Abstract
Purpose:
We sought to demonstrate the feasibility of a lower-cost, portable method for qualitative and quantitative analysis of the corneal endothelium using a smartphone and slit lamp biomicroscope.
Methods:
In this study at a single academic center, we recruited healthy participants to undergo imaging of the corneal endothelium using both a smartphone-based method and a specular microscope. Participants first had their eyes imaged with a CellChek® NSP-9900 Specular Microscope (Konan™ Medical, Inc.; Irvine, CA, USA). For image capture on the smartphone, a beam of light approximately 0.2mm in diameter was directed to the center of the cornea with a slit-lamp biomicroscope to achieve specular reflection. With 40x zoom on the slit-lamp and 4K video mode set on an iPhone 7 Plus held to an ocular, the corneal endothelium was recorded until the hexagonal pattern of cells was identified, and the sharpest frame from the video selected.
Results:
Videos were analyzed from 14 sets of eyes (average length 2 minutes 40 seconds). The average intraclass correlation coefficient was 0.67 (95% CI 0.43–0.82). The mean difference between smartphone endothelial cell count and specular endothelial cell count was −209 cells/mm2 (standard deviation = 483 cells/mm2), which did not achieve significance (p=0.14). Bland-Altman analysis with simple linear regression showed no proportional bias when comparing the two modalities (coefficient =−0.20; t-value = −0.42; P = 0.68).
Conclusion:
Smartphone specular microscopy is capable of qualitative and quantitative analysis of the corneal endothelium. Further refinement to standardize the light source and automate analysis will increase feasibility.
Introduction
Abnormalities of the cornea pose a significant burden on the well-being and quality of life of patients and are common in the outpatient ophthalmologic setting. Analysis of claims data from a large, national managed-care network in the United States found an estimated overall prevalence of 897 per 100,000 per persons, 60 percent of which were endothelial in origin.1 Diagnosis of these diseases relies upon both gross clinical presentation and imaging techniques. Since the cells of the corneal endothelium are not mitotically active, it is important for clinicians to track the density of these cells over time to monitor dystrophic progression.
Specular microscopy in particular is an imaging modality that provides a magnified view of the corneal endothelium, allowing for perioperative evaluation of the corneal endothelium and preparation of tissue in eye banks for cornea transplantation.2,3 Imaging of the corneal endothelium through a specular microscope or confocal microscope, while relatively straightforward, requires a regular electrical supply, a separate computer with operating system, and equipment that requires significant financial investment (USD 25,000–30,000).4 The cost is prohibitive for many medical practices in the United States, as the indications for which Medicare will reimburse are narrow and limited to suspected endothelium problems (such as cornea dystrophy or edema) or cataract surgery following keratoplasty.5 Specular microscopy for quantitative assessment of endothelial cell density (ECD), therefore, may be less accessible or feasible in low-resource or low-surgical volume settings in the United States and abroad.
In contrast, smartphones are battery powered, portable and rechargeable, and contain both hardware and software in one package. Their ubiquitous distribution throughout the world6 makes such technology a good candidate for development of imaging technology for wide distribution, especially in underserved areas in both the United States and abroad. The rapid spread of smartphone technology over the past decade has had a significant impact on the healthcare industry, as the mobile health (mHealth) industry is estimated to be utilized by 500 million of 1.5 billion smartphone users worldwide.7 When paired with a slit lamp, standard clinical equipment can be outfitted or retrofitted with low-energy usage LED lamps, providing an opportunity for development of corneal imaging technology with decreased cost and increased portability compared to standard specular microscopes. Previous studies have demonstrated the feasibility of using a smartphone to image the corneal endothelium.8 However, there exists a lack of evidence with regard to how imaging with a smartphone-based method compares to the clinical standard of specular microscopy with regard to quantitative analysis of ECD.
In this study, we present a method for imaging of the corneal endothelium using a smartphone and slit lamp biomicroscope. We then describe a human subjects research study in which the corneas of healthy volunteers were imaged and graded using both this novel method and the clinical standard using a specular microscope. Lastly, we employed statistical analysis to test both the internal inter-rater reliability of this new method among graders of different levels of training in addition to correlating results using this novel method to the current clinical standard.
