Abstract
Typically, peripheral T-cell lymphoma (PTCLs) prognosis is estimated using overall survival before treatment. However, these estimates cannot show how prognosis evolves with the changing hazard rate over time. Patients (n = 650) with newly diagnosed PTCLs were enrolled retrospectively. After a median follow-up of 5.4 years, angioimmunoblastic T-cell lymphoma, peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS) and NK/T cell lymphoma had initially lower 3-year conditional overall survival (COS3; i.e., the 3-year conditional overall survival was defined as the probability of surviving an additional 3 years) and higher hazards of death (26–44.3%). However, after 2 years, the COS3 increased and the death risk decreased over time, whereas anaplastic lymphoma kinase-positive anaplastic large-cell lymphoma constantly had a lower risk over time (0–19.5%). For patients with complete remission after initial treatment, prognosis varied by histological subtypes, with PTCL, NOS having a negative impact. Our data suggested that the risk stratification using the International Prognostic Index might not accurately predict the COS3 for survivors of PTCLs. The COS3 provided time-dependent prognostic information for PTCLs, representing a possible surrogate prognosis indicator for long-term survivors after systemic chemotherapy.
Keywords: lymphoma, T-cell, peripheral, survival analysis, prognosis
INTRODUCTION
Peripheral T-cell lymphomas (PTCLs) are relatively rare and heterogeneous types of aggressive malignancies, accounting for only 10–30% of all cases of non-Hodgkin lymphomas (NHLs) [1–5]. Except for anaplastic lymphoma kinase (ALK)-positive anaplastic large-cell lymphoma (ALK+ALCL), the majority of subtypes have poor prognosis, with a 5-year overall survival (OS) of about 14–56% [6–9].
Previous studies of PTCLs made general predictions of prognosis based on clinical information at diagnosis [10, 11]. However, these estimates become less relevant as prognosis evolves. There are limited data for its prognostic value for patients surviving for a certain period of time. Conditional overall survival, a method providing more accurate information on survival outcome during follow-up, has been proposed [12, 13]. Generally, conditional survival estimates increase with the number of surviving years, especially for patients with advanced-stage disease [14]. These time-dependent statistics of conditional survival for patients over time since treatment can provide more accurate information during follow-up, and have been applied to lymphomas such as Hodgkin lymphoma [15], diffuse large B-cell lymphoma (DLBCL) [16], and NK/T cell Lymphoma (NK/TCL) [17].
However, there are limited data on the conditional survival for PTCLs. This retrospective study aimed to determine the spectrum of conditional survival and to estimate the annual hazards of death for patients with PTCLs.
RESULTS
Baseline characteristics
Baseline characteristics are summarized in Table 1. The median age was 45 years (interquartile range [IQR], 32–59 years), with a ratio of males to females of 2.3:1. NK/TCL was the most frequent subtype, accounting for 43.1%. In addition, 405 (62.3%) patients had advanced disease, and 303 (46.6%) patients were divided into the low-risk group according to the International Prognostic Index (IPI).
Table 1. Patient characteristics.
| Total | AITL | PTCL, NOS | NK/TCL | ALK+ALCL | ALK-ALCL | Others | |
| Overall | 650 | 91 | 65 | 280 | 55 | 33 | 126 |
| Sex | |||||||
| Male | 455 (70.0%) | 57 (62.6%) | 49 (75.4%) | 205 (73.2%) | 41 (74.5%) | 24 (72.7%) | 82 (65.1%) |
| Female | 195 (30.0 %) | 34 (37.4%) | 16 (24.6%) | 75 (26.8%) | 14 (25.5%) | 9 (27.3%) | 44 (34.9%) |
| Age (yr) | |||||||
| < 60 | 502 (77.2%) | 44 (48.4%) | 49 (75.4%) | 242 (86.4%) | 52 (94.5%) | 17 (51.5%) | 98 (77.8%) |
| ≥ 60 | 148 (22.8%) | 47 (51.6%) | 16 (24.6%) | 38 (13.6%) | 3 (5.5%) | 16 (48.5%) | 28 (22.2%) |
| Stage | |||||||
| I-II | 245 (37.7%) | 1 (1.1%) | 10 (15.4%) | 163 (58.2%) | 18 (32.7%) | 20 (60.6%) | 33 (26.2%) |
| III-IV | 405 (62.3%) | 90 (98.9%) | 55 (84.6%) | 117 (41.8%) | 37 (67.3%) | 13 (39.4%) | 93 (73.8%) |
| LDH* | |||||||
| < ULN | 402 (61.8%) | 38 (41.8%) | 36 (55.4%) | 194 (69.3%) | 32 (58.2%) | 22 (66.7%) | 80 (63.5%) |
| ≥ ULN | 248 (38.2%) | 53 (58.2%) | 29 (44.6%) | 86 (30.7%) | 23 (41.8%) | 11 (33.3%) | 46 (36.5%) |
| Extranodal sites | |||||||
| < 2 | 437 (67.2%) | 73 (80.2%) | 45 (69.2%) | 161 (57.5%) | 41 (74.5%) | 26 (78.8%) | 91 (72.2%) |
| ≥ 2 | 213 (32.8%) | 18 (19.8%) | 20 (30.8%) | 119 (42.5%) | 14 (25.5%) | 7 (21.2%) | 35 (27.8%) |
| ECOG | |||||||
| < 2 | 584 (89.8%) | 74 (81.3%) | 59 (90.8%) | 257 (91.8%) | 49 (89.1%) | 32 (97/0%) | 113 (89.7%) |
| ≥ 2 | 66 (10.2%) | 17 (18.7%) | 6 (9.2%) | 23 (8.2%) | 6 (10.9%) | 1 (3.0%) | 13 (10.3%) |
| Risk groups | |||||||
| Low | 303 (46.6%) | 19 (20.9%) | 21 (32.3%) | 154 (55%) | 31 (56.4%) | 19 (57.6%) | 59 (46.8%) |
| Low-intermediate | 192 (29.5%) | 28 (30.8%) | 23 (35.4%) | 76 (27.1%) | 16 (29.1%) | 7 (21.2%) | 42 (33.3%) |
| High-intermediate | 111 (17.1%) | 29 (31.9%) | 17 (26.2%) | 37 (13.2%) | 5 (9.1%) | 5 (15.2%) | 18 (14.3%) |
| High | 44 (6.8%) | 15 (16.5%) | 4 (6.2%) | 13 (4.6%) | 3 (5.5%) | 2 (6.1%) | 7 (5.6%) |
AITL: angioimmunoblastic T-cell lymphoma; ALK: anaplastic lymphoma kinase positive; ALCL: anaplastic large cell lymphoma; PTCL: peripheral T-cell lymphoma; NKTCL: NK/T cell lymphoma. PTCL, NOS: peripheral T-cell lymphoma, unspecified. LDH: lactate dehydrogenase; ULN: upper limit of normal; ECOG: Eastern Cooperative Oncology Group.
