Abstract
rRNA is the most single abundant polymer in most cells. Mammalian rRNAs are nearly twice as large as those of prokaryotes. Differences in rRNA size are due to expansion segments, which contain extended tentacles in metazoans. Here we show the termini of an rRNA tentacle of Homo sapiens contains 10 tandem G-tracts and forms highly stable G-quadruplexes in vitro. We characterized rRNA of the H. sapiens large ribosomal subunit by computation, circular dichroism, UV melting, fluorescent probes, nuclease accessibility, electrophoretic mobility shifts and blotting. We investigated Expansion Segment 7 (ES7), oligomers derived from ES7, intact 28S rRNA, 80S ribosomes and polysomes. We used mass spectrometry to identify proteins that bind to rRNA G-quadruplexes in cell lysates, which include helicases (DDX3, CNBP, DDX21, DDX17) and heterogeneous nuclear ribonucleoproteins (hnRNPs). Finally, by multiple sequence alignments, we observe that G-quadruplex-forming sequences are a general feature of LSU rRNA of Chordata but not, as far as we can tell, other species. Chordata ribosomes present polymorphic tentacles with the potential to switch between inter- and intramolecular G-quadruplexes. To our knowledge G-quadruplexes have not been reported previously in ribosomes.
Keywords: rRNA, expansion segments, G-tracts, chordates, helicases, polysomes
INTRODUCTION
Cytosolic ribosomes are the most abundant assemblies in any cell, containing over 80% of cellular RNA [1]. Ribosomes are built on a ‘common core’ [2] of rRNA with universal structure and function in all extant species. Common core rRNA is approximated by prokaryotic rRNA; around 90% of prokaryotic rRNA is contained in the common core.
The LSU of eukaryotic ribosomes contains additional rRNA in a secondary shell that surrounds the common core. The rRNA of the eukaryotic shell is composed of expansion segments (ESs) that attach to common core rRNA at a handful of specific sites [3–6]. ESs are the most variable rRNA structures over phylogeny. In S. cerevisiae, ESs are important in ribosome biogenesis [7] and chaperone association [8, 9]. Beckman and coworkers recently described a 3D structure in which S. cerevisiae ESs associate with and localize N-terminal acetylases [10].
ESs of chordates contain “tentacles” (Figure 1) [11]. These tentacles reach a zenith in primates and birds, extending for hundreds of Ångstroms from the ribosomal surface. Here we observe G-quadruplexes in rRNA tentacles of human ribosomes. Tandem G-tracts are found in the tentacles of expansion segments 7 and 27 of H. sapiens ribosomes (ES7HS and ES27HS). ES7HS contains ten tandem G-tracts in tentacle a and four in tentacle b. ES27HS contains six tandem G-tracts in tentacle a, two sets of three in tentacle b, and four within a base helix (Table 1).
Figure 1.
Model of the secondary structure of the LSU rRNA of Homo sapiens. G-quadruplex-forming regions (defined by G≥3N1–7G≥3N1–7G≥3N1–7G≥3) are highlighted. a) Expansion segment ES7HS is orange. Tentacles a, b and d of ES7HS are indicated. G-quadruplex-forming regions of ES7HS are GQES7-a (red, in tentacle a) and GQES7-b (cyan, in tentacle b) and are boxed by dashed lines. Expansion segment ES27HS is green with purple G-tracts. Helix 63, at the base of ES27HS, contains a G-quadruplex motif (purple). Tentacles a and b of ES27HS are indicated. b) An expanded view of GQES7-b indicates the nucleotide sequence. c) An expanded view of GQES7-a indicates the nucleotide sequence.
Table 1.
G-quadruplex-forming regions within ES7 and ES27 rRNA
| ES7 | Nucleotides | |
|---|---|---|
| tentacle a | ||
| Homo sapiens | GGGGGCGGGCUCCGGCGGGUGCGGGGGUGGGCGGGCGGGGCCGGGGGUGGGGUCGGCGGGGG | 587–648 |
| Pan troglodytes | GGGGGCGGGCUCCGGCGGGUGCGGGGGUGGGCGGGCGGGGCCGGGGGUGGGGUCGGCGGGGG | 583–644 |
| Mus musculus | GGGCGGGGCCGGGGGUGGGGUCGGCGGGGG | 627–656 |
| Gallus gallus | GGGGCGGGGCGGGCCCAGGGGGGGCGGGCGGGCCGGGG | 557–594 |
| tentacle b | ||
| Homo sapiens |
GGGAGGGCGCGCGGGUCGGGG |
829–849 |
| Pan troglodytes | GGGAGGGCGCGCGGGUCGGGG | 816–836 |
| tentacle d | ||
| Mus musculus | GGGCGGGCGUGGGGGUGGGGGCCGGG | 907–932 |
| Gallus gallus | GGGGCGCGGGGGCGGGGGGGGUCGGG | 933–958 |
| ES27 | ||
| tentacle a | ||
| Homo sapiens | GGGGGAGCGCCGCGUGGGGGCGGCGGCGGGGGGAGAAGGGUCGGGGCGGCAGGGG | 3095–3149 |
| tentacle b | ||
| Homo sapiensa | GGGGGCGGGGAGCGGUCGGGCGGCGGCGGUCGGCGGGCGGCGGGGCGGGG | 3373–3422 |
| Helix 63 | ||
| Homo sapiens | GGGCUGGGUCGGUCGGGCUGGGG | 2896–2918 |
) This sequence falls outside the G≥3N1–7G≥3N1–7G≥3N1–7G≥3 motif.
G-quadruplexes are favored by tandem G-tracts separated by short non-specific sequences. To investigate the possibility that G-tracts in rRNA tentacles form G-quadruplexes, we used computation, circular dichroism, fluorescent probes, thermal melting, nuclease accessibility, electrophoretic mobility shift assays (EMSA), dot blotting, Western blotting, and pull-down assays combined with stable isotope labeling of amino acids in cell culture (SILAC) and Mass Spectrometry. To investigate phylogenetic distribution of ribosomal G-quadruplexes we conducted Multiple Sequence Alignments (MSAs) and database analysis.
The results indicate that G-quadruplexes form in oligomers composed of sequences derived from ES7HS. In addition, G-quadruplexes form in intact ES7HS and in purified human 28S rRNA. We present data supporting formation of G-quadruplexes in 80S ribosomes and in polysomes. SILAC experiments show that known G-quadruplex-binding proteins associate with the G-tracts of ES7HS. MSAs indicate that G-quadruplex-forming sequences are found in ES7s of all chordates.
RESULTS
ES7 and ES27 of the human LSU contain G-quadruplex-forming sequences.
The propensity of an RNA to form G-quadruplexes can be estimated from sequence - by lengths of guanine tracts and the lengths and compositions of loops regions. The program QGRS Mapper [12] provides “G-scores”, which quantitate this propensity. We have identified ES7 and ES27 as the primary regions in the human LSU with sequences that appear to be capable of forming G-quadruplexes. The computational results suggest that G-quadruplexes can form near the termini of the longest rRNA tentacles of these two ESs. In tentacles a and b of ES7HS, two regions, here named GQES7-a and GQES7-b (Figure 1), meet the G-quadruplex consensus (G≥3N1–7G≥3N1–7G≥3N1–7G≥3). The G-scores of GQES7-a and GQES7-b are in the range of well-established RNA G-quadruplexes.
The sequence 5’ GGGGCCGGGGGUGGGGUCGGCGGGG 3’ (nts 623–647, from within GQES7-a, Figure 1, Table 1) gives a G-score of 60. The sequence 5’ GGGUGCGGGGGUGGGCGGG 3’ (nts 603–621, also within GQES7-a) gives a G-score of 40. The sequence 5’ GGGAGGGCGCGCGGGUCGGGG 3’ (nts 829–849 within GQES7-b, Figure 1, Table 1) gives a G-score of 38. Differences in the number of tandem G-tracts (10 in GQES7-a and 4 in GQES7-b), the lengths of the G-tracts, and G-scores suggest more stable and more extensive G-quadruplex formation in GQES7-a than in GQES7-b. The G-tracts of GQES7-a are longer than those of GQES7-b and there are more of them. A greater propensity of GQES7-a over GQES7-b for G-quadruplex formation is seen in all experiments below.