Materials and Methods
Human Subjects Data Acquisition
We sought and received Institutional Review Board approval for a human subjects study recruiting healthy participants without known ocular comorbidity via self-report. Healthy participants were recruited from an academic medical center and medical school campus; patients who had any ocular comorbidities were excluded. Informed, written consent was obtained and participants were compensated with a gift card in the amount of $10 for their participation.
First, the central corneas of each participant were imaged using a CellChek® NSP-9900 Specular Microscope (Konan™ Medicathanks l, Inc.; Irvine, CA, USA). Next, subjects were positioned in front of a SL-D701 photo slit-lamp biomicroscope (Topcon Medical Systems, Inc.; Oakland, NJ, USA) operated by a licensed ophthalmologist with specialization in cornea and external disease. The beam on the slit lamp was reduced to 0.2 mm in diameter, set to maximum brightness, and directed toward the center of the cornea at an angle to achieve specular reflection. An iPhone 7 Plus (Apple Inc., Cupertino, CA, USA) was then freely held by a member of the study team and directed to the ocular of the slit lamp adjusted to 40x zoom. The iPhone was set to record video at 4K (3840×2160p) settings at 30 frames per second to capture videos of the endothelial cell layer (Video 1). The position of the iPhone relative to the ocular was optimized, and then the optical settings on the slit lamp were adjusted to achieve the angle where the specular reflection was brightest on the screen. At this point, the manual focus on the iPhone was employed by tapping on the screen at the area where the cells were visualized to optimize focus on the endothelial layer. Once the characteristic hexagonal pattern of endothelial cells became visible to the observer through the phone with acceptable quality, video recording was stopped. An example of an image acquired using this method compared to the specular microscope, and qualitative pathological features that can be identified, can be seen in Figure 1.
Figure 1.
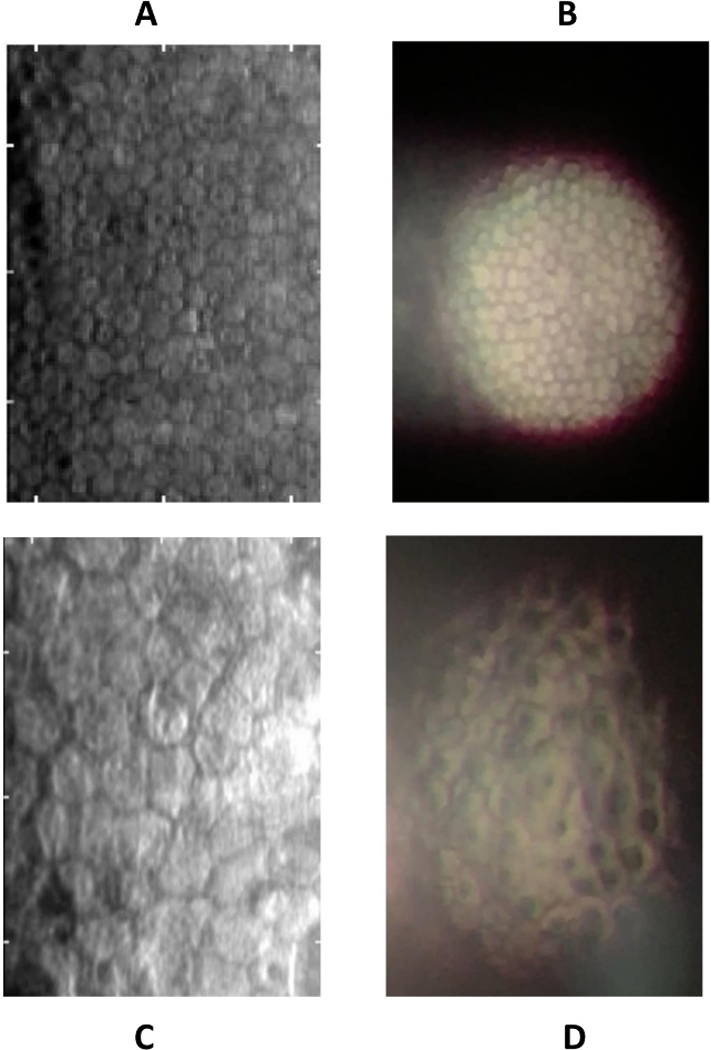
Comparison of Konan specular microscope to smartphone imaging. In a healthy cornea (A), standard specular microscopy reveals characteristic hexagon-shaped endothelial cells. Smartphone specular microscopy (B) demonstrates a circular field of view, with reflective artifact of specular reflection to the left. Qualitative analysis of pathology is seen in a case of posterior polymorphous corneal dystrophy visualized using the standard specular microscope (C), which reveals large endothelial cells and a few apparent opacities. Smartphone specular microscopy highlights intracellular opacities not seen in images of normal corneas.
