Abstract
Purpose of review
To highlight mechanisms of elevated risk of atherosclerotic cardiovascular disease (ASCVD) among people living with HIV (PLWH), discuss therapeutic strategies, and opportunities for primary prevention.
Recent findings
HIV-associated ASCVD risk is likely multifactorial and due to HIV-specific factors and traditional risk factors even in the setting of treated and suppressed HIV disease. While a growing body of evidence suggests that inflammation and immune activation are key drivers of atherogenesis, therapies designed to lower inflammation including colchicine and low-dose methotrexate have not improved secondary cardiovascular endpoints among PLWH. Statins continue to be the mainstay of management of hyperlipidemia in HIV, but the impact of newer lipid therapies including PCSK-9 inhibitors on ASCVD risk among PLWH is under investigation. Aside from the factors mentioned above, health care disparities are particularly prominent among PLWH and thus likely contribute to increased ASCVD risk.
Summary
Our understanding of mechanisms of elevated ASCVD risk in HIV continues to evolve, and the optimal treatment for CVD in HIV aside from targeting traditional risk factors remains unknown. Future studies including novel therapies to lower inflammation, control of risk factors, and implementation science are needed to ascertain optimal ways to treat and prevent ASCVD among PLWH.
Keywords: HIV infection, atherosclerotic cardiovascular disease, cardiovascular primary prevention, antiretroviral therapy, disparities
Introduction
People living with HIV (PLWH), even treated with antiretroviral therapy (ART), have elevated risk of atherosclerotic cardiovascular disease (ASCVD). Risk factors among PLWH include HIV-specific factors such as chronic inflammation and immune activation and ART-associated metabolic changes, traditional cardiovascular risk factors, and disparities. In this Review, we will explore mechanisms of ASCVD among PLWH and targets for prevention.
Epidemiology of HIV & Atherosclerotic Cardiovascular Disease
With early ART initiation as recommended for all PLWH, HIV infection has become a chronic disease.(1) A meta-analysis of 793,635 PLWH with 3.5 million person-years follow-up demonstrated that PLWH are twice as likely to develop CVD.(2) The global burden of HIV-associated CVD has tripled over the past 20 years, now accounting for 2.6 million disability adjusted life-years.(2) The median age of PLWH is projected to increase from 44 years in 2010 to 57 years in 2030, and the prevalence of CVD is projected to increase from 28% to 78% over that period.(3)
HIV infection is associated with premature ASCVD; namely, PLWH develop acute coronary syndromes (ACS) a decade younger than people without HIV.(4) The risk of acute myocardial infarction (AMI) among PLWH on ART is 1.4 to 2.2 times higher compared to matched controls.(2, 5–7) PLWH also have higher risk of ACS recurrence.(8) Type 2 AMI (supply-demand mismatch) occurs more frequently among PLWH as compared to Type 1 AMI (acute spontaneous plaque rupture and atherothrombosis), and the mortality among PLWH is higher after Type 2 AMI compared to Type 1 AMI.(9, 10) Even in people without HIV, there are limited data regarding optimal prevention and management of Type 2 AMI.(11, 12)
HIV-Associated Inflammation and Immune Activation
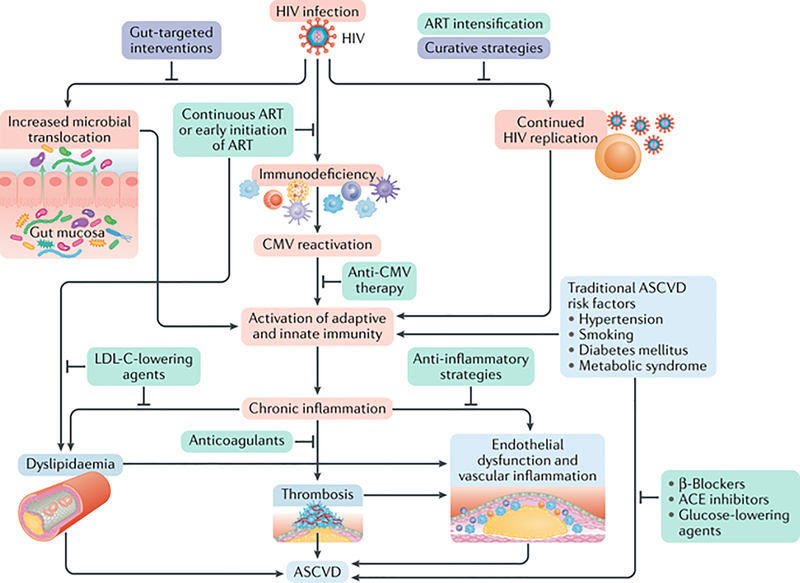
HIV infection, even treated, results in chronic inflammation and immune activation that drives atherosclerosis. The mechanisms of atherogenesis in HIV are complex, not well-understood, and beyond the scope of this Review, so we briefly highlight a few key mechanisms as shown in Figure 1.(13)
Fig 1. Mechanisms of Atherosclerotic Cardiovascular Disease among People Living with HIV.
Mechanisms of ASCVD include HIV infection itself, and the resulting increased microbial translocation, immunodeficiency, Cytomegalovirus (CMV) reactivation, chronic inflammation and immune activation, dyslipidemia, and traditional risk factors. Pathophysiologic mechanisms are highlighted in red, potential therapeutic targets with supporting evidence in green, and potential future areas of investigation are in purple. Reproduced with permission from Springer Nature.
