Abstract
circRNAs are present in human ovarian tissue, but how they regulate ovarian function remains unknown. In the current study, we investigated the levels of circRNAs in granulosa cells (GCs) derived from human follicular fluid, explored their correlation with female ovarian reserve function and clinical outcomes of assisted reproduction technique (ART), and investigated their effects on the biological functions of GC cell lines (COV434) in vitro. We identified that the levels of circDDX10 in GCs decreased gradually with aging (P < 0.01) and was positively correlated with AMH (r = 0.45, P < 0.01) and AFC (r = 0.32, P < 0.01), but not with FSH and estradiol (P > 0.05). Additionally, circDDX10 was related to the number of oocytes obtained, and good quality embryo rates. Silencing circDDX10 in GCs could markedly up-regulate the expression of apoptosis-related factors, reduce cell proliferation activity, inhibit the expression of steroid hormone synthesis-related factors, and prohibit the synthesis of estradiol. On the contrary, over-expression of circDDX10 had the opposite effect. circDDX10 is expected to become a novel biomarker for predicting the outcomes of ART, and may participate in the regulation of ovarian function by affecting the proliferation and apoptosis of GCs and steroid hormone synthesis.
Keywords: circDDX10, ovary, granulosa cell, assisted reproductive technology, aging
INTRODUCTION
Ovarian senescence, characterized by the reduction of the quantity and quality of oocytes, is the key factor affecting the outcomes of assisted reproductive technology (ART) [1]. Granulosa cells (GCs), as an important component of follicles, provide a basic follicular microenvironment that affects oocyte development [2]. Apoptosis of GCs is closely related to follicular atresia, which is crucial to follicle selection [3]. Moreover, various active peptides secreted by GCs, such as inhibin, activin, follistatin, etc., can also regulate follicle development [4]. Many studies have tried to use the transcriptome of GCs as a non-invasive method to deeply understand the microenvironment around oocytes, as well as predict the developmental potential of oocytes [5, 6]. However, due to species heterogeneity and the instability of linear RNAs, there is a lack of consistency between studies.
Non-coding RNAs (ncRNAs), including miRNA, piRNA, and lncRNA, are key members of the epigenetic regulation network [7, 8]. Recently, researchers have discovered their involvement in the regulation of ovarian function [9, 10]. More importantly, a new type of ncRNA, circular RNA (circRNA), has been identified in the ovary of various mammals. This may relate to ovarian activation and oviposition [11–13]. circRNAs have already been proven as a new kind of biomarker in various human diseases due to their particular biological properties. For example, circRNAs are resistant to RNase R digestion as compared to their linear transcripts [14]. Interestingly, one study recently reported circRNA profiles in human GCs during maternal aging and tried to uncover age-related circRNA variations that potentially reflect decreased oocyte competence [15]. They revealed that age-related up-regulation of circRNA_103827 and circRNA_104816 might be potential indicators of a compromised follicular micro-environment. This could be used to predict IVF prognosis, although the mechanism remains to be explored [15]. Another study demonstrated that circINHA promoted GCs proliferation and inhibited GCs apoptosis via connective tissue growth factor as a competing endogenous RNA that directly bound to miR-10a-5p [16]. More recently, researchers found that loss of hsa_circ_0118530 inhibited KGN cell (human granulosa-like tumor cell line) injury by sponging miR-136 [17]. In our previous work, we explored the potential roles of circRNAs in human ovarian senescence using human ovarian samples [18]. We found the ovary-derived circRNA, circDDX10, was highly expressed in human GCs, and that it gradually decreased with aging. Bioinformatics demonstrated that circDDX10 could act as miRNA sponges to participate in the process of ovarian aging.
The aim of this study was to further elucidate the role of ovary-derived circDDX10 on the biological behaviours of GCs that could potentially reflect oocyte competence, thus offering novel insights into the complex molecular network of ovarian senescence.
RESULTS
Baseline information of the participants
We collected 239 human follicular fluid samples. After extracting GCs by method of Percoll density gradient centrifugation, a total of 210 women were enrolled in this study. The participants’ demographics and baseline characteristics, including age, BMI, duration of infertility, female baseline hormone level, ovarian stimulation protocol, and treatment method are presented in Table 1.
Table 1. Demographic and clinical characteristics of the enrolled participants (n = 210).
| Variable | Mean | SD | n (%) |
| Age, mean | 31.7 | 4.7 | |
| 20–25 | 17 (8.1) | ||
| 26–30 | 75 (35.7) | ||
| 31–35 | 73 (34.8) | ||
| 36–40 | 37 (17.6) | ||
| > 40 | 8 (3.8) | ||
| BMI a | 22.3 | 3.6 | |
| < 18.5 | 24 (11.5) | ||
| 18.5–24.9 | 142 (68.3) | ||
| 25-27.9 | 26 (12.5) | ||
| > 28 | 16 (7.7) | ||
| Duration of infertility (years) b | 4.0 | 3.2 | |
| Types of infertility | |||
| Primary infertility | 111 (52.9) | ||
| Secondary infertility | 99 (47.1) | ||
| Female baseline hormone level | |||
| FSH (IU/l) | 7.5 | 2.6 | |
| LH (IU/l) | 5.2 | 3.4 | |
| Estradiol (pg/ml) | 50.8 | 43.7 | |
| Progesterone (ng/ml) | 0.7 | 1.0 | |
| AMH (ng/ml) c | 3.6 | 2.8 | |
| AFC d | 19.7 | 11.3 | |
| Ovarian stimulation protocol | |||
| Long protocol | 59 (28.1) | ||
| Prolonged protocol | 85 (40.5) | ||
| Antagonist protocol | 66 (31.4) | ||
| Treatment method | |||
| IVF | 154 (73.3) | ||
| ICSI | 56 (26.7) | ||
| Sperm source | |||
| Husband | 153 (72.9) | ||
| Donor | 57 (27.1) |
aBMI was recorded in 208 participants. bDuration of infertility was documented in 187 participants. cAMH was detected in 189 participants. dAFC was monitored only in 141 participants. AFC, antral follicle count; AMH, anti-Müllerian hormone; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; PPOS, progestin-primed ovarian stimulation; SD, standard deviation.
