Abstract
In this issue of Neuron, Gatto et al. (2021) demonstrate that tactile reflexes are driven by excitatory modules defined by location, while Peirs et al. (2021) show that the circuits implicated in the conversion of touch to pain are defined by the nature of the injury.
Walking along the beach, you step on a sharp object. The first thing you do is withdraw your foot; then you realize it hurt. Nevertheless, you continue walking and get sunburned. Hours later, someone brushing against your arm evokes a similar response to the sharp object piercing your foot—withdrawal followed by “ouch!” How does our nervous system encode this sensory information (sharp object/brush) and evoke appropriate motor responses (withdrawal), and why do we become more sensitive after injury? In this issue of Neuron, Gatto et al. (2021) and Peirs et al. (2021) use behavioral analyses to examine the neural substrates encoding somatosensory information, providing new insight into how spinal cord neurons are organized to evoke appropriate responses.
How do we define the neural substrates of behavior? The study of spinal cord circuits is well suited to exploring this question. The direct link between sensory input (touch, pain, itch) and behavioral output (muscle contraction, withdrawal, locomotion) affords an experimental tractability that spinal cord neurobiologists have leveraged for decades. From this work, two prominent ideologies have emerged: (1) labeled lines, i.e., defined circuits for each sensory modality, and (2) population coding, where crosstalk between different neuronal populations encodes sensory information. In an attempt to demystify how circuits encode information, recent work has aimed to identify functionally distinct neuronal populations by exploiting their unique molecular signatures (Osseward and Pfaff, 2019; Luo et al., 2018). Gain- and loss-of-function studies targeting “distinct” neuronal populations—defined by differential expression of molecular markers—often results in selective impairment, supporting a labeled line model. However, many so-called distinct populations regulate the same behavior, and single modalities are capable of activating heterogeneous populations (Gatto et al., 2019; Smith and Ross, 2020). Can functionally discrete somatosensory pathways be circumscribed by molecular signature, or are we really just disrupting components of broader spinal circuits working in concert? Are populations we manipulate really subpopulations at all?
To understand how sensory information is processed, it is important to establish where modality-specific information is encoded. Using activation markers, Gatto et al. (2021) highlight a location specificity to the processing of scratch and withdrawal reflexes, with LI-II neurons excited by scratch and LIIi-IV activated during withdrawal reflexes. However, neurons in similar regions are excited by different stimuli too. Previously, Peirs et al. (2015) showed that two neuronal populations, labeled by calretinin (CR) and protein kinase C gamma (PKCγ), are preferentially activated following inflammatory and neuropathic injury, respectively. So, is it location or neuronal subtype that determines function?
Now, Peirs et al. (2021) investigate how four excitatory neuron classes contribute to the development of mechanical allodynia (when touch turns to pain). The first major finding confirms that LII CR- and PKCγ-expressing neurons are preferentially involved in the expression of mechanical allodynia following inflammatory and neuropathic injury, respectively. In this instance, two molecularly distinct populations within the same region transmit the same information; however, the circumstances in which they do so depends on the injury. Given that touch information is also transmitted to the deep dorsal horn, the group determined the role of two populations within this region. They show that cholecystokinin (CCK)-expressing neurons are important for punctate and dynamic allodynia following both inflammatory and neuropathic injury. Additionally, a subset of these neurons characterized by transient expression of vesicular glutamate transporter 3 (tVGluT3) conveys primarily dynamic mechanical allodynia. This suggests that within an excitatory CCK module, functionally heterogeneous populations exist, each with their own defined roles. Together, this study shows that while inflammatory and neuropathic injury can both result in touch turning into pain, the neuronal circuits underlying this final output are activated under different conditions. Further, numerous functionally distinct subcomponents are likely to work together to ensure full expression of mechanical allodynia phenotypes (Figures 1B and 1C).
