Abstract
Background
Group B streptococci (GBS) are β-hemolytic, Gram-positive bacteria associated with fetal injury, preterm birth, spontaneous abortion, and neonatal infections. A key factor promoting GBS virulence is the β-hemolysin/cytolysin, a pigmented ornithine rhamnolipid (also known as granadaene) associated with the bacterial surface.
Methods
A previous study indicated that GBS produce small structures known as membrane vesicles (MVs), which contain virulence-associated proteins. In this study, we show that GBS MVs are pigmented and hemolytic, indicating that granadaene is functionally active in MVs.
Results
In addition, MVs from hyperhemolytic GBS induced greater cell death of neutrophils, T cells, and B cells compared with MVs from isogenic nonhemolytic GBS, implicating MVs as a potential mechanism for granadaene-mediated virulence. Finally, hemolytic MVs reduced oxidative killing of GBS and aggravated morbidity and mortality of neonatal mice infected with GBS.
Conclusions
These studies, taken together, reveal a novel mechanism by which GBS deploy a crucial virulence factor to promote bacterial dissemination and pathogenesis.
Keywords: granadaene, group B streptococcus, hemolysin, immune evasion, membrane vesicles
The group B streptococcus hemolytic pigment, granadaene, is released in membrane vesicles, which prevent oxidative killing and promote in vivo infection.
Annually, at least 4 million preterm births or stillbirths and over 300 000 neonatal infections are attributable to group B streptococcus ([GBS] or Streptococcus agalactiae), a β-hemolytic, Gram-positive bacterium that commonly colonizes the female lower genital tract [1, 2]. Group B streptococcus is typically transmitted to the fetus via ascending infection, in which the bacteria traffic from the lower genital tract into the amniotic cavity, greatly increasing the risk of preterm birth, fetal injury, and stillbirth. In addition, neonates can acquire GBS through the aspiration of infected vaginal fluids during birth, leading to severe infections including pneumonia, meningitis, or sepsis. A major determinant promoting invasive GBS infection is the β-hemolysin/cytolysin, which is a pigmented ornithine rhamnolipid [3] also known as granadaene [4]. Several studies have shown that granadaene facilitates GBS dissemination by weakening host barriers at the maternal-fetal interface [3, 5], lung [6–8], and brain [9, 10]. Furthermore, the hemolytic pigment is cytotoxic to several host immune cells, including macrophages [11], neutrophils [5], mast cells [12], T cells, and B cells [13].
Hemolytic activity of GBS is associated with the bacterial cell surface, and previous studies have shown that direct contact between GBS and red blood cells (RBCs) is required for hemolysis [14] and that GBS membrane fragments are pigmented [15]. Beyond this, little experimental evidence exists on whether or how granadaene may be released from the bacterial cell. A recent study demonstrated that GBS produce membrane vesicles (MVs), which are small spherical buds originating from the bacterial cell membrane [16]. Group B streptococcus MVs were found to contain several GBS surface-associated virulence proteins, including hyaluronidase and metalloproteinases, and intra-amniotic injection caused weakening of choriodecidual membranes and fetal injury in mice [16]. In other pathogens, MVs have been shown to act as vehicles for toxins and effector molecules, delivering these factors to host cell targets [2, 17, 18]. We hypothesized that granadaene via MVs may exacerbate GBS infection. Our studies indicate that MVs isolated from hyperhemolytic (HH) GBS are pigmented, hemolytic, and cytotoxic to several host cells. Furthermore, we show that hemolytic MVs aggravate morbidity and mortality in neonatal mice infected with nonhemolytic (NH) GBS. These findings, taken together, reveal a novel mechanism of granadaene delivery during GBS infection and further elucidate the function of this key virulence factor.
MATERIALS AND METHODS
Ethics Statement
Written informed patient consent for donation of human blood was obtained with approval from the Seattle Children’s Research Institute Institutional Review Board (protocol no. 11117) per the Principles in the WMA Declaration of Helsinki and Department of Health and Human Services Belmont Report. Children under the age of 18 were not recruited for blood donation.
All animal experiments were approved by the Seattle Children’s Research Institutional Animal Care and Use Committee (protocol IACUC00036) and performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Eighth Edition).
Bacterial Strains
Group B streptococcus ΔcovR and GBSΔcovRΔcylE were derived from the wild-type strain (WT) A909, as previously described [9, 19]. A909 is a clinical isolate obtained from a human neonate and is classified as serotype Ia [20]. All GBS liquid cultures were grown in tryptic soy broth ([TSB] Difco Laboratories) at 37°C, 5% CO2. Lactococcus lactis pcylX-K and L lactis pEmpty generated previously [21] were grown in TSB at 37°C, 5% CO2 with 5 μg/mL chloramphenicol (Sigma-Aldrich).
