Abstract
Inhibition of autophagy, the major cellular recycling pathway in mammalian cells, is a promising strategy for the treatment of triple-negative breast cancer (TNBC). We previously reported SBI-0206965, a small molecule inhibitor of unc-51-like autophagy activating kinase 1 (ULK1), which is a key regulator of autophagy initiation. Herein, we describe the design, synthesis, and characterization of new dual inhibitors of ULK1 and ULK2 (ULK1/2). One inhibitor, SBP-7455 (compound 26), displayed improved binding affinity for ULK1/2 compared with SBI-0206965, potently inhibited ULK1/2 enzymatic activity in vitro and in cells, reduced the viability of TNBC cells and had oral bioavailability in mice. SBP-7455 inhibited starvation-induced autophagic flux in TNBC cells that were dependent on autophagy for survival and displayed synergistic cytotoxicity with the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib against TNBC cells. These data suggest that combining ULK1/2 and PARP inhibition may have clinical utility for the treatment of TNBC.
Graphical Abstract

INTRODUCTION
Macroautophagy (hereafter referred to as autophagy) is a highly conserved pathway in eukaryotic cells that mediates the coordinated lysosome-dependent disassembly and recycling of cellular components. By degrading damaged organelles, misfolded or toxic proteins, and pathogens, autophagy maintains cellular homoeostasis and promotes cell survival under stress.1–3 Autophagic flux is maintained at a basal level in all cells, but it can be further increased by various stressors, such as elevated levels of reactive oxygen species (ROS), deprivation of nutrients, and exposure to chemotherapeutic agents.4
Autophagy is a multistep pathway initiated through the ULK preinitiation complex, which consists of unc-51-like autophagy activating kinase 1 (ULK1) or its homologue ULK2, autophagy related protein 13 (ATG13), ATG101 and focal adhesion kinase (FAK) family-interacting protein (FIP200) (Figure S1).5,6 Autophagy is stimulated under stress conditions by activation of AMP-activated protein kinase (AMPK) and inhibited under nonstressed conditions by the mechanistic target of rapamycin (mTOR).7,8 AMPK activates autophagy by two mechanisms: first, by inhibiting mTOR activity via phosphorylation of the mTOR complex components raptor and tuberous sclerosis complex 2 (TSC2); and second, by directly phosphorylating ULK1/2 at multiple sites N-terminal to the mTOR-phosphorylation site, triggering ULK1/2 activation. Conversely, mTOR suppresses autophagy by directly phosphorylating and inhibiting ULK1/2 function (Figure S1).
ULK1/2 are the only serine/threonine (S/T) kinases in the core autophagy pathway, and their involvement at the first committed step in the autophagy cascade makes them attractive druggable targets for inhibiting autophagy. ULK1/2 play crucial roles in autophagy by phosphorylating the other three proteins in the ULK1/2 preinitiation complex (ATG13, ATG101, and FIP200)9–11 and proteins in the downstream Beclin1 initiation complex: Beclin1 (encoded by the gene BECN1), VPS34, ATG9, and ATG16L1.12
As a major cellular recycling process, autophagy plays important roles in numerous diseases, including cancer.13,14 Autophagy promotes tumor growth in several ways: by providing nutrients and small molecule building blocks to support the elevated metabolic demands of tumor cells, regulating oxidative stress,15–18 and contributing to the development of drug resistance.19–23 Some tumor types require a higher basal level of autophagy than normal cells, which suggests that autophagy inhibition may be a feasible therapeutic approach for certain cancers.24 