Data Review and Analysis
Images captured with the specular microscope were analyzed for endothelial cell density of at least 20 contiguous cells using the Flex Center method in the image obtained from each eye by three independent graders (one undergraduate and two medical students). The calculated cell densities were averaged together for analysis. Next, each recorded video was reviewed frame-by-frame by a member of the study team to determine the ideal image for analysis. Images were selected with discernable hexagonal endothelial cells, high contrast and demarcation between the visible cell area and surrounding dark borders, and a complete circular field of view. Each image was captured using the screenshot function on the iPhone, and then imported to a computer for analysis.
Adobe Photoshop® CS, Version 6.0 (Adobe Systems, Inc.; San Jose, CA, USA) was used to analyze images captured from the iPhone, and multiple graders of different training levels independently analyzed each image (one undergraduate student, one medical student, and one ophthalmologist). The work flow for image analysis is illustrated in Figure 2. Using the “count” tool in Photoshop, graders counted at least 20 individual hexagonal cells. Graders were instructed to adjust the brightness and contrast settings as they counted the cells in the case that some cells were hard to discern. For accuracy, they were further instructed to refrain from including cells that were not clearly discernable; this means that there were cells in the captured image that were intentionally not counted. In order to account for these cells, graders were instructed to measure the relative proportion of cells in the field of view that were included in analysis. In order to accomplish this, the Elliptical Marquee and Lasso Tools were used to capture the total area of the field of view compared to the area within which cells were counted, as seen in Figure 2. Thus, the “extrapolated cell count” was calculated using the following relationship:
Figure 2.
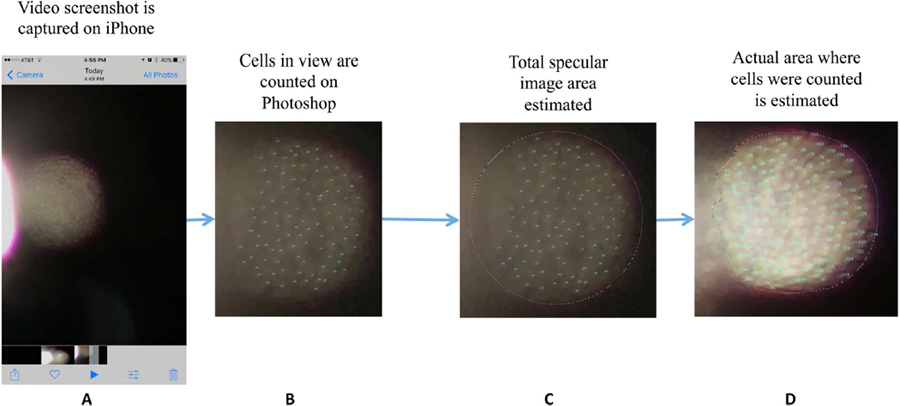
Image analysis for smartphone specular images. The screenshot is obtained on the iPhone (A) and then uploaded to the computer. In Photoshop, the Count tool is used to count the number of endothelial cells in contiguous areas (B). The Elliptical Marquee and Lasso tools are then used to measure the area of the total viewing area (C) and area counted (D), respectively.
Thus, the “extrapolated” number of cells is calculated by dividing the actual cell count by the ratio of the area that contained those cells to the total area that was visualized.