As a positive control for both computation and experiment, we used the G-quadruplex from the 5’-UTR of the mRNA of the ADAM10 metalloprotease [13]. This stable and well-characterized RNA G-quadruplex gives a G-score of 42. As negative controls, we used two mutant RNA oligomers (mtES7-a and mtES7-b) that are analogous to GQES7-a and GQES7-b in composition and length, with disrupted G-tracts (Table S.1). Neither gives a G-score. In several experiments we used yeast-tRNAphe as an additional negative control.
We focused our experiments primarily on ES7HS. However, the end of tentacle a of ES27HS contains the sequence 5’ GGGGAGAAGGGUCGGGGCGGCAGGG 3’ (nts 3124–3148, tentacle a), which gives a G-score of 40 (Figure 1, Table 1). ES27HS also contains a G-quadruplex-forming region within Helix 63, near the junction of tentacles a and b. Based on the high G-scores and our experimental observation of G-quadruplexes within ES7HS, we expect G-quadruplexes to form in ES27HS.
Circular dichroism.
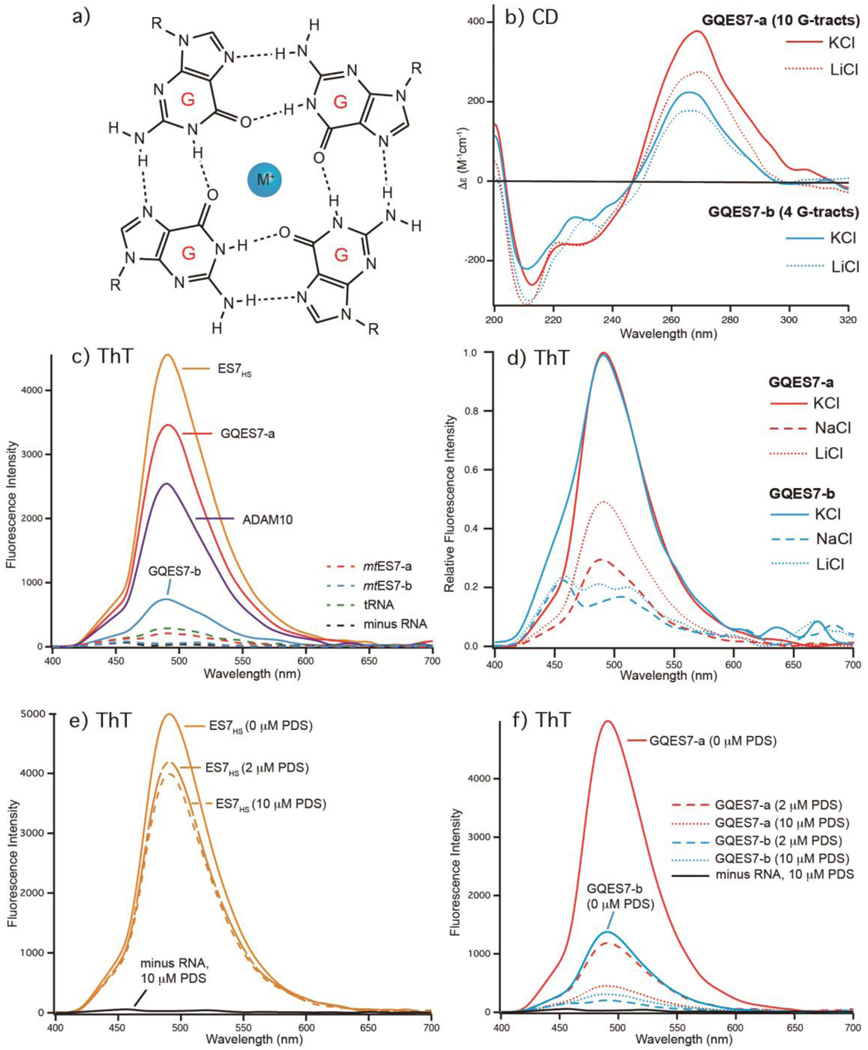
CD spectra of GQES7-a and GQES7-b indicate G-quadruplex formation, with the expected dependence on type of counterion. CD is used widely to study RNA and DNA G-quadruplexes [14–18]. CD spectra of GQES7-a and GQES7-b (Figure 2b) show the characteristic peak at 260 nm and trough at 240 nm. It is known that G-quadruplex formation is promoted by K+ and is inhibited by Li+ or Na+ [19]. The intensities of the 260 nm peaks of both GQES7-a and GQES7-b are attenuated when the monovalent cation is switched from K+ to Li+. GQES7-a gives a more intense CD signal than GQES7-b under all conditions.
Figure 2.
Formation of G-quadruplexes by rRNA fragments GQES7-a, GQES7-b, and ES7HS. a) G-quadruplexes preferentially coordinate K+. b) CD spectra of GQES7-a and GQES7-b in the presence of either K+ or Li+. c) Fluorescence emission at 487 nm of the G-quadruplex probe ThT in the presence of ES7HS, GQES7-a, GQES7-b, or positive control ADAM10. Negative controls (dashed) are tRNA, mtES7-a, mtES7-b and minus RNA. d) ThT fluorescence emission of GQES7-a and GQES7-b in the presence of various monovalent cations. Intensities of GQES7-a and GQES7-b are normalized in the presence of K+ to highlight cation-induced differences. e) PDS competes with ThT in association with ES7HS. f) PDS competes with ThT in association with GQES7-a and GQES7-b.
ThT fluorescence.
ThT is known to yield intense fluorescence at 487 nm upon association with G-quadruplexes [20, 21]. ThT fluorescence results here suggest formation of G-quadruplexes in GQES7-a, GQES7-b and intact ES7HS (Figure 2c). Intact ES7HS and GQES7-a give more intense ThT fluorescence signals than the positive control (ADAM10). The GQES7-b signal is less than that of ADAM10 but is significantly greater than the controls. ThT fluorescence of GQES7-a and GQES7-b is attenuated when the monovalent counterion is switched from K+ to Li+. (Figure 2d). Consistent with results of QGRS Mapper and CD spectroscopy, the ThT-induced fluorescence signal for GQES7-b is less than that of GQES7-a under all conditions.
The formation of G-quadruplexes by GQES7-a, GQES7-b and intact ES7HS is supported by competition assays with pyridostatin (PDS) (Figures 2e and 2f). PDS is a G-quadruplex stabilizer and a ThT competitor with a greater affinity than ThT for G-quadruplexes [22]. As expected if G-quadruplexes form in these rRNAs, PDS displaces ThT. In this series of experiments mtES7-a, mtES7-b and tRNA were used as negative controls, giving signals near background.
UV thermal melting.
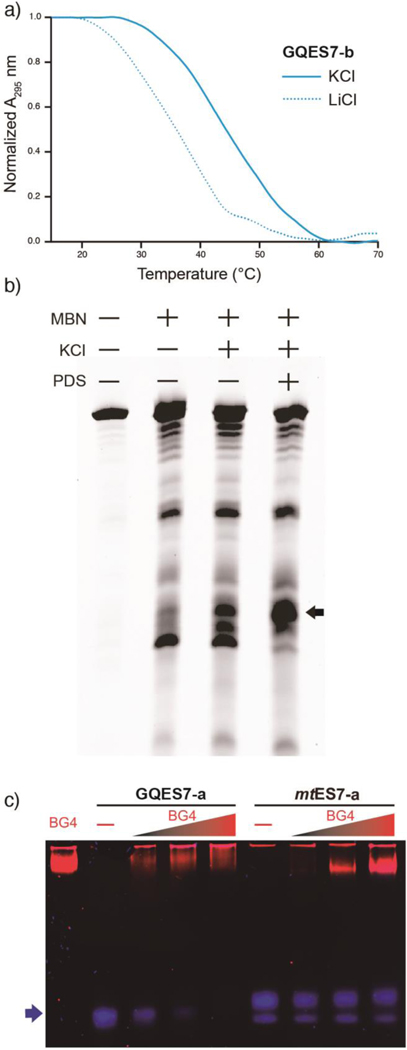
The melting of G-quadruplexes is distinguishable from melting of other RNA secondary structures. Melting of G-quadruplexes, but not other RNA structures, is accompanied by hypochromicity at 295 nm [23]. Melting of G-quadruplexes, but not other RNA structures, shows an acute dependence on type of monovalent counterion. The Tm’s of G-quadruplex melting are expected to be greater in K+ than in Li+.
