Abstract
Background
Bacterial flagellin is a major target of innate and adaptive immunity, both of which can promote and/or compensate for deficiencies in each other’s function.
Methods
To investigate the role of innate immune detection of flagellin irrespective of adaptive immunity, we examined the consequences of loss of Toll-like receptor 5 (T5) and/or Nod-like receptor 4 (N4) upon a Rag1-deficient background.
Results
Mice lacking Toll-like receptor 5 and Rag1 (T5/Rag-DKO) exhibited frequent lethal Pasteurellaceae-containing abscesses that prevented breeding of these mice. Mice lacking Toll-like receptor 5, Nod-like receptor 4, and Rag1 (T5/N4/Rag-TKO) also resulted in sporadic lethal abdominal abscesses caused by similar Pasteurellaceae. In the absence of such infections, relative to Rag1-KO, T5/N4/Rag-TKO mice exhibited microbiota encroachment, low-grade inflammation, microbiota dysbiosis, and, moreover were highly prone to Citrobacter infection and developed severe colitis when adoptively transferred with colitogenic T cells. Relative proneness of T5/N4/Rag-TKO mice to T-cell colitis was ablated by antibiotics while fecal microbiota transplant from T5/N4/Rag-TKO mice to wild-type mice transferred proneness to Citrobacter infection, indicating that dysbiosis in T5/N4/Rag-TKO mice contributed to these phenotypes.
Conclusions
These results demonstrate a critical role for innate immune detection of flagellin, especially in the intestinal tract and particularly in hosts deficient in adaptive immunity.
Keywords: Toll-like receptor 5 (TLR5), Nod-like receptor 4 (NLRC4), Pasteurellaceae infection, immune-mediated colitis, low-grade inflammation
This study showed that, in the absence of adaptive immunity, mice deficient in innate immunity to flagellin developed microbiota dysbiosis and occasional lethal Pasteurellaceae infections. Moreover, such mice were highly prone to pathogen infection and T-cell–induced colitis.
Controlled motility, which is mediated by flagella and utilized by a variety of pathogenic and commensal bacteria, is a central nexus of host-bacterial interactions [1, 2]. Specifically, bacteria employ numerous sensing pathways that dynamically regulate flagella expression while the primary component of flagella, flagellin, is a major target of both innate and adaptive immunity. Detection of picomolar concentration of flagellin by cell surface Toll-like receptor 5 (TLR5) drives canonical nuclear factor-κB (NF-κB)–mediated host defense gene expression while intracellular detection of flagellin by the Nod-like receptor 4 (NLRC4) inflammasome is thought to serve as a “red alert” that initiation of more potentially host-damaging immune responses, including production of interleukin-1β (IL-1β) and pyroptosis, are warranted [3]. Flagellin is also a major target of humoral immunity thus providing the basis of H-serotyping and is a dominant T-cell epitope in response to infection [4]. Furthermore, flagellin is a dominant antigenic target of adaptive immunity in Crohn disease [5]. Immune responses targeting flagellin are particularly active at mucosal surfaces, perhaps reflecting the range of bacteria that use flagella to reach and or adhere to the epithelium. Targeting of flagellin by adaptive immunity, especially intestinal secretory antiflagellin immunoglobulin A (IgA), serves to limit flagellin expression thus keeping flagellated bacteria at low levels in healthy hosts [6, 7]. Innate immune responses to flagellin are also particularly active in the intestinal mucosa. TLR5 is expressed on epithelial cells and CD11c intestinal phagocytes [8–10]. In contrast, TLR5 is not consistently expressed by most phagocytes in other locations. NLRC4 is also expressed by gut epithelial cells and intestinal macrophages [11, 12].
The importance of innate immunity to flagellin has been studied via use of knockout (KO) mice. Mice with complete or intestinal epithelial-specific deletion of TLR5 are prone to developing spontaneous intestinal inflammation that can, depending upon microbiota composition, manifest as overt colitis but more commonly takes the form of low-grade inflammation that promotes metabolic syndrome [13, 14]. Such inflammation is associated with alterations in microbiota composition and localization, specifically microbiota encroachment into the inner mucus layer [15]. These changes in microbiota resulting from loss of TLR5 are not restricted to flagellated bacteria but also reflect that genes induced by TLR5 activation (eg, antimicrobial peptides) will impact other bacteria and/or that such changes are, in part, a consequence of inflammation [16]. Some portion of proinflammatory phenotypes of TLR5 can be envisaged to involve adaptive immunity. Most simply, one might imagine that the increased levels of flagellated bacteria that can result from loss of TLR5 might eventuate in greater compensatory adaptive immune responses to flagellin, which although are likely protective from some infections, might increase potential for chronic inflammation. Additionally or alternatively, loss of TLR5 on regulatory T cells may promote colitis by reducing the immunosuppressive capacity of these cells [17].
Loss of NLRC4 has not been observed to cause spontaneous phenotypes but makes mice prone to chemical-induced colitis and infection by motile pathogens [18]. Interestingly, combined loss of TLR5 and NLRC4 alleviates rather than exacerbates spontaneous and some challenge-induced TLR5-KO phenotypes, thus suggesting that some aspects of TLR5-KO phenotypes are promoted by aberrant inflammasome activation rather than reflecting an essential role of innate immune detection of flagellin per se [18]. More generally, the lack of a marked overt phenotype in TLR5/NLRC4-double KO (DKO) mice seemed in accord with the notion that adaptive immunity provides redundancy for innate sensing of flagellin. Hence, we sought to examine the role of innate immunity to flagellin in the absence of adaptive immunity. We generated and examined mice lacking TLR5 or TLR5 and NLRC4 upon a Rag1-KO background. We found that, in the absence of adaptive immunity, loss of innate immunity to flagellin results in changes in gut microbiota that confer proneness to infection and colitis.
