Abstract
Stem cell-mediated regenerative endodontics has reached the human clinical trial phase; however, many issues still exist preventing such technology to be a widely used clinical practice. These issues are not straightforward and are complicated. And they should be because pulp regeneration is dealing with a small dead-end space. More so, when regeneration is needed, the space is often heavily infected. The true standard of pulp regeneration should be everything except generation of some fibrous connective tissue and amorphous mineral deposit. As of now, we are still far short of reaching the standard – complete vascularized and innervated pulp regeneration with newly formed tubular dentin in all types of teeth. Thus, we need to go back to the bench and use established animal models or create new animal models to tackle those issues. This paper will address several key issues including the possibility of pulp regeneration in small canals of molar teeth by enhancing the neovascularization, and whether the organized tubular dentin can be generated on the canal walls. Data from our semi-orthotopic tooth fragment mouse model have shown that complete pulp regeneration using dental pulp stem cells (DPSCs) in small canal has been inconsistent due to limited blood supply. This inconsistency is similar in our orthotopic miniature swine model, although in some cases vascularized pulp-like tissue can be formed throughout the canal space after DPSC transplantation. Furthermore, no tubular dentin was observed in the orthotopic pulp regeneration, despite DPSCs have the capacity to generate some tubular dentin-like structure in the hydroxyapatite/tricalcium phosphate-mediated ectopic pulp/dentin formation model in mice. Potential strategies to be tested to address these regeneration issues are discussed herein.
Keywords: dental pulp stem cells, mini-swine, pulp regeneration, tooth fragment model, orthotopic model, neovascularization
Introduction
Previously we have discussed the classification of regenerative endodontics based on whether there is use of exogenously delivered cells or not, i.e., cell-based vs cell-free approaches (1). In this article, we mainly focus on the stem cell-based regenerative endodontics. De novo regeneration of completely lost pulp via stem cell-based approach has been demonstrated in large animals (2, 3) as well as in humans (4, 5). Despite such progress, two areas of obstacles are likely to limit its clinical applications. One is the type of teeth that can receive regenerative endodontic treatment that warrents predictable and successful pulp-dentin regeneration. So far, immature teeth with open apex are considered better candidates for such treatment because open apex would allow better blood supply. Therefore, mature teeth especially molars are unlikely to benefit from this technology. The other is that even if regenerative treatment can apply to all teeth and conditions, can this new technology surpass the already highly successful non-surgical endodontic techniques by filling the canals with gutta-percha (6–9)? Any new technology must outperform existing ones to be prevalent. This article will discuss how the obstacles may be overcome by developing new strategies.
Using a large animal model for preclinical studies is a prerequisite for pulp-dentin regeneration research. In small animals, we can relatively more conveniently test many aspects of pulp-dentin regeneration. For example, the ectopic model which uses hydroxyapatite-tricalcium phosphate (HA/TCP) for pulp-dentin complex formation (10, 11). We can also use the semi-orthotopic mouse model with which the complete pulp regeneration with new dentin depositing along the canal walls in a human tooth fragment can be observed. This is semi-orthotopic because the pulp regeneration takes place within the root canal space in situ while the tooth is inside a mouse (10, 12, 13). Orthotopic de novo pulp regeneration in large animal models has been tested and demonstrated in dogs and mini-swine including the use of multi-rooted teeth with the formation of vascularized pulp-like tissue (13). Although complete pulp and dentin-like tissue regeneration can be observed in those large animal models, there was less than 50% of cases showing full regeneration with modest tissue quality in the mini-swine model. Those teeth tested in swine were multi-rooted premolars having similar root and canal morphologies to human molars.
In order to expand pulp regeneration technology to a broader use, for example, applicable to all tooth types and conditions, further research is needed to overcome challenges including disinfection efficiency of the heavily infected root canal, and blood supply during regeneration.
Why regeneration, not traditional endodontic treatment?
Here we list the most important reasons why we need to develop pulp-dentin regeneration technology.
To avoid tooth loss: Current endodontic treatment is very aggressive and damaging to tooth structures. Subsequent restoration further causes more structure loss rendering the tooth susceptible to fracture from traumatic injuries (14, 15).