Isolation of Membrane Vesicles
Membrane vesicles MVs were isolated from WT and mutant strains of GBS using methods previously described [16], with slight modifications. Cultures (15 mL) of WT GBS, GBSΔcovR, GBSΔcovRΔcylE, L lactis pcylX-K, and L lactis pEmpty were grown to OD600 nm of 1. Bacterial cultures were centrifuged (2000 ×g) for 30 minutes at 4°C. Supernatants were collected and passed through a 0.22-µm syringe-driven filter (EMD Millipore) to remove residual bacterial cells. Then, the filtrate was added to a 10-kDa Amicon Ultra-15 filter device (EMD Millipore), which was centrifuged at 4000 ×g for 15 minutes. The concentrated solute was recovered, and the MVs were pelleted by ultracentrifugation (150 000 ×g for 3 hours at 4°C). The supernatant was removed without disturbing the pellet. The pellets containing MVs were resuspended in sterile phosphate-buffered saline (PBS) and were normalized among all strains to 5 mg/mL. For detailed methods regarding scanning electron microscopy of MVs and bacteria, see Supplementary Materials.
Hemolysis Assay
Membrane vesicle resuspension (10 µL) was sonicated (10 minutes) and then spotted on a blood agar plate (Remel) and allowed to dry for approximately 10 minutes. The plate was incubated at 37°C in 5% CO2 overnight and then examined for a zone of hemolysis. The activity of proteinase K (PK) was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) before hemolysis assays (see Supplementary Materials). To test the effect of PK on MV hemolysis, 13.5 µL sonicated MV resuspension was mixed with 1.5 µL PK (2.5 mg/mL, [final] = 0.25 mg/mL) or PBS and incubated for 1 hour. Then, 10 µL was spotted and allowed to dry for 10 minutes. The plate was incubated at 37°C in 5% CO2 overnight then analyzed for hemolysis. Plates were placed on a light box, and photographs were captured with the digital SLR camera and processed using Photoshop CC (Adobe).
To quantify hemolytic activity of MVs, a hemolytic assay was performed as described previously with purified pigment/control extracts [3]. In brief, human RBCs in PBS were coincubated with 10 µL sonicated MV resuspensions (5 mg/mL) from GBSΔcovR (or NH control MVs) in the presence of PK (0.25 mg/mL) or PBS for 1 hour at 37°C. Hemoglobin release in cell supernatants was measured, and percentage hemolysis was determined relative to Triton X-100 (0.1%)-treated positive controls and PBS-treated negative controls.
Cytotoxicity Assay
Primary human neutrophils, CD4+ T cells, CD8+ T cells, and B cells were isolated as described previously [5, 13]. Neutrophils were seeded into 96-well plates at 2.5 × 105 cells/well in 90 µL Roswell Park Memorial Institute (RPMI)-G medium, and T and B cells were seeded at 2.5 × 106 cells/well in 90 µL RPMI-G medium. Membrane vesicles (10 µL, 5 mg/mL) isolated from GBSΔcovR and GBSΔcovRΔcylE were added to seeded cells and allowed to incubate at 37°C (neutrophils = 3 hours, T cells and B cells = 1 hour). As positive and negative controls, neutrophils were incubated in 0.1% Triton X-100 (Sigma-Aldrich) or sterile PBS, respectively. Cells were analyzed for cytotoxicity by the presence of cytoplasmic lactate dehydrogenase (LDH) in cell supernatants using the colorimetric LDH kit (Clontech), per the manufacturer’s instructions. Percentage cytotoxicity was calculated by normalizing to PBS-treated cells (0% cell death) and Triton X-100-treated cells (100% cell death), as described previously [3, 5, 11].
Oxidative Killing Assay
Membrane vesicles isolated from GBSΔcovR or GBSΔcovRΔcylE were resuspended in sterile PBS at 5 mg/mL, and 1.23 mg of each MV type was coincubated with 0.06% H2O2 (Sigma-Aldrich) for 45 minutes while rocking (500 μL total volume). Phosphate-buffered saline (no MVs) + 0.06% H2O2 and PBS-only (no MVs, no H2O2) conditions were included as positive and negative controls, respectively. Meanwhile, GBSΔcovRΔcylE overnight cultures were subcultured in TSB, grown to mid-log phase (OD600 0.3), washed twice in sterile PBS, and normalized to approximately 2 × 108 colony-forming units (CFU)/mL in PBS. Then, approximately 1 × 108 CFU (500 μL) was added to the preincubated MVs (and controls), bringing the final H2O2 concentration to 0.03%, as previously described for oxidative killing assays with GBS [22]. The mixtures incubated at 37°C, 5% CO2 for 1 hour, and then 1000 units of catalase (from bovine liver; Sigma-Aldrich) were added to each reaction condition to quench remaining H2O2 (as previously described [22]), and surviving CFU were enumerated by dilution plating onto TSA.