METHODS
T5/N4/Rag-triple KO (TKO) mice were generated by crossing Rag1-KO mice with TLR5−/−/NLRC4−/−(T5/N4-DKO) mice. Rag1-KO, T5/Rag-DKO, and T5/N4/Rag-TKO mice were bred and housed at Georgia State University under approved animal protocols (institutional animal care and use committee number A17047). Male Rag1-KO and T5/N4/Rag-TKO mice at 8 months old were euthanized, the splenocytes and colon lamina propria cells were isolated and analyzed by flow cytometry. The subcutaneous abscess and colon were fixed in 10% phosphate-buffered formalin and processed for H&E staining. Total RNA was isolated from colon using TRIzol (Invitrogen), and the expression levels of zonula occludens-1 (ZO-1), Claudin-1, monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor-α (TNF-α) were analyzed by using quantitative reverse transcription polymerase chain reaction (qRT-PCR). Fecal samples were collected to analyze LCN-2 by enzyme-linked immunosorbent assay (ELISA), gut microbiota composition by16S rRNA sequencing, and bacteria localization by fluorescence in situ hybridization staining. Abscesses were harvested from T5/N4/Rag-TKO mice under aseptic conditions and the content was cultured in Luria-Bertani (LB) broth prior to analysis by PCR with Pasteurellaceae-specific primers: 5′-CATAAGATGAGCCCAAG-3′; 5′-GTCAGTACATTCCCAAGG-3′. Further details of the materials and methods are given in the Supplementary Material.
RESULTS
Frequent Lethality in T5/Rag-DKO Mice
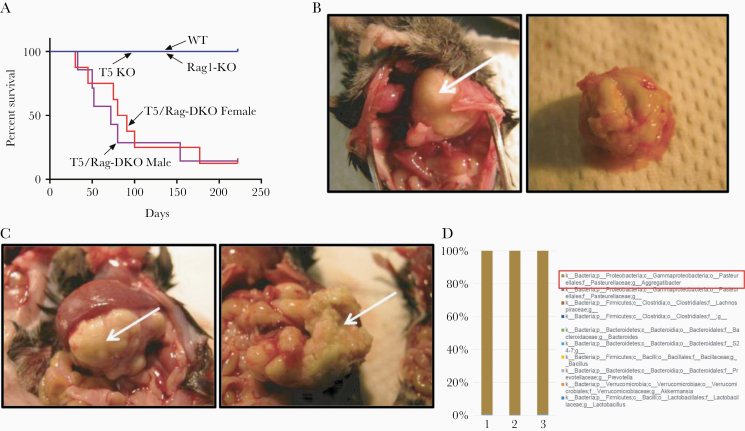
We sought to test the hypothesis that the proneness of TLR5−/−, herein referred to as T5-KO mice, to develop inflammatory phenotypes, namely colitis and metabolic syndrome, might result from compensatory and/or dysregulated adaptive immunity. We crossed T5-KO and Rag1-KO mice and subsequently bred their offspring to each other in an attempt to generate closely related breeding colonies of T5-KO, Rag1-KO, T5/Rag-DKO, and wild-type (WT) mice. While mice with these genotypes, and intermediate heterozygotes, were generated in proportions in accord with the expected mendelian ratios, T5/Rag-DKO rarely successfully bred with each other thus preventing development of a stable colony of these mice. Rather, in contrast to their T5-KO and Rag1-KO littermates and cousins, which uniformly survived well into middle age, T5/Rag-DKO had a generally sickly/runted appearance and most male and female T5/Rag-DKO mice died before 3 months of age (Figure 1A). Deaths in T5/Rag-DKO mice were frequently in mice displaying visible protrusions, most of which were associated with presence of abscesses in various organs, for example, lung (Figure 1B) or pancreas (Figure 1C). Microscopic examination of the abscess fluid indicated it was filled with white blood cells and bacteria. Interrogation of the bacterial species content of 3 distinct abscesses from 3 different mice by 16S sequencing indicated that over 99% of the bacteria in such abscesses were Aggregatibacter from the Pasteurellaceae family (Figure 1D). These results suggest that in the absence of adaptive immunity, TLR5 plays an important role in protecting against some common normally nonpathogenic bacteria, but inability to stably maintain T5/Rag-DKO mice hindered further investigation.
Figure 1.
Deletion of TLR5 from Rag1-KO mice was frequently lethal. A, Survival curves of closely related TLR5−/− (T5-KO), Rag1−/− (Rag1-KO), and TLR5−/−/Rag1−/− (T5/Rag-DKO) mice (n > 20 mice per condition). B and C, Macroscopic image of representative abscesses in lung (B) and pancreas (C), which were only observed in T5/Rag-DKO mice. D, Results of 16s rRNA sequencing of abscess content. Abbreviations: DKO, double knockout; TLR5, Toll-like receptor 5; WT, wild type.