Source: Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nature Reviews Cardiology. 2019;16(12):745–759.(13)
Immunodeficiency with CD4+ T cell depletion and detectable viremia are associated with the highest risk of AMI and cardiovascular mortality.(14) Even with viral suppression, transcription of HIV-encoded genes induces inflammation, endothelial dysfunction, and endothelin 1 production which drive atherogenesis.(13) Elite controllers have higher rates of inflammatory markers and atherosclerosis compared to uninfected individuals.(15) Other studies have demonstrated that elite controllers and long-term non-progressors have elevated monocyte activation markers but similar levels of atherosclerosis.(16, 17) Among treated individuals, measurements of viral reservoir were independently associated with incident plaque development, providing additional evidence that HIV disease itself accelerates CVD.(18)
PLWH have elevated biomarkers of inflammation including CRP, IL-1ß, IL-6, sTNF-αR1 sTNF-αR2, monocyte activation including CCL2, soluble CD163, soluble CD14, and endothelial dysfunction (ICAM-1).(19) Inflammatory markers are associated with arterial and lymph node inflammation(20, 21) and subclinical atherosclerosis.(22) Inflammatory and coagulation biomarkers are strongly associated with mortality and cardiovascular events among PLWH.(23–29) Given the strong predictive value of IL-6 and D-dimer for non-AIDS conditions and death in treated HIV, a 25% lowering of these markers has been predicted to reduce serious non-AIDS events and death by up to 37%.(30) Given the role of inflammation in HIV disease pathogenesis and HIV-associated ASCVD, anti-inflammatory strategies may be even more beneficial than in the general population. However, as discussed below, pathways of inflammation in HIV are complex and likely distinct from the general population and thus, one size does not fit all.
Inflammation is a therapeutic target for ASCVD even in people without HIV. In a randomized placebo-controlled trial (CANTOS) that included 10,061 patients with prior AMI and elevated CRP, a monoclonal antibody against IL-1ß, canakinumab, decreased CRP, IL-6 and cardiovascular events, but was associated with higher incidence of fatal infection.(31, 32) A single dose of canakinumab safely reduced inflammatory markers and arterial inflammation among PLWH,(33) and we are currently conducting a trial of canakinumab among PLWH with cardiovascular risk factors (NCT02272946).
The Cardiovascular Inflammation Reduction Trial randomized 4,786 people with stable atherosclerosis to low-dose methotrexate versus placebo for secondary prevention, with no difference in cardiovascular events or reduction in inflammatory markers.(34) In a randomized, placebo-controlled trial that included 176 PLWH, methotrexate did not improve endothelial function or inflammatory biomarkers,(35) although it did reduce CD8+ T cells and improve novel brachial artery ultrasound measures.(36)
In the general population, low-dose colchicine has shown promise in reducing cardiovascular events in patients with stable coronary artery disease(37) and after AMI.(38) A recent randomized, placebo-controlled trial that included 81 PLWH tested the effect of colchicine (0.6mg daily) on plasma inflammatory markers and coronary endothelial function found no difference after 8 weeks.(39) Reasons why colchicine was not effective this study include small sample size, short duration, and possibly inclusion of individuals without CAD or recent AMI.
Co-infection with cytomegalovirus may also plays a significant role in atherosclerosis among PLWH on ART(15, 40) and efforts are underway to evaluate the impact of anti-CMV strategies on inflammatory markers and CV risk in HIV (ACTG 5383, evaluation of letermovir in HIV). Gut microbial translocation is present in HIV, associated with elevated inflammatory markers, and may contribute to atherosclerosis, but attempts to target this mechanism have not consistently lowered inflammatory markers.(41)
ART-Specific Mechanisms
Initiation with ART, particularly protease inhibitor (PI) based regimens are associated with risk of AMI,(42, 43) likely through alterations in lipids, insulin resistance, and lipodystrophy, which are discussed later. Newer ART regimens may have a lower risk of AMI than older regimens, and the population-attributable risk of ASCVD from ART is low.(44) Furthermore, high ART adherence is association with partial normalization of biomarkers of inflammation and immune activation.(45) The impact of integrase inhibitors which have been associated with weight gain(46) on ASCVD risk remains unknown.(47)
Traditional Risk Factors
Traditional risk factors remain a major contributor to cardiovascular morbidity and mortality among PLWH due to high prevalence and inadequate control.(48) Notably, less than 2% of Veterans in the large VACS VC cohort (who have access to care) have optimal control of traditional risk factors.(6) Prevention of hypertension, hyperlipidemia, and smoking would prevent 40% of AMI among PLWH.(48) Nurse-led interventions are currently being tested and may provide evidence for strategies to improve management of traditional risk factors.(49)
Hypertension
Hypertension is the leading cause of cardiovascular disease worldwide including among PLWH.(50) A global meta-analysis found that 35% of PLWH on ART have hypertension compared to 30% among people without HIV and that the prevalence is increasing.(51) Initiation of ART is associated with risk of hypertension.(52, 53) People with both hypertension and HIV have 2-fold risk of AMI compared to people with only HIV or hypertension.(7, 54) Similarly, one study found that men with non-advanced HIV and hypertension had a three-fold higher death rate compared to men with HIV without hypertension.(55)
Mechanisms of hypertension specific to HIV include ART-associated lipodystrophy and renal disease, direct ART effects, immune suppression or reconstitution, gut microbial translocation, chronic inflammation, and activation of the renin-angiotensin aldosterone system (RAAS).(50)
PLWH have high plasma renin activity, possibly related to structural similarity between renin and HIV-protease, production of renin by CD4+ T cells stimulated by HIV-1, and renin-stimulated viral reproduction.(50, 56–58) Several small trials of telmisartan, an angiotensin receptor blocker and PPAR-γ agonist, showed impressive blood pressure reductions.(59–61) A study of losartan demonstrated decreased blood pressure but no improvement in inflammation or T-cell recovery.(62) Currently, there are no HIV-specific guidelines regarding screening or treatment of hypertension.