One example is triple-negative breast cancer (TNBC), which accounts for about 15–20% of all breast cancer cases.24 TNBC cells do not express clinically significant levels of estrogen receptors, progesterone receptors, or of the human epidermal growth factor receptor 2 (HER2), thus rendering them insensitive to endocrine or anti-HER2-targeted treatments.25,26 Both basic research and clinical studies support the hypothesis that autophagy inhibition is a promising therapeutic strategy for TNBC.27 However, the only drugs available for clinical studies are the weak autophagy inhibitors chloroquine (CQ) and hydroxychloroquine (HCQ),28,29 which are not autophagy-specific and have poor toxicity profiles resulting in serious adverse side effects. Thus, we considered that small molecule ULK1/2 inhibitors that block autophagy initiation may hold promise for the treatment of TNBC.30
We previously reported SBI-0206965 (compound 1 in Table 1, Figure S2), a first-generation chemical probe that inhibits ULK1 enzymatic activity in vitro and in cells, as demonstrated by inhibition of phosphorylation of the downstream substrates VPS34 and Beclin1.11 While SBI-0206965 has proven to be a valuable autophagy probe,31–33 we were keen to improve its on-target potency and drug-like properties, including oral bioavailability. Herein, we describe new dual ULK1/2 inhibitors that resulted from a combination of a structure-based and rational design. We demonstrate that the optimized autophagy inhibitor SBP-7455 (26) selectively blocks autophagic flux in human TNBC cell lines and exhibits favorable PK and PD properties in vivo. In addition, we show that compound 26 acts synergistically with poly(ADP-ribose)polymerase (PARP) inhibitors to kill TNBC cells, suggesting that combination therapy with ULK1/2 inhibitors may have utility for the treatment of drug-resistant TNBC.
Table 1.
Structures of ULK1/2 Inhibitors and IC50 Values Measured by ADP-Glo and NanoBRET Assaysa
 |
(*)Compounds 1 and 26 are SBI-0206965 and SBP-7455, respectively. ADP-Glo and NanoBRET assay results are presented as the means ± SD of three independent experiments performed in triplicate (ADP-Glo) or duplicate (NanoBRET).
RESULTS AND DISCUSSION
SAR Studies.
To obtain a structure-based pharmacophore for improving the ULK1/2 potency of SBI-0206965 (Figure S2), we obtained crystals of the ULK2 kinase domain (1-277) bound to SBI-0206965. The kinase domains of ULK1 and ULK2 share high sequence identity (78%) with near-complete structural correspondence of residues in the interlobe region. We recently reported that several small molecule kinase inhibitors exhibit comparable potencies against recombinant ULK1 and ULK2.34 By utilizing a similar strategy, crystals of the T150D ULK2 kinase domain bound to SBI-0206965 were obtained at a resolution of 2.7 Å (Figure 1A–C and Table S1). The T150D mutant of ULK2 was used to mimic the active form of the kinase domain because it more readily forms high-quality crystals than the wild-type (WT) protein. The crystallized form of ULK2 bound to SBI-0206965 adopted face-to-face interactions with a symmetrical protomer counterpart in which the activation loop and C helix of the two protomers interdigitated.34
Figure 1.

Crystal structure of the kinase domain of ULK2 bound to chemical probe SBI-0206965 (1). (A) Crystal structure of SBI-0206965 bound to the interlobe ATP site in the kinase domain of ULK2 at 2.7 Å (PDB ID: 6YID). (B) SBI-0206965 forms hydrogen bonds to Cys88 in the hinge region. (C) Detailed interactions with hinge residue atoms and Lys39 are indicated. (D) In silico docking of compound 3 to the crystal structure of ULK2.