Characteristics and biological properties of circDDX10
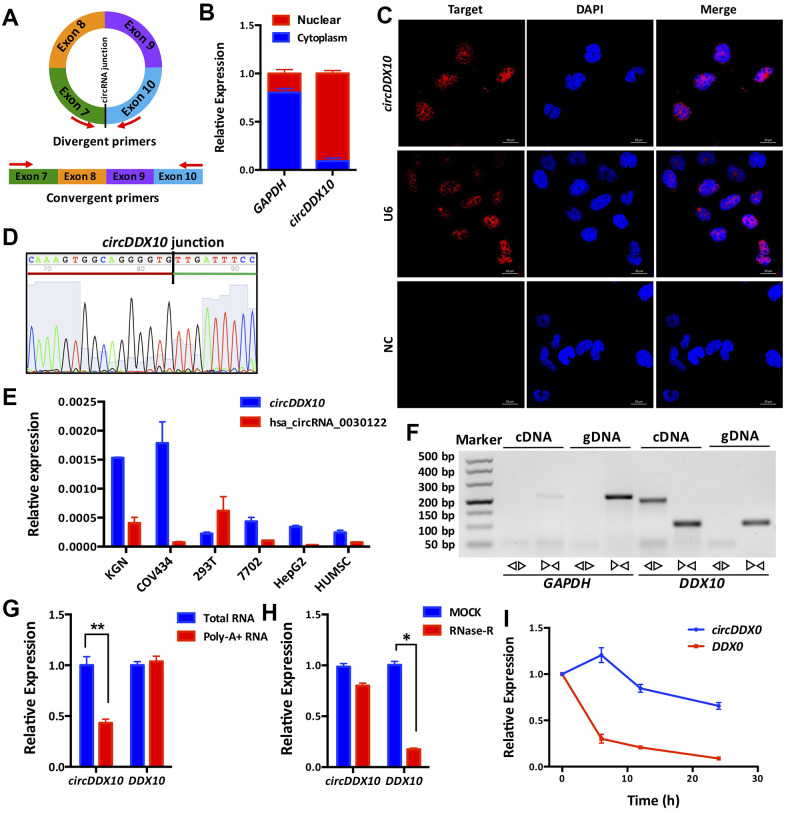
Similar to the common circRNAs, circDDX10 is back-spliced from “head” to “tail” of exon 7 and 10 of its host gene DDX10 (Figure 1A, see detailed information of circDDX10 in Supplementary Table 1). By isolating the nuclear and cytoplasmic RNA from GCs followed by quantitative RT–PCR (qRT–PCR), we found that circDDX10 was mainly located in the nucleus (Figure 1B). This was further confirmed by FISH and Sanger sequencing (Figure 1C, 1D). As derived from the ovary tissue, circDDX10 was highly enriched in GC lines (KGN and COV434) but not in other cell lines (293T, 7702, HepG2 and HUMSC), suggesting its tissue-specific expressing pattern (Figure 1E). We then validated the general biological characteristics of circDDX10. As the results demonstrated, circDDX10 should be amplified in vitro by using back-to-back primers and cDNA as a template. However, it could not be amplified using face-to-face primers and/or gDNA as a template (Figure 1F). circRNA was successfully reverse-transcribed and amplified in vitro using total RNA, but could not be amplified after reverse transcription using Poly-A+ RNA (Figure 1G). Similar to other circRNAs, circDDX10 was also resistant to RNase R digestion, whereas its corresponding linear transcript was rapidly degraded by RNase R (Figure 1H). The level of circDDX10 was stable within 24 h at room temperature, while its corresponding linear transcript rapidly degraded (Figure 1I).
Figure 1.
Subcellular localization, expression levels and biological properties of circDDX10 in granulosa cells (GCs). (A) Schematics of back-to-back and face-to-face primers (black vertical line indicates the back-splicing site; red arrow represents the amplification direction of the upstream and downstream primers). (B) CircDDX10 nucleoplasm distribution in COV434 (granulosa cell line). (C) Subcellular localization of circDDX10 in COV434 using FISH technique. (U6 was used as a positive control for nuclear. NC, negative control. Bar = 20 μm). (D) Sanger sequencing of circDDX10. (Black vertical line indicates the back-splicing site. Red horizontal line representing 3' end and green horizontal line represents 5' end). (E) The relative expression levels of circDDX10 in different cell lines (hsa_circRNA_0030122 was used as control). (F) Properties of back-splicing. (gDNA and cDNA were used as templates. And back-to-back primers and face-to-face primers were used for amplification. GAPDH as an internal control). (G) poly-A- RNA characterization. (Total RNA and poly-A+ RNA were used for reverse transcription into cDNA, respectively). (H) Properties of resistance to RNase R digestion. (I) Properties of stability. All experiments were repeated for three times and the data were expressed as mean ± standard deviation. *, P < 0.05; **, P < 0.01.
Expression levels of circDDX10 in GCs from human follicular fluids
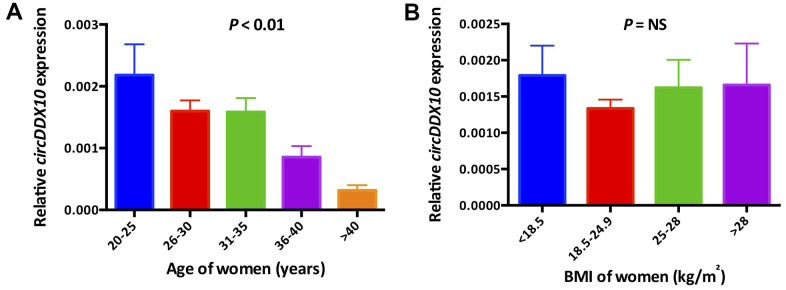
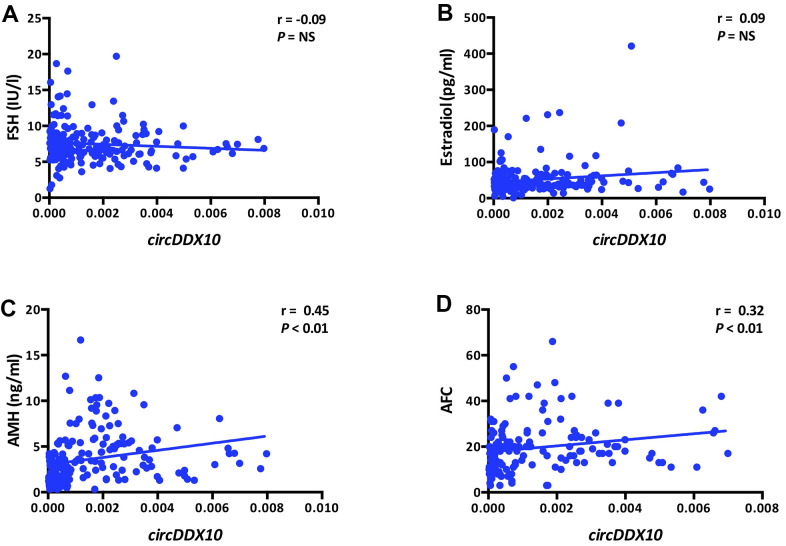
The expression level of circDDX10 in GCs from human follicular fluids decreased steadily with aging (P < 0.01, Figure 2A), whereas no significant differences were discovered with BMI (P > 0.05, Figure 2B). Further analysis demonstrated that there was no remarkable correlation between the level of circDDX10 in GCs from human follicular fluids and FSH or estradiol (both P > 0.05, see in Figure 3A, 3B). However, it was positively correlated with AMH (r = 0.45, P < 0.01, Figure 3C) and antral follicle count (AFC) (r = 0.32, P < 0.01, Figure 3D).
Figure 2.
The expression levels of circDDX10 in granulosa cells from 210 human follicular fluid stratified according to female age (A) and body mass index (B). BMI, body mass index.
Figure 3.
Correlations between the levels of circDDX10 in granulosa cells (GCs) from human follicular fluid and reproductive hormones (FSH, estradiol and AMH, A–C) and antral follicle counts (AFC, D). Serum FSH, LH, estradiol and progesterone levels were tested in all women (n = 210). AMH level and antral follicle count were assayed in 189 and 141 women, respectively.
Association between circDDX10 levels and ovarian reserve
A subgroup analysis of circDDX10 expression levels of the 210 human follicular GC samples divided by the quartiles (Q1, Q2, Q3, Q4) showed significant differences among the age, AMH, AFC, and infertility years (P < 0.01). That is, the higher the age and the longer the infertility duration, the lower the expression level of circDDX10. Additionally, the higher the AMH levels and AFC, the higher the expression level of circDDX10. No significant differences were exhibited in the subgroups regarding BMI, FSH, and estradiol (P > 0.05, Table 2).