Mouse Model
C57BL/6J mice between 12 and 36 hours of age were pooled and randomly assigned to dams. Each group of neonates was then designated to an experimental group (HH MVs + NH GBS, PBS + NH GBS, HH MVs + PBS, NH MVs + NH GBS, or PBS + PBS). According to the assigned experimental group, neonates were injected (intraperitoneal [I.P.]) with 50 µL HH MVs (5 mg/mL from GBSΔcovR) or PBS and 10 µL NH GBS (GBSΔcovRΔcylE, 108 CFU/mL) or PBS and returned to their assigned dam. For the survival study, mice were monitored twice daily for 7 days for signs of morbidity and mortality. Moribund neonates were euthanized. Sample sizes for the survival study are as follows: n = 6 HH GBS MVs + NH GBS; n = 6 PBS + NH GBS; n = 5 HH GBS MVs + PBS; n = 9 NH MVs + NH GBS; n = 7 PBS + PBS. Randomly selected neonates from each experimental group (n = 1 NH GBS MVs + NH GBS; n = 1 PBS + NH GBS; n = 4 HH GBS MVs + NH GBS; n = 2 HH GBS MVs + PBS; and n = 1 PBS + PBS) were euthanized via decapitation at 24 hours postinoculation, and lungs were analyzed by hematoxylin and eosin (H&E) staining. For detailed methods of tissue preparation, H&E staining, and analysis, see Supplementary Materials. In addition, mice from the HH GBS MVs + NH GBS (n = 6) and NH GBS MVs + NH GBS (n = 7) groups were euthanized by decapitation 24 hours after inoculation, and lungs were processed into single-cell suspension and analyzed by flow cytometry. For detailed methods on cell preparation, antibody staining, analysis, and gating, see Supplementary Materials.
Statistical Analysis
A P < .05 was considered significant. Unless otherwise noted, an unpaired t test or one-way analysis of variance with Tukey’s posttest was used to compare groups in in vitro assays. Survival data was plotted on a Kaplan-Meier curve and analyzed using the log-rank test. GraphPad Prism (version 7.03) was used for all statistical tests.
RESULTS
Group B Streptococci Hemolytic Pigment, Granadaene, Is Released in Membrane Vesicles
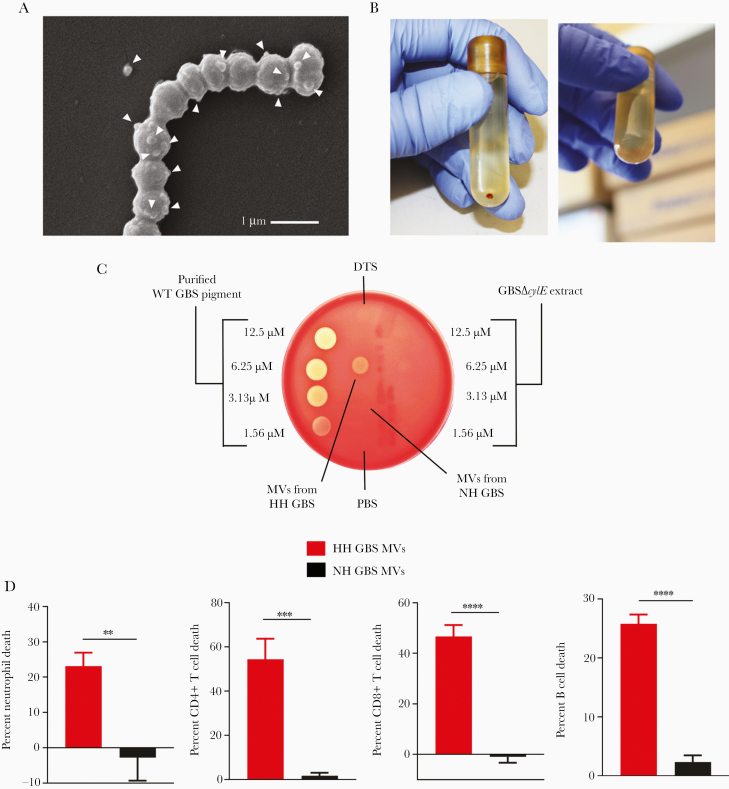
To test the hypothesis that GBS hemolytic pigment associates with MVs, we isolated MVs from GBS strains that overexpress the hemolytic pigment, namely, GBSA909ΔcovR (lacks the hemolysin repressor CovR/S) and its isogenic NH strain GBSΔcovRΔcylE. It is notable that HH GBS strains, including those containing mutations in the covR/S 2 component system exhibit increased virulence [3, 9, 23] and have been isolated from women in preterm labor [3] as well as from patients with other manifestations of GBS infections [23–26]. Using scanning electron microscopy, we confirmed that these GBS strains (ΔcovR and ΔcovRΔcylE) produce MVs; these are seen as spherical structures proximal to and arising from GBS (Figure 1A, Supplementary Figure 1A and 1B), similar to those previously identified as MVs [16]. Ultracentrifugation of MVs from GBSA909ΔcovR yielded a small pellet that was red/orange in color, unlike MVs from GBSΔcovRΔcylE, indicating the presence of pigment in the MVs (Figure 1B, Supplementary Figure 1A). Membrane vesicle pellets were resuspended in PBS and then analyzed for hemolysis by spotting on red blood agar. We found that MVs from HH GBS were indeed hemolytic, whereas MVs from NH GBS were not (Figure 1C). Of note, significant hemolysis was not observed in MVs isolated from mildly hemolytic WT GBS (Supplementary Figure 2).
Figure 1.