Dyslipidemia
Hyperlipidemia prevalence among PLWH is estimated to be 28–80% with hypertriglyceridemia most common.(63) First-generation PIs, NRTIs, and NNRTIs generally increase triglyceride levels and can increase LDL-C levels.(13) Newer ARTs that improve lipid profiles and surrogate markers of atherogenesis include integrase inhibitors [dolutegravir & raltegravir], C-C chemokine receptor 5 (CCR5)-co-receptor antagonists [maraviroc], and second-generation PI [atazanavir].(64, 65)
Statins effectively lower LDL-C among PLWH and are the first line pharmacologic treatment for hyperlipidemia.(66) A small trial demonstrated that atorvastatin reduces non-calcified plaque volume and high-risk plaques among PLWH at 1 year.(67) Until the results from REPRIEVE (NCT02344290), a large multicenter randomized-controlled trial of pitavastatin versus placebo among PLWH at low to moderate ASCVD risk, there are no trials of statins powered for clinical events among PLWH.(68)
The 2018 ACC/AHA Cholesterol Guidelines include HIV as a risk-enhancing feature that prompts statin initiation among adults age 40–75 without diabetes with a 10-year risk of 7.5–19.9% of ASCVD using the pooled cohort equations.(69) The European AIDS Clinical Society Guidelines recommend a target LDL-C <2.0 mmol/L for PLWH with established ASCVD, type 2 diabetes, or 10-year risk ≥10% for primary prevention, and a target <1.4 mmol/L for secondary prevention.(70) An estimated 50% of PLWH qualify for statins, yet many are not prescribed statins.(71) Switching to an integrase-inhibitor based ART regimen may improve lipids at a small risk of virologic failure,(72, 73) but rosuvastatin initiation improves lipids more without that risk.(74) Drug-drug interactions are an important consideration, but atorvastatin and rosuvastatin have only modest interactions with most ART regimens.(75, 76)
PLWH achieve less LDL-C lowering than expected on statin therapy(77) with minimal evident short-term vascular benefit.(78) PLWH intolerant of statins or with insufficient LDL-C lowering response may be treated with ezetimibe, which is safe but less effective.(79) Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors are highly effective at lowering LDL-C, but expensive. The BEIJERINCK trial randomized 464 PLWH to evolocumab versus placebo; it reduced LDL-C by 56.9% from baseline to 24 weeks compared to placebo among PLWH and was well-tolerated.(80) The EPIC-HIV trial (NCT03207945), by our group, is examining whether PCSK-9 inhibition with alirocumab improves arterial inflammation and endothelial function. Bempedoic acid, which lowers LDL-C among people without HIV treated with statins (81) and among people intolerant of statins,(82) has not been studied among PLWH.
Hypertriglyceridemia, although common among PLWH, does not independently predict ASCVD events, so treatment of hypertriglyceridemia among PLWH remains uncertain.(1) Icosapent ethyl may be a promising treatment not yet studied among PLWH. Among people without HIV, icosapent ethyl compared to mineral-oil placebo lowered triglycerides,(83) reduced ischemic events(84) and decreased inflammatory markers among those with elevated high sensitivity C-reactive protein.(85) In contrast, marine n-3 fatty acids (combined eicosapentaenoic acid and docosahexaenoic acid) did not reduce major cardiovascular events in two large trials among people without HIV(86, 87) or improve inflammatory markers among PLWH.(88)
Lipodystrophy, Diabetes, Metabolic Syndrome, and Chronic Kidney Disease
Older ART regimens increase risk of lipodystrophy, diabetes, and the metabolic syndrome, but there are mixed data regarding risk of diabetes with newer ART regimens.(89, 90) Lipodystrophy is a syndrome of central adiposity from dorsocervical fat accumulation, increased or preserved visceral fat and peripheral fat loss in some patients taking older protease inhibitors and NRTIs [didanosine and stavudine].(41) Lipodystrophy is associated with the metabolic syndrome, which is associated with cardiovascular events and mortality among PLWH.(91) Chronic kidney disease is highly associated with cardiovascular risk.(92)
Smoking
Smoking is highly prevalent among PLWH. One study from Denmark found that PLWH were twice as likely to smoke as people without HIV, and the population-attributable risk of death from smoking was 61.5% among PLWH compared to 34.2% among those without HIV.(93) Similarly, a study in California found that 43.3% of PLWH had a smoking history compared to 29.0% of controls.(5) Smoking cessation nearly halves the incident rate ratio of AMI compared after 3 years of abstinence.(94) Behavioral interventions increase abstinence rates by 50% among PLWH,(95) and pharmacologic smoking cessation tools including nicotine replacement, buproprion, and varenicline have similar safety and efficacy among PLWH.(96)
Diet, Physical Activity, Alcohol and Other Substance Use
Diet, physical activity, unhealthy alcohol use and other substance use are important contributing factors in the development of ASCVD among PLWH not covered in this Review.
Disparities
PLWH are a vulnerable group and face significant stigma. In the US, historically marginalized racial and ethnic groups including African Americans have much higher rates of HIV infection and lower rates of viral control; African Americans make up 41% of PLWH in the US and 41% of new HIV diagnoses despite only making up 13% of the population.(97) Neighborhood poverty is also associated with unsuppressed viral load.(98)
African Americans with HIV have a higher prevalence of ASCVD risk factors than non-African Americans with HIV.(99) Racial disparities in care further impact treatment of ASCVD risk factors among PLWH. Black veterans living with HIV are less likely to have hypertension, diabetes, and lipids controlled than white veterans.(100) Racism is causally related to cardiovascular disease,(101) and perceived discrimination based on sexual orientation and gender identity also contribute.(102, 103) These disparities translate into increased odds of CVD hospitalizations (OR 1.45, 95% CI 1.39–1.51) among African American PLWH compared to white PLWH, for example, but are less studied among other socially stigmatized groups.(104)
Sex and gender differences also modify risk among PLWH. Most research on ASCVD among PLWH has focused on men, but women have a higher excess risk of cardiovascular disease (relative risk of 3.0 for women compared to 1.4 for men).(54) Sex-related differences in monocyte activation are associated with noncalcified plaque.(105) Intersectionality of marginalized identities may further exacerbate cardiovascular risk through activation of stress pathways.(106),(107)
The majority of PLWH (25.6 million out of 37.9 million) live in sub-Saharan Africa. The burden of cardiovascular disease attributable to HIV is highest in sub-Saharan Africa and Asia Pacific.(2) Access to newer ART regimens and prevalence of traditional ASCVD risk factors varies by geography, with lower smoking rates and dyslipidemia but more hypertension in sub-Saharan Africa compared to Europe and North America.(108) Different approaches need to be studied in a global context to reduce risk of ASCVD among PLWH.(109) If new therapies are effective, cost-effectiveness and global access need to be prioritized.