The UV melting profile of GQES7-b demonstrates the characteristic hypochromic shift at 295 mm and the expected salt dependence (Figure 3a). The Tm of the melting transition is increased by around 9 °C when the counterion is switched from Li+ (Tm≅36 °C) to K+ (~45 °C). We were unable to observe a melting transition under any conditions for GQES7-a. Even under low concentrations of Li+ the Tm of GQES7-a appears to be greater than experimentally accessible temperatures. The observed differences between melting behaviors of GQES7-a and GQES7-b are consistent with the greater G-quadruplex propensity of GQES7-a than of GQES7-b observed by GQRS Mapper, CD spectroscopy and ThT fluorescence.
Figure 3.
a) UV thermal melting profile of GQES7-b at 295 nm. Before melting RNA was annealed in the presence of either 100 mM KCl or 100 mM LiCl. b) ES7HS cleavage by mung bean nuclease. ES7 was annealed with or without KCl and with or without PDS. The black arrow indicates cleaved rRNA. c) EMSA of the BG4 antibody with GQES7-a and its non-G-quadruplex-forming mutant mtES7-a, visualized on a native gel. GQES7-a and mtES7-a RNAs were loaded at a constant strand concentration with increasing concentrations of BG4 antibody. The RNA (arrow) is blue and the protein is red.
Mung bean nuclease cleavage.
In the conventional secondary model of H. sapiens LSU rRNA (Figure 1), G-quadruplex-forming sequences are represented as doubled-stranded and are paired with C-rich strands. If these tentacles form G-quadruplexes, the C-rich strands would be dissociated, presumably as single strands. As an additional test for G-quadruplexes in intact ES7HS rRNA, we examined cleavage by mung bean nuclease (MBN, Figure 3b). MBN preferentially cleaves single-stranded RNA or DNA and would cleave ES7HS rRNA more rapidly if G-quadruplexes form than if they do not. MBN cleavage has been used previously to test for G-quadruplexes in DNA [24]. The results here show that MBN cleaves ES7HS most rapidly under G-quadruplex-stabilizing conditions (Figure 3b). Addition of K+ increases the extent of cleavage (at constant time). Addition of PDS to K+ further increases the extent of cleavage. The simplest interpretation of the MBN results is that ES7HS exists as a mixture of duplex and G-quadruplex forms and that the equilibrium is shifted by the type of counterion and by G-quadruplex stabilizers. In a negative control, extent of MBN hydrolysis of tRNA did not increase upon addition of K+ and/or PDS (Figure S.2).
Antibody binding.
BG4 is an antibody developed by Balasubramanian and coworkers [25, 26] that binds to a variety of G-quadruplex types but not to other nucleic acids such as RNA hairpins, single-stranded or double-stranded DNA. Here, to test for G-quadruplex formation in GQES7-a, an EMSA was performed with BG4 (Figure 3c). BG4 was also used for dot blotting experiments with GQES7-a, GQES7-b, and intact ES7HS (Figure 4). We observe binding of BG4 to GQES7-a, GQES7-b, and intact ES7HS (Figure 4a–b). Consistent with the results above, BG4 binds more tightly to GQES7-a than to GQES7-b.
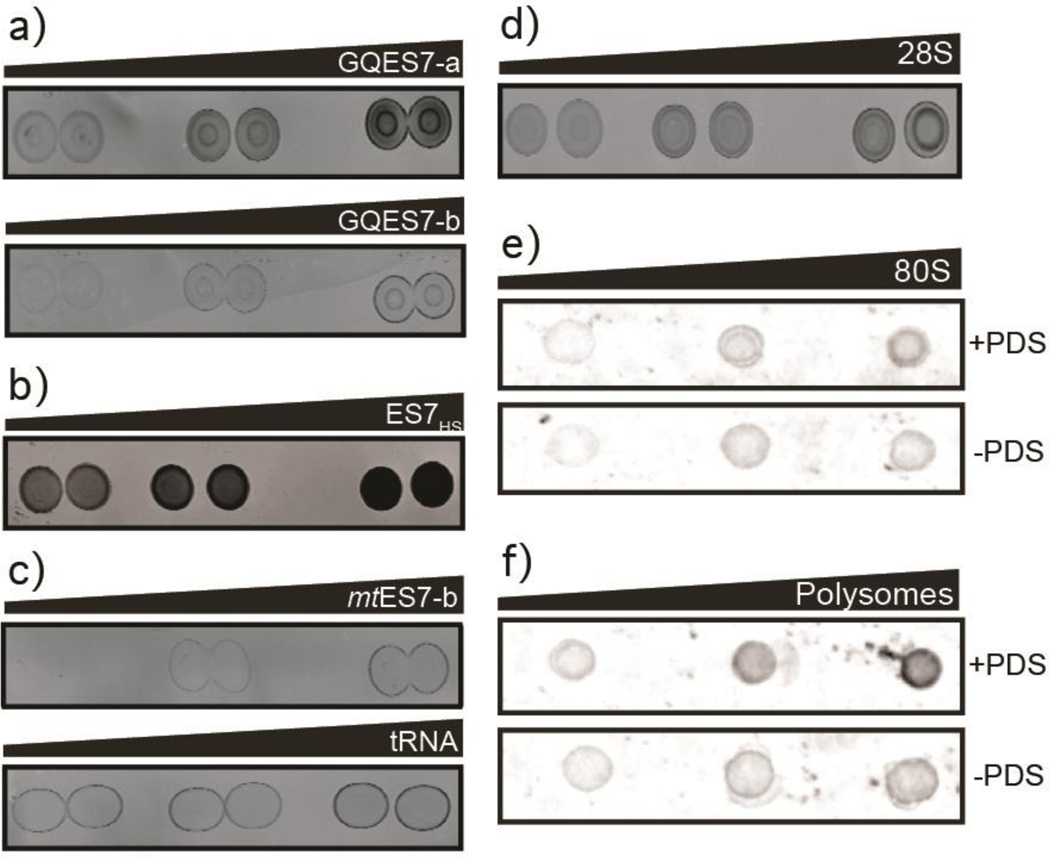
Figure 4.
Dot blots performed with the BG4 antibody on a) GQES7-a and GQES7-b, b) intact ES7HS, c) the negative controls mtES7-b and tRNA, d) the 28S rRNA extracted from HEK293T cells and on e) human 80S ribosomes and f) polysomes purified from HEK293 cells. All samples were incubated in the presence of 50mM KCl and ribosomes and polysomes were further analyzed with or without 10 μM PDS, which stabilizes G-quadruplexes. Samples were loaded onto the membrane in increasing amounts from left to right.
The experiments presented above are consistent with in vitro formation of G-quadruplexes by ES7HS and by oligomers derived from ES7HS. Below we investigate whether intact H. sapiens 28S rRNA can form G-quadruplexes when protein-free or when assembled in ribosomes. The 28S rRNA was extracted from HEK293T cells and dot blotting was performed with BG4 (Figure 4d). The results suggest that 28S rRNA forms G-quadruplexes.
To determine if 28S rRNA from H. sapiens forms G-quadruplexes when assembled in intact ribosomes, dot blotting was also performed with purified 80S human ribosomes and with polysomes (Figure 4e and 4f). The results show that the BG4 antibody binds preferentially to intact human ribosomes and polysomes in a concentration-dependent manner. PDS enhances binding of the antibody, as expected for G-quadruplex formation. The observation of more extensive binding of the antibody to polysomes than to monomer ribosomes suggests formation of intermolecular G-quadruplexes in polysomes (i.e., that G-quadruplexes link tentacles of adjacent ribosomes).
G-quadruplex sequences in ribosomes throughout Chordata.
Focusing specifically on translation, we have developed the SEREB Database [2], which contains fully curated and cross-validated sequences of rRNAs from all major phyla, yet samples the tree of life in a sparse, efficient and unbiased manner. Here we extended the SEREB database, increasing the number of chordate species from 10 to 17, for a fine-grained analysis of ES7.
G-quadruplex-forming sequences in chordate ES7s.
Our MSA confirms that the lengths of rRNA tentacles of eukaryotes are variable, reaching maxima in species such as G. gallus and H. sapiens (Figure 5). Aligned sequences of relevant segments of ES7s of various eukaryotes demonstrate G-quadruplex-forming sequences in chordates, indicated by the motif G≥3N1–7G≥3N1–7G≥3N1–7G≥3 (number of G tracts (n) >3). The motif is observed near the termini of ES7 tentacles in all warm-blooded chordates, although the exact locations and specific sequences are variable. The maximum number of tandem G-tracts in tentacle a of ES7 is ten in human and chimpanzee and eight in rat and chicken. Fish, reptiles and amphibians appear to lack the G-tract motif in ES7 tentacles.
Figure 5.