Membrane vesicles (MVs) isolated from hemolytic group B streptococci (GBS) are hemolytic and cytolytic. (A) Hyperhemolytic (HH) GBS (GBSΔcovR) were centrifuged, fixed, and analyzed by scanning electron microscopy. Arrowheads indicate MVs, which are seen as spherical structures emerging from the surface of bacterial cells. (B) Pelleted MVs from HH GBS (GBSΔcovR) or nonhemolytic (NH) GBS (GBSΔcovRΔcylE) are shown. (C) Membrane vesicles from HH GBS (GBSΔcovR) or NH GBS (GBSΔcovRΔcylE) were resuspended in phosphate-buffered saline (PBS), sonicated, and 10 µL was spotted onto red blood agar. Purified GBS pigment and equivalent amount of GBSΔcylE extract in DTS at various dilutions were spotted (10 µL) for comparison. (D) Primary human neutrophils, CD4+ T cells, CD8+ T cells, or B cells were incubated with MVs from HH GBS or NH GBS (final concentration = 0.5 mg/mL), and cell death was measured by LDH release into the supernatant relative to Triton X-100 (0.1%)- and PBS-treated controls. Mean and standard error of the mean are shown from 3 experiments performed in technical triplicate. Groups were compared with unpaired, 2-tailed Student’s t test. Neutrophils, P = .0042; CD4+ T cells, P = .0005; CD8+ T cells, P < .0001; B cells, P < .0001. **, P < .01; ***, P < .001; ****, P < .0001.
We then examined whether MVs from HH GBS are cytolytic to host immune cells similar to live bacteria and purified granadaene. Accordingly, MVs from HH GBSΔcovR (HH GBS MVs) or NH GBSΔcovRΔcylE (NH GBS MVs) were coincubated with primary human neutrophils, CD4+ T cells, CD8+ T cells, or B cells, and cell death was measured by LDH release in cell supernatants. We found that MVs from HH GBS caused significantly greater cell death in all cell types compared with MVs from isogenic, NH GBS (Figure 1D). These data, taken together, demonstrate for the first time that GBS pigment is released from the bacterial cell surface with MVs, which induce hemolysis and cytolysis to host cells.
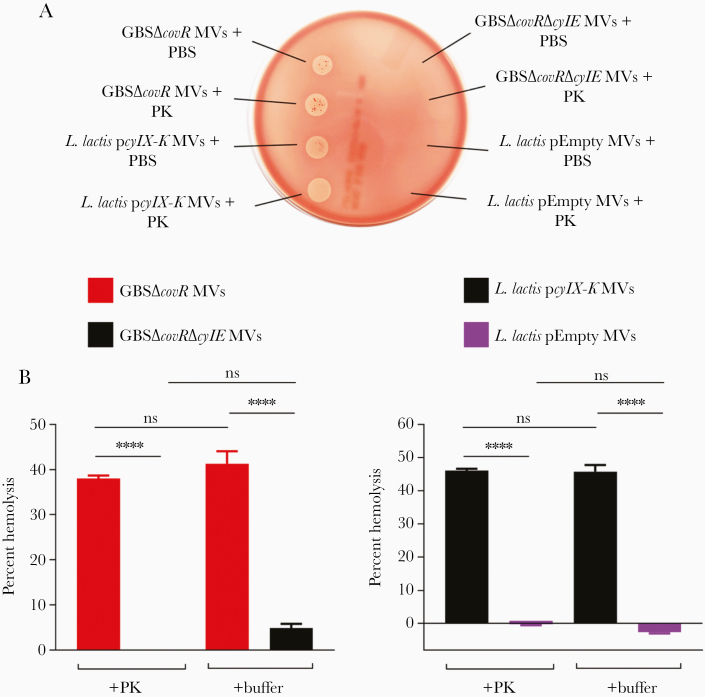
Next, we asked whether the release of granadaene in MVs was dependent on a GBS-specific factor. To test this, we used the Gram-positive bacterium L lactis expressing the GBS cyl operon (strain L lactis pcylX-K), which heterologously expresses granadaene as described recently [21]. We also included the NH/nonpigmented L lactis strain with only the vector (L lactis pEmpty), which does not produce granadaene [21]. After isolating MVs from these strains as described above, we noted that MVs from L lactis pcylX-K were pigmented and hemolytic on red blood agar (Figure 2, Supplementary Figure 1C), whereas MVs from L lactis pEmpty were neither pigmented nor hemolytic (Supplementary Figure 1C). Taken together, these findings demonstrate that granadaene is released in MVs from Gram-positive bacteria, and its association with MVs did not require any GBS-specific factor.
Figure 2.
Membrane vesicles (MVs) are hemolytic after treatment with protease. (A) The MVs from GBSΔcovR, GBSΔcovRΔcylE, Lactococcus lactis pcylX-K, or L lactis pEmpty were treated with proteinase K ([PK] final concentration 0.25 mg/mL) or an equivalent volume of phosphate-buffered saline (PBS) for 1 hour at 37°C, and 10 µL of each was spotted on blood agar. (B) The MVs from GBSΔcovR, GBSΔcovRΔcylE, L lactis pcylX-K, or L lactis pEmpty were mixed with human erythrocytes in the presence of PBS or PK (0.25 mg/mL) for 1 hour, and percentage hemolysis was determined relative to Triton X-100 (0.1%)-treated positive controls. One-way analysis of variance with Tukey’s posttest was used. Mean and standard error of the mean from 3 experiments performed in triplicate are shown. ****, P < .0001; ns, P ≥ .05.