Sporadic Infectious Abscesses in T5/N4/Rag-TKO Mice
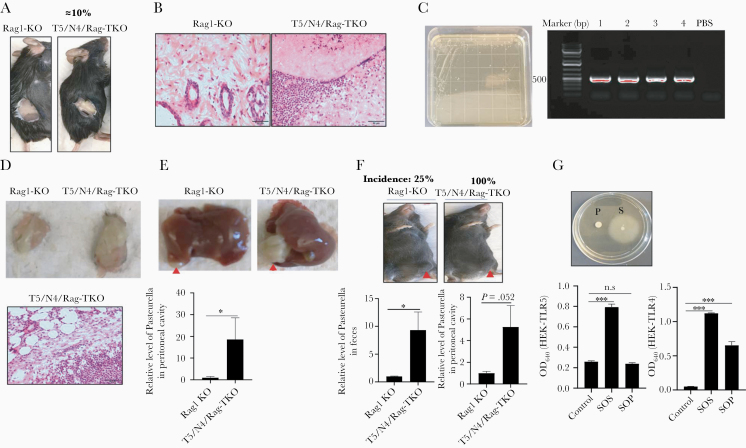
Mice lacking both TLR5 and NLRC4, that is T5/N4-DKO mice, lack spontaneous inflammatory phenotypes exhibited by T5-KO mice, likely reflecting a role for inflammasome activation in driving the latter’s inflammatory phenotypes [19]. We thus hypothesized that mice lacking TLR5, NLRC4, and Rag1 might lack the problems exhibited by T5/Rag-DKO, thus allowing more extensive study of loss of innate immunity to flagellin amidst absence of adaptive immunity. Hence, we crossed TLR5−/−/NLRC4−/−, whose generation was previously described, to Rag1-KO mice. Subsequent crossing of offspring generated (T5/N4/Rag-TKO) and other genotypes at the expected mendelian ratios. Analogous to the case for T5/Rag-DKO, attempts to establish a breeding colony of T5/N4/Rag-TKO mice were stymied by unsuccessful breeding, often associated with abdominal abscesses that dictated euthanasia. However, a small number of mice produced offspring, thus allowing generation of a breeding colony in which such abscesses were less frequent. Specifically, initial litters produced by breeding T5/N4/Rag-TKO mice to each other exhibited abscesses in about 10% of mice (Figure 2A), a prevalence that gradually declined to about 2%–3% in subsequent generations. In contrast, no abscesses were observed in the closely related Rag1-KO mice generated in this crossing (Figure 2A) or in other colonies of Rag1-KO mice used by the authors. Histopathologic analysis of abscesses found the presence of neutrophils and apparent presence of bacteria (Figure 2B). Isolation and culture of abscess fluid in ambient conditions yielded similar-looking colonies, 4 of which were randomly selected and identified by PCR to be Pasteurella species using previously verified Pasteurella-specific primers (Figure 2C) [20]. Sequencing of the PCR product of 16S rRNA genes indicated 97.4% identity to Rodentibacter pneumotropicus, which is known to frequently colonize the murine digestive and respiratory tracts [21]. To investigate the role of innate immunity to flagellin in protecting against this bacteria, broth culture of this Pasteurella isolate were administered to Rag1-KO and T5/N4/Rag-TKO mice subcutaneously (Figure 2D), intraperitoneally (Figure 2E), or by oral gavage (Figure 2F), all of which showed more severe infection in mice lacking innate immunity to flagellin. Specifically, relative to Rag1-KO, T5/N4/Rag-TKO displayed larger and more frequent abscesses as well as higher numbers of colony forming units (CFU). Such abscesses resulting from direct administration of Pasteurella were indistinguishable from those appearing spontaneously in T5/N4/Rag-TKO mice. These results suggest that loss of innate immunity to flagellin, combined with complete loss of adaptive immunity, can result in translocation of Pasteurella from the intestine throughout the abdominal cavity wherein its ability to persist in absence of adaptive immunity results in lethal abscess. Such proneness to infection did not likely reflect a direct role for TLR5 and NLRC4 in recognizing Pasteurella directly, in that it lacked flagellar-mediated motility and was not capable of activating TLR5 in vitro (Figure 2G).
Figure 2.
T5/N4/Rag-TKO mice exhibited sporadic Pasteurellaceae infections. A, Representative example of subcutaneous abscesses, which were exhibited by about 10% of first-generation T5/N4/Rag-TKO mice and 0 of more than 100 Rag1-KO mice. B, H&E image of skin from the region where abscesses were observed. C, Culture of abscess content on LB agar. Four single colonies were randomly selected and subjected to PCR, which confirmed they were Pasteurellaceae species. D, Colonies from (C) were grown in LB and 3 ×106 CFU subcutaneously injected into Rag1-KO and T5/N4/Rag-TKO mice. Tissue at injection site was harvested 8 days post injection and abscess from T5/N4/Rag-TKO mice was subjected to H&E staining. E, Macroscopic images of a representative peritoneal abscesses after intraperitoneal injection of Pasteurellaceae (arrowhead, abscess), and relative level of Pasteurellaceae in peritoneal cavity as measure by qPCR. F, Macroscopic image of representative abscesses in skin after oral gavage of Pasteurellaceae, and relative level of Pasteurellaceae in feces and peritoneal cavity as measured by qPCR. G, Isolated Pasteurellaceae (P) was assayed for motility on semisolid plate containing 0.3% agar with Salmonella (S) as positive control. Superannuants of cultured Pasteurellaceae were assayed for levels of bioactive flagellin and lipopolysaccharide via use of TLR5 and TLR4 reporter cells. Data are means ± SEM. Abbreviations: CFU, colony forming unit; LB, Luria-Bertani; N4, Nod-like receptor 4; NS, nonsignificant; OD, optical density; PBS, phosphate-buffered saline; qPCR, quantitative polymerase chain reaction; SOP, superannuant of Pasteurellaceae; SOS, superannuant of Salmonella; T5, Toll-like receptor 5; TKO, triple knockout; TLR, Toll-like receptor. *P ≤ .05, or ***P ≤ .001. n.s, not significant.