*The upper limit of normal for LDH was defined as 240 IU/L.
Chemotherapy with an anthracycline-based regimen was administered to 528 (81.2%) patients, and radiotherapy was administered to 247 (38%) patients. The objective response rate (ORR) was 63.3%, with complete remission (CR) occurring in 42.8% (Table 2).
Table 2. First-line therapy regimens and response to initial therapy.
| Total | AITL | PTCL, NOS | NK/TCL | ALK+ALCL | ALK-ALCL | Others | |
| Number | 650 | 91 | 65 | 280 | 55 | 33 | 126 |
| First-line treatment | |||||||
| CHOP | 211 (32.5%) | 44 (48.4%) | 18 (27.7%) | 53 (18.9%) | 19 (34.5%) | 15 (45.5%) | 62 (49.2%) |
| CHOP-EP/PEP | 30 (4.6%) | 0 (0%) | 21 (32.3%) | 1 (0.4%) | 4 (7.3%) | 2 (6.1%) | 2 (1.6%) |
| CHOPE | 99 (15.2%) | 24 (26.4%) | 14 (21.5%) | 8 (2.9%) | 29 (52.7%) | 12 (36.4%) | 12 (9.5%) |
| COP | 12 (1.8%) | 0 (0%) | 0 (0%) | 2 (0.7%) | 0 (0%) | 0 (0%) | 10 (7.9%) |
| ASP-containing | 217 (33.4%) | 1 (1.1%) | 2 (3.1%) | 199 (71.1%) | 0 (0%) | 0 (0%) | 15 (11.9%) |
| CHOP/GemOx | 17 (2.6%) | 11 (12.1%) | 1 (1.5%) | 0 (0%) | 1 (1.8%) | 1 (3.0%) | 3 (2.4%) |
| CHOPE/GDP | 13 (2.0%) | 6 (6.6%) | 2 (3.1%) | 0 (0%) | 0 (0%) | 3 (9.1%) | 2 (1.6%) |
| Other CT regimens | 51 (7.8%) | 5 (5.5%) | 7 (10.8%) | 17 (6.1%) | 2 (3.6%) | 0 (0%) | 20 (15.9%) |
| Combined RT | 247 (38%) | 2 (2.2%) | 14 (21.5%) | 192 (68.6%) | 3 (5.5%) | 9 (27.3%) | 27 (21.4%) |
| Response to initial therapy | |||||||
| CR | 278 (42.8%) | 36 (39.6%) | 18 (27.7%) | 143 (51.1%) | 36 (65.5%) | 15 (45.5%) | 30 (23.8%) |
| PR | 133 (20.5%) | 23 (25.2%) | 14 (21.5%) | 38 (13.6%) | 7 (12.7%) | 6 (18.2%) | 45 (35.7%) |
| SD/PD | 165 (25.4%) | 28 (30.1%) | 28 (43.1%) | 57 (20.4%) | 8 (14.5%) | 7 (21.2%) | 37 (29.4%) |
| Unknown | 74 (11.1%) | 4 (4.4%) | 5 (7.7%) | 42 (15%) | 4 (7.3%) | 5 (15.2%) | 14 (11.1%) |
| 5-year OS | 50.0% | 35.0% | 28.0% | 58.0% | 82.0% | 66.0% | 40.0% |
CHOP: cyclophosphamide doxorubicin vincristine prednisolone; CHOPE: CHOP plus etoposide; CHOP-EP/PEP: CHOP plus etoposide and cisplatin; COP: cyclophosphamide vincristine prednisolone; ASP-containing: COEPL (cyclophosphamide, vincristine, etoposide, prednisone and pegaspargase), CHOPL (CHOP plus pegaspargase), and LOP (pegaspargase, vincristine and prednisone); CHOPE/GemOx: CHOPE alternating with gemcitabine and oxaliplatin; CHOPE/GDP (CHOPE alternating with gemcitabine, cisplatin and dexamethasone); CT: chemotherapy; Other CT regimens: ESHAP (ESHAP: cytarabine, cisplatin, etoposide, methylprednisolone; COPP (cyclophosphamide vincristine procarbazine prednisolone); COMP (cyclophosphamide vincristine methotrexate prednisolone); CHEP (cyclophosphamide adriamycin etoposide prednisolone); Hyper CVAD/MA: cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate, and cytarabine); ITE (pirarubicin ifosfamide etoposide); FND (fludarabine mitoxantrone dexamethasone); GDP (gemcitabine dexamethasone cisplatin), SMILE (dexamethasone methotrexate ifosfamide l-asparaginase etoposide), and oral thalidomide. RT: radiation therapy; CR: complete response; PR: partial response; SD/PD: stable disease/progressive disease; AITL: angioimmunoblastic T cell lymphoma; ALK: anaplastic lymphoma kinase positive; ALCL: anaplastic large cell lymphoma; PTCL: peripheral T-cell lymphoma; NKTCL: NK/T cell lymphoma. PTCL, NOS: peripheral T-cell lymphoma, not otherwise specified.