G-tracts are observed in ES7 tentacles. a) Conventional secondary structural models of ES7 from various eukaryotes. G-tracts within the G≥3N1–7G≥3N1–7G≥3N1–7G≥3 motif are highlighted in red. b) Sequence alignment of ES7 tentacle a showing G-quadruplex-forming sequences are common in chordates. Individual G-tracts in both panels are labeled with Greek symbols. Nucleotides are colored by type. G’s within G-tracts are dark red. Other G’s are pink. All nucleotides are numbered in accordance with H. sapiens 28S rRNA. Sizes of eukaryotic ES7 secondary structures are not to scale. Complete species nomenclature is provided in Table S.3.
G-quadruplex-forming sequences in chordate ESs other than ES7.
The extended SEREB Sequence Database suggests that G-quadruplex-forming sequences are universal to chordates (Table S.2). Several chordate species present G-quadruplex-forming sequences in tentacles other than ES7 (Table S.2). These G-quadruplex-forming sequences are not shown in Figure 5. In addition, repeated G-tracts outside of the motif can form G-quadruplexes [27, 28]. Therefore, additional G-quadruplexes cannot be excluded in these ribosomes. It is possible that G-tracts with n<4 form intermolecular G-quadruplexes with other tentacles or with other ribosomes as in polysomes.
Absence of G-quadruplex sequences in non-chordate rRNAs.
To determine the phylogenic distribution of G-quadruplexes in LSU rRNA, we inspected highly curated sequences of 20 non-chordate eukaryotes from the SEREB database [2]. Thus far we can find no evidence of G-quadruplex-forming sequences in ribosomes of non-chordate eukaryotes.
RNA remodeling proteins bind to rRNA G-quadruplex sequences.
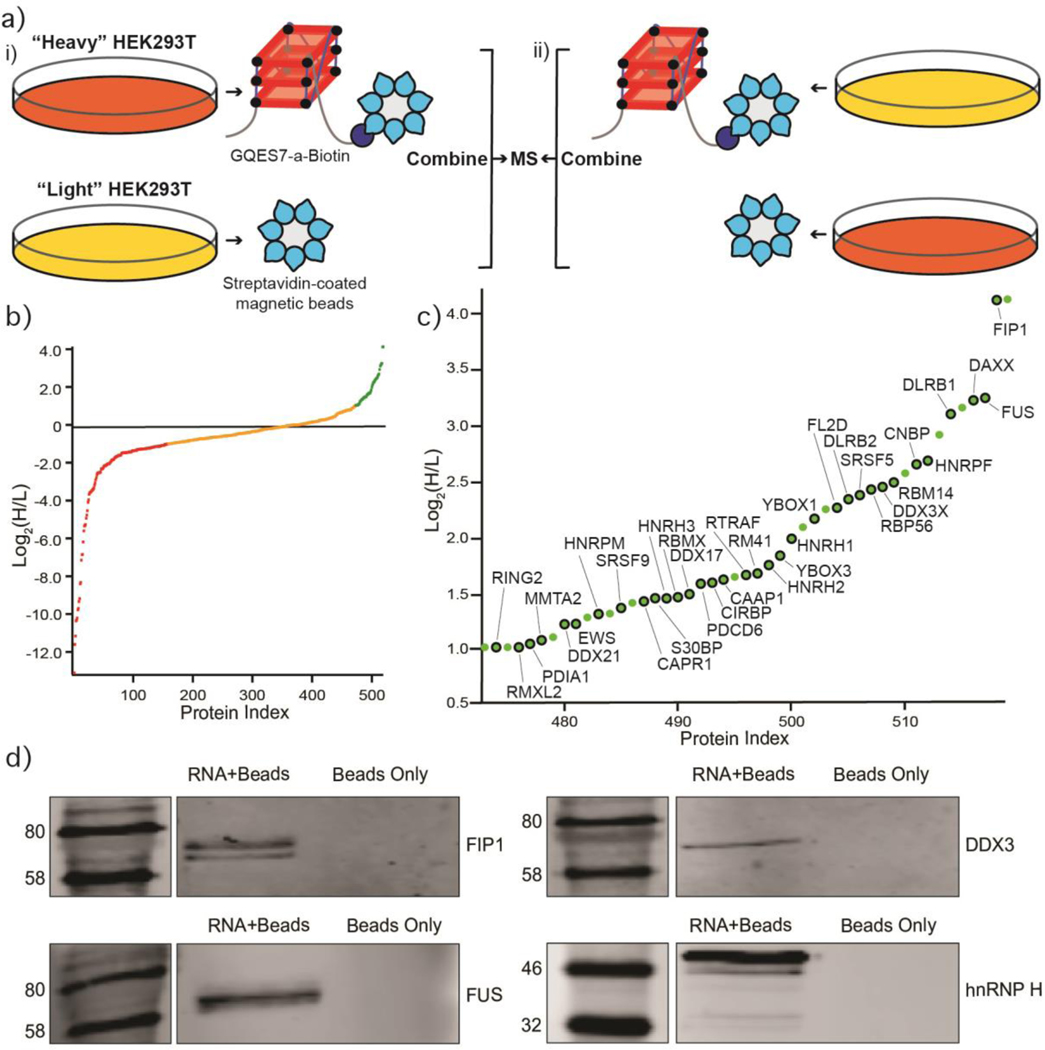
The localization of G-quadruplex-forming sequences to ribosomal tentacles suggests the possibility of interaction with non-ribosomal proteins. To identify the proteins that bind to rRNA G-quadruplexes, we performed pull-down experiments and used stable isotope labeling with amino acids in cell culture (SILAC) for protein quantification. We focused on GQES7-a, the longest and most stable G-quadruplex-forming region in human rRNA (Figure 6). GQES7-a rRNA was linked on the 3’ end to biotin (GQES7-a-Biotin) and associated proteins in human cell lysates were pulled down and analyzed by mass spectrometry. The biotinylation of GQES7-a does not disrupt the G-quadruplexes (Figure S.3). Known G-quadruplex-binding proteins were pulled down by this assay including CNBP, YBOX1, hnRP F, hnRP H, DDX21, DDX17, DDX3X [29–35]. A significant number of helicases were identified (DDX3X, CNBP, DDX21, DDX17), all of which have been reported to unfold G-quadruplexes. In addition, a significant number of heterogeneous nuclear ribonucleoproteins (hnRNPs) were shown in this experiment to bind to GQES7-a, including hnRNP G-T/RMXL2, hnRNP M, hnRNP G/RBMX, hnRNP H2, hnRNP H, hnRNP F, hnRNP H3, and FUS. hnRNPs are a family of RNA-binding proteins with functions including pre-mRNA processing and transport of mRNAs to ribosomes [36]. Several of these proteins have been previously identified as ribosome-binding proteins [37].
Figure 6.
Identification of GQES7-a-binding proteins. a) Scheme of the SILAC experiment. “RNA+beads” samples were combined in HEK293T grown in heavy media (panel i). The “Beads Only” control sample was combined in HEK293T grown in light media. To verify the proteins identified by this method, the experiment was performed using reverse labeling (panel ii). b) Scatter plot representing fold enrichment of the proteins binding to GQES7-a in “Heavy” HEK293T. Color representation indicates specific proteins that bound more tightly to GQES7-a than to the beads (green), to the beads than to GQES7-a (red) or bound to the beads and GQES7-a to a similar extent (orange). c) A close-up view of the green region of the scatter plot represented in panel b. Dots with a black contour are used to indicate proteins that appeared in the green region of the two replicate experiments described in panel a. d) Western blotting analyses of the eluted proteins from the RNA pull-down of HEK293T. All four blotted proteins (FIP1, FUS, DDX3 and hnRNP H) eluted from the GQES7-a sample (RNA+Beads) but not from the control (Beads Only), confirming the SILAC results.
To support results of the pull-down experiments, Western blotting was performed with four of the proteins obtained in the pull-down experiments (Figure 6d). We assayed a DEAD-box RNA helicase (DDX3X), a heterogeneous nuclear ribonucleoprotein (hnRNP H), the RNA-binding protein FUS, and a pre-mRNA polyadenylation stimulator (FIP1). hnRNP H and DDX3X have been previously identified as G-quadruplex-binding proteins. All four proteins bind to GQES7-a in the Western blot, suggesting we have tapped an uncharacterized pool of G-quadruplex-binding proteins.