Risk Stratification for Primary Prevention
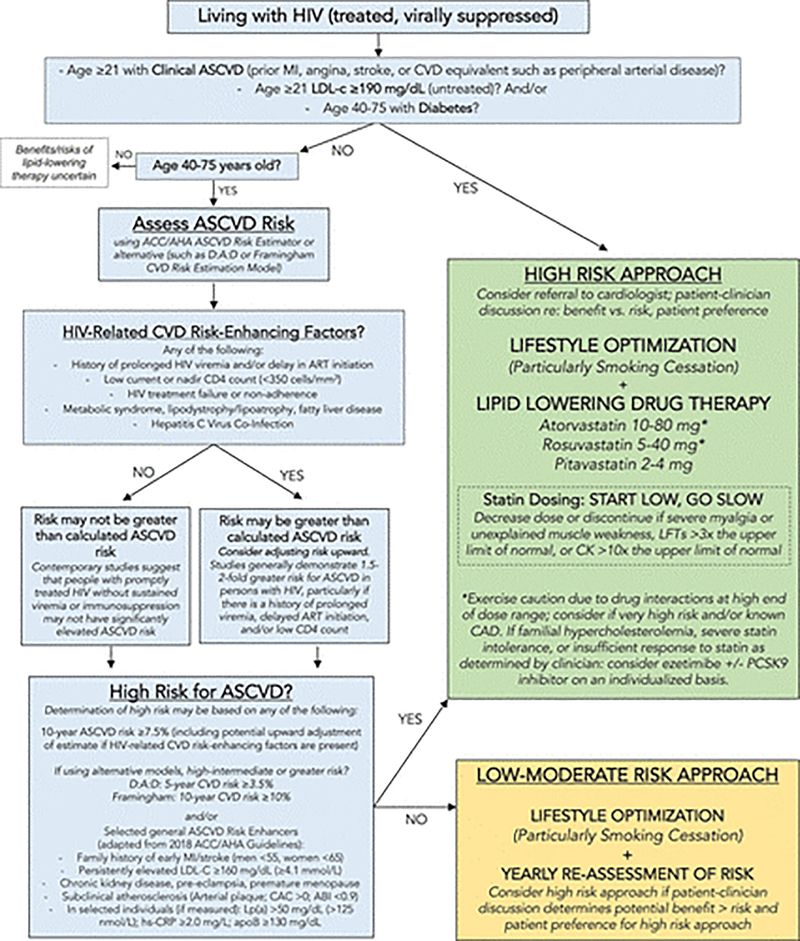
The paradigm for primary prevention of ASCVD is matching treatment intensity with estimated risk. Current guidelines favor the pooled cohort equations, which underestimate risk of ASCVD among PLWH, with poor discrimination and calibration similar to the Framingham Heart Study ASCVCD.(110) The D:A:D models provide HIV-specific risk estimators but were derived in white European cohorts.(111) The risk stratification and management algorithm developed by the AHA Scientific Statement committee in 2019 is presented in Figure 2.(1) There are insufficient data to recommend routine measurement of subclinical atherosclerosis through assessment of coronary artery calcification, arterial plaque, or ankle-brachial indices, but they may help with risk stratification among PLWH.(1, 112) Proteomics or imaging-based approaches may improve risk stratification in the future.
Fig 2. Primary Prevention Management Algorithm for ASCVD Primary Prevention among People Living with treated HIV.
This is the 2019 American Heart Association Scientific Statement pragmatic algorithm summarizing recommendations for management of people with treated HIV to prevent ASCVD. For people with uncontrolled HIV, the priority is appropriate HIV therapy to achieve viral suppression. ABI indicates ankle-brachial index; ACC/AHA, American College of Cardiology/American Heart Association; apoB, apolipoprotein B; ART, antiretroviral therapy; CAC, coronary artery calcium; CAD, coronary artery disease; CK, creatine kinase; CVD, cardiovascular disease; D:A:D, Data Collection on Adverse Events of Anti-HIV Drugs; hs-CRP, high sensitivity C-reactive protein; LFT, liver function test; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein A; and PCSK9, proprotein convertase subtilisin-kexin type 9. Reproduced with permission from the American Heart Association.
Source: Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98-e124. (1)
Lack of HIV-Specific Management Recommendations
There are few HIV-specific recommendations for ACSVD prevention.(1) No prospective primary prevention trials powered for clinical events of common therapies such as statins and aspirin have yet been completed in HIV. As the pendulum has shifted away from aspirin for primary prevention in the general population, the net benefit of aspirin for primary prevention among PLWH is uncertain. Nonetheless, PLWH are prescribed aspirin and statins less than people without HIV, which may contribute to excess risk.(113)
Conclusion
HIV is a major risk factor for ASCVD due to HIV-associated mechanisms and excess traditional risk factors. Additional investigations are needed to identify therapeutic targets and test interventions particularly related to HIV-specific mechanisms such as immune activation and chronic inflammation, and the impact of HIV-curative strategies on CV risk. In addition, how to best improve management of traditional risk factors and address disparities among PLWH at risk for ASCVD remains an important area for future work.
Key Points:
Inflammation and immune activation are key drivers of elevated ASCVD risk among PLWH, but impact of anti-inflammatory therapies on CV risk in HIV and clinical endpoints in HIV remains unknown.
Statins continue to be the mainstay of management of hyperlipidemia; PCSK-9 inhibitors effectively lower LDL-C among PLWH. Whether or not attainment of lower clinical cutpoints for LDL will translate into improvement clinical outcomes or reduction in incident CVD in HIV remains unknown.
There is emerging evidence that disparities are present in HIV-associated ASCVD risk factors and may contribute to increased risk in ASCVD.
Acknowledgments
Financial support and sponsorship
Dr Durstenfeld is supported by US National Institutes of Health grant no. 5T32HL007731-28. Dr Hsue is supported by US National Institutes of Health grant no. 2K24AI112393-06.