Basal Phenotype of T5/N4/Rag-TKO Mice
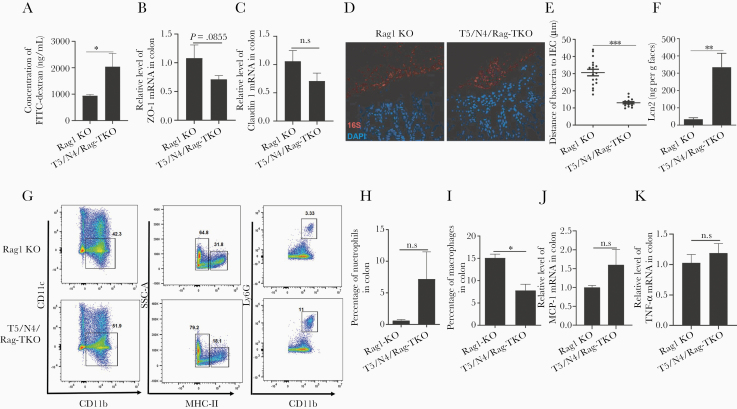
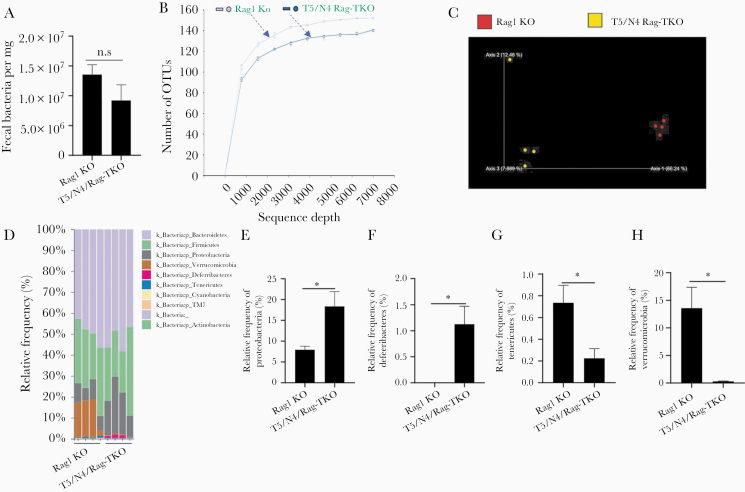
While the majority of T5/N4/Rag-TKO mice lacked visible abscesses, or any other overt indices of disease, they exhibited a clear reduction in body mass relative to closely related age-matched sex-matched Rag1-KO, but otherwise did not display notable differences in nonnormalized or normalized organ sizes (Supplementary Figure 1). Nor did T5/N4/Rag-TKO mice display gross, histopathological evidence of colitis and did not differ significantly from Rag1-KO in levels of Pasteurella species in feces (Supplementary Figure 2). However, T5/N4/Rag-TKO mice exhibited some abnormalities in gut barrier function. Specifically, T5/N4/Rag-TKO mice exhibited a modest but statistically significant increase in gut permeability to orally administered fluorescein isothiocyanate (FITC)-dextran (Figure 3A), which associated with a modest nonsignificant reduction in colonic ZO-1 and Claudin-1 (Figure 3B and 3C). Furthermore, T5/N4/Rag-TKO mice displayed microbiota encroachment (Figure 3D), exhibiting a more than 2-fold reduction in the average distance between the epithelium and the closest bacteria observed by confocal microscopy (Figure 3E). Such microbiota encroachment and modestly impaired barrier function are features of low-grade inflammation. Accordingly, levels of fecal lipocalin-2, which is frequently used as an indicator of low-grade inflammation [22], was significantly elevated in T5/N4/Rag-TKO mice relative to Rag1-KO mice (Figure 3F). Furthermore, colons of T5/N4/Rag-TKO mice displayed elevated numbers of neutrophils, but not macrophages (Figure 3G–3I), as well as a slight increase in the expression of MCP-1 and TNF-α compared with Rag1-KO mice (Figure 3J and 3K). In further accord with a state of low-grade colon inflammation, 16S-based analysis of microbiota revealed that T5/N4/Rag-TKO mice had marked alterations in gut microbiota composition relative to their Rag1-KO controls. Specifically, while the total levels of gut bacteria did not differ significantly between the groups (Figure 4A), there was a clear difference in α-diversity (ie, species richness) and β-diversity (ie, species/phyla composition) as indicated by the number of operational taxonomic units and principal coordinates analysis, respectively (Figure 4B and 4C). This difference was readily evident at phylum level, which showed an increased abundance of Proteobacteria, Deferrabacteres, and a decrease in the relative abundance of Tenericutes and Verrucomicrobia in T5/N4/Rag-TKO mice (Figure 4D–4H). Furthermore, linear discriminant analysis effect size identified a variety of taxa whose abundance differed between Rag1-KO and T5/N4/Rag-TKO mice (Supplementary Figure 3). T5/N4/Rag-TKO mice also displayed evidence of mild systemic inflammation, including splenomegaly (normalized or nonnormalized) as well as a statistically significant elevation in the percentage of splenic neutrophils that was not associated with differences in splenic macrophages nor dendritic cells (Supplementary Figure 4), and a modest nonsignificant increase in other white blood cell counts (Supplementary Figure 5). Together, these results suggest that despite lacking overt disease, T5/N4/Rag-TKO mice developed perturbations in the mucosal immune system-microbiota relationship, which was not observed when TLR5 and NLRC4 were ablated in mice with functioning adaptive immune systems (data not shown).
Figure 3.