DISCUSSION
The results presented here suggest that G-quadruplexes are far more profuse than previously conceived. rRNA is the most abundant macromolecule in most cells. Human LSU rRNA and rRNA of other chordates contain sequences with strong propensity to form G-quadruplexes (Figure 1). We have identified ten tandem G-tracts in tentacle a of human ES7 rRNA and four in tentacle b. These tandem G-tracts form stable G-quadruplexes under a variety of conditions in vitro. Computation, ThT fluorescence, CD spectroscopy, UV melting, EMSAs, nuclease digestion and blotting with a G-quadruplex antibody provide a consistent picture of the propensities of various regions of 28S rRNA to form G-quadruplexes.
G-quadruplex-forming sequences have been shown previously to cluster within regulatory mRNA regions such as 5′ and 3′ untranslated regions [38] and within the first intron [39]. The extent to which such G-quadruplexes form in vivo remains uncertain. Bartel and coworkers suggest that mRNA G-quadruplexes are globally unfolded by unwinding factors in eukaryotic cells [40]. By contrast, Wong and Monchaud support a model in which G-quadruplexes continuously form and unfold in vivo [41]. However, rRNAs were explicitly excluded from both of these investigations; the extent of G-quadruplex formation in vivo remains an open question.
In our view, the following merit a ribosome centric reinvestigation of G-quadruplex structure and function in vivo:
inherent flexibility and polymorphism of rRNA tentacles,
their ability to extend hundreds of Ångströms from the ribosomal surface,
the large number of tandem G-tracts on some rRNA tentacles,
high stability of tentacle G-quadruplexes in vitro, and
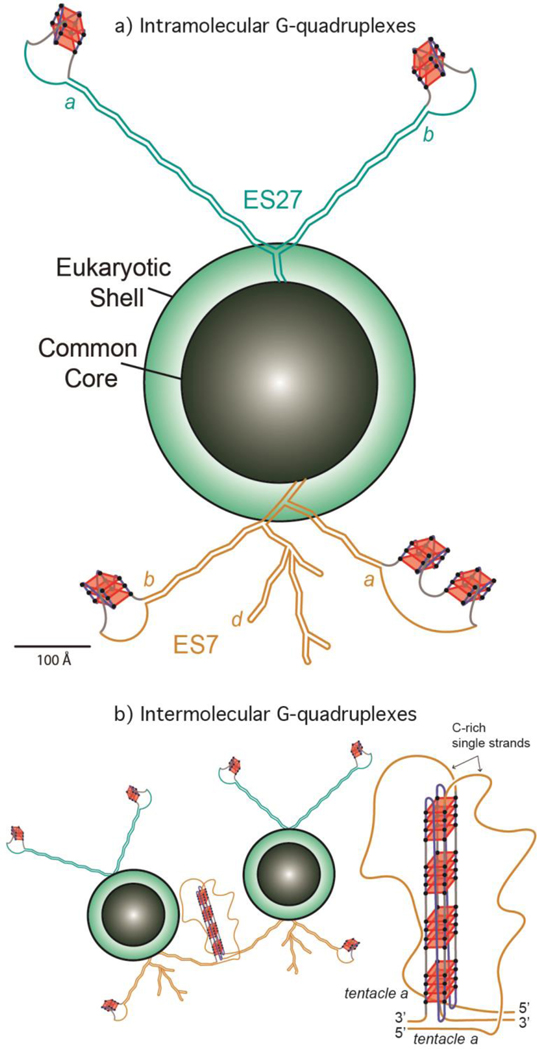
extreme concentrations of rRNA on the rough ER and in polysomes. Our results suggest that nature’s most complex organisms have evolved long rRNA tentacles with unexpected structural polymorphism, including the ability to form G-quadruplexes (Figure 7).
Figure 7.
a) Schematic representation of the common core, the eukaryotic shell and the tentacles of the LSU of the Homo sapiens ribosome. G-quadruplexes are indicated on ES7 and ES27. The lengths of ES7HS (orange) and ES27HS (green) tentacles are roughly scaled to the size of the common core. The G-quadruplexes represented in tentacle b of ES27HS do not fall within the G≥3N1–7G≥3N1–7G≥3N1–7G≥3 motif and are speculative. The G-quadruplex region found in Helix 63 of ES27HS is not indicated here. b) Schematic representation of interactions between ribosomes via intermolecular G-quadruplexes. G-tracts on ES7 tentacle a from different ribosomes contribute to the formation of G-quadruplexes (an expanded view is presented on the right).
G-quadruplex-forming rRNA sequences appear to be a universal feature of tentacles of chordate ribosomes. We have inferred the locations of tandem rRNA G-tracts in various species by Multiple Sequence Alignments. The specific sequences and exact locations of the G-quadruplexes on tentacles are variable across phylogeny. We searched the SEREB database and thus far could find no evidence of G-quadruplex-forming sequences outside of the Chordata phylum. The SEREB database is specifically designed for analysis of rRNA, and includes species from all major phyla, and samples the tree of life in a sparse, efficient and accurate manner [2]. It contains complete and highly curated rRNA sequences.
The preferential localization of rRNA G-quadruplexes near the termini of rRNA tentacles suggests these regions are loci for association of specific cytosolic proteins or for assembly of ribosomes. Here we identified multiple human RNA helicases and other RNA remodeling proteins that bind to rRNA G-quadruplexes. (Figure 6) These proteins could be participants in G-quadruplex regulation on ribosomes.
Our observation here that polysomes appear to form more extensive G-quadruplexes than monomer ribosomes suggests a role for inter-ribosomal G-quadruplexes in closely associated ribosomes (Figure 7b). Our work points to the possibility that, inside cells, ribosomes present polymorphic tentacles that can switch between unimolecular and multimolecular G-quadruplexes and duplex forms. In this model, surfaces of ribosomes contain multivalent docking sites for G-quadruplex-specific proteins and for nucleic acid assemblies, including in polyribosomes. It has been shown that G-quadruplexes can form phase separated RNA gels [42]. It is conceivable that densely packed ribosomes on the rough ER and in polysomes are surrounded by phase separated G-quadruplexes gels, composed of rRNA tentacles.
G-quadruplex-forming sequences have been described in genes encoding rRNA, where they are proposed to influence transcription [43] and bind to the nucleolar protein nucleophosmin [44]. These studies have focused on the external and internal transcribed regions (ETS and ITS) and are not part of the assembled ribosome. Moore and co-workers reported tetramerization of an oligomer containing a single G-tract, derived from E. coli 5S rRNA [45]. The results here highlight potential ribosomal functionality associated with the large rRNA tentacles of mammals and birds. The conservation of rRNA G-quadruplex-forming sequences throughout Chordata, albeit at various locations in rRNA tentacles, suggests significant functions.
METHODS
ES7HS spans nucleotides 436 to 1311 of the H. sapiens LSU rRNA and contains sequences that we call GQES7-a (nts 583–652) and GQES7-b (nts 825–853). RNAs corresponding to ES7HS, GQES7-a and GQES7-b were synthesized in vitro by transcription (HiScribe™ T7 High Yield RNA Synthesis Kit, New England Biolabs). For cation-dependent experiments, precautions were used to remove contaminating cations that stabilize G-quadruplexes. RNAs were ethanol precipitated from 800 mM LiCl, 10 mM Tris-HCl, pH 7.5. The RNA pellets were resuspended in 1 mM LiCl, 10 mM Tris-HCl, pH 7.5 were dialyzed extensively against the same buffer using Slide-a-Lyzer dialysis cassettes (MWCO 3,500, Pierce) at 4°C. mtES7-a and mtES7-b were ordered as RNA oligomers. Baker’s yeast tRNAs were purchased from Roche. RNA purity was monitored by 8 M Urea 5% acrylamide gel in TBE buffer. Complete sequences of ES7HS, GQES7-a, GQES7-b, mtES7-a and mtES7-b are contained in Table S.1.
HEK293T 28S rRNA extraction and purification.
HEK293T cells were grown to 60% confluency after which total RNA was extracted with TRI Reagent® (Sigma-Aldrich). 28S rRNA was extracted from an agarose gel by running the rRNA into wells in the center of the gel, where the rRNA was extracted with a pipette. The rRNA was precipitated in 5 M ammonium acetate-acetic Acid, pH 7.5 with excess ethanol. 28S rRNA purity was monitored on 1% agarose gels (Figure S.1).
Thioflavin T (ThT) fluorescence.