Overall survival, conditional survival, and annual hazard
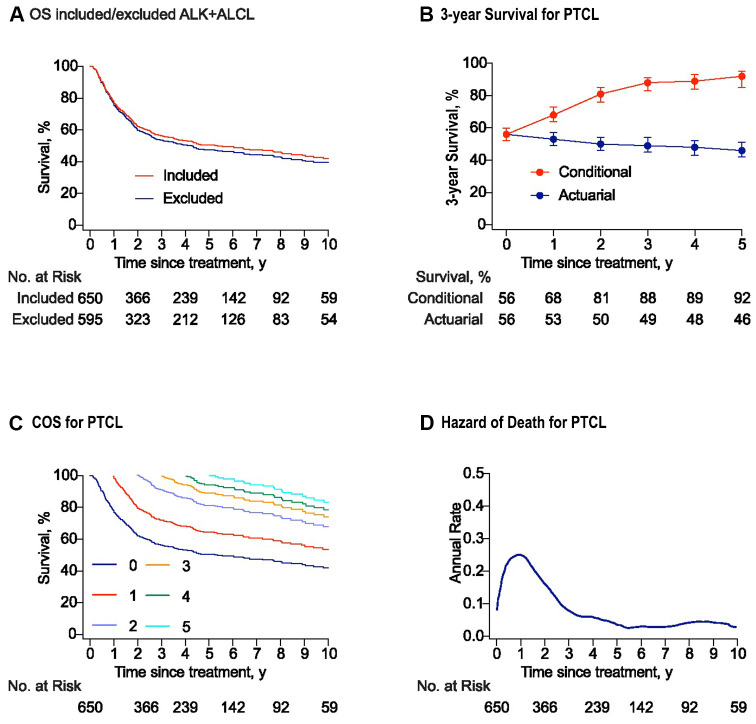
With a median follow-up of 5.4 years, 320 (49.2%) patients died, and the majority of deaths (n = 235, 73.4%) occurred within the first two years after treatment. The 5-year OS rates were 50.3% (95% confidence interval [CI], 46.4–54.4%) for the whole cohort, and 47.5% (95%, CI: 45.3–49.7%) for those patients without ALK+ALCL, respectively (Figure 1A).
Figure 1.
Survival and conditional survival curves for patients with peripheral T-cell lymphomas (PTCLs). (A) The overall survival curves for the whole study and the cohort with PTCL excluded anaplastic lymphoma kinase positive anaplastic large-cell lymphoma (ALK+ALCL). (B) Three-year conditional and 3-year actuarial survival with error bars of 95% confidence intervals (CIs) for the whole study cohort. For example, the 3-year actuarial survival rate at 2 years was the 5-year survival rate estimated at baseline. All the actuarial survival rates were calculated at the time point of starting treatment. (C) Conditional survival curves for patients who have survived for 1 year, 2 years, 3 years, 4 years, and 5 years from the time of treatment are shown. (D) Smoothed hazard plots for the annual rate of death for PTCLs since treatment.
There-year conditional survival (COS3) was defined as the probability of surviving an additional 3 years for patients who had already survived for a certain time. For the whole cohort, the COS3 rates increased for the survivors, while actuarial OS decreased over time (Figure 1B). The conditional survival probabilities for patients with PTCLs increased obviously in the first two years after treatment and then increased slightly in the following years (Figure 1C). The COS3 at year 2 (the probability of reaching the landmark of year 5) increased to 81% (Δ 31% compared with 5-year OS). In addition, the overall annual death hazard of 24% in the first year was the highest, which then decreased to about 10% at year 2 (range: 0–24.2%). Moreover, the hazard after five years post- treatment showed a stable trend, with an annual death hazard of about 5% (Figure 1D).