To restore tooth functions: Pulpless teeth cannot regain any tooth structure and have no sensation to irritations, rendering caries progression unnoticed by patients. Tooth loss is higher for endodontically treated teeth than non-treated due to secondary caries and complex restoration associated problems (16, 17).
Pulp-dentin regeneration will allow endodontic therapy to enter a new era by reversing the diseased tooth condition back to its previous more natural state.
Have we been practicing “regenerative” endodontics clinically?
While stem cell-mediated pulp regeneration is only at the clinical trial stage, non-cell-based approaches have been practiced for more than a decade. This procedure is termed “revascularization” or “revitalization”. The latter is adopted by the European Society of Endodontology (18). Investigators/clinicians have been rigorously studying the tissues generated in the canal space histologically after revitalization procedures especially human teeth. A significant number of reports have confirmed that the generated tissues are not pulp or dentin, but fibrous tissue or cementum and bone (for review, see (19)). To be more precise, it perhaps is more suited to term this procedure “revitalization endodontics” or “conservative endodontics” than regenerative endodontics.
Ultimate goals of regenerative endodontics
To make the pulp regeneration technology more widely applicable for endodontic practice, it should be able to apply to all types of permanent teeth with various conditions: i) immature or mature, ii) large or small canals, iii) anterior or posterior, iv) vital or necrotic, and v) new or retreatment cases. The regenerated pulp should be identical to the natural and functional with: i) complete regeneration from apex to coronal chamber, ii) complete and stable vascularity, iii) re-established sensory perception and complete reinnervation into predentin and dentinal tubules, and iv) capability of producing new tubular dentin integrating to the existing dentin.
Key challenges that prevent reaching the ultimate goal
1). Disinfection efficiency
It is a well-known fact that obtaining a bacteria-free status after disinfection is highly challenging due to the complex morphology of the root canal system (20, 21). Traditional endodontic treatment uses gutta-percha plus a sealer to fill and seal the root canal space which seems to effectively prevent remaining bacteria to cause further infection. However, when root canal space is filled with stem cells instead of gutta-percha, the remaining hidden bacteria in the root canal system after disinfection is problematic (22). A case with heavily infected root canal with periapical lesions is likely to leave bacteria in the canals system especially dentinal tubules after disinfection. Here, we present a case of mandibular posterior teeth of mini-swine (Fig.1A, B). The teeth were first infected to establish periapical lesions by removing the pulp tissue and filling the canals with periodontal plaque. After 5 weeks of infection, periapical lesions were developed as shown in Fig. 1C,D. The disinfected the canals with standard NaOCl irrigation along with intracanal medication using tri-antibiotic paste (minocycline, metronidazole, ciprofloxacin, each 0.1 mg/mL) led to healing of the periapical bone with minimal apical radiolucency (Fig. 1E,F). Therefore, implantation with swine DPSCs into the canal system was performed. However, ~8 weeks following the cell implantation, periapical lesions of 3rd premolar recurred. Thus, the regeneration process was deemed not successful and animal euthanized right after radiographs were taken (Fig. 1G,H). Histological analysis showed an inflammation in the root canal with apical resorption and inflammation (data not shown).The dentinal tubules were heavily infected with microbes (Fig. 1I,J). Regardless that the failure in this swine study could result from leakage of the coronal filling, histologic studies of human teeth underwent revitalization treatment and showed that the presence of bacterial biofilm or bacterial invasion of dentinal tubules appears to be the cause of clinical failure (23, 24). Improved strategies for root canal disinfection are certainly needed, for example, the use of irrigation activation techniques that may maximize root canal disinfection. Among various methods including sonic, ultrasonic, negative apical pressure irrigation, etc., we have tested photon-induced photoacoustic streaming (PIPS) to enhance the disinfection of the root canal including dentinal tubule space (25, 26). Further improving the disinfection protocol, for example nanobubble technologies (27), may one day reach a level sufficient enough to allow pulp-dentin regeneration without further recurred infection from remaining microbes in the root canal system.
Fig 1.