Although we utilized the 0.2 mm light beam setting, we postulated that the true diameter of the beam may be slightly different. To determine the area of the light beam from the machine, the machine was “calibrated” by using the cell density as measured from the specular microscope to estimate the area of the field of view on the smartphone images using the relationship:
The ECD from the specular microscope allowed us to estimate the area of the field of view by employing the relationship between cell density and cell count. We averaged together this calculated area from the right eye images to calibrate a standardized slit lamp area to determine the approximate cell density in the left eye. This calculation workflow is described visually in Supplemental Figure 1. This “calculated” ECD was then compared to the “actual” ECD in the left eye as assessed by the CellChek machine.
Data were analyzed using IBM® SPSS® Statistics for Windows, version 25 (IBM Corporation, Armonk, NY, USA), with p values <0.05 considered statistically significant. Specifically, Intraclass Correlation Coefficients were calculated to compare cell count results obtained from the three different graders of the iPhone images using a Two-Way Mixed model for Absolute Agreement. This model was chosen to account for potential systematic differences between graders. We then used Bland-Altman analysis to compare ECD as calculated from the smartphone images versus the Konan machine to determine level of agreement between our novel method and the specular microscope value.
Results
Study participation was limited to healthy volunteers without known ocular comorbidity. A total of 20 participants were recruited for this study, of which data from 14 were included in the final analysis, meaning six participants were excluded. Data for three participants were lost due to data transfer issues and could not be accessed for analysis. One participant had a pre-existing corneal epithelial defect and was subsequently excluded. Lastly, the images from two participants did not have a round field of view due to them being enrolled as study techniques were being refined and were consequently excluded from the analysis.
Participants ranged in age from 20 to 30 years old (mean age 24). Videos were obtained for all subjects in each eye. The average video length for images collected in the right and left eye were 165 seconds (2 minutes, 45 seconds) and 156 seconds (2 minutes, 36 seconds) respectively. Screenshots of acceptable quality as outlined in the Methods section were obtained for 28 of the 28 videos. The mean slit lamp area from the smartphone images was 0.06 mm2 (standard deviation [SD] 0.02 mm2, 95% confidence interval [CI] 0.026 to 0.087 mm2). The average number of cells counted on the specular microscope images (inclusive of all graders and each eye) was 111.85 cells (SD 30.65 cells, 95% CI 51.77 to 171.92 cells). The average number of cells counted on the smartphone images was 158.66 cells (SD 45.17, 95% CI 70.11 to 249.17).
Extrapolated cell counts were compared among graders to determine the level of reliability of the entire counting and analysis procedure. The highest concordance in extrapolated cell count was observed between the undergraduate grader and medical student grader (Table 1). The average intraclass correlation coefficient was 0.67 (95% CI 0.43–0.82), indicating substantial agreement between raters.9
Table 1.
Inter-Item Correlation Matrix Regarding the Number of Cells Counted on iPhone Specular Images
| Medical Student | Undergraduate Student | Ophthalmologist | |
|---|---|---|---|
| Medical Student | 1.000 | 0.589 | 0.484 |
| Undergraduate Student | 0.589 | 1.000 | 0.386 |
| Ophthalmologist | 0.484 | 0.386 | 1.000 |
1.000 represents perfect agreement, and lower numbers represent lower agreement.
We compared ECD as estimated from the smartphone images (ECD-S) to the ECD calculated by the Konan specular microscope (ECD-K) using two-way t-testing, Bland-Altman plots, and linear regression. Mean calculated ECD-S was 2735.65 cells/mm2 (SD 463.94). The mean difference between ECD-K and ECD-S was −209 cells/mm2 (SD 484 cells/mm2). The mean absolute value difference between the two measurements was 399 cells/mm2, or a 13.8% difference (SD = 385 cells/mm2). There was no statistically significant difference between ECD-S and ECD-K by two-way paired t-test (p-value = 0.14). We then performed Bland-Altman plot analysis—a widely used test designed to explore the level of agreement between two measurements10—to compare the ECD-S and ECD-K (Figure 3). There were eight points above the average mean difference line and six below. In order to determine if this data represented a statistically significant proportional bias, a linear regression analysis was performed, where the dependent variable was the difference between ECD measurements and the independent variable was the mean ECD measurement. The null hypothesis is that the coefficient for the mean ECD value was zero. If the null hypothesis is rejected, there is proportional bias; if we fail to reject the null hypothesis, then it is concluded that there is no proportional bias. In our analysis, we failed to reject the null hypothesis (coefficient =−0.20; t value = −0.42; p value= 0.68). This means that our data did not have a statistically significant trend where more data points were above or below the mean difference line in a way that was proportionally biased in either direction.