T5/N4/Rag-TKO mice display reduced barrier function, microbiota encroachment, and increased levels of neutrophils. A, Measure of serum FITC 3 hours following oral gavage with FITC-dextran. B and C, Assay of colon expression of indicated tight junction genes by qRT-PCR. D and E, Assessment of bacterial localization in Carnoy-fixed colon by confocal microscopy: (D) representative fluorescent image of bacteria in the colon and (E) average distance of closest bacteria per high-powered field. F, Fecal Lcn-2 measured by ELISA. G, Representative flow cytometry plot included neutrophils (CD45+CD11b+ MHCII-Ly6G+) and macrophages (CD45+CD11b+ MHCII+). H and I, Percentage of neutrophils (H) and macrophages (I) in colon. J and K, Relative level of MCP-1 (J) and TNF-α (K) in the colon analyzed by qRT-PCR. Data are means ± SEM, n = 3–4 mice per condition. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay; FITC, fluorescein isothiocyanate; IEC, intestinal epithelial cell; Lcn2, lipocalin-2; MCP-1, monocyte chemoattractant protein-1; N4, Nod-like receptor 4;NS, nonsignificant; qRT-PCR, quantitative reverse transcription polymerase chain reaction; SSC, side scatter; T5, Toll-like receptor 5; TKO, triple knockout; TNF-α, tumor necrosis factor-α; ZO-1, zonula occludens-1. *P ≤ .05, **P ≤ .01 or ***P ≤ .001. n.s, not significant.
Figure 4.
Deletion of TLR5 and NLRC4 from Rag1-KO mice alters gut microbiota composition. A, The total bacteria in feces was analyzed by qPCR, calculated using a standard curve. B, Rarefaction curves showing the observed OTU. C, Fecal microbiota composition was analyzed by 16S RNA sequencing, global composition as expressed by unweighted UniFrac PCoA analysis. D–H, Relative abundance of bacteria at the phylum level. Data are means ± SEM, n = 4 mice per condition. Abbreviations: KO, knockout; N4, Nod-like receptor 4; NLRC4, Nod-like receptor 4; NS, nonsignificant; OTU, operational taxonomic unit; PCoA, principal coordinates analysis; qPCR, quantitative polymerase chain reaction; T5, Toll-like receptor 5; TKO, triple knockout; TLR5, Toll-like receptor 5. *P ≤ .05. n.s, not significant.
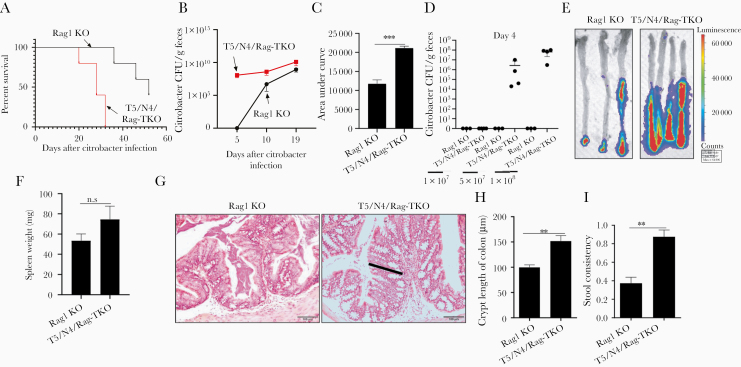
Microbiota Alterations in T5/N4/Rag-TKO Mice Promote C. rodentium Infection
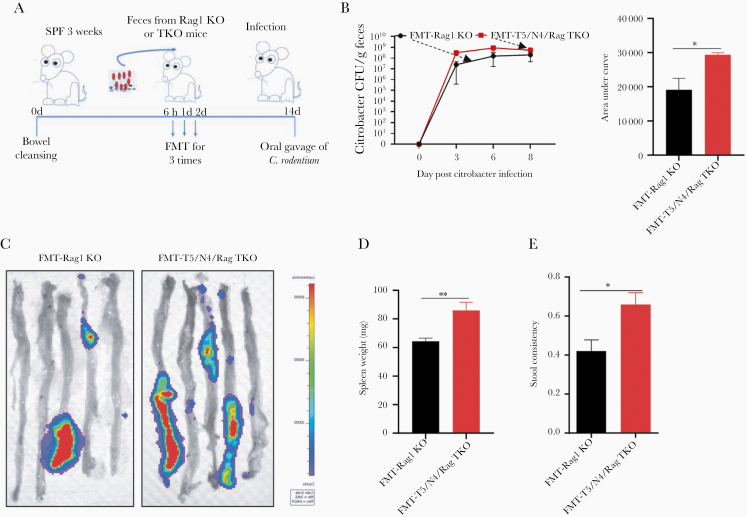
In accord with previous studies, administration of C. rodentium to Rag1-KO mice resulted in chronic infection and death. However, such mortality was markedly accelerated in T5/N4/Rag-TKO mice (Figure 5A). The increased mortality of T5/N4/Rag-TKO mice was associated with higher levels of C. rodentium colonization, especially at early time points (5 days) wherein C. rodentium did not achieve detectable levels in Rag1-KO mice at the moderate inoculating dose utilized (Figure 5B and 5C). Moreover, lower doses of C. rodentium were also sufficient to uniformly result in colonization in T5/N4/Rag-TKO mice 4 days post inoculation, while colonization was below detection limits in Rag1-KO mice (Figure 5D). Levels of colonization assessed by measuring fecal CFU correlated with levels via intestinal bioluminescence imaging (Figure 5E), splenomegaly (Figure 5F), and with extent of intestinal inflammation as assayed by colonic hyperplasia (Figure 5G and 5H) and stool consistency (Figure 5I). Thus, in the absence of adaptive immunity, loss of innate immunity to flagellin resulted in high proneness to C. rodentium infection. We initially hypothesized that this reflected a direct role for recognition of C. rodentium flagellin. However, analogous to the case for the Pasteurella that spontaneously infected T5/N4/Rag-TKO mice, the C. rodentium strain that we utilized appeared to lack flagella expression (no detectable motility in soft agar). Hence, we hypothesized that the alterations in microbiota composition exhibited by T5/N4/Rag-TKO mice might contribute to loss of microbiota-mediated colonization resistance to infection. To test this possibility, we performed fecal transplants from T5/N4/Rag-TKO and Rag1-KO mice to WT mice, using a recently developed protocol with polyethylene glycol and reported to transfer microbiota composition with high fidelity, even to mice raised in conventional conditions, which enables normal immune system development [23]. We observed that, relative to mice receiving fecal transplants from Rag1-KO mice, recipients of microbiotas from T5/N4/Rag-TKO mice displayed a significantly higher level of C. rodentium at early time points following inoculation (Figure 6A–6C), which was associated with splenomegaly (Figure 6D) and greater loss of stool consistency (Figure 6E). These results suggest that the alterations in the microbiota composition of T5/N4/Rag-TKO mice contributed to their proneness to infection.