Conditional survival and annual hazards stratified by subtypes
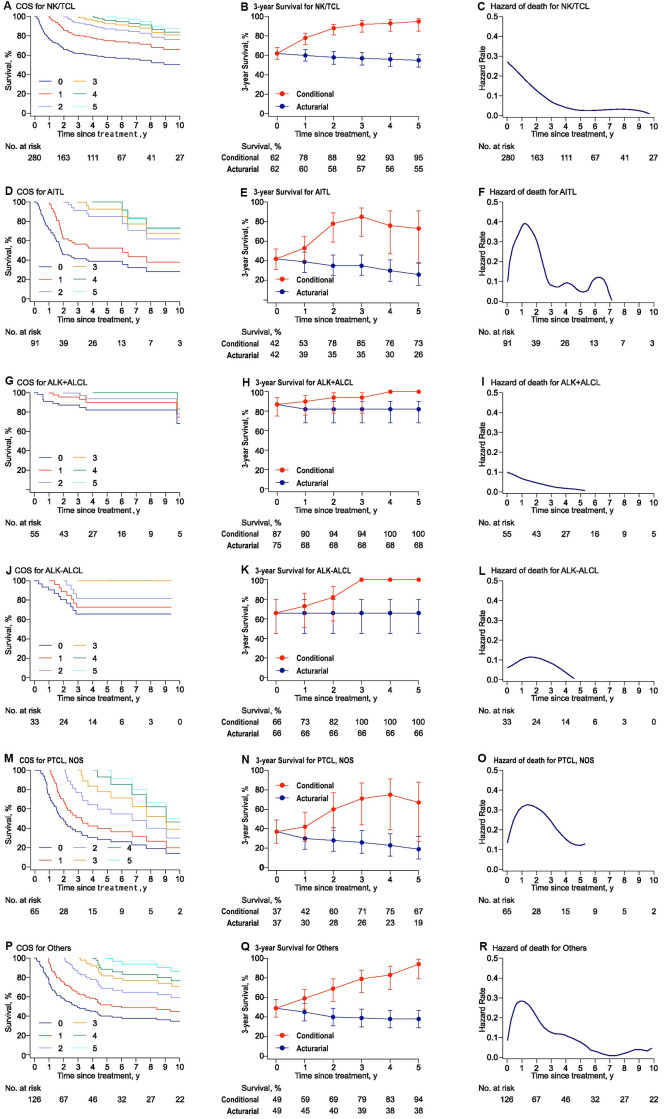
Heterogeneous change in COS3 and the annual hazard of death varied among histological subtypes (Supplementary Figure 2). In general, COS estimates increased obviously over time in almost all subtypes. For 2-year survivors, the COS3 and annual hazard of death were 88% and < 7% for NK/TCL, 78% and < 10% for angioimmunoblastic T-cell lymphoma (AITL), 94% and <1% for ALK+ALCL, 82% and 20% for ALK-ALCL, 60% and 31% for peripheral T-cell Lymphoma, not otherwise specified (PTCL, NOS), and 69% and 16.5% for other histological types (Others), respectively (Figure 2).
Figure 2.
Conditional survival, actuarial survival, and annual hazards stratified by histologic subtypes for PTCLs. (A), (D), (G), (J), (M), and (P) presented the conditional survival curves for patients who have already survived a certain time for each subtype. (B), (E), (H), (K), (N), and (Q) showed the three-year conditional and 3-year actuarial survival with error bars of CIs. (C), (F), (I), (L), (O), and (R) demonstrated the smoothed hazard plots for the annual rate of death for each subtype. AITL: angioimmunoblastic T-cell lymphoma; ALK: anaplastic lymphoma kinase; ALCL: anaplastic large cell lymphoma; PTCL: peripheral T-cell lymphoma; NKTCL: NK/T cell lymphoma. PTCL, NOS: peripheral T-cell lymphoma, not otherwise specified.
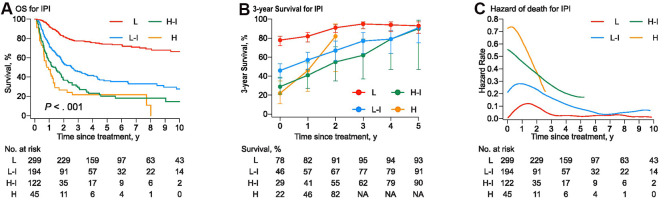
Conditional survival and annual hazards based on risk-stratification
The probability of survival increased more strikingly in higher-risk patients as time accrued (Figure 3). The COS3 and annual hazard of death at year 2 were 91% and 5.7% for the low-risk group, 67% and 16% for the low-intermediate risk group, 55% and 25% for the high-intermediate risk group, and 82% and 22% for high-risk group (Supplementary Table 1). For the high-intermediate patients who survived four years after inductive therapy, the COS3 was comparable with that of the low-intermediate risk group (79%). The 5-year survivors in PTCLs, except for the high-risk group, attained an equivalent favorable COS3 (all > 90% at year 5).
Figure 3.
(A) The overall survival curves were stratified into four groups by the International Prognostic Index (IPI). (B) Three-year conditional survival with error bars of 95% CIs for patients who have survived for 1 year, 2years, 3 years, 4 years, and 5 years from the time of treatment. (C) Smoothed hazard plots for the annual rate of death since treatment.
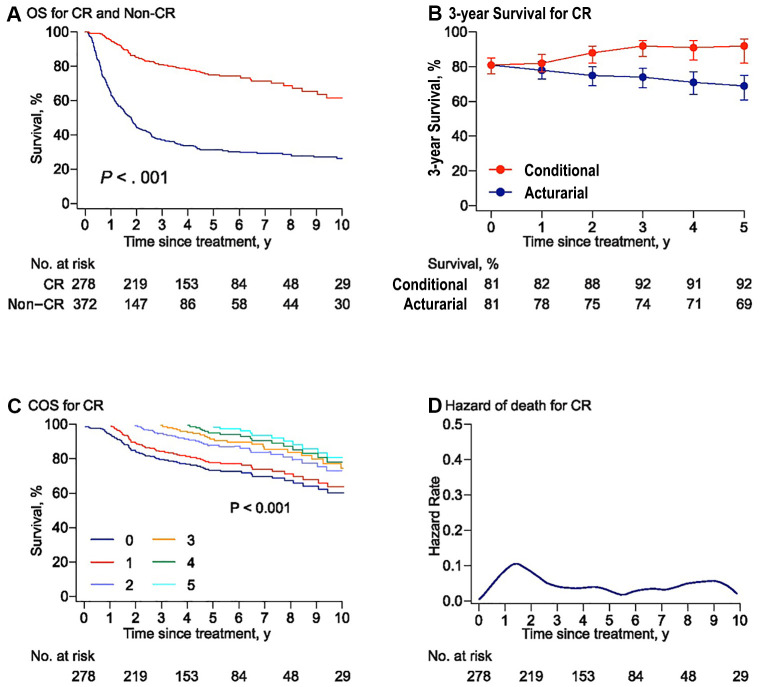
Conditional survival and annual hazards of death for patients with CR
Compared with those patients who could not achieve CR after initial treatment, patients who achieved CR had a better COS3 (Figure 4B, 4C). The annual hazard of death was present throughout and the highest risk of death was observed within the first year after treatment (about 13%) and was comparably stable at a low rate (< 5%) after year 3 (range: 0–13.5%, Figure 4D). For 2-year survivors, patients with PTCL, NOS had a worse prognosis, with a COS3 rate of 67% compared with those with ALK+ALCL (95%), NK/TCL (88%), and ALK-ALCL (85%, Supplementary Table 2).