Figure 3.
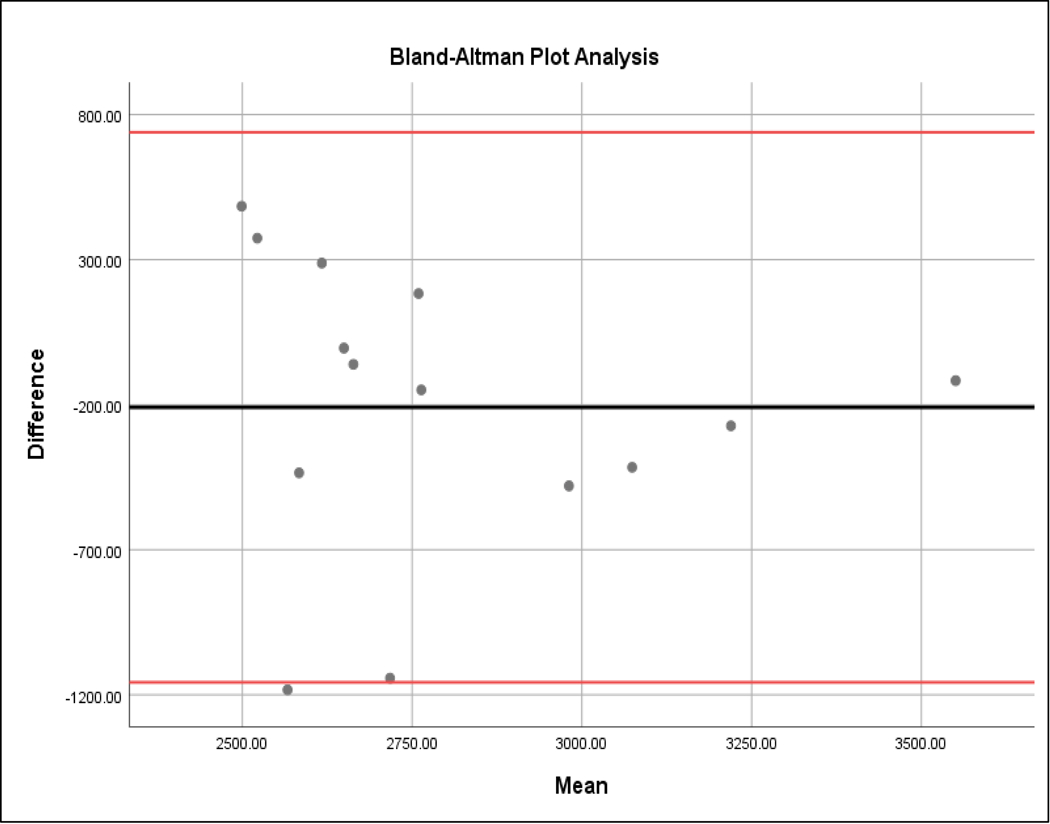
Bland-Altman plot relating the difference between smartphone and Konan ECD values. On the x-axis is the mean of the smartphone ECD and Konan ECD measurements (cells/mm2). On the y-axis is the difference between the two measurements (i.e. Smartphone count – Konan count in cells/mm2). The solid middle horizontal line represents the mean difference of −209 cells/mm2, and the upper and lower red lines represent the 95% confidence interval (−1011 to 573). Eight data points lie above the median while six are below.
Discussion
In this study, we demonstrate qualitative and quantitative analysis of the corneal endothelium using a smartphone and slit lamp biomicroscope.
Qualitatively, the visibility of cell borders allows the clinician to appreciate polymorphism and the appearance of opacities such as guttae. We demonstrate in Figure 1 an example of pathology in posterior polymorphic corneal dystrophy, in which the smartphone highlights intracellular opacities that are seemingly less visible with the clinical standard specular microscope. Further analysis in eyes with pathology, including corneal edema, will be necessary to determine additional advantages that may exist with this technique.