Abbreviations
- PLWH
People living with HIV
- ASCVD
atherosclerotic cardiovascular disease
- CVD
cardiovascular disease
- ART
antiretroviral therapy
- ACS
acute coronary syndrome
- AMI
acute myocardial infarction
- RAAS
renin-angiotensin aldosterone system
- LDL-C
low density lipoprotein cholesterol
- PIs
protease inhibitors
- NRTIs
nucleoside reverse transcriptase inhibitors
- NNRTIs
non-nucleoside reverse transcriptase inhibitor
- PCSK-9
Proprotein convertase subtilisin/kexin type 9
Footnotes
Conflicts of interest
Dr. Priscilla Hsue has received honoraria from Gilead and Merck.
References and Recommended Reading
- 1.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98–e124.•• American Heart Association Scientific Statement with expert recommendations for management and research priorities.
- 2.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation. 2018;138(11):1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. The Lancet Infectious diseases. 2015;15(7):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109(3):316–9. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. Journal of acquired immune deficiency syndromes (1999). 2014;65(2):160–6. [DOI] [PubMed] [Google Scholar]
- 6.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. Journal of acquired immune deficiency syndromes (1999). 2015;68(2):209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armah KA, Chang CC, Baker JV, Ramachandran VS, Budoff MJ, Crane HM, et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boccara F, Mary-Krause M, Potard V, Teiger E, Lang S, Hammoudi N, et al. HIV Infection and Long-Term Residual Cardiovascular Risk After Acute Coronary Syndrome. J Am Heart Assoc. 2020;9(17):e017578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinstein MJ, Nance RM, Delaney JAC, Heckbert SR, Budoff MJ, Drozd DR, et al. Mortality following myocardial infarction among HIV-infected persons: the Center for AIDS Research Network Of Integrated Clinical Systems (CNICS). BMC Med. 2019;17(1):149.• This study showed that mortality after Type 2 AMI is higher than Type 1 AMI among PLWH in CNICS.
- 10.Crane HM, Paramsothy P, Drozd DR, Nance RM, Delaney JA, Heckbert SR, et al. Types of Myocardial Infarction Among Human Immunodeficiency Virus-Infected Individuals in the United States. JAMA Cardiol. 2017;2(3):260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFilippis AP, Chapman AR, Mills NL, de Lemos JA, Arbab-Zadeh A, Newby LK, et al. Assessment and Treatment of Patients With Type 2 Myocardial Infarction and Acute Nonischemic Myocardial Injury. Circulation. 2019;140(20):1661–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandoval Y, Jaffe AS. Type 2 Myocardial Infarction: JACC Review Topic of the Week. Journal of the American College of Cardiology. 2019;73(14):1846–60. [DOI] [PubMed] [Google Scholar]
- 13.Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nature reviews Cardiology. 2019;16(12):745–59.• This review covers the pathophysiology and mechanisms in more depth than we were able to in the current Review.
- 14.Drozd DR, Kitahata MM, Althoff KN, Zhang J, Gange SJ, Napravnik S, et al. Increased Risk of Myocardial Infarction in HIV-Infected Individuals in North America Compared With the General Population. Journal of acquired immune deficiency syndromes (1999). 2017;75(5):568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20(18):2275–83. [DOI] [PubMed] [Google Scholar]
- 16.Brusca RM, Hanna DB, Wada NI, Blankson JN, Witt MD, Jacobson LP, et al. Subclinical cardiovascular disease in HIV controller and long-term nonprogressor populations. HIV Med. 2020;21(4):217–27.• This study of 134 elite controllers and 135 long-term non progresors found that they had elevated monocyte activation markers sCD163 and sCD14 compared to uninfected controls, less carotid plaque than viremic controls but similar carotid plaque to ART-suppressed or uninfected controls.
- 17.Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26(18):2409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin MM, Ma Y, Scherzer R, Rahalkar S, Martin JN, Mills C, et al. Association of Viral Persistence and Atherosclerosis in Adults With Treated HIV Infection. JAMA Netw Open. 2020;3(10):e2018099.••This study found that measurements of viral persistence in treated HIV disease are independently associated with incident carotid plaque development.
- 19.Subramanya V, McKay HS, Brusca RM, Palella FJ, Kingsley LA, Witt MD, et al. Inflammatory biomarkers and subclinical carotid atherosclerosis in HIV-infected and HIV-uninfected men in the Multicenter AIDS Cohort Study. PloS one. 2019;14(4):e0214735.•• This study from MACS found significant associations between inflammatory and monocyte activation biomarkers and carotid plaque among 452 PLWH compared to 276 HIV uninfected controls.
- 20.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. Jama. 2012;308(4):379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tawakol A, Ishai A, Li D, Takx RA, Hur S, Kaiser Y, et al. Association of Arterial and Lymph Node Inflammation With Distinct Inflammatory Pathways in Human Immunodeficiency Virus Infection. JAMA Cardiol. 2017;2(2):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14(6):385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. Journal of acquired immune deficiency syndromes (1999). 2010;55(3):316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PloS one. 2012;7(9):e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. The Journal of infectious diseases. 2014;210(8):1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.So-Armah KA, Tate JP, Chang CH, Butt AA, Gerschenson M, Gibert CL, et al. Do Biomarkers of Inflammation, Monocyte Activation, and Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? Journal of acquired immune deficiency syndromes (1999). 2016;72(2):206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada NI, Bream JH, Martinez-Maza O, Macatangay B, Galvin SR, Margolick JB, et al. Inflammatory Biomarkers and Mortality Risk Among HIV-Suppressed Men: A Multisite Prospective Cohort Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63(7):984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker JV, Sharma S, Grund B, Rupert A, Metcalf JA, Schechter M, et al. Systemic Inflammation, Coagulation, and Clinical Risk in the START Trial. Open Forum Infect Dis. 2017;4(4):ofx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, et al. Relevance of Interleukin-6 and D-Dimer for Serious Non-AIDS Morbidity and Death among HIV-Positive Adults on Suppressive Antiretroviral Therapy. PloS one. 2016;11(5):e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–31. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). European heart journal. 2018;39(38):3499–507. [DOI] [PubMed] [Google Scholar]
- 33.Hsue PY, Li D, Ma Y, Ishai A, Manion M, Nahrendorf M, et al. IL-1beta Inhibition Reduces Atherosclerotic Inflammation in HIV Infection. Journal of the American College of Cardiology. 2018;72(22):2809–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380(8):752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsue PY, Ribaudo HJ, Deeks SG, Bell T, Ridker PM, Fichtenbaum C, et al. Safety and Impact of Low-dose Methotrexate on Endothelial Function and Inflammation in Individuals With Treated Human Immunodeficiency Virus: AIDS Clinical Trials Group Study A5314. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019;68(11):1877–86.• In a randomized, placebo-controlled trial that included 176 PLWH, low dose methotrexate had increased safety events (below the noninferiority threshold of 15%) and did not significantly improve endothelial function or inflammatory biomarkers.