Table 2. Association between the levels of circDDX10 in granulosa cells (GCs) from human follicular fluids and the baseline reproductive parameters.
| Parameters | Quartile of the levels of circDDX10 in GC from human follicular fluid | P value | |||
| Q1 | Q2 | Q3 | Q4 | ||
| n = 52 | n = 53 | n = 53 | n = 52 | ||
| Age (years) | 32.9 (31.6–34.2) | 33.7 (32.2–35.1) | 30.2 (29.1–31.2) | 29.9 (28.8–31.0) | < 0.01 |
| BMI (kg/m2) | 22.3 (21.3–23.3) | 22.3 (21.3–23.3) | 22.8 (21.9–23.7) | 21.7 (20.6–22.8) | NS |
| FSH (IU/l) | 7.8 (7.0–8.6) | 7.9 (7.1–8.7) | 7.0 (6.6–7.4) | 7.4 (6.6–8.1) | NS |
| Estradiol (pg/ml) | 44.7 (36.8–52.6) | 47.8 (39.3–56.3) | 49.6 (38.3–60.8) | 61.6 (43.1–80.1) | NS |
| AMH (ng/ml)a | 2.0 (1.7–2.4) | 2.5 (1.8–3.1) | 5.5 (4.5–6.6) | 4.4 (3.7–5.1) | < 0.01 |
| AFCb | 14.9 (12.2–17.6) | 16.9 (13.1–20.7) | 25.4 (20.2–30.6) | 21.4 (18.7–24.1) | < 0.01 |
| Duration of infertility (years)c | 4.6 (3.5–5.7) | 5.0 (3.9–6.1) | 3.4 (2.8–4.0) | 3.1 (2.2–4.0) | < 0.01 |
aAMH was detected in 189 participants. bAFC was monitored only in 141 participants. cDuration of infertility was recorded in 187 participants. Data are shown as mean (95% CI). AFC, antral follicle count; AMH, anti-Müllerian hormone; GC, granulosa cell; NS, not statistically significant; Q, quartile.
Correlation of circDDX10 levels and assisted reproductive technology outcomes (ART)
Considering the ART outcomes, we found remarkable differences in the number of oocytes retrieved in each subgroup. The number of oocytes retrieved in Q3 and Q4 were significantly higher than those in the Q1 and Q2 subgroups (P < 0.01). In terms of normal fertilization rate, no significant difference was observed between the groups (P > 0.05), regardless of the overall fertilization rate, the IVF cycle, or the ICSI cycle, respectively. As for the good quality embryo rate, similar findings were observed. Specifically, the good quality embryo rate in the Q3 and Q4 groups were significantly higher than in the Q1 group (P < 0.05). Nevertheless, no significant differences were shown regarding the positive βhCG rate and clinical pregnancy rate, although they appeared to be positively correlated as a whole (P > 0.05, Table 3).
Table 3. Association between the levels of circDDX10 in granulosa cells (GCs) from human follicular fluids and reproductive outcomes of assisted reproductive technology (ART).
| Parameters | Quartile of the level of circDDX10 in GC from human follicular fluid | P value | |||||||
| Q1 | % | Q2 | % | Q3 | % | Q4 | % | ||
| Oocytes retrieved | |||||||||
| Total | 483 | – | 461 | – | 683 | – | 642 | – | – |
| Mean | 9.9 (7.8–11.9) a | – | 9.4 (7.7–11.1) a | – | 13.4 (11.7–15.) b | – | 12.6 (10.9–14.3) b | – | < 0.01 |
| Normal fertilization rate | |||||||||
| Total | 266/452 | 58.8 | 284/451 | 63.0 | 404/640 | 63.1 | 367/597 | 61.5 | NS |
| IVF cycles | 142/268 | 53.0 | 209/337 | 62.0 | 301/496 | 60.7 | 298/505 | 59.0 | NS |
| ICSI cycles | 124/184 | 67.4 | 75/114 | 65.8 | 103/144 | 71.5 | 69/92 | 75.0 | NS |
| Good quality embryo rate | |||||||||
| Total | 126/266 a | 47.4 | 156/284 a, b | 54.9 | 259/404 b, c | 64.1 | 240/367 c | 65.4 | < 0.01 |
| IVF cycles | 74/142 a | 52.1 | 124/209 a, b | 59.3 | 202/301 b | 67.1 | 196/298 b | 65.8 | < 0.01 |
| ICSI cycles | 52/124 a | 41.9 | 32/75 a, b | 42.7 | 57/103 a, b | 55.3 | 44/69 b | 53.6 | < 0.05 |
| β-hCG positive rate | |||||||||
| Total | 14/23 | 60.9 | 18/25 | 72.0 | 22/28 | 78.6 | 21/25 | 84.0 | NS |
| IVF cycles | 10/17 | 58.8 | 13/18 | 72.2 | 16/21 | 76.2 | 19/21 | 90.5 | NS |
| ICSI cycles | 4/6 | 66.7 | 5/7 | 71.4 | 6/7 | 85.7 | 2/4 | 50.0 | NS |
| Clinical pregnancy rate * | |||||||||
| Total | 9/23 | 39.1 | 11/21 | 52.4 | 19/27 | 70.4 | 18/25 | 72.0 | NS |
| IVF cycles | 8/17 | 47.1 | 8/15 | 53.3 | 13/20 | 65.0 | 16/21 | 76.2 | NS |
| ICSI cycles | 1/6 | 16.7 | 3/6 | 50.0 | 6/7 | 85.7 | 2/4 | 50.0 | NS |
Mean oocytes retrieved are shown as mean (95% CI). a–c, denote the differences between groups (Kruskal-Wallis test and Bonferroni method were used for multiple comparisons, respectively). * A total of five patients’ clinical pregnancy outcomes were unclear. Normal fertilization rates were calculated as: number of 2PN/number of oocytes inseminated. Good quality embryo rates were calculated as: number of good quality embryos/number of 2PN embryos. GC, granulosa cell; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; NS, not statistically significant; Q, quartile; 2PN, two pronuclei.
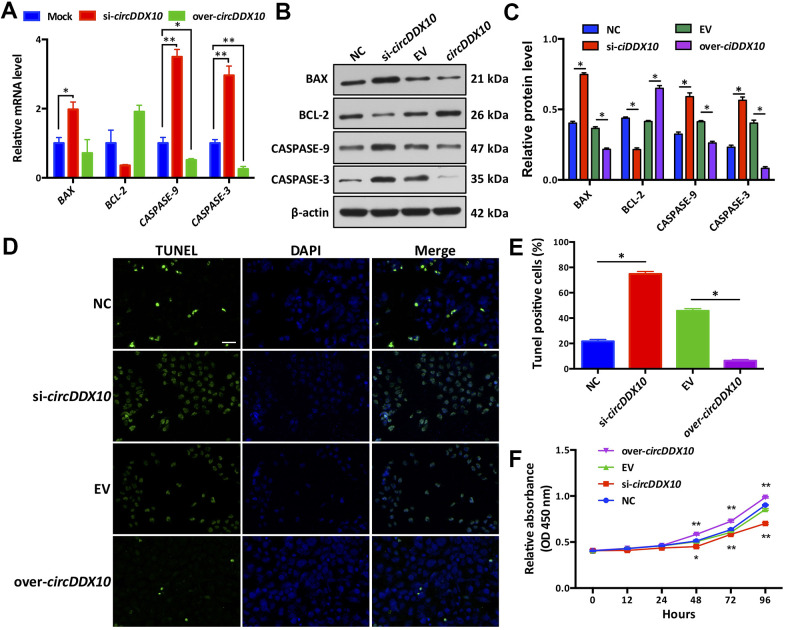
circDDX10 modulated apoptosis and proliferation of GCs
Silencing circDDX10 resulted in the up-regulation of apoptosis-related genes BAX, CASPASE-3, and CASSASE-9 in GCs (P < 0.05), but it had no significant effect on the expression of BCL-2 (P > 0.05). Overexpression of circDDX10 significantly inhibited the levels of CASPASE-3 and CASSASE-9 (P < 0.05), while no remarkable changes were demonstrated on the levels of BAX and BCL-2 (P > 0.05, Figure 4A). Similar changes on the translational level were confirmed. Low expression of circDDX10 promoted the expression of apoptosis-related proteins BAX, CASSASE-3, and CASSASE-9 in GCs, while inhibiting the expression of the BCL-2 protein. Overexpression of circDDX10 played an opposite role (Figure 4B, 4C). Results of TUNEL further supported the above findings (Figure 4D, 4E).