Figure 5.
Deletion of TLR5 and NLRC4 from Rag1-KO mice increases susceptibility to Citrobacter colonization. Mice were orally administered 2 × 108 CFU C. rodentium. A, Survival. B, Fecal C. rodentium CFU. C, Cumulative shedding as assessed by area under curve. D, Dose of administered C. rodentium was varied as indicated and fecal CFU measured on day 4. E, Use of bioluminescent imaging to assess C. rodentium infection at 10 days post inoculation. F, Spleen weight. G, H&E staining of colon (black bar: crypt). H, Colon crypt length. I, stool consistency. Data are means ± SEM, n = 3–5 mice per condition. Abbreviations: CFU, colony forming unit; KO, knockout; N4, Nod-like receptor 4; NLRC4, Nod-like receptor 4; T5, Toll-like receptor 5; TKO, triple knockout; TLR5, Toll-like receptor 5. **P ≤ .01, ***P ≤ .001. n.s, not significant.
Figure 6.
Transplant of feces from T5/N4/Rag-TKO mice conferred increased susceptibility to Citrobacter. A, WT C57BL/6 mice (3 weeks old) were subjected to a bowel cleansing followed at 6 h, 1 d, and 2 d, by administration of fecal suspension generated from Rag1-KO (FMT-Rag1-KO) or T5/N4/Rag-TKO mice. B, Fecal C. rodentium CFU over time and cumulative shedding as assessed by area under curve. C–E, Mice were euthanized on day 10 post inoculation to enable bioluminescent assessment of C. rodentium infection (C), spleen weight (D), and stool consistency (E). Data are means ± SEM, n = 5 mice per condition. Abbreviations: SPF, specific-pathogen-free; FMT, fecal microbiota transplantation; CFU, colony forming unit; N4, Nod-like receptor 4; T5, Toll-like receptor 5; TKO, triple knockout; WT, wild type. *P ≤ .05, **P ≤ .01.
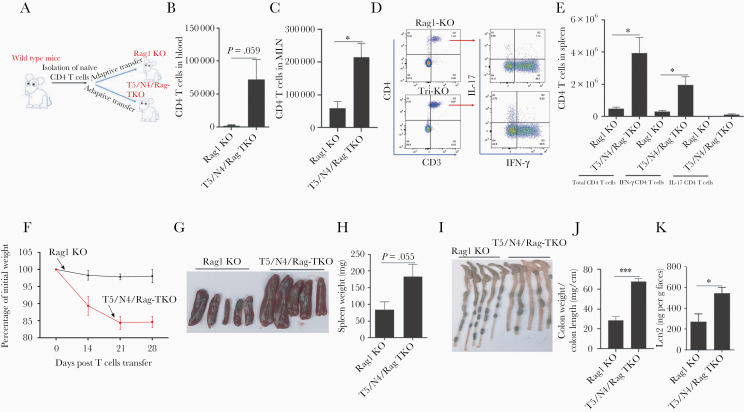
Loss of TLR5 and NLRC4 Results in Higher Susceptibility to T-Cell–Mediated Colitis
Lastly, we utilized T5/N4/Rag-TKO mice to examine the notion that lack of innate immunity to flagellin might result in microbiota-mediated alterations in adaptive immunity, which may drive the susceptibility to colitis that we previously reported in TLR5-KO mice [14, 19]. Specifically, we sought to measure the extent to which lack of TLR5 and NLRC4 would impact exogenously administered colitogenic T cells and subsequent development of colitis. Rag1-KO and T5/N4/Rag-TKO mice were intravenously administered 1 × 106 CD45RBhigh CD4 T cells that had been isolated from WT mice (Figure 7A). Two weeks post transfer, T cells were present in peripheral blood of T5/N4/Rag-TKO mice at markedly higher levels than in Rag1-KO recipients (Figure 7B). Assay of T cells in the mesenteric lymph nodes and spleen, measured 4 weeks post administration, also showed clearly higher levels of total CD4 T cells in both compartments in T5/N4/Rag-TKO mice (Figure 7C–7E). Further analysis of T cells in spleen showed elevated interferon-γ–expressing CD4 T cells in T5/N4/Rag-TKO mice relative to Rag1-KO mice (Figure 7E). Such enhanced expansion of the administered T cells correlated with markedly increased severity in the extent of colitis developed by these mice (Figure 7F–7K). Specifically, relative to Rag1-KO recipients of CD45RBhigh cells, T5/N4/Rag-TKO mice displayed markedly greater weight loss (Figure 7F), splenomegaly (Figure 7G and 7H), colon shortening/thickening (Figure 7I and 7J), and fecal lipocalin 2 levels (a fecal colitis marker; Figure 7K), all of which are established indicators of colitis in this and other colitis models. Such exacerbation of colitis was completely prevented by administration of broad-spectrum antibiotics, consistent with a central role played by the intestinal microbiota in driving this phenotype (Supplementary Figure 6). These results suggest that impaired innate immunity to flagellin promotes development of inflammation, in part, as a result of dysbiosis, which promotes exacerbated adaptive immune responses that drive chronic inflammation.