Mini-swine pulp regeneration in periapical lesion model. (A, B) Mandibular left 2nd, 3rd and 4th premolars pre-op. The teeth were pulpectomized and the canals filled with periodontal plaque and sealed with composite. (C, D) Five weeks after canal infection, periapical lesions developed; disinfection initiated. (E, F) Five weeks after disinfection, lesions largely healed; implantation of swine DPSCs performed. (G, H) Eight and a half weeks after cell implantation, lesions recurred, particularly in 3rd premolar. Animal euthanized, teeth extracted, fixed and decalcified for paraffin embedded sectioning. (I, J) Deparaffinized sections were processed for Gram stain (Hardy Diagnostic Gram staining kit) showing bacteria invasion in dentinal tubules (Gram-positive bacteria -- blue; Gram-negative bacteria -- pink to red). Scale bars: (G) 100 μm, (H) 50 μm.
2). Blood supply during regeneration
It has been well-recognized that de novo cell-based regeneration of tissues requires good blood supply for a successful outcome. The culprit of the root canal system is that a good blood supply is almost impossible to acquire due to small apex opening. Immature teeth with large open apex are the type of teeth being considered possible for pulp regeneration. Using tooth fragment model in mice has proven that when the apex is ~2–3 mm and the root canal is large and short (~6 mm), complete pulp regeneration is possible (12). When the apex is narrowed down to 1 mm and canal length to 11 mm, nanofibrous microsphere injectable scaffolds encapsulated with vascular endothelial growth factors can help achieving complete and vascularized pulp regeneration (28). Orthotopic pulp regeneration using dogs has shown that the apical size of ~#70 of anterior teeth can also allow complete regeneration if using a subpopulation of DPSCs that produce angiogenic factors (2). In a human clinical trial, stem cell-based pulp regeneration on immature teeth showed good neovascularization (4). The question is whether this result can be achieved when treating long roots including multi-rooted teeth and even smaller foramen, especially most endodontic cases we deal with clinically the apex is not enlarged more than #40 (~0.4 mm). A rapid vasculature formation within the canals space after stem cell transplantation is needed. One approach is to implant both dental pulp stem cells (DPSCs) and endothelial cells (ECs) (29–31). hoping that the ECs will form vasculature in the canal before the angiogenesis taking place from the periapical blood vessel system into the canal. However, this is impractical as ECs are difficult to obtain from the same host. Taking the advantage of DPSC endothelial differentiation potential, we can angiogenically induce DPSCs to become endothelial-like cells (DPSC-ECs) before implanting into the root canal which may accelerate the vascularization of pulp regeneration. Previously we have tested in vitro that both angiogenically induced human and mini-swine DPSCs appear to perform similarly in tubule formation comparing to ECs (32). We then tested in vivo by injecting GFP-labeled DPSC-ECs carried by Matrigel into the subcutaneous space of immunocompromised mice forming a Matrigel plug which is a standard in vivo angiogenesis/neovascularization assay. After 7 days, we found that the mouse blood vessels sprouted and invaded into the Matrigel area trying to connect with the premature vascular networks formed by the implanted DPSC-ECs (Fig. 2A,B). This is a proof-of-principle experiment using an ectopic neovascularization mouse model. A new study needs to be development that can simulate such biological process occurring orthotopically.
Fig 2.
Immunohistochemistry of vascular formation in Matrigel plug in vivo. hDPSC-ECs-GFP were generated by transduction of hDPSCs with pLenti-EF1a-GFP followed by angiogenic induction for 7 days. hDPSC-ECs-GFP were mixed in Matrigel and injected into the dorsum subcutaneously of SCID mice for 7 d. GFP was stained by anti-GFP shown in brown. (A) Lower magnification showing hDPSC-ECs-GFP forming vascular-like networks (red arrowheads). (B) Higher magnification showing the junction of Matrigel (G) and mouse tissue (T) indicated by the dotted red lines. Blue arrows indicate ingrown vessels from mouse tissues, red arrows indicate vessels formed by hDPSC-ECs-GFP connecting with vessels from the mouse (in areas of dotted circles). Some vessels contain RBCs are seen. Scale bars: (A) 500 μm; (B) 100 μm.
The strategy is worthy of testing for pulp regeneration. The question to be answered is how quickly and stable the transplanted DPSC-ECs can establish vascular network in the canal space and anastomose with the sprouting new vessels from the periapex. Schematic illustration in Fig. 3. depicts the aspired outcome using this approach. If the transplanted DPSC-ECs in the canal space formed vascular network (Fig. 3A), it requires simultaneous angiogenic sprouting from the periapical blood vessels and anastomosing with the DPSC-EC vascular network in order to obtain blood perfusion (Fig. 3B).