- 36.Stein JH, Yeh E, Weber JM, Korcarz C, Ridker PM, Tawakol A, et al. Brachial Artery Echogenicity and Grayscale Texture Changes in HIV-Infected Individuals Receiving Low-Dose Methotrexate. Arteriosclerosis, thrombosis, and vascular biology. 2018;38(12):2870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383(19):1838–47. [DOI] [PubMed] [Google Scholar]
- 38.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381(26):2497–505. [DOI] [PubMed] [Google Scholar]
- 39.Hays AG, Schar M, Barditch-Crovo P, Bagchi S, Bonanno G, Meyer J, et al. A randomized, placebo-controlled, double-blinded clinical trial of colchicine to improve vascular health in people living with HIV. AIDS. 2021.•• A randomized, placebo-controlled trial that included 81 PLWH tested the effect of low dose colchicine (0.6mg daily) on plasma inflammatory markers and coronary endothelial function via hand-grip augmented measurement of coronary blood flow on MRI found no difference between colchicine and placebo after 8 weeks.
- 40.Knudsen A, Kristoffersen US, Panum I, Hansen YB, Skottrup PD, Hasbak P, et al. Coronary artery calcium and intima-media thickness are associated with level of cytomegalovirus immunoglobulin G in HIV-infected patients. HIV Med. 2019;20(1):60–2. [DOI] [PubMed] [Google Scholar]
- 41.Hsue PY. Mechanisms of Cardiovascular Disease in the Setting of HIV Infection. Can J Cardiol. 2019;35(3):238–48. [DOI] [PubMed] [Google Scholar]
- 42.Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. [DOI] [PubMed] [Google Scholar]
- 43.Group DADS, Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–35. [DOI] [PubMed] [Google Scholar]
- 44.Smit M, van Zoest RA, Nichols BE, Vaartjes I, Smit C, van der Valk M, et al. Cardiovascular Disease Prevention Policy in Human Immunodeficiency Virus: Recommendations From a Modeling Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(5):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castillo-Mancilla JR, Brown TT, Palella FJ Jr., Macatangay BJC, Breen EC, Jacobson LP, et al. Partial Normalization of Biomarkers of Inflammation and Immune Activation Among Virally Suppressed Men With HIV Infection and High ART Adherence. Open Forum Infect Dis. 2020;7(4):ofaa099.• ART adherence is associated with improvement in inflammatory and immune activation markers.
- 46.Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis. 2020;33(1):10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Team NBftRS, editor Assoication between Integrase Inhibitors (InSTIs) and Cardiovascular Disease CROI; 2021.
- 48.Althoff KN, Gebo KA, Moore RD, Boyd CM, Justice AC, Wong C, et al. Contributions of traditional and HIV-related risk factors on non-AIDS-defining cancer, myocardial infarction, and end-stage liver and renal diseases in adults with HIV in the USA and Canada: a collaboration of cohort studies. The Lancet HIV. 2019;6(2):e93–e104.• This study found that traditional risk factors contribute the greatest population attributable risk to HIV-associated myocardial infarction.
- 49.Okeke NL, Webel AR, Bosworth HB, Aifah A, Bloomfield GS, Choi EW, et al. Rationale and design of a nurse-led intervention to extend the HIV treatment cascade for cardiovascular disease prevention trial (EXTRA-CVD). Am Heart J. 2019;216:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV-Infected Adults: Novel Pathophysiologic Mechanisms. Hypertension. 2018;72(1):44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens. 2017;11(8):530–40. [DOI] [PubMed] [Google Scholar]
- 52.Nduka CU, Stranges S, Sarki AM, Kimani PK, Uthman OA. Evidence of increased blood pressure and hypertension risk among people living with HIV on antiretroviral therapy: a systematic review with meta-analysis. J Hum Hypertens. 2016;30(6):355–62. [DOI] [PubMed] [Google Scholar]
- 53.Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. Aids. 2005;19(9):953–60. [DOI] [PubMed] [Google Scholar]
- 54.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. Journal of Clinical Endocrinology & Metabolism. 2007;92(7):2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bloomfield GS, Hogan JW, Keter A, Holland TL, Sang E, Kimaiyo S, et al. Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: a retrospective analysis of electronic health records. BMC Infect Dis. 2014;14:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srinivasa S, Fitch KV, Wong K, Torriani M, Mayhew C, Stanley T, et al. RAAS Activation Is Associated With Visceral Adiposity and Insulin Resistance Among HIV-infected Patients. Journal of Clinical Endocrinology & Metabolism. 2015;100(8):2873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tzoupis H, Leonis G, Megariotis G, Supuran CT, Mavromoustakos T, Papadopoulos MG. Dual inhibitors for aspartic proteases HIV-1 PR and renin: advancements in AIDS-hypertension-diabetes linkage via molecular dynamics, inhibition assays, and binding free energy calculations. J Med Chem. 2012;55(12):5784–96. [DOI] [PubMed] [Google Scholar]
- 58.Chandel N, Ayasolla K, Lan X, Rai P, Mikulak J, Husain M, et al. Renin modulates HIV replication in T cells. J Leukoc Biol. 2014;96(4):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vecchiet J, Ucciferri C, Falasca K, Mancino P, Di Iorio A, De Caterina R. Antihypertensive and metabolic effects of telmisartan in hypertensive HIV-positive patients. Antivir Ther. 2011;16(5):639–45. [DOI] [PubMed] [Google Scholar]
- 60.Ucciferri C, Falasca K, Vignale F, Di Nicola M, Vecchiet J. Long term effect of telmisartan in HIV-positive male patients with high blood pressure. Braz J Infect Dis. 2015;19(6):668–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lake JE, Seang S, Kelesidis T, Liao DH, Hodis HN, Stein JH, et al. Telmisartan to reduce cardiovascular risk in older HIV-infected adults: a pilot study. HIV Clin Trials. 2015;16(5):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker JV, Wolfson J, Collins G, Morse C, Rhame F, Liappis AP, et al. Losartan to reduce inflammation and fibrosis endpoints in HIV disease. AIDS. 2021;35(4):575–83.• This trial of losartan found that it improved blood pressure but not inflammatory markers among PLWH.