RNAs were prepared at a final concentration of 1 μM (strand) and annealed in 150 mM KCl, NaCl or LiCl, 10 mM Tris-HCl, pH 7.5, 2 μM ThT by cooling from 90°C to 25°C at 1°/min. RNAs were incubated at 4°C for 10 min and were loaded onto a Corning® 384 Well Flat Clear Bottom Microplate. Fluorescence from 300–700 nm, exciting at 440 nm were acquired on a BioTek Synergy™ H4 Hybrid plate reader. When appropriate, pyridostatin (PDS) was added to the desired concentration after the RNA was annealed.
Circular dichroism.
RNA at 1 μM (strand) in 150 mM KCl or LiCl and 10 mM Tris-HCl (pH 7.5) was annealed as described above. CD spectra were acquired at 20 °C on a Jasco J-810 spectropolarimeter using 1 mm cuvettes. Data from 200–320 nm was acquired at a rate of 100 nm/min with 1 sec response, a bandwidth of 5 nm, and averaged over three measurements. The buffer spectrum was subtracted. Smoothing was performed with Igor Pro. The observed ellipticity (𝜃, mdeg) was normalized [46] using the expression Δ𝜀 = 𝜃/(32,980 × 𝑐 × 𝑙), where c is the molar strand concentration of the RNA and l is the path length of the cuvette in centimeters.
UV thermal melting.
Absorbance measurements were collected at 295 nm using a Varian Cary-1E UV spectrophotometer. RNA samples (800 μL, final OD260 of 0.50 units) in 10 mM Tris-HCl, pH 7.5 and 100 mM KCl or LiCl were annealed as described above and added to 1 cm path-length quartz cuvettes. Samples were then heated from 15°C to 90°C and cooled at the same rate at 0.5°C/min. Data was recorded every 0.5°C.
EMSAs.
The anti-G-quadruplex BG4 antibody was purchased from Absolute Antibody (Catalog #: Ab00174–1.1). GQES7-a (3 μM) rRNA or the negative control mtES7-a RNA were annealed in 20 mM Hepes-Tris, pH 7.5, 50 mM KCl. GQES7-a rRNA or mtES7-a RNA were combined with various concentrations of BG4 at a final RNA concentration of 1 μM RNA (strand). RNA-protein mixtures were incubated at room temperature for 20 min in 50 mM KCl. RNA-protein interactions were analyzed by 5% native-PAGE. Gels were visualized following a dual fluorescent dye protocol [47] with a Azure imager c400 (Azure Biosystems).
rRNA - BG4 antibody dot blotting.
RNAs were annealed in the presence of 50 mM KCl and were diluted 1x, 2x and 4x. GQES7-a, GQES7-b, mtES7-b, tRNA: 3.2 μM, 1.6 μM 0.8 μM. ES7HS: 1.4 μM, 0.7 μM, 0.35 μM. 28S rRNA: 55 nM, 27.5 nM, 13.7 nM. RNAs were loaded onto nitrocellulose membranes and dried at room temperature for 30 min. The membranes were blocked for 1 h at room temperature. BG4 antibody was added (1:2,000 dilution) and incubated with gentle rocking for sixty min at room temperature. The membrane was washed for ten min twice with 1X TBST and incubated for sixty min with an appropriate fluorescent secondary antibody anti-mouse (1:10,000 dilution) (Biotium, #20065–1). The membrane was washed for ten min twice with 1X TBST and was imaged on a Li-Cor Odyssey Blot Imager. Intact 80S ribosomes and polysomes were purified from HEK293, which were incubated 5 min in 10 μg/mL cycloheximide at 37°C. Lysis buffer (10 mM NaCl, 10 mM MgCl2, 10 mM Tris-HCl, pH 7.5, 1% Triton X-100, 1% sodium deoxycholate, 0.2 U/mL DNase I, RNase inhibitor, 1mM dithiothreitol, 10 μg/mL cycloheximide) was used to scrap the cells. Nuclei and cell debris were removed by centrifugation and the supernatant was transferred to a 15–50% sucrose gradient containing 100 mM NaCl, 10 mM MgCl2, 30 mM Tris-HCl, pH 7.5 and centrifuged by ultracentrifugation. Purified 80S ribosomes and polysomes were then incubated at room temperature for 15 min in the presence of 50 mM KCl with or without 10 μM PDS. Ribosomes or polysomes were added iteratively in 30-min intervals to the same site on a nitrocellulose membrane (0.9 μg, 2.7 μg, 4.5 μg). The membrane was then treated as described above. BG4 was added to a final dilution of 1:1,000 and the secondary antibody was added to a final dilution of 1:5,000.
Mung bean nuclease (MBN) probing.
ES7HS and tRNA were prepared at 100 ng/μL and annealed in the presence/absence of 100 mM KCl, 15 mM Tris-HCl (pH 7.5) by cooling from 90°C to 25°C, at 1°/min. PDS was added to the annealed RNA to a final concentration of 2 μM. One unit of MBN was added per μg of RNA and samples were incubated at 30°C for 30 min. SDS was added to a final concentration of 0.01% to denature the nuclease and RNA was purified by ethanol precipitation. The extent of RNA cleavage was determined on an 8 M urea 5% acrylamide (19:1 acrylamide/bisacrylamide) gel stained with ethidium bromide.
ES7 secondary structures.
Secondary structures of human and D. melanogaster ES7 were obtained from RiboVision [48]. Nucleotides of G-quadruplex regions in P. troglodytes, M. musculus and G. gallus (Table 1) were numbered as in Bernier [2], subtracting the nucleotides from the 5.8S rRNA.
Phylogeny and Multiple Sequence Alignments.
The SEREB MSA [2] was used as a seed to align additional eukaryotic ES7 sequences to increase the density of eukaryotic species in the MSA. The 28S rRNA sequences in the SEREB MSA were used to search [49] the NCBI databases [50] for LSU rRNA sequences. The SEREB database has sequences from 10 chordate species; seven additional chordate species from 7 new orders were added to the ES7 tentacle a MSA (Figure 7, Table S.3). Sequences without intact ES7 tentacle a were excluded. Sequences with partial 28S rRNA were marked as partial. Sequences inferred from genomic scaffolds were marked as predicted (Table S.3). The extended database was queried for G-quadruplex-forming sequences.
Sequences were incorporated into the SEREB-seeded MSA using MAFFT [51] and adjusted manually using BioEdit [52]. Manual adjustments incorporated information from available secondary structures. In some cases, the positions of G-tracts in sequences with large gaps relative to H. sapiens are not fully determined, as they can be aligned equally well with flanking G-tracts in the MSA. Alignment visualization was done with Jalview [53]. The phylogenetic tree and the timeline of clade development were inferred from TimeTree [54].
Analysis of the entire LSU was performed on SEREB sequences, which are highly curated and always complete. This procedure ensured that negative results indicate absence of G-quadruplex-forming sequences from intact rRNAs rather than absence from rRNA fragments that lack the appropriate regions. G-quadruplex-forming sequences are not detected in any of the 20 non-chordate members of the SEREB database.
SILAC.
HEK293T cells were cultured in SILAC media - “heavy” or “light” Dulbecco’s Miodified Eagle Media (DMEM) (Thermo Scientific) supplemented with 10% dialyzed fetal bovine serum (FBS) (Corning) and 1% penicillin-streptomycin solution (Sigma) in a humidified incubator at 37 °C with 5% carbon dioxide. The heavy media contained 0.798 mM L-lysine (13C6 and 15N2, Cambridge Isotope Laboratories) and 0.398 mM L-arginine (13C6, Cambridge Isotope Laboratories). The light media had the same concentrations of normal lysine and arginine (Sigma). Media were supplemented with 0.2 mg/mL proline (Sigma) to prevent arginine-to-proline conversion. Heavy and light cells were grown for at least six generations. Once the confluency reached 80%, cells were harvested by scraping, washed twice with ice-cold PBS (Sigma), lysed in a buffer containing 10 mM HEPES pH=7.4, 200 mM potassium chloride, 1% Triton X-100, 10 mM magnesium chloride (all from Sigma) and 1 pill/10 mL cOmplete ULTRA tablet protease inhibitor (Roche), and incubated on an end-over-end shaker at 4 oC for 1 hour. Lysates were centrifuged at 25,830 g at 4 oC for 10 minutes, and the supernatants were collected and kept on ice.