Figure 7.
Deletion of TLR5 and NLRC4 from Rag1-KO mice promotes expansion of exogenously administered effector T cells, thus exacerbating colitis. A and B, Rag1-KO and T5/N4/Rag-TKO mice were intravenously injected with 1 × 106 WT CD45RBhigh CD4+ T cells. B, Peripheral blood levels of CD4 T cells 2 weeks post transfer. C–E, Four weeks post transfer, mice were euthanized enabling assay of total CD4 T cells in mesenteric lymph node and spleen, and analysis of IFN-γ and IL-17–expressing CD4 T cells after stimulating splenocytes with stimulation cocktail. F–K, Development of colitis was assayed by tracking weight loss (F), spleen appearance/mass (G and H), colon appearance (I), colon weight/length ratio (J), and levels of fecal lipocalin-2 (K). Data are means ± SEM, n = 3–5 mice per condition. Abbreviations: IFN-γ, interferon-γ; IL-17, interleukin-17; Lcn2, lipocalin-2; MLN, mesenteric lymph node; N4, Nod-like receptor 4; NLRC4, Nod-like receptor 4; T5, Toll-like receptor 5; TKO, triple knockout; TLR5, Toll-like receptor 5; WT, wild type. *P ≤ .05, ***P ≤ .001.
DISCUSSION
Ability to manage, and protect oneself from, the large diverse microbial community that colonizes the intestine is essential for health. This requires stable maintenance of commensal and beneficial microbial populations as well as resisting colonization of the pathogenic microbes that are occasionally orally ingested. These tasks are mediated by a multifaceted mucosal immune system, which has considerable redundancy in terms of targeting multiple motifs on most microbes and use of multiple immune mechanisms to target select microbial structures. Such redundancy likely explains why many discrete genetic abnormalities in genes that mediate immune system function often do not by themselves result in overt phenotypes. However, the observation that numerous individual genetic polymorphisms increase risk of developing infectious and chronic inflammatory diseases demonstrates that redundancy is clearly not absolute. The flagellin receptor, TLR5, illustrates this concept in that its dysfunction increases proneness to infection by flagellated pathogens, in both mice and humans [16, 18, 24, 25]. Moreover, TLR5-deficient mice are prone to developing chronic inflammatory diseases, including colitis and metabolic syndrome, although the extent to which they do so is dependent on the composition of microbiota that they acquire [13, 14]. However, in many conditions, TLR5-deficient mice and humans lack an overt phenotype, which we hypothesized reflected immune system redundancy. This study explored this possibility by generating mice lacking TLR5 and NLRC4, which activates the inflammasome in response to detection of flagellin, on an adaptive immune-deficient (Rag1-KO) background. We found that such loss of immunity to flagellin resulted in infections by normally nonpathogenic microbes and proneness to infection by the mouse pathogen Citrobacter rodentium. It also resulted in enhanced expansion of exogenously administered effector T cells and subsequent potentiation of colitis driven by these cells. These phenotypes could be explained, at least in part, by alterations in gut microbiota composition that developed as result of the lack of innate and adaptive immunity to flagellin. Thus, redundancy in the immune system’s ability to detect flagellin is not merely a means of combatting motile pathogens but is generally important in managing the gut microbiota.
Adaptive immunity, especially secretory IgA, is known to play a key role in suppressing levels of flagellated bacteria [6]. Hence, we hypothesized that the spontaneous infections we observed in T5/Rag-DKO and T5/N4-Rag-TKO likely reflected expansion or translocation of flagellated bacteria that would have been kept in check had innate or adaptive immunity to flagellin been functional. However, bacteria present in the abscesses were nonflagellated, which was also true of the Citrobacter strain that more readily colonized T5/N4-Rag-TKO mice. This result is reminiscent of finding that TLR5-KO mice are, at least in some environments, resistant to Salmonella infection [10]. However, that such resistance extends to aflagellate Salmonella argued such resistance was driven by basal phenotype, rather than reflecting that activation of TLR5 during infection aids Salmonella [16]. Such nonspecific resistance correlated with extent of antimicrobial gene expression and thus provided a logical potential underlying mechanism. A more general example of this concept is the observation that mice expressing human defensin 5 (HD-5) are highly resistant to Salmonella infection, even though this antimicrobial peptide lacks direct potent activity against this and other gram-negative bacteria [26]. This phenotype may reflect that HD-5 elicits activation of host defense gene expression [27]. Additionally, expression of HD-5, like loss of TLR5, alters gut microbiota composition, which can be envisaged as contributing to their resistance to colonization by Salmonella [28]. Conversely, that T5/N4-Rag-TKO mice exhibited altered microbiota composition and fecal transplant from these mice to WT mice conferred increased susceptibility to Citrobacter colonization suggests a role for microbiota in this phenotype. Such altered gut microbiota is potentially a consequence of their elevated intestinal expression of lipocalin-2, which has direct antimicrobial activity and impacts microbiota composition. However, irrespective of its potential role in shaping microbiota, we presume that elevated lipocalin-2, and other aspects of basal phenotype, are in fact a consequence of altered microbiota composition. In any case, we speculate that the alteration in microbiota composition resulting from loss of TLR5 and NLRC4 reflects that events activated by these receptors, such as secretion of antimicrobial peptides, will broadly impact gut microbes irrespective of whether individual microbes are flagellated.
