultaneous measurement of cortisol and cortisone in human saliva using liquid chromatography-tandem mass spectrometry: application in basal and stimulated conditions. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(29):3771-3775. [DOI] [PubMed] [Google Scholar]
- 23. Baid SK, Sinaii N, Wade M, Rubino D, Nieman LK. Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: a comparison of assays to establish hypercortisolism. J Clin Endocrinol Metab. 2007;92(8):3102-3107. [DOI] [PubMed] [Google Scholar]
- 24. Rubinstein G, Osswald A, Hoster E, et al. Time to diagnosis in Cushing’s syndrome: a meta-analysis based on 5367 patients. J Clin Endocrinol Metab. 2020;105(3):1-11. [DOI] [PubMed] [Google Scholar]
- 25. Wallace WH, Crowne EC, Shalet SM, et al. Episodic ACTH and cortisol secretion in normal children. Clin Endocrinol (Oxf). 1991;34(3):215-221. [DOI] [PubMed] [Google Scholar]
- 26. Robert Bjerknes OBK, Njølstad PR, Bland JD, Knudtzon J. Utredning ved mistanke om Cushing syndrom. Pediatrisk Endokrinologi 1998;12:67-78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.