Fig 3.
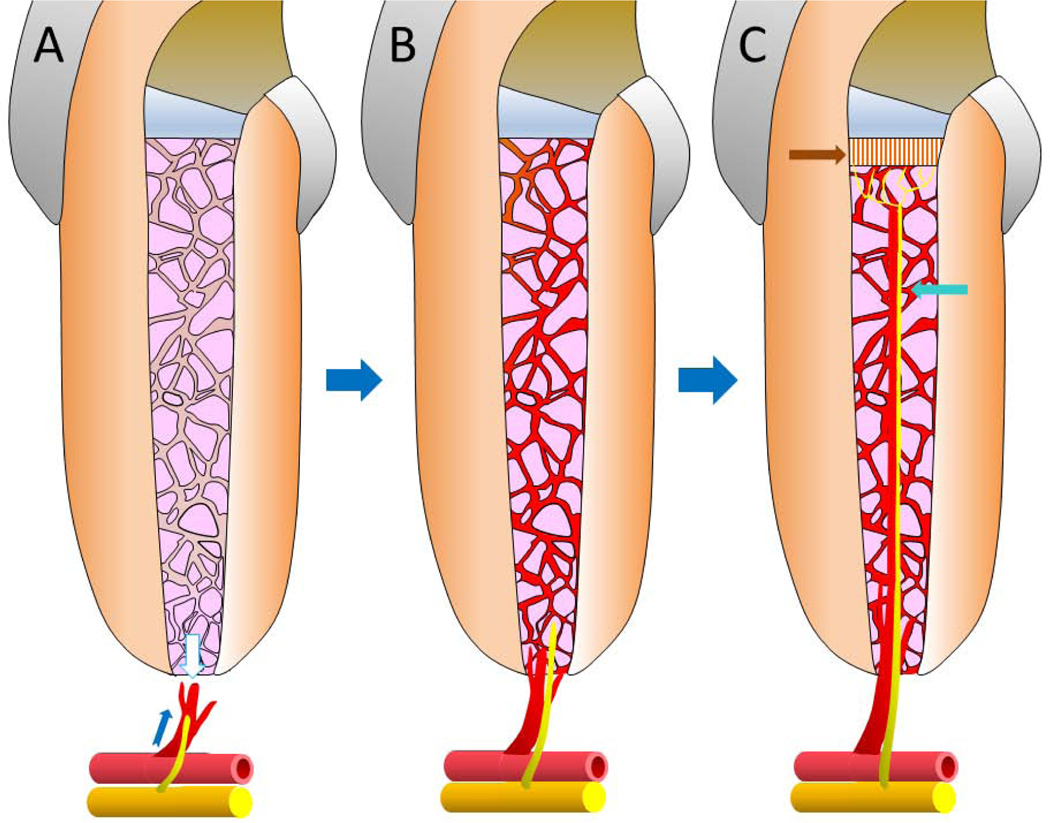
Schematic illustration of the potential process during stem-cell based pulp regeneration. (A) DPSC-ECs carried by scaffold are implanted into the canal space and the tooth sealed with cement. The DPSC-ECs form vascular networks in the canal space. The apical part of the formed network is reaching out (white arrow) to the newly sprouting vessels at the periapex (blue arrow) in order to establish anastomosis. The nerve fibers at the periapex also branch out and extend into the canal space. (B) If the anastomosis occurs, blood perfusion takes place allowing the maturation of the newly formed blood vessel network by DPSC-ECs to establish and mature, thus supplying nutrition to the implanted stem cells. Pulp regeneration can then continue to progress. The sprouting nerve fibers also continue to extend into the canal. (C) Some implanted DPSCs will differentiate into odontoblast-like cells and produce tubular dentin bridge underneath the cement (indicated by the brown arrow). The innervation will also extend (blue arrow) and eventually reach the dentin bridge.