Figure 4.
Risk-dependent conditional survival and annual hazard for patients with CR. (A) Overall survival curves for the patients who achieved or failed to obtain complete remission (CR) to initial chemotherapy. The overall survival rate was 75% and 31% at 5 years in the CR group and non-CR group, respectively. Survival comparison was made using the log-rank test. (B) Three-year conditional and 3-year actuarial survival with error bars of 95% confidence intervals (CIs) for the patients with CR. (C) Conditional survival curves for patients with CR who have survived for 1 year, 2 years, 3 years, 4 years, and 5 years from the time of treatment are shown. (D) Smoothed hazard plots for the annual rate of death for patients with CR since treatment.
DISCUSSION
The disease burden of NHLs has been increasing in China over the last decade [18]. The present study presented the changes in survival probability based on a large-scale study of patients with PTCLs in the real world. The five-year OS of 50.3% in our study was favorable in comparison with other published studies [19, 20]. The main possible reason might be the large proportion of NK/TCL (43%) in our cohort. Specifically, the prognosis of early-stage NK/TCL has been improved by radiotherapy and chemotherapy regimens containing asparaginase in the past decade [21–23].
Our results revealed that the survival possibility increased dynamically with elapsed time after treatment for patients with PTCLs. COS3 increased markedly for most histological subtypes. Further analysis showed a histology-dependent pattern: AITL, PTCL, NOS, and NK/TCL had an initially higher hazard of death that decreased over time, whereas ALK+ALCL had a constantly lower risk. These findings represented a markedly heterogeneous spectrum of prognosis in PTCLs over time, providing time-dependent survival information for clinicians to make rational decisions on patients’ intervention selection and follow-up guidance.
The heterogenous prognosis of PTCLs varies by histological subtypes [7]. For example, patients with AITL were associated with poor prognosis, with a 5-year OS rate of less than 40% [3, 24, 25]. However, for AITL, the 3-year survival probability for two-year survivors reached 78% in our study. Furthermore, our results of a low annual hazard of death and a COS3 more than 90% for patients who still survived at 2 years after treatment, demonstrated a good long-term outcome in ALK+ALCL. Therefore, the second year after treatment could be a critical time point in the prognosis of patients with PTCLs, which might be associated with relapse [26].
Similar to the observations in previous research, a high IPI score and a lack of CR after systemic therapy resulted in inferior OS in PTCLs [27, 28]. Risk stratification could predict the initial prognosis of patients with PTCLs in this study, which was consistent with previous studies [29, 30]. Despite the heterogeneous prognoses in each risk category over time, the conditional survival is improved and the annual hazard of death decreased, more strikingly in the higher risk groups. At 5 years after treatment, the non-high-risk groups attained an equivalent and favorable COS3 (all > 90% at year 5). However, the results for COS3 in the high-risk group implied that risk stratification based on the IPI score might not be appropriate to predict conditional survival for patients with PTCLs. A possible explanation might be that the individual prognostic factors of IPI lost their predictive value when patients had already survived for a period of time [12]. Many patients with poor prognostic factors died in the first few years. Those who had initially unfavorable predictors but survived for a certain time after treatment were likely to achieve a better prognosis in the following years. Therefore, the results highlighted the importance of improving the current prognosis model for PTCLs. A more accurate prognostic scoring system, including genetic stratification, should be considered for survivors with PTCLs, as noted by previous studies [11, 31, 32]. Furthermore, the COS3 could be used as a surrogate end point to validate the accuracy of novel and advanced evaluation systems of risk stratification, from a dynamic perspective.
Our results further suggest that the patients who achieved CR after induction therapy and survived for more than two years might have an encouraging subsequent OS, similar to the results of a previous study [33]. A tendency for plateauing for the annual hazard of death in patients who achieved CR was observed after three years. However, our finding also suggests that the histological subtypes with initially inferior prognosis, such as PTCL, NOS, still retained a higher hazard of death in patients who achieved CR. As far as we know, durable remissions are uncommon with CHOP-based chemotherapy in some PTCLs [34]. A higher death risk in the previous two years might be associated with early relapse. Generally, our data provides information for the evaluation of therapeutic opportunities, such as new drugs or regimens, including allografts, at specific time points for patients who respond to intensive therapies, [35–38] and suggest that assessment of the COS3 might be used to stratify patients who achieved CR but remained at a high risk of early or late relapse.