Our results show that SBI-0206965 bound to the ATP binding site of ULK2 adopts an orientation that formed a specific bidentate hydrogen bond between the trisubstituted pyrimidine scaffold and backbone residue Cys88 in the hinge region. The compact binding mode of SBI-0206965 conformed to the dimensions of the ATP binding site with a high degree of competitive occupancy. The amide substituent of the N-methylbenzamide ring extended toward the catalytic residues of the DFG loop. The trimethoxyphenyl ring was located along the hinge toward the solvent-exposed region of the binding pocket. The bromine of the pyrimidine scaffold filled a malleable “back pocket” cavity proximal to the gatekeeper residue Met85. The trimethoxyphenyl and pyrimidinyl rings of SBI-0206965 adopted nearly coplanar geometries, while the N-methylbenzamide ring was rotated ~6° out of plane. Overall, SBI-0206965 adopted a Type I binding mode that targeted the ULK1/2-active conformation.35–37
The currently reported structures of inhibitors bound to ULK1 or ULK2 suggest the presence of common specificity-determining features that belie the observed binding pocket plasticity.34 In all compounds with high potency, the cyclopropyl and trifluoromethyl groups, or the halogen atoms located at the 5-position of the pyrimidine are placed into the back pocket of ULK1/2. This is evident in the structures of MRT67307 or MRT68921 bound to ULK2 (PDB ID: 6QAU and 6QAV, respectively).34 ULK1/2 accepts diverse Type I scaffolds; the reported pyrazolylquinazoline (Shokat compound, Figure S2) even binds to ULK1 in a flipped orientation (PDB ID: 4WNO).38 However, the structure of SBI-0206965 bound to ULK2 revealed multiple adjacent sites for expanded interaction, providing the opportunity to construct second-generation autophagy inhibitors using structure-based design.
Optimization and Characterization of ULK Inhibitors.
Using the hinge-binding pyrimidine scaffold of SBI-0206965 as the basis for next-generation ULK inhibitors, we conducted a focused biochemical screen on a small number of 2,4,5-trisubsitituted pyrimidine compounds and identified the benzo[d][1,3]dioxole 2 and the related N4-cyclopropyl derivative 3 as promising candidates for further optimization (Scheme 1A). Because compound 3 possessed a 5-trifluoromethyl to insert in the hydrophobic back pocket and convenient 2,4-diamine substituents to explore space in and around the active site, we used the crystal structure of ULK2 to model the interactions of 3 within the ligand binding site (Figure 1D). Flexible ligand docking with compound 3 was performed using the ICM docking algorithm.39 The data obtained from these docking studies supported a binding mode in which nitrogen in the 1-position of the pyrimidine and the amino group in the 2-position of the pyrimidine ring form hydrogen-bonding interactions with the backbone atoms of Cys88 in the hinge region. These data are in agreement with the co-crystal structure of ULK2 bound to SBI-0206965, which showed the corresponding 2-aminopyrimidine moiety of SBI-0206965 in a similar position, undergoing similar interactions. The docking data indicated that the trifluoromethyl group in the 5-position of the pyrimidine ring in 3 occupied the hydrophobic back pocket formed by the side chains of Val23, Ala37, Lys39, Val69, Met85, and Leu138, thus providing additional favorable interactions compared with the bromo group at the corresponding position in SBI-0206965. The docking data also suggested that the cyclopropylamine moiety in the 4-position of the pyrimidine ring of 3 formed hydrophobic interactions with the sidechains of Val15 and Val23 and with backbone atoms of Gly16 within the β1 and β2 sheets, respectively. Finally, the benzo[d][1,3]dioxole moiety was found to undergo hydrophobic interactions with the side chains of Val15, Tyr87, and Leu138 and with backbone atoms of Asp89, Gly90, and Gly91. Notably, the benzo[d][1,3]dioxole moiety was not involved in any specific hydrogen bonding interactions, suggesting that it could be replaced with other moieties that might add additional polar interactions or could be truncated to improve ligand efficiency.
Scheme 1. SAR Strategy Around ULK1/2 Fragmentsa.

a(A) Compounds 2 and 3 identified in a screen for ULK1 inhibition, with IC50 values (ADP-Glo assay) and (B) a synthetic scheme for N4-cyclopropyl-5-(trifluoromethyl)pyrimidine ULK inhibitors.