In addition to studying innate immunity to flagellin in a reductionist yet in vivo model, a central goal driving this study was to investigate the role of adaptive immunity in the proneness of TLR5-KO to developing colitis. We had hoped to examine evidence of colitis in T5/Rag-TKO as a means of investigating the extent to which such proneness required adaptive immunity. However, inability to maintain such mice precluded this approach. Nor was examining basal phenotype in T5/N4-Rag-TKO mice especially informative in that both they and the closely related T5/N4-DKO lacked histopathological evidence of colitis. However, generation of T5/N4-Rag-TKO provided a useful means to address the extent to which lack of innate immunity to flagellin would impact development of colitogenic effector T cells. We observed that absence of TLR5 and NLRC4 greatly accelerated expansion of such cells and the colitis they caused. Thus, these results suggest that inability to manage microbiota as a result of select discrete innate immune deficiencies results in expansion of effector T cells that can eventuate in colitis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grants numbers DK099071 and DK083890 to A. T. G.). J. Z. is supported by the American Diabetes Association (grants number 1-19-JDF-077). B.C. is supported by a Starting Grant from the European Research Council, an Innovator Award from the Kenneth Rainin Foundation, and a Chaire d’Excellence from Paris University.
Potential conflict of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gewirtz AT. Flag in the crossroads: flagellin modulates innate and adaptive immunity. Curr Opin Gastroenterol 2006; 22:8–12. [DOI] [PubMed] [Google Scholar]
- 2. Vijay-Kumar M, Gewirtz AT. Flagellin: key target of mucosal innate immunity. Mucosal Immunol 2009; 2:197–205. [DOI] [PubMed] [Google Scholar]
- 3. Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol 2007; 29:275–88. [DOI] [PubMed] [Google Scholar]
- 4. McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol 2000; 164:986–93. [DOI] [PubMed] [Google Scholar]
- 5. Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest 2004; 113:1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cullender TC, Chassaing B, Janzon A, et al. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe 2013; 14:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tran HQ, Ley RE, Gewirtz AT, Chassaing B. Flagellin-elicited adaptive immunity suppresses flagellated microbiota and vaccinates against chronic inflammatory diseases. Nat Commun 2019; 10:5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 2001; 167:1882–5. [DOI] [PubMed] [Google Scholar]
- 9. Sanders CJ, Moore DA 3rd, Williams IR, Gewirtz AT. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J Immunol 2008; 180:7184–92. [DOI] [PubMed] [Google Scholar]
- 10. Uematsu S, Jang MH, Chevrier N, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol 2006; 7:868–74. [DOI] [PubMed] [Google Scholar]
- 11. Franchi L, Kamada N, Nakamura Y, et al. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol 2012; 13:449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang B, Chassaing B, Shi Z, et al. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science 2014; 346:861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010; 328:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest 2007; 117:3909–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell Toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 2014; 147:1363–77.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vijay-Kumar M, Aitken JD, Kumar A, et al. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect Immun 2008; 76:1276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol 2005; 175:8051–9. [DOI] [PubMed] [Google Scholar]
- 18. Carvalho FA, Nalbantoglu I, Aitken JD, et al. Cytosolic flagellin receptor NLRC4 protects mice against mucosal and systemic challenges. Mucosal Immunol 2012; 5:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carvalho FA, Nalbantoglu I, Ortega-Fernandez S, et al. Interleukin-1β (IL-1β) promotes susceptibility of Toll-like receptor 5 (TLR5) deficient mice to colitis. Gut 2012; 61:373–84. [DOI] [PubMed] [Google Scholar]
- 20. Bootz F, Kirschnek S, Nicklas W, Wyss SK, Homberger FR. Detection of Pasteurellaceae in rodents by polymerase chain reaction analysis. Lab Anim Sci 1998; 48:542–6. [PubMed] [Google Scholar]
- 21. Benga L, Knorr JI, Engelhardt E, et al. Current distribution of Rodentibacter species among the mice and rats of an experimental facility. J Am Assoc Lab Anim Sci 2019; 58:475–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 2012; 7:e44328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Roy T, Debédat J, Marquet F, et al. Comparative evaluation of microbiota engraftment following fecal microbiota transfer in mice models: age, kinetic and microbial status matter. Front Microbiol 2018; 9:3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andersen-Nissen E, Hawn TR, Smith KD, et al. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J Immunol 2007; 178:4717–20. [DOI] [PubMed] [Google Scholar]
- 25. Hawn TR, Verbon A, Lettinga KD, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires’ disease. J Exp Med 2003; 198:1563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 2003; 422:522–6. [DOI] [PubMed] [Google Scholar]
- 27. Ishikawa C, Tanabe H, Maemoto A, et al. Precursor processing of human defensin-5 is essential to the multiple functions in vitro and in vivo. J Innate Immun 2010; 2:66–76. [DOI] [PubMed] [Google Scholar]
- 28. Bevins CL, Salzman NH. The potter’s wheel: the host’s role in sculpting its microbiota. Cell Mol Life Sci 2011; 68:3675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.