Ten μg of GQES7-a-Biotin RNA was annealed as described above in the presence of 10 mM Tris-HCl, pH 7.5, and 100 mM KCl. Twenty μL of magnetic streptavidin-coated beads (GE Healthcare) were washed with the lysis buffer (10 mM HEPES, pH 7.4, 200 mM KCl, 1% Triton X-100, 10 mM MgCl2, protease inhibitors). Annealed RNA was then added to the washed beads and incubated at 4oC for 30 min with gentle shaking. For control experiments, no RNA was added. SILAC cell lysates were incubated with 0.5 mg E. coli tRNA per 1 mg protein at 4oC for 30 min with gentle shaking. “RNA+Beads” and control “Beads Only” samples were transferred into the SILAC cell lysates: “RNA+Beads” were added to the Heavy cell lysate and “Beads Only” was added to “Light” HEK293T cell lysate. As a replicate, “RNA+Beads” was added “Light” cell lysate and “Beads Only” was added to “Heavy” cell lysate. 200 U/mL of RNasin was added and the lysates were incubated at 4 oC for 2 hrs with gentle shaking. Samples were centrifuged, the supernatant was discarded, and the pelleted beads were washed with lysis buffer with increasing KCl concentrations (0.4 M, 0.8 M, 1.6 M). After the three washes, 100 μL of the elution buffer (100 mM Tris-HCl, pH 7.4, 1% SDS, 100 mM DTT) was added to one of the two samples and then combined with the beads of the corresponding sample. “RNA+Beads” in “Heavy” lysates were combined with “Beads Only” in “Light” lysates and “RNA+Beads” in “Light” lysates were combined with “Beads Only” in “Heavy” lysates. The combined samples were boiled and then briefly centrifuged. Beads were discarded and samples were analyzed with an online LC-MS system.
Mass spectrometry.
Eluted proteins were diluted 10 times with 50 mM HEPES pH=7.4 and were alkylated with 28 mM iodoacetamide (Sigma) for 30 minutes at room temperature in the dark. Proteins were precipitated by methanol-chloroform, and the pellets were resuspended in digestion buffer containing 50 mM HEPES pH=8.8, 1.6 M urea, and 5% acetonitrile (ACN) (all from Sigma). After digestion with sequencing-grade modified trypsin (Promega) at 37 °C for 16 hours, reactions were quenched with 1% trifluoroacetic acid (TFA, Fisher Scientific) and purified with StageTip. Peptides were dissolved in 10 μL 5% ACN and 4% FA solution, and 1 μL was loaded to a Dionex UltiMate 3000 UHPLC system (Thermo Fisher Scientific) with a microcapillary column packed inhouse with C18 beads (Magic C18AQ, 3 μm, 200 Ǻ, 75 μmx16 cm). A 110-minute gradient of 3–22% ACN containing 0.125% FA was used. The peptides were detected with an LTQ Orbitrap Elite Hybrid Mass Spectrometer (Thermo Fisher Scientific) controlled by Xcalibur software (version 3.0.63). MS/MS analysis was performed with a data-dependent Top20 method. For each cycle, a full MS scan in the Orbitrap with the automatic gain control (AGC) target of 106 and the resolution of 60,000 at 400 m/z was followed by up to 20 MS/MS scans in the ion trap for the most intense ions. Selected ions were excluded from further sequencing for 90 seconds. Ions with singly or unassigned charge were not sequenced. Maximum ion accumulation times were 1,000 ms for each full MS scan and 50 ms for each MS/MS scans. The spectra were searched against a human protein database downloaded from UniProt using the SEQUEST algorithm (version 28) [55]. The following parameters were used: 20 ppm precursor mass tolerance; 1.0 Da fragment ion mass tolerance; trypsin digestion; maximum of 3 missed cleavages; differential modifications for methionine oxidation (+15.9949 Da), heavy lysine (+8.0142 Da), and heavy arginine (+6.0201 Da); fixed modification for cysteine carbamidomethylation (+57.0215 Da). The false discovery rates (FDR) were evaluated and controlled by the target-decoy method. Linear discriminant analysis (LDA) was used to filtered the peptides to <1% FDR based on parameters such as XCorr, ACn, and precursor mass error. An additional filter was used to control the protein FDR to <1%. For SILAC quantification, the S/N ratios of both heavy and light peptides must be greater than 3. Otherwise, one of the two versions of the peptides (heavy or light) must have the S/N ratio greater than 10. Other peptides that did not pass these criteria were removed. The final protein ratio was calculated from the median value of the peptides from each parent protein. The raw files are publicly accessible at http://www.peptideatlas.org/PASS/PASS01260, Username: PASS01260, Password: TL3854zn.
Western Blotting.
Samples were electrophoresed on 12% SDS-PAGE gels and transferred to a nitrocellulose membrane overnight. Membranes were blocked for 1 hour at room temperature with gentle shaking and then incubated for another hour with primary antibodies: 1:200 dilution of FIP1 (mouse monoclonal, sc-398392), DDX3 (mouse monoclonal, sc-81247), FUS (mouse monoclonal, sc-47711), or hnRNP H (mouse monoclonal, sc-32310). Antibodies were obtained from Santa Cruz Biotechnology. Membranes were washed three times with 1X TBST and secondary antibody CF680 goat anti-mouse IgG (H+L) (Biotium, 20065) was added (1:5,000 dilution). Membranes were washed three times with 1X TBST and imaged on a Li-Cor Odyssey Blot Imager.
Supplementary Material
- Human ribosomal RNA can form G-quadruplexes in vitro.
- Human G-quadruplex-forming regions are located near the termini of rRNA tentacles of expansion segments 7 and 27 and are exposed on the ribosomal surface.
- Their surface localization suggests potential functions on non-ribosomal protein recruitment and/or polysome assembly.
- G-quadruplex-forming rRNA sequences are observed throughout the phylum Chordata.
ACKNOWLEDGEMENTS
The authors thank Drs. Jonathan B. Chaires and Aaron Engelhart for helpful discussions, Dr. Lizzette M. Gómez Ramos for designing the negative G-quadruplex controls and Ahmad M. Mohamed for the technical support on CD and UV spectroscopy. Purified 80S ribosomes and polysomes were a gift from Immagina BioTechnology. This work was supported by NASA (NNX16AJ28G and NNX16AJ29G to LDW) and the National Institutes of Health (R01GM118803 to RW).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest with the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- [1].Milo R, Phillips R. Cell biology by the numbers. New York, New York: Garland Science; 2016. [Google Scholar]
- [2].Bernier CR, Petrov AS, Kovacs NA, Penev PI, Williams LD. Translation: The universal structural core of life. Mol. Biol. Evol. 2018;34:2065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ware VC, Tague BW, Clark CG, Gourse RL, Brand RC, Gerbi SA. Sequence analysis of 28S ribosomal DNA from the amphibian Xenopus laevis. Nucleic Acids Res. 1983;11:7795–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clark CG, Tague BW, Ware VC, Gerbi SA. Xenopus laevis 28S ribosomal RNA: A secondary structure model and its evolutionary and functional implications. Nucleic Acids Res. 1984;12:6197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hassouna N, Michot B, Bachellerie JP. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Res. 1984;12:3563–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gerbi SA. Expansion segments: Regions of variable size that interrupt the universal core secondary structure of ribosomal RNA. In: Zimmermann RA, Dahlberg AE, editors. Ribosomal RNA—structure, evolution, processing, and function in protein synthesis. Boca Raton, FL: CRC Press; 1996. p. 71–87. [Google Scholar]
- [7].Ramesh M, Woolford JL Jr. Eukaryote-specific rRNA expansion segments function in ribosome biogenesis. RNA. 2016;22:1153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leidig C, Bange G, Kopp J, Amlacher S, Aravind A, Wickles S, et al. Structural characterization of a eukaryotic chaperone--the ribosome-associated complex. Nat. Struct. Mol. Biol. 2013;20:23–8. [DOI] [PubMed] [Google Scholar]
- [9].Gumiero A, Conz C, Gesé GV, Zhang Y, Weyer FA, Lapouge K, et al. Interaction of the cotranslational hsp70 ssb with ribosomal proteins and rRNA depends on its lid domain. Nat. Commun. 2016;7:13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Knorr AG, Schmidt C, Tesina P, Berninghausen O, Becker T, Beatrix B, et al. Ribosome-nata architecture reveals that rRNA expansion segments coordinate n-terminal acetylation. Nat. Struct. Mol. Biol. 2018;26:35–9. [DOI] [PubMed] [Google Scholar]
- [11].Melnikov S, Ben-Shem A, Garreau de Loubresse N, Jenner L, Yusupova G, Yusupov M. One core, two shells: Bacterial and eukaryotic ribosomes. Nat. Struct. Mol. Biol. 2012;19:560–7. [DOI] [PubMed] [Google Scholar]