Figure 4.
Effect of circDDX10 on apoptosis and viability of granulosa cells (GCs). (A) Expression levels of apoptosis-related genes. Mock was a negative control group; (B, C) Expression level of apoptosis-related proteins (200×, bar = 100 μm). (D, E) Cell apoptosis was determined by TUNEL. (F) CCK-8 kit was used to detect the cell viability of each group in vitro for 24 h, 48 h, 72 h and 96 h, respectively. NC was a siRNA negative control and EV was an empty vector control group. Each set of experiments was repeated for three times. *, P < 0.05; **, P < 0.01.
We then observed the effects of circDDX10 on the proliferation activity of granulosa cells by using a CCK-8 kit. As the results demonstrated, no significant differences were exhibited on the proliferation activities among the four groups before 48 h of culturing in vitro (P > 0.05). However, this situation changed after 48 h of culturing. The proliferation activity of GCs in the si-circDDX10 group significantly decreased, while a steady increase of proliferation activity of GCs in the over-circDDX10 group was exhibited (P < 0.05, Figure 4F).
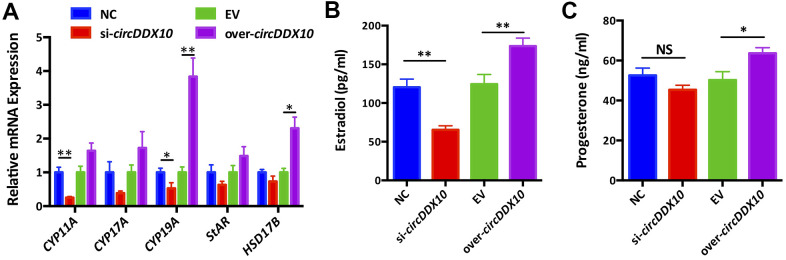
circDDX10 regulated steroidogenesis of GCs
Silencing circDDX10 significantly inhibited the expression levels of CYP11A1 and CYP19A1 (P < 0.05), but it did not change the levels of CYP17A1, StAR, and HSD17B1 (P > 0.05). Overexpression of circDDX10 promoted the levels of CYP19A1 and HSD17B1 (P < 0.05), but no significant changes were exhibited in the expressions of CYP11A1, CYP17A1, and StAR (P > 0.05, Figure 5A).
Figure 5.
The effect of circDDX10 on steroid hormone synthesis of granulosa cells (GCs). (A) The effect of circDDX10 on the expression of steroid hormone synthesis related genes. The effect of circDDX10 on the levels of estradiol (B) and progesterone (C). NC was a siRNA negative control and EV was an empty vector control. Each set of experiments was repeated for three times. *, P < 0.05; **, P < 0.01.
We further detected the changes of estradiol and progesterone levels in the supernatants obtained from each group using chemiluminescence immunoassay. The results demonstrated that the concentration of estradiol was significantly down-regulated in the si-circDDX10 group (Figure 5B, 5C, P < 0.01). However, no significant effect on the concentration of progesterone was observed (P > 0.05). Conversely, overexpression of circDDX10 significantly up-regulated the estradiol (P < 0.01) and progesterone levels (P < 0.05).
DISCUSSION
Age has been a major factor affecting clinical ART outcomes [19]. This study found that the expression level of circDDX10 in human GCs from follicular fluid was negatively correlated with age, but not with BMI. This is consistent with the results of the RNA sequencing in our previous work [18]. That is, circDDX10 shows a down-regulation trend in the aged ovarian tissue, which suggests that circDDX10 may be one of the factors involved in the regulation of ovarian function.
AMH is expressed in GCs of pre-antral follicles and antral follicles. Compared with FSH, AMH is not affected by menstrual cycle, and its expression is more stable [20]. AMH released from GCs of antral follicles is measurable in serum, and its concentration has been shown to be proportional to the number of follicles developed in the ovary [21]. Currently, AMH is recognized as the gold standard for ovarian reserve function and predicting ovarian response to stimulating drugs [20, 22]. Evidences have shown that antral follicle count (AFC) is associated with female reproductive age in fertile women [23]. This study found that the expression level of circDDX10 in GCs derived from human follicular fluid has a positive correlation with serum AMH levels and AFC in infertile women. This suggests that it may be closely related to ovarian reserve function. However, the linear relationship seemed not so strong. To some extent, this could be due to the biological differences between individuals. Moreover, some of the patients presented with polycystic ovarian morphology (PCOM) in response to ovarian stimulation. Lastly, the limited cases included in this study also made our findings not strong enough. At present, the specific relationship between circDDX10 and AMH expressed in GCs is still unclear, and whether there is an interaction between them needs further investigation.
Recently, Cheng et al. [15] discovered and identified two circRNAs (circRNA_103827 and circRNA_104816) in human GCs from follicular fluid that may be associated with reproductive aging. Bioinformatics analysis revealed these two circRNAs were closely related to glucose metabolism. The mitotic cycle and ovarian steroid hormone synthesis may be potential indicators of an impaired follicular microenvironment. Among them, the expression level of circRNA_103827 was significantly up-regulated with age. It was also closely related to the good quality embryo rate and live birth rate in IVF cycles, but its specific mechanism of action has not yet been elucidated. However, by comparing the sequencing results of this study, we did not find the two circRNAs identified by Cheng et al., which may be related to the specimens used in this study and the methods adopted for sequencing. In this study, ovarian cortex specimens were used for sequencing, whereas Cheng et al. used GC specimens derived from follicular fluids. In our study, we used circRNA high-throughput sequencing methods, while Cheng et al. used gene chip technology. There might also be differences in the methods of bioinformatics analysis, prediction, and screening. Our results showed that the expression level of circDDX10 in human follicular GCs was correlated with the number of eggs obtained and the good quality embryo rates. In the high expression levels of the circDDX10 groups (Q3 and Q4), the number of eggs obtained and good quality embryo rates were higher than those in lower level groups (Q1 and Q2). In terms of pregnancy outcomes, βhCG positive rate and clinical pregnancy rate were not statistically different. This may be related to the lack of follow-up period and the limited number of cases included in this study. Therefore, more studies are still needed to clarify the correlation between the levels of circDDX10 and the clinical pregnancy outcomes of ART.