Hemolytic Activity of Membrane Vesicles Containing Granadaene Is Independent of Proteins
To confirm that protein or peptides do not contribute to the hemolytic activity observed in MVs, we tested hemolysis of MVs in the presence of PK. Membrane vesicles isolated from HH GBS and L lactis pcylX-K were subjected to PK (0.25 mg/mL) treatment for 1 hour at 37°C. As a control, the activity of the PK used in these studies was verified by incubating it (at 0.25 mg/mL) with 100 µg of bovine serum albumin. All samples were analyzed for protein content SDS-PAGE, and the data shown in Supplementary Figure 3 confirm that proteins present in the MVs are susceptible to degradation by PK. Next, MVs isolated from HH GBS or L lactis pcylX-K, with and without PK treatment, were spotted onto a red blood agar plate or mixed with human RBCs for hemolysis assays. Nonhemolytic GBS MVs and L lactis pEmpty were included as controls. We observed that treatment with PK did not diminish hemolytic activity in MVs isolated from HH GBS or L lactis pcylX-K (Figure 2B and 2C). Taken together, these data indicate that hemolytic activity observed with MVs did not require any MV-associated proteins.
Hemolytic Membrane Vesicles Prevent Oxidative Killing of Group B Streptococci
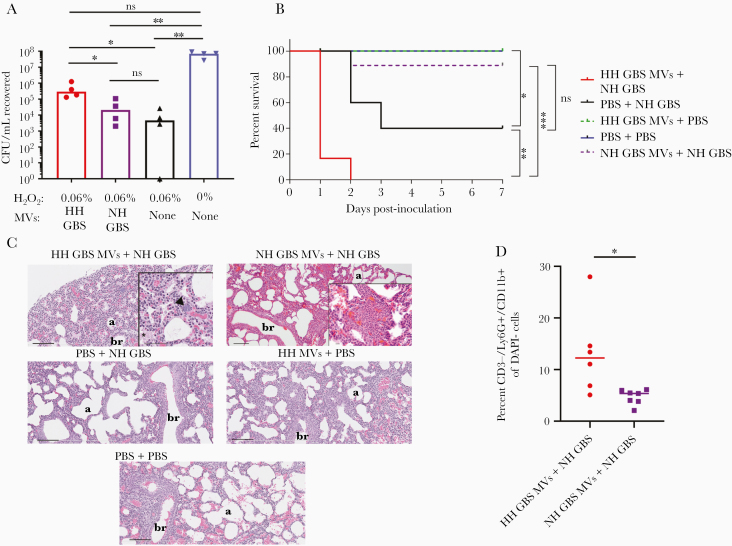
Next, we asked whether hemolytic MVs protect GBS against antimicrobial host defenses encountered during infection, such as reactive oxygen species (ROS) that are often produced by macrophages and neutrophils. Based on previous observations that the hemolytic pigment of GBS has antioxidant properties [22], and that MVs from Helicobacter pylori promote bacterial survival against ROS [27], we hypothesized that MVs derived from HH GBS may protect GBS against oxidative killing. To test this hypothesis, we treated NH GBS (GBSΔcovRΔcylE) with H2O2 that was preincubated with either HH GBS MVs or NH GBS MVs. As controls, GBS was exposed to H2O2 alone (ie, without MVs) or PBS only. After 1 hour, we quenched residual ROS with catalase and then enumerated surviving CFU. We observed that significantly more GBS survived H2O2 that had been pretreated with HH MVs compared with H2O2 pretreated with NH MVs (Figure 3A). These findings show that hemolytic pigment in MVs can dampen killing of GBS by H2O2, a major constituent of the oxidative burst [28].
Figure 3.
Hemolytic membrane vesicles (MVs) dampen oxidative killing and exacerbate group B streptococci (GBS) pathogenesis in neonatal mice. (A) Membrane vesicles isolated from hyperhemolytic (HH) GBSΔcovR or nonhemolytic (NH) GBSΔcovRΔcylE were preincubated with 0.06% H2O2 for 45 minutes and then incubated with NH GBS at 37°C for 1 hour. After incubation, remaining H2O2 was quenched with 1000 units of catalase, and surviving colony-forming units (CFU) were enumerated by dilution plating onto TSA. A group consisting of no MVs + H2O2 and no MVs + no H2O2 were included as positive and negative controls, respectively. Treatment groups were compared using a Kruskal-Wallis test with multiple comparisons corrected for false discovery. Hyperhemolytic GBS MVs + H2O2 vs no MVs + H2O2, P = .0170; HH GBS MVs + H2O2 vs NH GBS MVs + H2O2, P = .0395. Nonhemolytic GBS MVs + H2O2 vs no MVs + H2O2, P > .9999; HH GBS MVs + H2O2 vs phosphate-buffered saline (PBS) only, P = .0822; NH GBS MVs + H2O2 vs PBS only, P = .0032; no MVs + H2O2 + PBS, P = .0015. Line at median. (B) Kaplain-Meier plot depicts survival of neonatal mice inoculated with MVs alone or GBS strains alone or GBS strains with MVs, as indicated. Survival between groups was compared using the log-rank test. PBS + PBS (blue) vs PBS + NH GBS (black), P = .0316; PBS + NH GBS (black) vs HH GBS MVs + NH GBS (red), P = .0033; NH GBS MVs + NH GBS (purple) vs HH GBS MVs + NH GBS MVs (red), P = .0002; NH GBS MVs + NH GBS (purple) vs PBS + NH GBS MVs (black), P = .0610. (C) Lungs from neonatal mice in each treatment group were harvested at 24 hours posttreatment, fixed, sectioned, and stained for hematoxylin and eosin. In the HH GBS MVs + NH GBS group, the arrow represents an aggregate of small basophilic circular structures (consistent with bacteria), and the asterisk indicates eosinophilic material within the alveoli (fibrin). Scale bar is 100 µm for all images except for insets. Scale bar for inset in HH GBS MVs + NH GBS is 50 µm, and scale bar for inset in NH GBS MVs + NH GBS is 10 µm. “b” indicates bronchiole (letter is placed in the lumen) and “a” indicates alveolar air space. (D) Lungs from neonatal mice inoculated with HH GBS MVs + NH GBS and NH GBS MVs + NH GBS were harvested 24 hours after inoculation, processed into single-cell suspensions, stained, and analyzed by flow cytometry. Percentage neutrophils (CD3−/Ly6G+/CD11b+) of viable (DAPI−) cells were compared using a Mann-Whitney test (P = .0140). Line at median. For all statistical comparisons, ns indicates P > .05, * indicates P < .05, ** indicates P < .01, and *** indicates P < .001.