There were several limitations of our study. First, the existence of heterogeneity of treatment patterns over a long-time span made it difficult to analyze the potential impacts of therapeutic approaches on outcomes of patients with PTCLs. Second, the central pathology of the histological subtypes could not be reviewed. Third, because of the small sample size in some histologic subtypes, the sample size should be further expanded to confirm the results. In conclusion, PTCL is a heterogeneous disease with multiple subtypes and varying clinical outcomes among patients who survive after a certain time. Multidisciplinary collaboration is necessary sometimes for better outcome, as rarer conditions in other disease [39, 40].
However, an apparent additional three-year survival improvement was observed in most subtypes after two years, which was associated with a reduced annual hazard of death. Accordingly, the follow-up interval might be varied among different histological subtypes of PTCLs based on our data. In addition, a significant difference was observed when evaluating the prognosis of patients with PTCLs using the traditional OS method and the COS3 method. Therefore, these results indicated that a conditional survival strategy and annual hazard analyses might be more accurate and helpful during additional survival years, especially for patients with initially inferior histological subtypes. Generally, the results suggest that COS3 might be useful in clinical decision making, novel prognosis model assessment, biomarker validation, patient counseling, disease surveillance, and clinical trial improvement in patients with PTCLs.
MATERIALS AND METHODS
Patients
The study was approved by the ethics committee of Peking University Cancer Hospital and Institute, and an exemption for individual informed consent from the patients was granted because of the anonymous nature of the data.
A total of 679 patients newly diagnosed with PTCLs at Peking University Cancer Hospital and Institute who were treated with combination chemotherapy from January 1997 to January 2016 were reviewed. Twenty-nine cases were excluded because of ambiguous histological type (n = 13) and incomplete clinical data (n = 16). Finally, 650 cases were enrolled (Supplementary Figure 1). The disease stage was determined using the Ann-Arbor staging system, and risk stratification was performed on the International Prognostic Index (IPI) [41]. Response to the treatment was reported accordingly [42, 43].
OS was defined as the time from treatment to death from any cause or the last follow-up. Conditional overall survival was defined as the probability of surviving an additional number of years, given that the patient had already survived a certain number of years since primary treatment [14, 44]. For example, the 2-year COS3 was defined as the probability of surviving an additional 3 years for a patient who had already survived 2 years (surviving to the landmark of 5 years since treatment).
Statistical analysis
Median follow-up time was estimated on OS using the reverse Kaplan–Meier method. The survival probability was estimated using the Kaplan–Meier method, and survival differences between groups were tested using the log-rank test for statistical significance. The annual hazard of death was defined as the rate of death during a certain year after treatment for surviving patients. Smoothed hazard estimates were calculated based on the Kernel–Epanechnikov smoothing procedure [45].
All statistical tests were two-sided, with an alpha level of 0.05 as the significance cutoff. All analyses were conducted using IBM SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA) and R version 3.2.5 (https://www.r-project.org).
Supplementary Material
Acknowledgments
The authors wish to thank all participating patients, hemato-oncologists, pathologists, statisticians, and the colleagues in Peking University Cancer Hospital and Institute College for their invaluable contributions to this study.
Abbreviations
- AITL
angioimmunoblastic T-cell lymphoma
- ALCL
anaplastic large cell lymphoma
- ALK
anaplastic lymphoma kinase positive
- COS
conditional survival
- COS3
3-year conditional survival
- ECOG
Eastern Cooperative Oncology Group
- IPI
International Prognostic Index
- NK/TCL
natural killer/T-cell lymphoma
- OS
overall survival
- PTCL, NOS
peripheral T-cell lymphoma, not otherwise specified
- PTCLs
peripheral T-cell lymphomas
Footnotes
AUTHOR CONTRIBUTIONS: HG and XJ are joint first authors. JZ obtained funding. YS designed the study. HG, XJ, WL, and XW collected the data. HG and XJ was involved in data cleaning, mortality follow-up, and verification. HG, LM, and XL analyzed the data. HG drafted the manuscript. HG, YS, WL, and JZ contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. All authors have read and approved the final manuscript. WL and YS are the study guarantors.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: This work was carried out by grants from the Capital’s Funds for Health Improvement and Research (grant no. 2018-1-2151).