Proliferation and differentiation of GCs is an important part of follicular development [24]. After the primordial follicles are activated, the GCs then express FSHR. FSHR-conjugated G protein on GCs stimulates hormone-sensitive adenylate cyclase after stimulation with FSH. This results in increased intracellular cAMP levels, which further activate the downstream PKA signaling pathway [25, 26]. Apoptosis of GCs is closely related to follicular atresia and it is critical for the selection of dominant follicles, affecting the function of normal ovarian function [3, 27]. BAX and BCL-2 are important genes that regulate GC apoptosis. The BCL-2 protein has anti-apoptotic functions, while the BAX protein can accelerate the induction of GC apoptosis. The dynamic balance is very important for the normal development of follicles [28, 29]. The mechanism of apoptosis is tightly regulated by members of the BCL-2 protein family. In general, the BAX protein forms a heterodimer with BCL-2 to exert biological effects, and the ratio of intracellular BCL-2/BAX can be used as a predictor of cell fate. When the concentration of the BCL-2 protein in the cells is increased, the proportion of BCL-2/BAX is also increased, and the amount of the BCL-2/BAX heterodimer is increased, as well. This can inhibit the occurrence of apoptosis. Conversely, when the proportion of BCL-2/BAX decreases and intracellular mitochondrial membrane permeability increases, the process of apoptosis is accelerated [30, 31]. The CASPASE family consists of important apoptotic proteins, and the most important apoptosis effector protein is CASPASE-3, which can participate in various pathways to induce apoptosis. When intracellular death signalling occurs, it interacts with BCL-2 family members such as BAX, BAK, etc. This activates BCL-2, causing increased mitochondrial membrane permeability, decreased mitochondrial membrane potential, and an increased release of cytochrome C and other proteins. Pigment C forms a multimeric complex by binding to an apoptotic protein activating factor (APAF-1). This recruits the CASPASE-9 precursor to form apoptotic bodies, as well as initiates the CASSASE cascade to activate downstream CASSASE-3 and CASSASE-6. CASSASE-7 (the ultimate performer of apoptosis) ultimately triggers the occurrence of apoptosis [32, 33]. Several studies have confirmed the important role of the BCL-2 family in ovarian apoptosis. The number of follicles in BCL-2 deficient mice is reduced [34], while overexpression of BCL-2 leads to decreased follicular cell apoptosis and atresia [35]. In BAX-knockdown mice, the number of abnormal follicles increased and the number of GCs contained was too large [36]. The BAX protein was significantly expressed in the atretic follicles compared with healthy ovarian follicles [37]. In mice and pigs, CASPASE-9 and APAF-1 have been shown to cause follicular atresia [38, 39]. Luo et al. [40] found that GC activity significantly decreased after heat stress (43° C). Additionally, apoptosis-related gene CASPASE-3 mRNA and protein levels were found in a model of ovarian injury induced by heat stress in mice. The expression level significantly increased. The BAX/BCL-2 ratio also significantly increased. The results of this study demonstrated that the silencing of circDDX10 resulted in increased expression of pro-apoptotic factors BAX, CASPASE-3, and CASSASE-9 in GCs, as well as decreased expression of apoptotic factor BCL-2. Meanwhile, overexpression of circDDX10 significantly inhibited BAX and CASSASE- 3. The expression of CASSASE-9 increased the expression of BCL-2. The TUNEL results also support the above findings. This suggests that with the progression of ovarian aging, the decreased levels of circDDX10 can lead to the increased expression of apoptosis proteins BAX, CASSASE-3, and CASSASE-9, as well as decreased levels of anti-apoptotic protein BCL-2, thus inducing GC apoptosis. The increased apoptosis of GC in turn affects the development of oocytes, which may be one of the mechanisms by which circDDX10 regulates GC apoptosis and participates in ovarian aging.
Hormones can regulate the metabolic activities of various cells and affect the metabolism, growth, and development of organisms. Luteinizing hormone secreted by the pituitary gland during the mid-menstrual period accelerates the production of estradiol and progesterone in GCs, which are essential for uterine receptivity and embryo implantation [41, 42]. In the process of progressive occlusion of follicles, steroid hormones can also be involved in the apoptotic process of GCs as an important influencing factor. As one of the important steroid hormones, estradiol can significantly inhibit the apoptosis of antral follicles and early antral follicles [43]. Progesterone regulates GC apoptosis through a G-like protease pathway [44]. The GSK-3β and ERK1/2 signaling pathways play an important role in the regulation of granule/luteocyte steroid hormone synthesis [45, 46]. To explore the mechanism by which circDDX10 regulates the synthesis and secretion of steroid hormones, we examined the expression of five steroid hormone synthesis-related genes (CYP11A, CYP17A, CYP19A, StAR, and HSD17B). It is well known that StAR plays an important role in the transfer of cholesterol from the mitochondrial outer membrane to the mitochondrial inner membrane, thereby providing a substrate for the biosynthesis of steroid hormones. This is then converted to pregnenolone by the CYP11A enzyme [47, 48]. The conversion of androgen to estradiol requires the involvement of CYP19A [49]. HSD17B contributes to the conversion from estrone to estradiol [50]. It was reported that chemokine treatment can increase the phosphorylation of P38 MAPK in rat GCs, thereby up-regulating the transcription levels of StAR, CYP11A, and 3β-HSD [51]. In the Hsd17b1 knockout mouse model, researchers found that the ovarian Hsd17b enzyme activity was significantly reduced, suggesting a core role of Hsd17b1 in ovarian physiology. The loss of Hsd17b activity leads to an increase in the proportion of ovarian estrone/estradiol, a significant decrease in progesterone concentration in the ovary, and a significant change in the expression of StAR, Cyp11a1, Lhcgr, Hsd17b7, and Cyp17a1 [51]. Our study found that the silencing of circDDX10 induces downregulation of CYP11A1 and CYP19A1 genes involved in granulocyte steroid synthesis, whereas overexpression of circDDX10 promotes expression of steroid hormone synthesis-related genes CYP19A1 and HSD17B1. This in turn affects steroid hormone synthesis. Our results show that circDDX10 may affect the quality and developmental potential of oocytes by affecting steroid synthesis, which may be one of the mechanisms involved in the regulation of ovarian aging.
Advantages and limitations
In this study, the expression level of circDDX10 in the GCs from follicular fluid was detected. Its correlation with ovarian reserve function and clinical outcomes of ART was analyzed. The expression level of circDDX10, ovarian reserve function, and ART were obtained for the first time in GCs from follicular fluid. There is a close relationship between the number of eggs and good quality embryo rates. Additionally, circDDX10 may participate in the regulation of ovarian aging by regulating the proliferation and apoptosis of GCs, as well as the synthesis of steroid hormones. This provides a molecular mechanism for exploring non-invasive biomarkers of assisted reproduction and exploring ovarian aging. However, there are still some limitations in this study. First, the clinical follicular fluid specimens included are limited. Also, there are certain biological differences between individuals, which may interfere with the results. In addition, the follow-up period was not long enough for an analysis of clinical outcomes. Therefore, in the future, it is necessary to further extend the follow-up time by expanding the sample size and analyzing the correlation between the expression levels of circDDX10 and ART clinical outcomes (including abortion rate, live birth rate, etc.). Furthermore, we used the cell line COV434 instead of human primary GCs for in vitro studies. COV434 could not completely mimic the biological function of human primary GCs [52]. Therefore, the interpretations of these results should be taken with caution. Finally, this study only focused on the role of circDDX10 in the biological function of ovarian GCs. We have not observed the effect of circDDX10 on the developmental potential of oocytes. Therefore, more experiments are needed to further explore the interacting proteins or genes of circDDX10 in GCs. It is better for us to observe the effects of circDDX10 on oocyte development by constructing a technique such as the circDDX10 knockout model. Then, we can further clarify the specific molecular mechanism that circDDX10 participates in with ovarian aging.
CONCLUSIONS
In conclusion, we first identified circDDX10, an ovary-derived circRNA, which was stably expressed in GCs from human follicular fluids. Being closely related to female age, ovarian reserve, oocytes retrieved, as well as good quality embryo rates of women undergoing ART treatment, circDDX10 could be a novel and valuable biomarker for predicting ART outcomes. By modulating the apoptosis/proliferation activity and steroid hormone biosynthesis, circDDX10 might participate in the process of ovarian aging. However, in order to further uncover the mechanisms of ovarian aging, more studies on molecular levels are still needed to further elucidate the roles of circDDX10 in ovarian aging.