Hemolytic Membrane Vesicles Exacerbate Group B Streptococci Pathogenesis in Neonatal Mice
Because our in vitro data indicated that hemolytic MVs promote GBS survival against ROS, we hypothesized that hemolytic MVs contribute to GBS pathogenesis in vivo. To test this, neonatal mice between 12 and 36 hours of age were inoculated (I.P.) with either (1) HH MVs alone, (2) HH MVs in the presence of NH GBS, (3) NH MVs with NH GBS, (4) NH GBS alone, or (5) control saline. The mice were observed for morbidity and mortality symptoms for up to 7 days postinoculation. We found that neonatal mice inoculated with HH MVs alone did not succumb to the challenge (Figure 3B). However, the presence of HH MVs aggravated morbidity and mortality of neonatal mice treated with NH GBS when compared with mice inoculated with NH GBS alone or NH MVs + NH GBS (Figure 3B). Because previous work has shown that I.P. infection of GBS can cause lung infection in neonatal rats [29], histological examination of H&E-stained sections was performed on the lungs from mice obtained 24 hours postinoculation. Of the 4 mice inoculated with HH MVs + NH GBS, one set of lungs had a few clusters of small basophilic structures consistent with bacteria, which were not observed in the other groups. In addition, there was mild eosinophilic acellular material within the alveoli (consistent with fibrin) in the lungs of this neonate, which was generally minimal to absent in the lungs of neonates from the other groups (Figure 3C). Two other mice in this group had minimal focal neutrophilic inflammation, in the lung in one mouse and in the mediastinal tissues of another mouse. Although focal mild neutrophilic inflammation was observed in the lung of a mouse treated with NH MVs + NH GBS, flow cytometric analysis indicated that there were more neutrophils in the lungs of neonatal mice treated with HH MVs + NH GBS (Figure 4D). These findings show that MVs from HH GBS reduced survival and potentially exacerbated lung injury and/or bacteremia in neonates infected with NH GBS. These data, taken together, indicate that hemolytic GBS MVs can promote GBS pathogenesis and neonatal morbidity and mortality.
DISCUSSION
Group B streptococci remain a leading etiological agent of infection in human newborns and are associated with preterm birth, stillbirth, and neonatal sepsis [1, 30–41]. A major barrier to the development of new prevention strategies is the lack of understanding of virulence factors important for GBS pathogenesis. In this work, we add new insight into a critical GBS virulence factor, the hemolytic pigment, and show how GBS may package and deploy this toxin to overcome host defenses and promote infection.
In 2013, Whidbey et al [3] showed that the GBS pigment (granadaene) is hemolytic in the presence of starch, demonstrating that the pigment and β-hemolysin of GBS were one in the same. Although this work represented a major advance in our understanding of GBS pathogenesis, the requirement for starch in purified pigment for hemolytic activity remained a mystery, because GBS does not produce starch per se and yet are hemolytic. In the present study, we observed that MVs isolated from hemolytic GBS or L lactis expressing the GBS cyl genes [21] are hemolytic and cytolytic, even when MV-associated proteins are degraded by PK (Figures 1 and 2). These findings demonstrate that exogenous, high-molecular-weight stabilizers such as starch are not essential for hemolysis and cytolysis when the pigment is associated with the bacterial membrane or membrane components. In addition, these data show that proteinaceous components of the bacterial membrane are also themselves not necessary for hemolytic activity, suggesting that positioning within the membrane may provide sufficient stabilization for pigment activity.