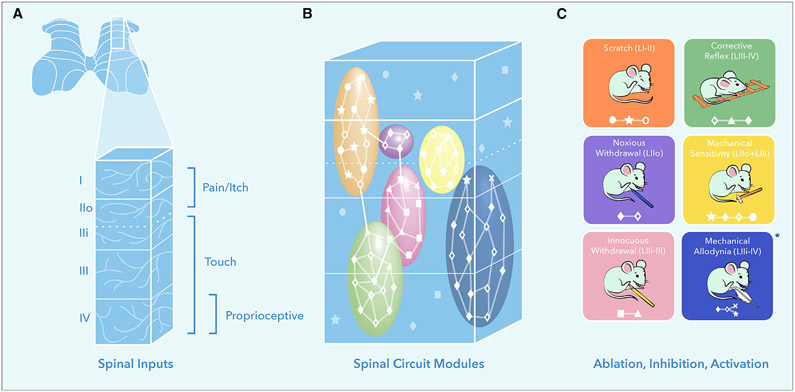
Figure 1. The Functional Organization of Excitatory Neurons within the Dorsal Horn.
(A) Sensory inputs to the spinal cord dorsal horn display a modular organization: LI-IIo, pain and itch; LIIi-IV, touch; and LIV, proprioceptive.
(B) Excitatory neurons within the dorsal horn are organized into spatially restricted modules that are molecularly heterogeneous.
(C) Neuronal manipulation studies reveal that reflex behaviors are evoked by molecularly heterogeneous populations within discrete excitatory modules. *In the case of mechanical allodynia distinct circuits are activated in a context-dependent manner. Symbols represent molecularly distinct neuronal populations.
Gatto et al. (2021) utilize intersectional genetic approaches to examine the contribution of eight classes of excitatory interneuron to sensorimotor reflexes. Carefully considered genetic combinations allowed for the manipulation of specific excitatory classes with similar and distinct locations. Manipulating two broad yet spatially distinct classes shows that the most superficial layers of the dorsal horn (LI-II) are involved in scratch reflexes, while the deeper layers (LII-IV) modulate withdrawal reflexes in response to noxious and innocuous mechanical stimuli. As LIIo is generally associated with pain processing and LIIi-III with touch, Gatto et al. (2021) sought to manipulate activity in these regions more precisely. They show that neurons within LIIi-III are involved in withdrawal reflexes in response to light touch and, by subtraction, that those in LIIo control the reflex in response to noxious stimuli. Interestingly, manipulating neurons restricted to one of these regions had no influence on mechanical sensitivity (withdrawal threshold). Thus, appropriate withdrawal requires excitatory neurons across LII-III to act in concert, with neurons spanning LIIo-IIi setting mechanical sensitivity and LIII neurons modulating motor output. Gatto et al. (2021) also show that the circuits underlying static and dynamic reflexes are distinct, with more superficial (LIIo-III) and deep (LIII-IV) populations showing preferential contributions to withdrawal and corrective reflexes.
Together, these data indicate that sensory information is processed by spatially organized ensembles. Like an orchestra, each section has its own role, working in synchrony to achieve the appropriate output. But are all the instruments in the string section the same? To answer this, Gatto et al. (2021) assess the contributions of non-overlapping excitatory populations within the same region. They show that heterogeneous populations of neurons contribute to scratch, withdrawal, and corrective reflexes (Figures 1B and 1C). Though this could be interpreted as redundancy, the data indicate that members of the same orchestral section play their own subtle role. For example, while two populations are involved in scratching, only one influences the speed of the scratch. This suggests a functional organization whereby location-dependent modules of heterogeneous excitatory neurons—each their own section of the orchestra—are working together to produce the right output—the symphony.