- 63.Libby PB RO; Mann DL; Zipes DP Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 2-volume set: Elsevier Health Sciences; 2007. [Google Scholar]
- 64.Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet (London, England). 2013;381(9868):735–43. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasa S, Grinspoon SK. Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. Eur J Endocrinol. 2014;170(5):R185–202. [DOI] [PubMed] [Google Scholar]
- 66.Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV. 2017;4(7):e284–e94. [DOI] [PubMed] [Google Scholar]
- 67.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. The Lancet HIV. 2015;2(2):e52–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grinspoon SK, Fitch KV, Overton ET, Fichtenbaum CJ, Zanni MV, Aberg JA, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J. 2019;212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Society EAC. EACS Guidelines Version 10.0. 2019.
- 71.Levy ME, Greenberg AE, Magnus M, Younes N, Castel A, Comm DCE. Evaluation of Statin Eligibility, Prescribing Practices, and Therapeutic Responses Using ATP III, ACC/AHA, and NLA Dyslipidemia Treatment Guidelines in a Large Urban Cohort of HIV-Infected Outpatients. Aids Patient Care and Stds. 2018;32(2):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eron JJ, Young B, Cooper DA, Youle M, DeJesus E, Andrade-Villanueva J, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. The Lancet. 2010;375(9712):396–407. [DOI] [PubMed] [Google Scholar]
- 73.Gatell JM, Assoumou L, Moyle G, Waters L, Johnson M, Domingo P, et al. Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS. 2017;31(18):2503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee FJ, Monteiro P, Baker D, Bloch M, Roth N, Finlayson R, et al. Rosuvastatin vs. protease inhibitor switching for hypercholesterolaemia: a randomized trial. HIV Med. 2016;17(8):605–14. [DOI] [PubMed] [Google Scholar]
- 75.Feinstein MJ, Achenbach CJ, Stone NJ, Lloyd-Jones DM. A Systematic Review of the Usefulness of Statin Therapy in HIV-Infected Patients. Am J Cardiol. 2015;115(12):1760–6. [DOI] [PubMed] [Google Scholar]
- 76.Myerson M, Malvestutto C, Aberg JA. Management of lipid disorders in patients living with HIV. J Clin Pharmacol. 2015;55(9):957–74. [DOI] [PubMed] [Google Scholar]
- 77.Burkholder GA, Muntner P, Zhao H, Mugavero MJ, Overton ET, Kilgore M, et al. Low-density lipoprotein cholesterol response after statin initiation among persons living with human immunodeficiency virus. J Clin Lipidol. 2018;12(4):988–98 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trevillyan JM, Dart A, Paul E, Cavassini M, Fehr J, Staehelin C, et al. Impact of rosuvastatin on atherosclerosis in people with HIV at moderate cardiovascular risk: a randomised, controlled trial. AIDS. 2021;35(4):619–24.• This trial found that initiation of rosuvastatin did not affect short term changes in atherosclerosis among PLWH at moderate cardiovascular risk, with a higher rate of adverse events in the rosuvastatin arm compared with placebo.
- 79.Wohl DA, Waters D, Simpson RJ Jr., Richard S, Schnell A, Napravnik S, et al. Ezetimibe alone reduces low-density lipoprotein cholesterol in HIV-infected patients receiving combination antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;47(8):1105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boccara F, Kumar PN, Caramelli B, Calmy A, Lopez JAG, Bray S, et al. Evolocumab in HIV-Infected Patients With Dyslipidemia: Primary Results of the Randomized, Double-Blind BEIJERINCK Study. Journal of the American College of Cardiology. 2020;75(20):2570–84.•• The BEIJERINCK trial randomized 464 PLWH to the PCSK-9 inhibitor evolocumab versus placebo; it reduced LDL-C by 56.9% from baseline to 24 weeks compared to placebo among PLWH and was well-tolerated.