MATERIALS AND METHODS
Sample collection and preparation
We collected follicular fluid samples from women undergoing oocyte retrieval for IVF/ICSI between February 2017 and March 2018 at the Center of Reproductive Medicine, Tongji Medical College, Huazhong University of Science and Technology. Participants were required to meet the following requirements: 1) age between 20 to 45 years old; 2) unable to conceive naturally for at least 1 year, regardless of male, female, both, or uncertain factors; 3) primary or secondary infertility; and 4) IVF or ICSI cycles fertilized by husband or donor sperm. The mean age of the participants was 35.4 ± 9.3 years old (range from 20 to 45, n = 239). Participants with polycystic ovary syndrome, premature ovarian failure, endometriosis, and other reproductive endocrine diseases, such as thyroid disease, diabetes, adrenal disease, etc., were excluded. The basic information of the patients (n = 210) who qualified for the final analysis is presented in Table 1. This study was approved by the ethics committee of the Tongji Medical College, Huazhong University of Science and Technology (NO. 2016 (04) on April 28, 2016, Wuhan, China. Written informed consent was obtained from each participant in this study.
Isolation, purification and identification of GCs
On the day of oocyte retrieval, all follicular fluid samples from the same patient were pooled after cumulus–oocyte complexes were isolated for conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) procedures. GCs were individually isolated from follicular fluid samples and the purity of the GCs was confirmed using similar methods as previously described [18]. Since they are highly specialized primary luteinized granulosa cells, which produces large amounts of progesterone and estradiol hormones, they could not proliferate neither spontaneously nor after stimulation with a mitogenic agent [53]. The harvested GCs were stored in TRIzol reagent (Life Technologies, CA, USA) at -80° C until RNA extraction.
Cell lines
The GC cell lines (KGN and COV434) were cultured, passaged, and cryopreserved according to previously described methods [54]. FSRH was used as a marker for identification of the GC cell line (Supplementary Figure 1). In this study, COV434 was obtained from a human granulosa cell tumor with specific biological characteristics, including the synthesis of 17 beta-estradiol in response to FSH, the absence of the LH receptor and luteinization capability, and the presence of specific molecular markers of apoptosis enabling the induction of follicular atresia [55]. The cultured COV434 cells were further divided into four groups according to the experimental design.
Plasmid constructs and transient transfections
The siRNA specific interference sequence (si-circDDX10) was designed and synthesized according to the reverse splicing site of circDDX10 (Suzhou GenePharma Co., Ltd, Suzhou, China). The overexpression vector of circDDX10 (over-circDDX10) was designed and constructed by Guangzhou Geneseed Biology Co., Ltd. The GCs (COV434 cell line) were transient transfected using Lipofectamin 2000 (Invitrogen, USA). The specificity of siRNA and the overexpression vector were confirmed by qRT-PCR (see in Supplementary Figures 2, 3). A specific interfering sequence of the linear transcript was also designed to exclude the influence of linear transcription on the experimental results. The ineffective interfering sequence was used as a negative control. All of the information from the oligo sequences are shown in Supplementary Table 2.
cDNA synthesis, PCR, electrophoresis and quantitative real-time PCR (qRT-PCR)
Total RNA was reverse transcribed using random primers with the PrimeScript RT reagent kit (RR047A, Takara, Dalian, China) following the manufacturer’s protocol. At least three pairs of divergent primers encompassing circRNA-specific back-splice junctions were designed for each of the candidate circRNAs. Details of the primer sequences are summarized in Supplementary Table 3. Only primers achieving a single peak in the melting curve were considered sufficient for the qRT-PCR validation. The qRT-PCR and validation experiments were performed using the methods as previously reported [18, 56].
Genomic DNA extraction
Genomic DNA (gDNA) of the ovary samples was extracted using the TIANamp Genomic DNA Kit (DP304, TIANGEN BIOTECH, Beijing, China) following the manufacturer’s instructions. The gDNA fraction was immediately transferred to a 2 ml microfuge tube and stored until further processing at –80° C.
RNase R treatment
Total RNA was incubated with RNase R (1 IU enzyme per μg RNA) in 1× RNase R buffer for 15 min at 37° C, and then quickly placed on ice. The control group was treated with an equal volume of ddH2O. DNase digestion, cDNA synthesis, and qRT-PCR procedures were performed as described above. qRT-PCR Ct values were calculated automatically and ∆Ct was termed as Ct (RNase R treatment) − Ct (mock treatment). The expression of circRNAs and mRNAs before the RNase R treatment were normalized as 1. 2−∆Ct was used to compare the expression of circRNAs and mRNAs after the RNase R digestion.
PolyA+ RNA extraction
PolyA+ RNA was enriched using the kit (MagnosphereTM UltraPure mRNA Purification Kit, Takara, Dalin, China), according to the manufacturer’s protocol manual. The polyA+ RNA fraction was immediately transferred to a 2 ml microfuge tube and stored at –80° C until the next step.
Detection of cellular proliferation and apoptosis
A CCK-8 cell proliferation detection kit ((DOJINDO, Shanghai)) was used to detect the proliferation activity of GCs after transient transfection at 0 h, 24 h, 48 h, 72 h and 96 h, respectively, following the manufacturer’s instructions. The GCs were formalin-fixed 72 h after transfection. The TUNEL method was used to detect the apoptosis of COV434 cells, according to the manufacturer's instructions (Roche, Switzerland).
Chemiluminescence immunoassay
The supernatant of the GCs was collected after centrifugation for 15min at 3500 rpm. Next, we quantified the levels of estradiol and progesterone in the supernatant by using Chemiluminescence Quantitative Immunoassay Kits (Beckman Coulter, USA), and we did so following the manufacturer's instructions as previously described [57].
Western blotting
The cultured GCs were lysed using RIPA protein extraction reagent (Beyotime, Beijing, China) supplemented with a protease inhibitor cocktail (Roche, CA, USA). Protein quantification was determined by the Bradford method (Beyotime, Shanghai, China). Approximately 40 μg of protein extract was separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a nitrocellulose membrane (Sigma-Aldrich, MO, USA). After blocking for 2 h, membranes were incubated with primary antibody (1:1000; GAPDH, #GB12002, Servicebio, China; Caspase-3, #9662, CST, USA; Caspase-9, #GB11053-1, Servicebio, China; Bax, #GB11007, Servicebio, China; Bcl-2, #ab59348, Abcam, USA) overnight at 4° C. Membranes were washed and incubated for 1.5 h with HRP conjugated secondary antibodies (1:2000; ab6721, Abcam, Cambridge, MA, USA). ECL chromogenic substrate was used to visualize the bands, and the band intensity was measured using Image J (version 1.49s, National Institutes of Health, USA).
Statistical analysis
Statistical analyses were performed using SPSS Statistics (version 23.0; IBM, Armonk, NY, USA) and GraphPad Prism 6.0 (version 6.0c; GraphPad Software, Inc., San Diego, CA, USA). All of the data are displayed as the mean ± SEM for triplicate independent measurements. One-way ANOVA was used to assess the differences between groups. Differences with P values < 0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the participants who provided follicular fluid samples in this study. The authors would also like to thank the American Journal Experts (Durham, North Carolina, U.S.A) for language editing.
Footnotes
AUTHOR CONTRIBUTIONS: Conception and design (HCC, WPX); performing experiments (HCC, YML, TLC, YZJ); data collection and analysis (HCC, YML); interpretation (all authors); drafting the manuscript (HCC); critical revision of the manuscript (HCC, PS, WPX).
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: This work was supported by the National Key Research and Development Program of China (2017YFC1002002) and the China Postdoctoral Science Foundation Grant (2019TQ0376).