Neuronal location provides a substantial clue as to what neurons do (Figure 1A) (Smith and Ross, 2020). If your neuron is located in LI-IIo, you’ll say, “it’s pain or itch,” in LIIi-III “probably touch,” and any deeper and it “must be motor!” How do spinal cord circuits make sense of this information? Both Gatto et al. (2021) and Peirs et al. (2021) show that indeed location and, by association, input type are critical factors in determining function. Their studies suggest that somatosensory reflexes are produced by the coordinated activity of spatially restricted, molecularly heterogeneous modules (Figure 1). This supports population coding, where the interaction between different populations determines the response. In contrast, both studies also provide evidence for specificity in molecularly defined populations. While loss of a subpopulation within an excitatory module may retain an obvious behavioral output, further analysis reveals more specific phenotypes. In a similar way, if you removed the entire percussion section from an orchestra, it would be obvious. However, you might get away with losing just the cymbals, until a key moment where the lack of a dramatic clash was apparent. As we reveal more functionally discrete populations, we must also embrace new assays and technologies, including computer vision and machine learning to identify more subtle behavioral transformations (Fried et al., 2020) and functional imaging and electrophysiology to correlate behavior with neuronal activity (Luo et al., 2018).
These exciting studies offer an array of future experiments that will further inform our understanding of how neuronal circuits process information. Do inhibitory interneurons similarly form spatially restricted modules? Given that the majority of inhibitory neurons’ input/output are restricted to the same lamina as their soma, such an arrangement seems likely. Do similar modules exist in the mediolateral aspect? The segregated arrangement of glabrous and hairy skin or restriction of C-LTMRs to lateral aspects of the lumbar spinal cord suggests so. While we know neurons receive an array of afferent, local, and descending input, we now must determine how they make sense of this convergent input and what makes them preferentially activated under different conditions (normal versus inflammatory versus neuropathic or swing versus stance phase). Answers to these questions will inform how the modules described by Gatto et al. (2021) and Peirs et al. (2021) encode information.
Earlier we asked: can functionally discrete somatosensory pathways be circumscribed by molecular signature? Are the populations we manipulate really subpopulations at all? It is tempting to suggest that neuronal circuits are arranged according to their molecular profile acting together to appropriately encode sensory information. However, how we define neuronal populations is continuously evolving. Lu and Perl (2005) proposed a similar modular structure within the dorsal horn, showing particular combinations of neurons to be repeatedly connected, while others were not. However, these neurons were defined by their morphological and electrophysiological profiles, not by molecular signature. Now, sequencing studies have granted us the ability to subdivide neuronal populations further than ever before. Are these populations really functionally distinct? How can we best leverage the genetic tools available to better understand circuits? Both studies underscore the need to move away from single gene tagging as our go-to approach for neuronal manipulation. But how do we determine the critical factors underlying circuit function? Combinatorial approaches assessing location, morphology, input, output, electrophysiological characteristics, molecular profile, and the functional relevance of each will likely be key. Understanding these factors will inform, first, what makes a subpopulation and, second, how different populations act in concert to ensure contextually relevant behaviors.
REFERENCES
- Fried NT, Chamessian A, Zylka MJ, and Abdus-Saboor I (2020). Improving pain assessment in mice and rats with advanced videography and computational approaches. Pain 161, 1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto G, Smith KM, Ross SE, and Goulding M (2019). Neuronal diversity in the somatosensory system: bridging the gap between cell type and function. Curr. Opin. Neurobiol 56, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto G, Bourane S, Ren X, Di Costanzo S, Fenton PK, Halder P, Seal RP, and Goulding MD (2021). A Functional Topographic Map for Spinal Sensorimotor Reflexes. Neuron 109, this issue, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, and Perl ER (2005). Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). J. Neurosci 25, 3900–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, and Svoboda K (2018). Genetic Dissection of Neural Circuits: A Decade of Progress. Neuron 98, 256–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osseward PJ 2nd, and Pfaff SL (2019). Cell type and circuit modules in the spinal cord. Curr. Opin. Neurobiol 56, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, and Seal RP (2015). Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 87, 797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Williams SG, Zhao X, Arokiaraj CM, Ferreira DW, Noh MC, Smith KM, Halder P, Corrigan KA, Gedeon JY, et al. (2021). Mechanical Allodynia Circuitry in the Dorsal Horn Is Defined by the Nature of the Injury. Neuron 109, this issue, 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, and Ross SE (2020). Making connections: recent advances in spinal cord dorsal horn circuitry. Pain 161 (Suppl 1), S122–S126. [DOI] [PubMed] [Google Scholar]