- 81.Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, et al. Effect of Bempedoic Acid vs Placebo Added to Maximally Tolerated Statins on Low-Density Lipoprotein Cholesterol in Patients at High Risk for Cardiovascular Disease: The CLEAR Wisdom Randomized Clinical Trial. Jama. 2019;322(18):1780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and Safety of Bempedoic Acid in Patients With Hypercholesterolemia and Statin Intolerance. J Am Heart Assoc. 2019;8(7):e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. [DOI] [PubMed] [Google Scholar]
- 84.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Effects of Icosapent Ethyl on Total Ischemic Events: From REDUCE-IT. Journal of the American College of Cardiology. 2019;73(22):2791–802. [DOI] [PubMed] [Google Scholar]
- 85.Miller M, Ballantyne CM, Bays HE, Granowitz C, Doyle RT Jr., Juliano RA, et al. Effects of Icosapent Ethyl (Eicosapentaenoic Acid Ethyl Ester) on Atherogenic Lipid/Lipoprotein, Apolipoprotein, and Inflammatory Parameters in Patients With Elevated High-Sensitivity C-Reactive Protein (from the ANCHOR Study). Am J Cardiol. 2019;124(5):696–701. [DOI] [PubMed] [Google Scholar]
- 86.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N Engl J Med. 2019;380(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Group ASC, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med. 2018;379(16):1540–50. [DOI] [PubMed] [Google Scholar]
- 88.Oliveira JM, Rondo PH, Lima LR, Fortuna ES, Yudkin JS. Effects of a Low Dose of Fish Oil on Inflammatory Markers of Brazilian HIV-Infected Adults on Antiretroviral Therapy: A Randomized, Parallel, Placebo-Controlled Trial. Nutrients. 2015;7(8):6520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rasmussen LD, Mathiesen ER, Kronborg G, Pedersen C, Gerstoft J, Obel N. Risk of Diabetes Mellitus in Persons with and without HIV: A Danish Nationwide Population-Based Cohort Study. PloS one. 2012;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mathabire Rucker SC, Tayea A, Bitilinyu-Bangoh J, Bermudez-Aza EH, Salumu L, Quiles IA, et al. High rates of hypertension, diabetes, elevated low-density lipoprotein cholesterol, and cardiovascular disease risk factors in HIV-infected patients in Malawi. AIDS. 2018;32(2):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nix LM, Tien PC. Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr HIV/AIDS Rep. 2014;11(3):271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ryom L, Lundgren JD, Ross M, Kirk O, Law M, Morlat P, et al. Renal Impairment and Cardiovascular Disease in HIV-Positive Individuals: The D:A:D Study. The Journal of infectious diseases. 2016;214(8):1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality Attributable to Smoking Among HIV-1-Infected Individuals: A Nationwide, Population-Based Cohort Study. Clinical Infectious Diseases. 2013;56(5):727–34. [DOI] [PubMed] [Google Scholar]
- 94.Petoumenos K, Worm S, Reiss P, de Wit S, d’Arminio Monforte A, Sabin C, et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*). HIV Med. 2011;12(7):412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Keith A, Dong YL, Shuter J, Himelhoch S. Behavioral Interventions for Tobacco Use in HIV-Infected Smokers: A Meta-Analysis. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2016;72(5):527–33. [DOI] [PubMed] [Google Scholar]
- 96.Pool ERM, Dogar O, Lindsay RP, Weatherburn P, Siddiqi K. Interventions for tobacco use cessation in people living with HIV and AIDS. Cochrane Database of Systematic Reviews. 2016(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muiruri C, Longenecker CT, Meissner EG, Okeke NL, Pettit AC, Thomas K, et al. Prevention of cardiovascular disease for historically marginalized racial and ethnic groups living with HIV: A narrative review of the literature. Prog Cardiovasc Dis. 2020;63(2):142–8.•• This outstanding review focuses on racial and ethnic disparities in HIV and cardiovascular disease.
- 98.Cope AB, Edmonds A, Ludema C, Cole SR, Eron JJ, Anastos K, et al. Neighborhood Poverty and Control of HIV, Hypertension, and Diabetes in the Women’s Interagency HIV Study. AIDS Behav. 2020;24(7):2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong C, Gange SJ, Buchacz K, Moore RD, Justice AC, Horberg MA, et al. First Occurrence of Diabetes, Chronic Kidney Disease, and Hypertension Among North American HIV-Infected Adults, 2000–2013. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64(4):459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richardson KK, Bokhour B, McInnes DK, Yakovchenko V, Okwara L, Midboe AM, et al. Racial Disparities in HIV Care Extend to Common Comorbidities: Implications for Implementation of Interventions to Reduce Disparities in HIV Care. J Natl Med Assoc. 2016;108(4):201–10 e3. [DOI] [PubMed] [Google Scholar]
- 101.Wyatt SB, Williams DR, Calvin R, Henderson FC, Walker ER, Winters K. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315–31. [DOI] [PubMed] [Google Scholar]
- 102.Panza GA, Puhl RM, Taylor BA, Zaleski AL, Livingston J, Pescatello LS. Links between discrimination and cardiovascular health among socially stigmatized groups: A systematic review. PloS one. 2019;14(6):e0217623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Udo T, Grilo CM. Cardiovascular disease and perceived weight, racial, and gender discrimination in U.S. adults. J Psychosom Res. 2017;100:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oramasionwu CU, Morse GD, Lawson KA, Brown CM, Koeller JM, Frei CR. Hospitalizations for cardiovascular disease in African Americans and whites with HIV/AIDS. Popul Health Manag. 2013;16(3):201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. The Journal of infectious diseases. 2013;208(11):1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rich AJ, Williams J, Malik M, Wirtz A, Reisner S, DuBois LZ, et al. Biopsychosocial Mechanisms Linking Gender Minority Stress to HIV Comorbidities Among Black and Latina Transgender Women (LITE Plus): Protocol for a Mixed Methods Longitudinal Study. JMIR Res Protoc. 2020;9(4):e17076.• This study is examining biopsychosocial mechanisms of gender minority stress on HIV comorbidities.
- 107.Gosiker BJ, Lesko CR, Rich AJ, Crane HM, Kitahata MM, Reisner SL, et al. Cardiovascular disease risk among transgender women living with HIV in the United States. PloS one. 2020;15(7):e0236177.• This study found elevated cardiovascular risk among transgender women with HIV.
- 108.So-Armah K, Benjamin LA, Bloomfield GS, Feinstein MJ, Hsue P, Njuguna B, et al. HIV and cardiovascular disease. The Lancet HIV. 2020;7(4):e279–e93.•• This outstanding review emphasized the global aspects of HIV-associated cardiovascular disease beyond the space available in this Review.
- 109.Feinstein MJ, Bogorodskaya M, Bloomfield GS, Vedanthan R, Siedner MJ, Kwan GF, et al. Cardiovascular Complications of HIV in Endemic Countries. Curr Cardiol Rep. 2016;18(11):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, et al. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation. 2018;137(21):2203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friis-Moller N, Ryom L, Smith C, Weber R, Reiss P, Dabis F, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214–23. [DOI] [PubMed] [Google Scholar]
- 112.Patel AA, Budoff MJ. Coronary Artery Disease in Patients with HIV Infection: An Update . Am J Cardiovasc Drugs. 2020.• This review emphasizes the opportunity that coronary computed tomography may provide to better characterize coronary artery disease among PLWH.
- 113.Ladapo JA, Richards AK, DeWitt CM, Harawa NT, Shoptaw S, Cunningham WE, et al. Disparities in the Quality of Cardiovascular Care Between HIV-Infected Versus HIV-Uninfected Adults in the United States: A Cross-Sectional Study. J Am Heart Assoc. 2017;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]