REFERENCES
- 1.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–90. 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park S, Ko YH. Peripheral T cell lymphoma in Asia. Int J Hematol. 2014; 99:227–39. 10.1007/s12185-014-1520-3 [DOI] [PubMed] [Google Scholar]
- 3.Vose J, Armitage J, Weisenburger D, and International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26:4124–30. 10.1200/JCO.2008.16.4558 [DOI] [PubMed] [Google Scholar]
- 4.Yang QP, Zhang WY, Yu JB, Zhao S, Xu H, Wang WY, Bi CF, Zuo Z, Wang XQ, Huang J, Dai L, Liu WP. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011; 6:77. 10.1186/1746-1596-6-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W, Liu J, Song Y, Wang X, Zhou M, Wang L, Ma J, Zhu J, and Union for China Leukemia Investigators of the Chinese Society of Clinical Oncology, Union for China Lymphoma Investigators of the Chinese Society of Clinical Oncology. Mortality of lymphoma and myeloma in China, 2004-2017: an observational study. J Hematol Oncol. 2019; 12:22. 10.1186/s13045-019-0706-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016; 66:443–59. 10.3322/caac.21357 [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Ji X, Song Y, Wang X, Zheng W, Lin N, Tu M, Xie Y, Ping L, Ying Z, Zhang C, Deng L, Wu M, et al. Improving survival of 3760 patients with lymphoma: experience of an academic center over two decades. Cancer Med. 2020; 9:3765–74. 10.1002/cam4.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armitage JO. The aggressive peripheral T-cell lymphomas: 2017. Am J Hematol. 2017; 92:706–15. 10.1002/ajh.24791 [DOI] [PubMed] [Google Scholar]
- 9.Ascani S, Zinzani PL, Gherlinzoni F, Sabattini E, Briskomatis A, de Vivo A, Piccioli M, Fraternali Orcioni G, Pieri F, Goldoni A, Piccaluga PP, Zallocco D, Burnelli R, et al. Peripheral T-cell lymphomas. Clinico-pathologic study of 168 cases diagnosed according to the R.E.A.L. Classification. Ann Oncol. 1997; 8:583–92. 10.1023/A:1008200307625 [DOI] [PubMed] [Google Scholar]
- 10.Mourad N, Mounier N, Brière J, Raffoux E, Delmer A, Feller A, Meijer CJ, Emile JF, Bouabdallah R, Bosly A, Diebold J, Haioun C, Coiffier B, et al. , and Groupe d ’ Etude des Lymphomes de l ’ Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008; 111:4463–70. 10.1182/blood-2007-08-105759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal J, Wright G, Wang C, Rosenwald A, Gascoyne RD, Weisenburger DD, Greiner TC, Smith L, Guo S, Wilcox RA, Teh BT, Lim ST, Tan SY, et al. , and Lymphoma Leukemia Molecular Profiling Project and the International Peripheral T-cell Lymphoma Project. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014; 123:2915–23. 10.1182/blood-2013-11-536359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spolverato G, Kim Y, Ejaz A, Alexandrescu S, Marques H, Aldrighetti L, Gamblin TC, Pulitano C, Bauer TW, Shen F, Sandroussi C, Poultsides G, Maithel SK, Pawlik TM. Conditional probability of long-term survival after liver resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 535 patients. JAMA Surg. 2015; 150:538–45. 10.1001/jamasurg.2015.0219 [DOI] [PubMed] [Google Scholar]
- 13.Jung SH, Lee HY, Chow SC. Statistical methods for conditional survival analysis. J Biopharm Stat. 2018; 28:927–38. 10.1080/10543406.2017.1405012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zabor EC, Gonen M, Chapman PB, Panageas KS. Dynamic prognostication using conditional survival estimates. Cancer. 2013; 119:3589–92. 10.1002/cncr.28273 [DOI] [PubMed] [Google Scholar]
- 15.Hapgood G, Zheng Y, Sehn LH, Villa D, Klasa R, Gerrie AS, Shenkier T, Scott DW, Gascoyne RD, Slack GW, Parsons C, Morris J, Pickles T, et al. Evaluation of the risk of relapse in classical Hodgkin lymphoma at event-free survival time points and survival comparison with the general population in British Columbia. J Clin Oncol. 2016; 34:2493–500. 10.1200/JCO.2015.65.4194 [DOI] [PubMed] [Google Scholar]
- 16.El-Asmar J, Rybicki L, Bolwell BJ, Kharfan-Dabaja MA, Dean R, Hamilton BK, Hanna R, Jagadeesh D, Kalaycio M, Pohlman B, Sobecks R, Hill BT, Majhail NS. Conditional long-term survival after autologous hematopoietic cell transplantation for diffuse large B cell lymphoma. Biol Blood Marrow Transplant. 2019; 25:2522–26. 10.1016/j.bbmt.2019.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Wu T, Zhu SY, Shi M, Su H, Wang Y, He X, Xu LM, Yuan ZY, Zhang LL, Wu G, Qu BL, Qian LT, et al. Risk-dependent conditional survival and failure hazard after radiotherapy for early-stage extranodal natural killer/T-cell lymphoma. JAMA Netw Open. 2019; 2:e190194. 10.1001/jamanetworkopen.2019.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Liu J, Song Y, Zeng X, Wang X, Mi L, Cai C, Wang L, Ma J, Zhu J, and Union for China Leukemia Investigators of the Chinese Society of Clinical Oncology, and Union for China Lymphoma Investigators of the Chinese Society of Clinical Oncology. Burden of lymphoma in China, 2006-2016: an analysis of the global burden of disease study 2016. J Hematol Oncol. 2019; 12:115. 10.1186/s13045-019-0785-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014; 124:1570–77. 10.1182/blood-2014-04-573089 [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm M, Smetak M, Reimer P, Geissinger E, Ruediger T, Metzner B, Schmitz N, Engert A, Schaefer-Eckart K, Birkmann J. First-line therapy of peripheral T-cell lymphoma: extension and long-term follow-up of a study investigating the role of autologous stem cell transplantation. Blood Cancer J. 2016; 6:e452. 10.1038/bcj.2016.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi M, Suzuki R, Oguchi M, Asano N, Amaki J, Akiba T, Maeda T, Itasaka S, Kubota N, Saito Y, Kobayashi Y, Itami J, Ueda K, et al. Treatments and outcomes of patients with extranodal natural killer/T-cell lymphoma diagnosed between 2000 and 2013: a cooperative study in Japan. J Clin Oncol. 2017; 35:32–39. 10.1200/JCO.2016.68.1619 [DOI] [PubMed] [Google Scholar]