Our findings in MVs support previous data indicating that the hemolytic pigment is localized to the bacterial surface [14, 15] and reveal a novel mechanism by which GBS may release this toxin from the bacterial cell. Previous work showed that virulence-associated proteins such as extracellular matrix-degrading enzymes are packaged in GBS MVs and likely contribute to placental membrane disruption, fetal injury, and preterm birth [16]. Our in vitro studies showing that hemolytic MVs cause cell death in several host immune cells suggested that hemolytic MVs alone may be pathogenic. However, because HH GBS MVs without bacteria did not induce death or lung injury in the neonatal mouse model, hemolytic MVs on their own may be insufficient to cause morbidity in complex host systems. On the other hand, our in vitro and in vivo findings suggest that the hemolytic pigment in MVs promotes GBS survival and pathogenesis, even for a NH strain (Figure 3). Furthermore, our in vivo data indicate that increased neutrophil recruitment by HH GBS MVs in addition to bacterial infection may intensify morbidity and mortality in neonates. It is notable that simultaneous isolation of hemolytic and NH GBS from human cases has been reported [23, 25, 42] including in neonates [42].
Membrane vesicles have recently gained appreciation for their ability to promote bacterial survival by interfering with host defenses. For instance, Staphylococcus aureus MVs promoted bacterial survival by via neutrophil cytotoxicity [43], MVs from Streptococcus pneumoniae inhibited opsonophagocytic killing by sequestering complement components [43], and catalase-containing MVs from H pylori decreased ROS-mediated killing [27]. Our data indicate that similar to H pylori, MVs from GBS facilitate bacterial survival from oxidative killing, although protection is dependent on the presence of the hemolytic pigment in MVs (Figure 3A). It is likely that release of hemolytic MVs by GBS quenches ROS produced by recruited neutrophils, thereby attenuating host defenses against GBS.
CONCLUSIONS
In summary, we identify a heretofore undescribed mechanism of pigment toxin-mediated virulence during GBS infection. Our results suggest that GBS releases the hemolytic pigment via MVs, which quench microbicidal oxidants and enable bacteria to survive. Reactive oxygen species production by neutrophils is critical to clearance of GBS by the host [5, 22, 44], and our findings provide new insight into how GBS overcomes hostile host environments and also lay a foundation for future studies examining the role of GBS MVs in immune evasion.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr. Anirban Banerjee for expert advice, Meghan Garrett and Shayla Nguyen for technical assistance, and Connie Hughes for administrative support.
Author contributions. B. A. and L. R. designed experiments. B. A., P. Q., V. S.-U., A. F., and A. B. performed experiments. B. A., J. M. S., and L. R. analyzed data. B. A. and L. R. wrote the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by funding from the National Institutes of Health ([NIH] Grants R01AI112619, R01AI133976, R01AI145890, and R21AI125907) and seed funds from Seattle Children’s Research Institute (to L. R). The NIH Training Grants T32AI007509 (Principal Investigator [P.I.] Dr. Lee Ann Campbell) and T32AI055396 (P.I. Dr. Ferric Fang) supported A. B. and A. F., respectively.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Seale AC, Bianchi-Jassir F, Russell NJ, et al. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis 2017; 65:200–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edmond KM, Kortsalioudaki C, Scott S, et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet 2012; 379:547–56. [DOI] [PubMed] [Google Scholar]
- 3. Whidbey C, Harrell MI, Burnside K, et al. A hemolytic pigment of group B streptococcus allows bacterial penetration of human placenta. J Exp Med 2013; 210:1265–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosa-Fraile M, Rodríguez-Granger J, Haidour-Benamin A, Cuerva JM, Sampedro A. Granadaene: proposed structure of the group B streptococcus polyenic pigment. Appl Environ Microbiol 2006; 72:6367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boldenow E, Gendrin C, Ngo L, et al. Group B streptococcus circumvents neutrophils and neutrophil extracellular traps during amniotic cavity invasion and preterm labor. Sci Immunol 2016; 1:eaah4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doran KS, Chang JC, Benoit VM, Eckmann L, Nizet V. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J Infect Dis 2002; 185:196–203. [DOI] [PubMed] [Google Scholar]
- 7. Nizet V, Gibson RL, Chi EY, Framson PE, Hulse M, Rubens CE. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun 1996; 64:3818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gibson RL, Nizet V, Rubens CE. Group B streptococcal beta-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr Res 1999; 45:626–34. [DOI] [PubMed] [Google Scholar]
- 9. Lembo A, Gurney MA, Burnside K, et al. Regulation of CovR expression in group B streptococcus impacts blood-brain barrier penetration. Mol Microbiol 2010; 77:431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doran KS, Liu GY, Nizet V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest 2003; 112:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whidbey C, Vornhagen J, Gendrin C, et al. A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury. EMBO Mol Med 2015; 7:488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gendrin C, Vornhagen J, Ngo L, et al. Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci Adv 2015; 1:e1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armistead B, Herrero-Foncubierta P, Coleman M, et al. Lipid analogs reveal features critical for hemolysis and diminish granadaene mediated group B streptococcus infection. Nat Commun 2020; 11:1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Platt MW. In vivo hemolytic activity of group B streptococcus is dependent on erythrocyte-bacteria contact and independent of a carrier molecule. Curr Microbiol 1995; 31:5–9. [DOI] [PubMed] [Google Scholar]