If without such anastomosis, the nutrition can only reach deep into the canal space through diffusion which is far less efficient. If the perfusion can occur rapidly, the newly formed vascular network in the canal space can be more stabilized and sustained so as to facilitate pulp regeneration. Currently, the orthotopic large animal studies only indicate that vascularization occurs during pulp regeneration (10), however, it is not necessarily consistent, nor do we know the kinetics of such neovascularization. The progression of vascular formation and the level of implanted DPSCs in becoming ECs during pulp regeneration are not clear. The use of DPSC-ECs plus DPSCs for pulp regeneration is at present only being proposed (32).
If pulp regeneration is successful, a new dentin bridge should form underneath the filling material in the pulp chamber. This possibility has been projected in the semi-orthotopic tooth fragment mouse model and orthotopic min-swine model where newly formed dentin bridge was observed underneath the cement (3, 12). Simultaneously, reinnervation should occur starting by sprouting of the nerve fibers at the periapex and extending into the canal space. This extension should run all the way to the newly formed dentin bridge and innervate as the normal nerve fibers would do, i.e., the branching nerve endings extend into the predentin tubule space. The innervation of the regenerated pulp is indicated by the direct histological finding in the orthotopic dog model studies (2) and positive pulp vitality test response in human trial studies (4, 5).
The ingrowth of the nerve fibers should be myelinated as they extend into the canal. The myelination may be facilitated by the Schwann cells proliferated from the periapex or differentiated from the implanted DPSCs which is known to potentially become Schwann cell-like expressing the cell markers (33).
3). Regeneration of tubular dentin
As we know the formation of dentin by odontoblasts is quite unique such that it creates dentinal tubules allowing the dentin sensitivity to exist. This process is difficult to recreate as evidenced by the pulp regeneration experiments using various study models. The hydroxyapatite/tricalcium (HA/TCP) mouse model was first reported by Gronthos et al when DPSCs were discovered (11). The formation of ectopic pulp-dentin complex in the mouse dorsal subcutaneous space revealed that the newly generated dentin-like mineral contains some dentinal tubule-like structures. After our extensive experiments using this model (3), we found that such tubular formation does not always occur. In fact, even the mineral tissue is not always formed. It could have to do with the quality of the DPSCs. Here in Fig. 4Aa, we present one experiment of HA/TCP model in which mini-swine DPSCs were tested. In an area of the sample with mineral tissue formation, dentinal tubule-like structures can be observed, although the tubules are not well-organized, nor well-compacted as those in natural dentin. Such tubular structures are even less observed in the tooth fragment mouse model (Fig. 4Ab) and never observed in the orthotopic min-swine model based on our experience (Fig. 4Ac). To truly regenerate functional dentin, newly differentiated odontoblasts need to produce regenerated dentin seamlessly integrating onto original dentin walls as depicted by the illustration in Fig. 4B which indicates that the newly differentiated odontoblasts need to extend their process into the existing dentinal tubules followed by producing dentin the same way during the dentin development. However, such evidence has only been reported by Oh et al demonstrating the use of copine 7, a preameloblast-derived factor, as a guiding force for DPSCs to become more authentic odontoblasts, thereby producing tubular dentin (34). More extensive investigation is needed to determine whether this approach can potentially be integrated into pulp regeneration protocols to consistently generate truly functional pulp and tubular dentin.
Fig. 4.

Dentinal tubules of regenerated dentin. (A) Current histological evidence of regenerative dentin (rD). (Aa) Hydroxyapatite/Tricalcium phosphate model (HA/TCP) for in vivo formation of ectopic pulp-dentin complex. Mini-swine DPSCs mixed with HA/TCP were implanted into SCID mice and samples harvested after 3 months. Formed mineral tissues contain dentinal tubule-like structures (arrowheads). (Ab) Tooth fragment model with mini-swine tooth and DPSCs implanted into SCID mice and samples harvested after 3 months. The rD contains many trapped cells and no dentinal tubule-like structures, except randomly formed irregular short curvy lines which could be tubules. (Ac) Orthotopic pulp regeneration in mini-swine showing formation of rD with trapped cells and lacking dentinal tubule structures. D: original dentin; Od; odontoblast-like cells lining against the mineral (blue arrows) Scale bars: (Aa) 50 μm; (Ab,c) 100 μm. (B) Schematics of tubular rD formation onto existing dentin of canal wall. (Ba) Implanted DPSCs differentiate into new Od and extend their cellular processes into the existing dentinal tubules. (Bb) Od deposit tubular rD on the dentin wall and DPSCs will regenerate pulp in canal space and some turn into Fbs. rP, regenerated pulp; pFbs, pulp fibroblasts. Newly differentiated odontoblasts produce regenerated tubular dentin seamlessly integrated onto original dentin walls. Growth factor (GFs), eg., CPNE7 is added in DPSCs/gel to fill the root canal space. The GFs along with naturally embedded factors in dentin D will guide DPSC differentiation into new Od.