- [12].Kikin O, D’Antonio L, Bagga PS. QGRS mapper: A web-based server for predicting G-quadruplexes in nucleotide sequences. Nucleic Acids Res. 2006;34:W676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lammich S, Kamp F, Wagner J, Nuscher B, Zilow S, Ludwig A-K, et al. Translational repression of the disintegrin and metalloprotease ADAM10 by a stable G-quadruplex secondary structure in its 5′-untranslated region. J. Biol. Chem. 2011;286:45063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].von Hacht A, Seifert O, Menger M, Schutze T, Arora A, Konthur Z, et al. Identification and characterization of RNA guanine-quadruplex binding proteins. Nucleic Acids Res. 2014;42:6630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fratta P, Mizielinska S, Nicoll AJ, Zloh M, Fisher EM, Parkinson G, et al. C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Scientific Reports. 2012;2:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Halder K, Wieland M, Hartig JS. Predictable suppression of gene expression by 5’-UTR-based RNA quadruplexes. Nucleic Acids Res. 2009;37:6811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arora A, Dutkiewicz M, Scaria V, Hariharan M, Maiti S, Kurreck J. Inhibition of translation in living eukaryotic cells by an RNA G-quadruplex motif. RNA. 2008;14:1290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kumari S, Bugaut A, Huppert JL, Balasubramanian S. An RNA G-quadruplex in the 5’ UTR of the NRAS proto-oncogene modulates translation. Nat Chem. Biol. 2007;3:218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choi KH, Choi BS. Formation of a hairpin structure by telomere 3’ overhang. Biochim. Biophys. Acta-Gene Struct. Expression. 1994;1217:341–4. [DOI] [PubMed] [Google Scholar]
- [20].Xu S, Li Q, Xiang J, Yang Q, Sun H, Guan A, et al. Thioflavin t as an efficient fluorescence sensor for selective recognition of RNA G-quadruplexes. Scientific Reports. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Renaud de la Faverie A, Guédin A, Bedrat A, Yatsunyk LA, Mergny J-L. Thioflavin t as a fluorescence light-up probe for G4 formation. Nucleic Acids Res. 2014;42:e65-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang S, Sun H, Chen H, Li Q, Guan A, Wang L, et al. Direct visualization of nucleolar G-quadruplexes in live cells by using a fluorescent light-up probe. Biochim Biophys Acta. 2018;1862:1101–6. [DOI] [PubMed] [Google Scholar]
- [23].Mergny J-L, Phan A-T, Lacroix L. Following g‐quartet formation by uv‐spectroscopy. FEBS Lett. 1998;435:74–8. [DOI] [PubMed] [Google Scholar]
- [24].Zheng K-w, Chen Z, Hao Y-h, Tan Z. Molecular crowding creates an essential environment for the formation of stable G-quadruplexes in long double-stranded DNA. Nucleic Acids Res. 2009;38:327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Biffi G, Di Antonio M, Tannahill D, Balasubramanian S. Visualization and selective chemical targeting of RNA G-quadruplex structures in the cytoplasm of human cells. Nat. Chem. 2014;6:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013;5:182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cang X, Šponer J, Cheatham TE III. Explaining the varied glycosidic conformational, G-tract length and sequence preferences for anti-parallel G-quadruplexes. Nucleic Acids Res. 2011;39:4499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li X-m, Zheng K-w, Zhang J-y, Liu H-h, Yuan B-f, Hao Y-h, et al. Guanine-vacancy–bearing G-quadruplexes responsive to guanine derivatives. Proc. Natl. Acad. Sci. USA. 2015;112:14581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Benhalevy D, Gupta SK, Danan CH, Ghosal S, Sun H-W, Kazemier HG, et al. The human cchc-type zinc finger nucleic acid-binding protein binds g-rich elements in target mRNA coding sequences and promotes translation. Cell Rep. 2017;18:2979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Khateb S, Weisman-Shomer P, Hershco-Shani I, Ludwig AL, Fry M. The tetraplex (CGG) n destabilizing proteins hnRNP a2 and cbf-a enhance the in vivo translation of fragile x premutation mRNA. Nucleic Acids Res. 2007;35:5775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Matunis MJ, Xing J, Dreyfuss G. The hnRNP f protein: Unique primary structure, nucleic acid-binding properties, and subcellular localization. Nucleic Acids Res. 1994;22:1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Caputi M, Zahler AM. Determination of the RNA binding specificity of the heterogeneous nuclear ribonucleoprotein (hnRNP) h/h′/f/2h9 family. J. Biol. Chem. 2001;276:43850–9. [DOI] [PubMed] [Google Scholar]
- [33].McRae EK, Booy EP, Padilla-Meier GP, McKenna SA. On characterizing the interactions between proteins and guanine quadruplex structures of nucleic acids. J. Nucl. Acids. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dardenne E, Espinoza MP, Fattet L, Germann S, Lambert M-P, Neil H, et al. RNA helicases DDX5 and DDX17 dynamically orchestrate transcription, miRNA, and splicing programs in cell differentiation. Cell Rep. 2014;7:1900–13. [DOI] [PubMed] [Google Scholar]
- [35].Herdy B, Mayer C, Varshney D, Marsico G, Murat P, Taylor C, et al. Analysis of NRAS RNA G-quadruplex binding proteins reveals DDX3X as a novel interactor of cellular G-quadruplex containing transcripts. Nucleic Acids Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chaudhury A, Chander P, Howe PH. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP e1’s multifunctional regulatory roles. RNA. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Simsek D, Tiu GC, Flynn RA, Byeon GW, Leppek K, Xu AF, et al. The mammalian ribo-interactome reveals ribosome functional diversity and heterogeneity. Cell. 2017;169:1051–65. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huppert JL, Bugaut A, Kumari S, Balasubramanian S. G-quadruplexes: The beginning and end of utrs. Nucleic Acids Res. 2008;36:6260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Eddy J, Maizels N. Conserved elements with potential to form polymorphic G-quadruplex structures in the first intron of human genes. Nucleic Acids Res. 2008;36:132133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guo JU, Bartel DP. RNA G-quadruplexes are globally unfolded in eukaryotic cells and depleted in bacteria. Science. 2016;353:aaf5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang SY, Lejault P, Chevrier S, Boidot R, Robertson AG, Wong JM, et al. Transcriptome-wide identification of transient RNA G-quadruplexes in human cells. Nat. Commun. 2018;9:4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jain A, Vale RD. RNA phase transitions in repeat expansion disorders. Nature. 2017;546:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Drygin D, Siddiqui-Jain A, O’Brien S, Schwaebe M, Lin A, Bliesath J, et al. Anticancer activity of cx-3543: A direct inhibitor of rRNA biogenesis. Cancer Res. 2009;69:7653–61. [DOI] [PubMed] [Google Scholar]
- [44].Chiarella S, De Cola A, Scaglione GL, Carletti E, Graziano V, Barcaroli D, et al. Nucleophosmin mutations alter its nucleolar localization by impairing G-quadruplex binding at ribosomal DNA. Nucleic Acids Res. 2013;41:3228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kim J, Cheong C, Moore PB. Tetramerization of an RNA oligonucleotide containing a GGGG sequence. Nature. 1991;351:331. [DOI] [PubMed] [Google Scholar]
- [46].del Villar‐Guerra R, Gray RD, Chaires JB. Characterization of quadruplex DNA structure by circular dichroism. Curr. Protoc. Nucleic Acid Chem. 2017:17.8. 1-.8. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shcherbakov D, Piendl W. A novel view of gel-shifts: Analysis of RNA-protein complexes using a two-color fluorescence dye procedure. Electrophoresis. 2007;28:749–55. [DOI] [PubMed] [Google Scholar]
- [48].Bernier C, Petrov AS, Waterbury C, Jett J, Li F, Freil LE, et al. Ribovision: Visualization and analysis of ribosomes. Faraday Discuss. 2014;169:195–207. [DOI] [PubMed] [Google Scholar]
- [49].Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped blast and psi-blast: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Database resources of the national center for biotechnology information. Nucleic Acids Res. 2017;45:D12–d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Katoh K, Standley DM. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics. 2016;32:1933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hall TA. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nucleic Acids Symp. Ser: [London: ]: Information Retrieval Ltd., c1979-c2000.; 1999. p. 95–8. [Google Scholar]
- [53].Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hedges SB, Dudley J, Kumar S. Timetree: A public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2. [DOI] [PubMed] [Google Scholar]
- [55].Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.