- 15. Merritt K, Jacobs NJ. Characterization and incidence of pigment production by human clinical group B streptococci. J Clin Microbiol 1978; 8:105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Surve MV, Anil A, Kamath KG, et al. Membrane vesicles of group B streptococcus disrupt feto-maternal barrier leading to preterm birth. PLoS Pathog 2016; 12:e1005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brameyer S, Plener L, Muller A, Klingl A, Wanner G, Jung K. Outer membrane vesicles facilitate trafficking of the hydrophobic signaling molecule CAI-1 between Vibrio harveyi cells. J Bacteriol 2018; 200:e00740–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog 2009; 5:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajagopal L, Vo A, Silvestroni A, Rubens CE. Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Mol Microbiol 2006; 62:941–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lancefield RC, McCarty M, Everly WN. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med 1975; 142:165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Armistead B, Whidbey C, Iyer LM, et al. The cyl genes reveal the biosynthetic and evolutionary origins of the group B streptococcus hemolytic lipid, granadaene. Front Microbiol 2019; 10:3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu GY, Doran KS, Lawrence T, et al. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci U S A 2004; 101:14491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sendi P, Johansson L, Dahesh S, et al. Bacterial phenotype variants in group B streptococcal toxic shock syndrome. Emerg Infect Dis 2009; 15:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Almeida A, Villain A, Joubrel C, et al. Whole-Genome Comparison Uncovers Genomic Mutations between group B streptococci sampled from infected newborns and their mothers. J Bacteriol 2015; 197:3354–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lupo A, Ruppen C, Hemphill A, Spellerberg B, Sendi P. Phenotypic and molecular characterization of hyperpigmented group B streptococci. Int J Med Microbiol 2014; 304:717–24. [DOI] [PubMed] [Google Scholar]
- 26. Whidbey C, Burnside K, Martinez RM, et al. A hyperhemolytic/hyperpigmented group B streptococcus strain with a CovR mutation isolated from an adolescent patient with sore throat. Clin Res Infect Dis 2015; 2:1018. [PMC free article] [PubMed] [Google Scholar]
- 27. Lekmeechai S, Su YC, Brant M, et al. Helicobacter pylori outer membrane vesicles protect the pathogen from reactive oxygen species of the respiratory burst. Front Microbiol 2018; 9:1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med 2002; 166:S4–8. [DOI] [PubMed] [Google Scholar]
- 29. Ferrieri P, Burke B, Nelson J. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect Immun 1980; 27:1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slattery MM, Morrison JJ. Preterm delivery. Lancet 2002; 360:1489–97. [DOI] [PubMed] [Google Scholar]
- 31. Katz J, Lee AC, Kozuki N, et al. ; CHERG Small-for-Gestational-Age-Preterm Birth Working Group . Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013; 382:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matorras R, Garcia Perea A, Omeñaca F, Usandizaga JA, Nieto A, Herruzo R. Group B streptococcus and premature rupture of membranes and preterm delivery. Gynecol Obstet Invest 1989; 27:14–8. [DOI] [PubMed] [Google Scholar]
- 33. Rubens CE, Gravett MG, Victora CG, Nunes TM; GAPPS Review Group . Global report on preterm birth and stillbirth (7 of 7): mobilizing resources to accelerate innovative solutions (Global Action Agenda). BMC Pregnancy Childbirth 2010; 10Suppl 1:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brigtsen AK, Jacobsen AF, Dedi L, Melby KK, Fugelseth D, Whitelaw A. Maternal colonization with group B streptococcus is associated with an increased rate of infants transferred to the neonatal intensive care unit. Neonatology 2015; 108:157–63. [DOI] [PubMed] [Google Scholar]
- 35. Verani JR, Spina NL, Lynfield R, et al. Early-onset group B streptococcal disease in the United States: potential for further reduction. Obstet Gynecol 2014; 123:828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 2008; 299:2056–65. [DOI] [PubMed] [Google Scholar]
- 37. Seale AC, Blencowe H, Bianchi-Jassir F, et al. Stillbirth with group B streptococcus disease worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bianchi-Jassir F, Seale AC, Kohli-Lynch M, et al. Preterm birth associated with group B streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ledger WJ. Perinatal infections and fetal/neonatal brain injury. Curr Opin Obstet Gynecol 2008; 20:120–4. [DOI] [PubMed] [Google Scholar]
- 40. Kohli-Lynch M, Russell NJ, Seale AC, et al. Neurodevelopmental impairment in children after group B Streptococcal disease worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schrag SJ, Zywicki S, Farley MM, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 2000; 342:15–20. [DOI] [PubMed] [Google Scholar]
- 42. Sigge A, Schmid M, Mauerer S, Spellerberg B. Heterogeneity of hemolysin expression during neonatal Streptococcus agalactiae sepsis. J Clin Microbiol 2008; 46:807–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Askarian F, Lapek JD Jr, Dongre M, et al. Staphylococcus aureus membrane-derived vesicles promote bacterial virulence and confer protective immunity in murine infection models. Front Microbiol 2018; 9:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doster RS, Sutton JA, Rogers LM, Aronoff DM, Gaddy JA. Streptococcus agalactiae induces placental macrophages to release extracellular traps loaded with tissue remodeling enzymes via an oxidative burst-dependent mechanism. mBio 2018; 99:e02084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.