Summary
The ultimate goal is to establish regenerative endodontic technology suited for all teeth that can be as successful as the current traditional endodontic treatment, thereby replacing the current protocol for most clinical cases.
We need to develop utterly effective disinfection protocol such that bacteria invading the complex root canal system including isthmus, grooves and dentinal tubules can be eliminated or killed. A condition after the disinfection must be able to allow regeneration to occur without recurrence of infection from the remaining bacteria in the root canal space.
We need to establish a protocol that allows timely vascularization during the pulp regeneration such that the transplanted stem cells in the coronal area (far away from apex) can receive blood supply in order to enable optimal cell growth and differentiation, thus to achieve consistent and complete pulp regeneration.
Significance.
Ultimate goal of regenerative endodontics is to restore pulp/dentin tissues to their natural state. Specific challenges are outlined in this article discussing the future research directions to overcome those challenges.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health R01 DE019156 (G.T.-J.H.), a grant from American Association of Endodontists Foundation and a Research Fund from the University of Tennessee Health Science Center.
Studies based on exempt protocols approved by the Medical Institutional Review Board (UTHSC:#19–06855 NHSR); no patient consents were needed. All animal procedures followed protocols approved by IACUC at UTHSC (#15–069-A and #15–070-A).
Footnotes
Declaration of interest:
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang GT, Garcia-Godoy F. Missing Concepts in De Novo Pulp Regeneration. J Dent Res 2014;93:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iohara K, Imabayashi K, Ishizaka R, et al. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A 2011;17:1911–1920. [DOI] [PubMed] [Google Scholar]
- 3.Zhu X, Liu J, Yu Z, et al. A Miniature Swine Model for Stem Cell-Based De Novo Regeneration of Dental Pulp and Dentin-Like Tissue. Tissue Eng Part C Methods 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xuan K, Li B, Guo H, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med 2018;10. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima M, Iohara K, Murakami M, et al. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther 2017;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alley BS, Kitchens GG, Alley LW, Eleazer PD. A comparison of survival of teeth following endodontic treatment performed by general dentists or by specialists. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98:115–118. [DOI] [PubMed] [Google Scholar]
- 7.Kojima K, Inamoto K, Nagamatsu K, et al. Success rate of endodontic treatment of teeth with vital and nonvital pulps. A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:95–99. [DOI] [PubMed] [Google Scholar]
- 8.Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: part 1: periapical health. Int Endod J 2011;44:583–609. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Anderson P, Bader J, et al. Outcomes of root canal treatment and restoration, implant-supported single crowns, fixed partial dentures, and extraction without replacement: a systematic review. J Prosthet Dent 2007;98:285–311. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Liu J, Yu Z, et al. A Miniature Swine Model for Stem Cell-Based De Novo Regeneration of Dental Pulp and Dentin-Like Tissue. Tissue Eng Part C Methods 2018;24:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences 2000;97:13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang GTJ, Yamaza T, Shea LD, et al. Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 2009;16:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakashima M, Iohara K, Bottino MC, Fouad AF, Nor JE, Huang GT. Animal Models for Stem Cell-Based Pulp Regeneration: Foundation for Human Clinical Applications. Tissue Eng Part B Rev 2019;25:100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dental Traumatology 2002;18:134–137. [DOI] [PubMed] [Google Scholar]