We found no statistically significant difference between the estimated cell density as calculated from our smartphone images and the Konan cell density count (p-value = 0.14). The mean absolute value difference of 13.8% between smartphone specular microscopy and standard specular microscopy was −209 cells/mm2; this difference is comparable to another comparison of two different models of non-contact specular microscopes—the Topcon SP3000P and the Konan Noncon Robo SP8000—reported by Gasser et al.11 In their series of 34 eyes of 18 consecutive patients presenting to the cornea clinic, the Konan microscope gave an average 187 cells/mm2 higher endothelial cell density (ECD) value than the Topcon, while both the Hexagonal cell ratio (HEX) and coefficient of value (CV) measurements demonstrated only weak correlation between the two instruments. This study demonstrated that there are differences in this imaging modality between validated instruments themselves, and potentially explains the difference between values deduced from the smartphone technique and the Konan machine. Further refinement can include protocol adjustments to consider the number of cells counted and optimize agreement between graders.
Inter-rater reliability analysis revealed that this process can be executed with satisfactory reproducibility. Our graders were of varying levels of clinical expertise with regard to ophthalmologic evaluation, from novice (undergraduate student) to more advanced (ophthalmologist), implying that the image analysis methodology can be executed by individuals at varying levels of medical expertise. We propose to further refine this method with the ultimate goal of expanding analysis of the corneal endothelium to areas where specular microscopes are less prevalent. Further investigation using this method must expand to a broader range of individuals in clinical settings, such as optical or eye bank technicians, nurses, or advanced practice providers.
There are both limitations and opportunities for improvement that can be identified from the results of our study. A challenge in obtaining images is the small area being targeted and obtaining the appropriate depth of focus. While eye banks may find it relatively straightforward to image tissue loaded and immobile in a viewing chamber, in our study we found that patients’ head movement in an anterior-posterior fashion can affect the ability to focus light. We addressed this challenge by using video from which images were extracted on a frame-by-frame basis and periodically brought back into focus by the recorder. Also, the average image acquisition time for our study was greater than two minutes, during which the participants were exposed to a bright light. However, all participants were asked and none expressed significant discomfort during this time, given that the beam of light was of minimal diameter. This average video length included optional times for patients to take several second breaks from video capture, which the majority of participants elected not to take. As the study progressed, the average video length was observed to decrease, likely due to refinement of the method with study team experience. Efforts are now underway to automate the process. A report by Daniel et al. demonstrated early “real-world” feasibility of neural networks automate endothelial cell density counts with good agreement when compared to manual counts.12
In this initial phase, this technique requires specific access to image processing software and a clinical standard specular microscope. Future refinement of this method could include a device with a standardized slit-beam size. Once the specific slit lamp beam area is determined and calibrated, the data used from the specular microscope is no longer a necessary input, and ECD measurements can be made independently. As this method is continually refined, a basic mobile phone application could be developed that would allow the examiner to take a video, quickly select a frame, count a number of cells and outline the area being analyzed; an endothelial cell density would be provided as output.
In this study, we sought to demonstrate feasibility, and therefore a small number of healthy, young volunteer subjects were enrolled. Pathology of the corneal endothelium can manifest in various ways, from changes in the physical shape of the cells to changes in cell density based on specific patterns of each disease. The relatively small sample size of 14 sets of eyes impacts the generalizability, applicability, and statistical significance of our study results, highlighting the opportunity for further study of this technique in a larger sample size of patients both with and without corneal disease.
In contrast to a standard specular microscope, the advantages of a smartphone include increased portability, potential for battery power, small space requirement, lower cost and high prevalence of slit lamps. Current limitations include the necessity of a smartphone with a high-resolution camera, slit lamp with adequate magnification, and technical skill required. In summary, we describe a novel method for visualizing and analyzing the corneal endothelium using a smartphone and slit lamp that has high interrater reliability and describes cell density comparable to clinical standard specular microscopy.
Supplementary Material
Supplemental Figure 1. An illustrative example of calibration method of slit-lamp beam area for smartphone specular microscopy. If unknown, the area of illumination from the slit lamp can be determined by dividing the total estimated number of cells in the smartphone image (A) by the cell density calculated from imaging by the specular microscope (B). This is then confirmed using a pair of images from the second eye, in which the area is used to calculate cell density from the smartphone image (C) and compared to the specular microscope (D).