REFERENCES
- 1.Laisk T, Tšuiko O, Jatsenko T, Hõrak P, Otala M, Lahdenperä M, Lummaa V, Tuuri T, Salumets A, Tapanainen JS. Demographic and evolutionary trends in ovarian function and aging. Hum Reprod Update. 2019; 25:34–50. 10.1093/humupd/dmy031 [DOI] [PubMed] [Google Scholar]
- 2.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015; 103:303–16. 10.1016/j.fertnstert.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 3.Ginther OJ. The theory of follicle selection in cattle. Domest Anim Endocrinol. 2016; 57:85–99. 10.1016/j.domaniend.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006; 132:191–206. 10.1530/rep.1.01074 [DOI] [PubMed] [Google Scholar]
- 5.Fragouli E, Lalioti MD, Wells D. The transcriptome of follicular cells: biological insights and clinical implications for the treatment of infertility. Hum Reprod Update. 2014; 20:1–11. 10.1093/humupd/dmt044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst EH, Nielsen J, Ipsen MB, Villesen P, Lykke-Hartmann K. Transcriptome analysis of long non-coding RNAs and genes encoding paraspeckle proteins during human ovarian follicle development. Front Cell Dev Biol. 2018; 6:78. 10.3389/fcell.2018.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischof O, Martínez-Zamudio RI. MicroRNAs and lncRNAs in senescence: A re-view. IUBMB Life. 2015; 67:255–67. 10.1002/iub.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki YW, Siomi MC, Siomi H. PIWI-interacting RNA: its biogenesis and functions. Annu Rev Biochem. 2015; 84:405–33. 10.1146/annurev-biochem-060614-034258 [DOI] [PubMed] [Google Scholar]
- 9.Bouckenheimer J, Assou S, Riquier S, Hou C, Philippe N, Sansac C, Lavabre-Bertrand T, Commes T, Lemaître JM, Boureux A, De Vos J. Long non-coding RNAs in human early embryonic development and their potential in ART. Hum Reprod Update. 2016; 23:19–40. 10.1093/humupd/dmw035 [DOI] [PubMed] [Google Scholar]
- 10.Barragán M, Pons J, Ferrer-Vaquer A, Cornet-Bartolomé D, Schweitzer A, Hubbard J, Auer H, Rodolosse A, Vassena R. The transcriptome of human oocytes is related to age and ovarian reserve. Mol Hum Reprod. 2017; 23:535–48. 10.1093/molehr/gax033 [DOI] [PubMed] [Google Scholar]
- 11.Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P, Celniker SE, Graveley BR, Lai EC. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep. 2014; 9:1966–80. 10.1016/j.celrep.2014.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao H, Xiong Q, Zhang F, Zhang N, Liu Y, Suo X, Li X, Yang Q, Chen M. Circular RNA profiling reveals chi_circ_0008219 function as microRNA sponges in pre-ovulatory ovarian follicles of goats (Capra hircus). Genomics. 2017; S0888-7543:30129–25. 10.1016/j.ygeno.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Shi W, Chen C. Differential circular RNAs expression in ovary during oviposition in honey bees. Genomics. 2019; 111:598–606. 10.1016/j.ygeno.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 14.Qu S, Zhong Y, Shang R, Zhang X, Song W, Kjems J, Li H. The emerging landscape of circular RNA in life processes. RNA Biol. 2017; 14:992–99. 10.1080/15476286.2016.1220473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng J, Huang J, Yuan S, Zhou S, Yan W, Shen W, Chen Y, Xia X, Luo A, Zhu D, Wang S. Circular RNA expression profiling of human granulosa cells during maternal aging reveals novel transcripts associated with assisted reproductive technology outcomes. PLoS One. 2017; 12:e0177888. 10.1371/journal.pone.0177888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo T, Zhang J, Yao W, Du X, Li Q, Huang L, Ma M, Li Q, Liu H, Pan Z. CircINHA resists granulosa cell apoptosis by upregulating CTGF as a ceRNA of miR-10a-5p in pig ovarian follicles. Biochim Biophys Acta Gene Regul Mech. 2019; 1862:194420. 10.1016/j.bbagrm.2019.194420 [DOI] [PubMed] [Google Scholar]
- 17.Jia C, Wang S, Yin C, Liu L, Zhou L, Ma Y. Loss of hsa_circ_0118530 inhibits human granulosa-like tumor cell line KGN cell injury by sponging miR-136. Gene. 2020; 744:144591. 10.1016/j.gene.2020.144591 [DOI] [PubMed] [Google Scholar]
- 18.Cai H, Li Y, Li H, Niringiyumukiza JD, Zhang M, Chen L, Chen G, Xiang W. Identification and characterization of human ovary-derived circular RNAs and their potential roles in ovarian aging. Aging (Albany NY). 2018; 10:2511–34. 10.18632/aging.101565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholtes MC, Hop WC, Alberda AT, Janssen-Caspers HA, Leerentveld RA, van Os HC, Zeilmaker GH. Factors influencing the outcome of successive IVF treatment cycles in attaining a follicular puncture. Hum Reprod. 1988; 3:755–59. 10.1093/oxfordjournals.humrep.a136779 [DOI] [PubMed] [Google Scholar]
- 20.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, La Marca A, Lambalk C, Mason H, Nelson SM, Visser JA, et al. The physiology and clinical utility of anti-mullerian hormone in women. Hum Reprod Update. 2014; 20:370–85. 10.1093/humupd/dmt062 [DOI] [PubMed] [Google Scholar]
- 21.Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006; 147:3228–34. 10.1210/en.2005-1588 [DOI] [PubMed] [Google Scholar]
- 22.Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-müllerian hormone. Reprod Biomed Online. 2015; 31:486–96. 10.1016/j.rbmo.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 23.Scheffer GJ, Broekmans FJ, Looman CW, Blankenstein M, Fauser BC, teJong FH, teVelde ER. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod. 2003; 18:700–06. 10.1093/humrep/deg135 [DOI] [PubMed] [Google Scholar]
- 24.Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci. 2004; 82:E40–52. 10.2527/2004.8213_supplE40x [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Jin Y, Liu Y, Shen C, Dong J, Xu J. SMAD3 regulates the diverse functions of rat granulosa cells relating to the FSHR/PKA signaling pathway. Reproduction. 2013; 146:169–79. 10.1530/REP-12-0325 [DOI] [PubMed] [Google Scholar]
- 26.Riccetti L, Sperduti S, Lazzaretti C, Casarini L, Simoni M. The cAMP/PKA pathway: steroidogenesis of the antral follicular stage. Minerva Ginecol. 2018; 70:516–24. 10.23736/S0026-4784.18.04282-X [DOI] [PubMed] [Google Scholar]
- 27.Du X, Zhang L, Li X, Pan Z, Liu H, Li Q. TGF-β signaling controls FSHR signaling-reduced ovarian granulosa cell apoptosis through the SMAD4/miR-143 axis. Cell Death Dis. 2016; 7:e2476. 10.1038/cddis.2016.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albamonte MS, Willis MA, Albamonte MI, Jensen F, Espinosa MB, Vitullo AD. The developing human ovary: immunohistochemical analysis of germ-cell-specific VASA protein, BCL-2/BAX expression balance and apoptosis. Hum Reprod. 2008; 23:1895–901. 10.1093/humrep/den197 [DOI] [PubMed] [Google Scholar]