- 22.Kim K, Chie EK, Kim CW, Kim IH, Park CI. Treatment outcome of angiocentric T-cell and NK/T-cell lymphoma, nasal type: radiotherapy versus chemoradiotherapy. Jpn J Clin Oncol. 2005; 35:1–5. 10.1093/jjco/hyi006 [DOI] [PubMed] [Google Scholar]
- 23.Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, Leung AY, Chim CS. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012; 120:2973–80. 10.1182/blood-2012-05-431460 [DOI] [PubMed] [Google Scholar]
- 24.Niitsu N, Okamoto M, Nakamine H, Aoki S, Motomura S, Hirano M. Clinico-pathologic features and outcome of Japanese patients with peripheral T-cell lymphomas. Hematol Oncol. 2008; 26:152–58. 10.1002/hon.853 [DOI] [PubMed] [Google Scholar]
- 25.de Leval L, Parrens M, Le Bras F, Jais JP, Fataccioli V, Martin A, Lamant L, Delarue R, Berger F, Arbion F, Bossard C, Copin MC, Canioni D, et al. Angioimmunoblastic T-cell lymphoma is the most common T-cell lymphoma in two distinct French information data sets. Haematologica. 2015; 100:e361–64. 10.3324/haematol.2015.126300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017; 129:1095–102. 10.1182/blood-2016-09-692541 [DOI] [PubMed] [Google Scholar]
- 27.Gleeson M, Peckitt C, Cunningham D, Gibb A, Hawkes EA, Back M, Yasar B, Foley K, Lee R, Dash J, Johnson H, O’Hara C, Wotherspoon A, et al. Outcomes following front-line chemotherapy in peripheral T-cell lymphoma: 10-year experience at The Royal Marsden and The Christie Hospital. Leuk Lymphoma. 2018; 59:1586–95. 10.1080/10428194.2017.1393671 [DOI] [PubMed] [Google Scholar]
- 28.Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, Villa D, Gascoyne RD, Connors JM, Savage KJ. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013; 31:1970–76. 10.1200/JCO.2012.44.7524 [DOI] [PubMed] [Google Scholar]
- 29.Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne RD, Armitage JO, Weisenburger DD, and International Peripheral T-Cell Lymphoma Project. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008; 111:5496–504. 10.1182/blood-2008-01-134270 [DOI] [PubMed] [Google Scholar]
- 30.Rüdiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA, Nathwani BN, Ullrich F, Müller-Hermelink HK, and Non-Hodgkin ’ s Lymphoma Classification Project. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 2002; 13:140–49. 10.1093/annonc/mdf033 [DOI] [PubMed] [Google Scholar]
- 31.Feldman AL, Law M, Remstein ED, Macon WR, Erickson LA, Grogg KL, Kurtin PJ, Dogan A. Recurrent translocations involving the IRF4 oncogene locus in peripheral T-cell lymphomas. Leukemia. 2009; 23:574–80. 10.1038/leu.2008.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, Feldman AL, Wada DA, Lu Y, Polk A, Briski R, Ristow K, Habermann TM, Thomas D, Ziesmer SC, Wellik LE, Lanigan TM, Witzig TE, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2014; 123:3007–15. 10.1182/blood-2013-12-544809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maurer MJ, Ellin F, Srour L, Jerkeman M, Bennani NN, Connors JM, Slack GW, Smedby KE, Ansell SM, Link BK, Cerhan JR, Relander T, Savage KJ, Feldman AL. International assessment of event-free survival at 24 months and subsequent survival in peripheral T-cell lymphoma. J Clin Oncol. 2017; 35:4019–26. 10.1200/JCO.2017.73.8195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004; 15:1467–75. 10.1093/annonc/mdh392 [DOI] [PubMed] [Google Scholar]
- 35.Schmitz N, Lenz G, Stelljes M. Allogeneic hematopoietic stem cell transplantation for T-cell lymphomas. Blood. 2018; 132:245–53. 10.1182/blood-2018-01-791335 [DOI] [PubMed] [Google Scholar]
- 36.Laribi K, Alani M, Truong C, Baugier de Materre A. Recent advances in the treatment of peripheral T-cell lymphoma. Oncologist. 2018; 23:1039–53. 10.1634/theoncologist.2017-0524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, Morschhauser F, Wilhelm M, Pinter-Brown L, Padmanabhan Iyer S, Shustov A, Nielsen T, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014; 7:11. 10.1186/1756-8722-7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanale MA, Horwitz SM, Forero-Torres A, Bartlett NL, Advani RH, Pro B, Chen RW, Davies A, Illidge T, Huebner D, Kennedy DA, Shustov AR. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014; 32:3137–43. 10.1200/JCO.2013.54.2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isik A, Ramanathan R. Approaches to the treatment of pilonidal sinus disease, clinical practice in 2019. Int Wound J. 2020; 17:508–09. 10.1111/iwj.13265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isik A, Isik N, Kurnaz E. Complete breast autoamputation: clinical image. Breast J. 2020; 26:2265–66. 10.1111/tbj.14072 [DOI] [PubMed] [Google Scholar]
- 41.International Non-Hodgkin ’ s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993; 329:987–94. 10.1056/NEJM199309303291402 [DOI] [PubMed] [Google Scholar]
- 42.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, et al. , and NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI sponsored international working group. J Clin Oncol. 1999; 17:1244. 10.1200/JCO.1999.17.4.1244 [DOI] [PubMed] [Google Scholar]
- 43.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, et al. , and International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–86. 10.1200/JCO.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 44.Zamboni BA, Yothers G, Choi M, Fuller CD, Dignam JJ, Raich PC, Thomas CR Jr, O’Connell MJ, Wolmark N, Wang SJ. Conditional survival and the choice of conditioning set for patients with colon cancer: an analysis of NSABP trials C-03 through C-07. J Clin Oncol. 2010; 28:2544–48. 10.1200/JCO.2009.23.0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han G, Schell MJ, Kim J. Improved survival modeling in cancer research using a reduced piecewise exponential approach. Stat Med. 2014; 33:59–73. 10.1002/sim.5915 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.