Supplemental Video 1. Video of image capture technique. Briefly, the slit-beam on the slit lamp is set to 0.2mm and maximum brightness, with 40x magnification. Specular reflection is achieved by shining the light at an angle at which the endothelial reflection can be captured. The hexagonal pattern of the endothelial cells is appreciated, and a single frame can be extracted from the video.
Acknowledgements
We would like to thank the David and Cynthia Tolsma Fund for their generosity in supporting this work, the Special Scholar Award from Research to Prevent Blindness, and our volunteer subjects for their gracious involvement.
Source of Funding
A.O.E. is supported by the Research to Prevent Blindness Sybil B. Harrington Special Scholar Award and the Tolsma family. He has ownership interest in Treyetech and LuckyVision, LLC.
Footnotes
Conflicts of Interest
The remaining authors have no financial disclosures or conflicts of interest to report.
References
- 1.Musch DC, Niziol LM, Stein JD, et al. Prevalence of Corneal dystrophies in the United States: Estimates from Claims Data. Invest Ophthalmol Vis Sci 2011;52:6959–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eghrari AO, Riazuddin SA, Gottsch LD. Fuchs Corneal Dystrophy. Expert Rev Ophthalmol 2010;5:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson AJ, Robinson FO, Frazer DG, et al. Corneal guttata: a comparative clinical and specular micrographic study. Eye 1999;13:737–743. [DOI] [PubMed] [Google Scholar]
- 4.Bonnell AJ, Cymbor M. Under the Specular Microscope. Review of Optometry August 15, 2012. Available at: https://www.reviewofoptometry.com/article/under-the-specular-microscope. Accessed 1 August 2019. [Google Scholar]
- 5.Center for Medicare & Medicaid Services. National Coverage Determination (NCD) for Endothelial Cell Photography (80.8) August 31, 1992. Available at: https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=213&ncdver=1&bc=AAAAQAAAAAAA&. Accessed 14 October 2019. [PubMed]
- 6.Pew Research Center. Smartphone Ownership and Internet Usage Continues to Climb in Emerging Economies February 22, 2016. Available at: http://s1.pulso.cl/wp-content/uploads/2016/02/2258581.pdf. Accessed August 10, 2019. [Google Scholar]
- 7.Research2guidance. Mobile Health Market Trends and Figures 2013–2017, the Commercialization of mHealth Applications 2013. Available at: https://research2guidance.com/wp-content/uploads/2015/08/Mobile-Health-Trends-and-Figures-2013-17-Preview.pdf. Accessed 1 April 2019. [Google Scholar]
- 8.Toslak D, Thapa D, Erol MK, et al. Smartphone-based imaging of the corneal endothelium at sub-cellular resolution. Journal of Modern Optics 2017;64: 1229–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viera AJ, Garrett JM. Understanding Interobserver Agreement: The Kappa Statistic. Family Medicine 2005;37:360–3. [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- 11.Gasser L, Reinhard T, Böhringer D. Comparison of corneal endothelial cell measurements by two non-contact specular microscopes. BMC Ophthalmol 2015;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel MC, Atzrodt L, Bucher F, et al. Automated segmentation of the corneal endothelium in a large set of ‘real-world’ specular microscopy images using the U-Net architecture. Scientific Reports 2019;9:4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. An illustrative example of calibration method of slit-lamp beam area for smartphone specular microscopy. If unknown, the area of illumination from the slit lamp can be determined by dividing the total estimated number of cells in the smartphone image (A) by the cell density calculated from imaging by the specular microscope (B). This is then confirmed using a pair of images from the second eye, in which the area is used to calculate cell density from the smartphone image (C) and compared to the specular microscope (D).
Supplemental Video 1. Video of image capture technique. Briefly, the slit-beam on the slit lamp is set to 0.2mm and maximum brightness, with 40x magnification. Specular reflection is achieved by shining the light at an angle at which the endothelial reflection can be captured. The hexagonal pattern of the endothelial cells is appreciated, and a single frame can be extracted from the video.