- 29.Felici MD, Carlo AD, Pesce M, Iona S, Farrace MG, Piacentini M. Bcl-2 and Bax regulation of apoptosis in germ cells during prenatal oogenesis in the mouse embryo. Cell Death Differ. 1999; 6:908–15. 10.1038/sj.cdd.4400561 [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Lin Y, Li H, Liu J, Sheng X, Zhang W. 2,5-hexanedione induces human ovarian granulosa cell apoptosis through BCL-2, BAX, and CASPASE-3 signaling pathways. Arch Toxicol. 2012; 86:205–15. 10.1007/s00204-011-0745-7 [DOI] [PubMed] [Google Scholar]
- 31.Ciucci A, Zannoni GF, Travaglia D, Scambia G, Gallo D. Mitochondrial estrogen receptor β2 drives antiapoptotic pathways in advanced serous ovarian cancer. Hum Pathol. 2015; 46:1138–46. 10.1016/j.humpath.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 32.Pirnia F, Schneider E, Betticher DC, Borner MM. Mitomycin C induces apoptosis and caspase-8 and -9 processing through a caspase-3 and Fas-independent pathway. Cell Death Differ. 2002; 9:905–14. 10.1038/sj.cdd.4401062 [DOI] [PubMed] [Google Scholar]
- 33.Julien O, Wells JA. Caspases and their substrates. Cell Death Differ. 2017; 24:1380–89. 10.1038/cdd.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of Bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995; 136:3665–68. 10.1210/endo.136.8.7628407 [DOI] [PubMed] [Google Scholar]
- 35.Hsu SY, Lai RJ, Finegold M, Hsueh AJ. Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology. 1996; 137:4837–43. 10.1210/endo.137.11.8895354 [DOI] [PubMed] [Google Scholar]
- 36.Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999; 21:200–03. 10.1038/5985 [DOI] [PubMed] [Google Scholar]
- 37.Kugu K, Ratts VS, Piquette GN, Tilly KI, Tao XJ, Martimbeau S, Aberdeen GW, Krajewski S, Reed JC, Pepe GJ, Albrecht ED, Tilly JL. Analysis of apoptosis and expression of Bcl-2 gene family members in the human and baboon ovary. Cell Death Differ. 1998; 5:67–76. 10.1038/sj.cdd.4400316 [DOI] [PubMed] [Google Scholar]
- 38.Robles R, Tao XJ, Trbovich AM, Maravel DV, Nahum R, Perez GI, Tilly KI, Tilly JL. Localization, regulation and possible consequences of apoptotic protease-activating factor-1 (Apaf-1) expression in granulosa cells of the mouse ovary. Endocrinology. 1999; 140:2641–44. 10.1210/endo.140.6.6931 [DOI] [PubMed] [Google Scholar]
- 39.Matsui T, Manabe N, Goto Y, Inoue N, Nishihara S, Miyamoto H. Expression and activity of Apaf1 and caspase-9 in granulosa cells during follicular atresia in pig ovaries. Reproduction. 2003; 126:113–20. 10.1530/rep.0.1260113 [DOI] [PubMed] [Google Scholar]
- 40.Luo M, Li L, Xiao C, Sun Y, Wang GL. Heat stress impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol Cell Biochem. 2016; 412:81–90. 10.1007/s11010-015-2610-0 [DOI] [PubMed] [Google Scholar]
- 41.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006; 12:731–46. 10.1093/humupd/dml004 [DOI] [PubMed] [Google Scholar]
- 42.Cavagna M, Mantese JC. Biomarkers of endometrial receptivity—a review. Placenta. 2003; 24:S39–47. 10.1016/s0143-4004(03)00184-x [DOI] [PubMed] [Google Scholar]
- 43.Billig H, Furuta I, Hsueh AJ. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology. 1993; 133:2204–12. 10.1210/endo.133.5.8404672 [DOI] [PubMed] [Google Scholar]
- 44.Peluso JJ, Pappalardo A. Progesterone regulates granulosa cell viability through a protein kinase G-dependent mechanism that may involve 14-3-3sigma. Biol Reprod. 2004; 71:1870–78. 10.1095/biolreprod.104.031716 [DOI] [PubMed] [Google Scholar]
- 45.Yu FQ, Han CS, Yang W, Jin X, Hu ZY, Liu YX. Role of ERK1/2 in FSH induced PCNA expression and steroidogenesis in granulosa cells. Front Biosci. 2005; 10:896–904. 10.2741/1584 [DOI] [PubMed] [Google Scholar]
- 46.Bai L, Chang HM, Cheng JC, Chu G, Leung PC, Yang G. Lithium chloride inhibits StAR and progesterone production through GSK-3β and ERK1/2 signaling pathways in human granulosa-lutein cells. Mol Cell Endocrinol. 2018; 461:89–99. 10.1016/j.mce.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 47.Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology. 1998; 139:303–15. 10.1210/endo.139.1.5694 [DOI] [PubMed] [Google Scholar]
- 48.Gasic S, Bodenburg Y, Nagamani M, Green A, Urban RJ. Troglitazone inhibits progesterone production in porcine granulosa cells. Endocrinology. 1998; 139:4962–66. 10.1210/endo.139.12.6385 [DOI] [PubMed] [Google Scholar]
- 49.Zheng X, Price CA, Tremblay Y, Lussier JG, Carrière PD. Role of transforming growth factor-beta1 in gene expression and activity of estradiol and progesterone-generating enzymes in FSH-stimulated bovine granulosa cells. Reproduction. 2008; 136:447–57. 10.1530/REP-07-0316 [DOI] [PubMed] [Google Scholar]
- 50.Kemiläinen H, Adam M, Mäki-Jouppila J, Damdimopoulou P, Damdimopoulos AE, Kere J, Hovatta O, Laajala TD, Aittokallio T, Adamski J, Ryberg H, Ohlsson C, Strauss L, Poutanen M. The hydroxysteroid (17β) dehydrogenase family gene HSD17B12 is involved in the prostaglandin synthesis pathway, the ovarian function, and regulation of fertility. Endocrinology. 2016; 157:3719–30. 10.1210/en.2016-1252 [DOI] [PubMed] [Google Scholar]
- 51.Hakkarainen J, Jokela H, Pakarinen P, Heikelä H, Kätkänaho L, Vandenput L, Ohlsson C, Zhang FP, Poutanen M. Hydroxysteroid (17β)-dehydrogenase 1-deficient female mice present with normal puberty onset but are severely subfertile due to a defect in luteinization and progesterone production. FASEB J. 2015; 29:3806–16. 10.1096/fj.14-269035 [DOI] [PubMed] [Google Scholar]
- 52.Jamieson S, Fuller PJ. Characterization of the inhibitor of kappaB kinase (IKK) complex in granulosa cell tumors of the ovary and granulosa cell tumor-derived cell lines. Horm Cancer. 2013; 4:277–92. 10.1007/s12672-013-0146-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuksel A, Bildik G, Senbabaoglu F, Akin N, Arvas M, Unal F, Kilic Y, Karanfil I, Eryılmaz B, Yilmaz P, Ozkanbaş C, Taskiran C, Aksoy S, et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum Reprod. 2015; 30:2926–35. 10.1093/humrep/dev256 [DOI] [PubMed] [Google Scholar]
- 54.Rosario R, Blenkiron C, Shelling AN. Comparative study of microRNA regulation on FOXL2 between adult-type and juvenile-type granulosa cell tumours in vitro. Gynecol Oncol. 2013; 129:209–15. 10.1016/j.ygyno.2012.12.034 [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Vollmer M, De Geyter M, Litzistorf Y, Ladewig A, Dürrenberger M, Guggenheim R, Miny P, Holzgreve W, De Geyter C. Characterization of an immortalized human granulosa cell line (COV434). Mol Hum Reprod. 2000; 6:146–53. 10.1093/molehr/6.2.146 [DOI] [PubMed] [Google Scholar]
- 56.Ouyang H, Chen X, Wang Z, Yu J, Jia X, Li Z, Luo W, Abdalla BA, Jebessa E, Nie Q, Zhang X. Circular RNAs are abundant and dynamically expressed during embryonic muscle development in chickens. DNA Res. 2018; 25:71–86. 10.1093/dnares/dsx039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai H, Chen L, Zhang M, Xiang W, Su P. Low expression of MFN2 is associated with early unexplained miscarriage by regulating autophagy of trophoblast cells. Placenta. 2018; 70:34–40. 10.1016/j.placenta.2018.08.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.