- 15.Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod 1989;15:512–516. [DOI] [PubMed] [Google Scholar]
- 16.Piwowarczyk A, Lauer HC, Sorensen JA. Microleakage of various cementing agents for full cast crowns. Dent Mater 2005;21:445–453. [DOI] [PubMed] [Google Scholar]
- 17.Caplan DJ, Cai J, Yin G, White BA. Root canal filled versus non-root canal filled teeth: a retrospective comparison of survival times. J Public Health Dent 2005;65:90–96. [DOI] [PubMed] [Google Scholar]
- 18.Galler KM, Krastl G, Simon S, et al. European Society of Endodontology position statement: Revitalization procedures. Int Endod J 2016;49:717–723. [DOI] [PubMed] [Google Scholar]
- 19.Lin LM, Ricucci D, Huang GT. Regeneration of the dentine-pulp complex with revitalization/revascularization therapy: challenges and hopes. Int Endod J 2014;47:713–724. [DOI] [PubMed] [Google Scholar]
- 20.McGurkin-Smith R, Trope M, Caplan D, Sigurdsson A. Reduction of intracanal bacteria using GT rotary instrumentation, 5.25% NaOCl, EDTA, and Ca(OH)2. J Endod 2005;31:359–363. [DOI] [PubMed] [Google Scholar]
- 21.Siqueira JF Jr., Magalhaes KM, Rocas IN. Bacterial reduction in infected root canals treated with 2.5% NaOCl as an irrigant and calcium hydroxide/camphorated paramonochlorophenol paste as an intracanal dressing. J Endod 2007;33:667–672. [DOI] [PubMed] [Google Scholar]
- 22.Verma P, Nosrat A, Kim JR, et al. Effect of Residual Bacteria on the Outcome of Pulp Regeneration In Vivo. J Dent Res 2017;96:100–106. [DOI] [PubMed] [Google Scholar]
- 23.Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM. Histologic Study of a Human Immature Permanent Premolar with Chronic Apical Abscess after Revascularization/Revitalization. J Endod 2014;40:133–139. [DOI] [PubMed] [Google Scholar]
- 24.Lin LM, Shimizu E, Gibbs JL, Loghin S, Ricucci D. Histologic and histobaceriologic observations of failed revascularization/revitalization therapy: a case report. J Endod 2014. [DOI] [PubMed] [Google Scholar]
- 25.Al Shahrani M, DiVito E, Hughes CV, Nathanson D, Huang GT. Enhanced removal of Enterococcus faecalis biofilms in the root canal using sodium hypochlorite plus photon-induced photoacoustic streaming: an in vitro study. Photomed Laser Surg 2014;32:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azim AA, Aksel H, Zhuang T, Mashtare T, Babu JP, Huang GT. Efficacy of 4 Irrigation Protocols in Killing Bacteria Colonized in Dentinal Tubules Examined by a Novel Confocal Laser Scanning Microscope Analysis. J Endod 2016;42:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iohara K, Nakashima M. The Utility of Nanobubbles for Removing the Smear Layer in Root Canal Treatment. Japanese Journal of Conservative Densitry 2019;62:159–164. [Google Scholar]
- 28.Li X, Ma C, Xie X, Sun H, Liu X. Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater 2016;35:57–67. [DOI] [PubMed] [Google Scholar]
- 29.Dissanayaka WL, Zhan X, Zhang C, Hargreaves KM, Jin L, Tong EH. Coculture of dental pulp stem cells with endothelial cells enhances osteo-/odontogenic and angiogenic potential in vitro. J Endod 2012;38:454–463. [DOI] [PubMed] [Google Scholar]
- 30.Dissanayaka WL, Zhu L, Hargreaves KM, Jin L, Zhang C. Scaffold-free Prevascularized Microtissue Spheroids for Pulp Regeneration. J Dent Res 2014;93:1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng Part A 2015;21:550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aksel H, Huang GT. Human and Swine Dental Pulp Stem Cells Form a Vascularlike Network after Angiogenic Differentiation in Comparison with Endothelial Cells: A Quantitative Analysis. J Endod 2017;43:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karaoz E, Demircan PC, Saglam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol 2011;136:455–473. [DOI] [PubMed] [Google Scholar]
- 34.Oh HJ, Choung HW, Lee HK, et al. CPNE7, a preameloblast-derived factor, regulates odontoblastic differentiation of mesenchymal stem cells. Biomaterials 2015;37:208–217. [DOI] [PubMed] [Google Scholar]