To fully characterize new ULK1/2 inhibitors and facilitate expansion of the SAR, we established a versatile testing platform. We used an in vitro end-point ADP-Glo kinase assay34,40 as the primary assay to measure the biochemical inhibitory potency of compounds (Table 1 and Figure S3). To determine on-target kinase engagement in live cells, we employed a bioluminescence energy transfer NanoBRET assay that we optimized for ULK1 (Table 1 and Figure S4).41 This quantitative technique relies on a proximity-based energy transfer between a protein tagged with a 19 kDa luciferase domain and cell-permeable bivalent fluorescent energy transfer (FRET) probe that emits a signal. A kinase inhibitor that is a cell penetrant competes with the fluorescent probe and reduces the signal.42 This assay was compatible with a high-throughput workflow and offered a reliable metric to compare the cellular potencies of compounds.43 In addition, protein thermal shift (PTS) assays were employed to confirm the biochemical potency and determine the relative ability of compounds to stabilize ULK1 or ULK2, as measured by a shift in protein-melting temperatures (Tm).44
The co-crystal structure of SBI-0206965 bound to ULK2, coupled with our in silico modeling data and previous structural studies34 around ULK1 and ULK2, suggested that improved potency could be elicited by systematic evaluation of arylsubstituents at the R2 position (Table 1). Analogues were synthesized in a modular approach by two sequential substitutions on the pyrimidine scaffold (Scheme 1B). Thus, 2,4-dichloro-5-(trifluoromethyl)pyrimidine was reacted with various alkyl amines under basic conditions to afford key intermediate A. Compound A was then reacted with a panel of aryl amines in acetic acid utilizing microwave irradiation to provide the target inhibitors (B) with diverse electronic and polar characteristics. The analogues were tested in dose—response mode in the ADP-Glo kinase assay (Figure S3), and the data for compounds with IC50 values better than 50 nM are shown in Table 1.
Since our docking studies revealed that the aniline moiety (R2) is directed toward solvent-exposed space, it is perhaps unsurprising that a range of aryl substituents were tolerated. For example, systematic expansion of the dioxole ring to the dioxine 5 or dioxepine 6 improved the IC50 compared with 3 (21 nM) to 14 and 7 nM, respectively, in the ADP-Glo assay (Table 1). On the other hand, the difluorodioxole derivative 4 (42 nM) was less potent than 3. The series of derivatives in which the dioxole moiety was replaced with a 5-membered heteroaromatic ring (7, 8, 9, 10, 11, and 15) showed subtle variations in potency. Thus, the indole (8), pyrrolopyridine (11), and benzoxazole (15) were approximately equipotent with 3, while the benzimidazole (7) and benzotriazole (10) derivatives were about 4-fold more potent (IC50 values 5 and 6 nM, respectively). We also tested analogues of 11 in which the cyclopropyl moiety (R1) was replaced with a cyclobutyl (12) or a methyl (13) group, resulting in no significant improvement in potency. Interestingly, in the series of 6,6-fused ring analogues (14, 16–19), all except the pyrido[1,4]oxazine derivative (14) were more potent than 3. Notably, the tetralone derivative (19) was the most potent analogue with an IC50 value of 3 nM in the ADP-Glo assay. Finally, in the series of six-membered ring aryl and heteroaryl derivatives (20–26), the potencies ranged from 13 to 50 nM in the ADP-Glo assay, with 21, 24, and 26 being the most potent. To verify the binding affinities and benchmark, the relative stabilization of protein compared with SBI-0206965, fifteen of the most potent analogues were tested in the PTS assay against the kinase domain of ULK1 (Table S2). The largest thermal shift observed was ΔTm = 9.5 °C for compound 26, compared with ΔTm = 8.3 °C for SBI-0206965. Interestingly, the PTS data followed a similar trend to the ADP-Glo data, with the more potent compounds in the ADP-Glo assay generally exhibiting a larger ΔTm in the PTS assay (Table S2).
We next utilized the ULK1 NanoBRET intracellular kinase assay to quantify the target engagement of compounds in live cells, marking the first report of a NanoBRET assay for ULK1 (Table 1 and Figure S4). The IC50 values, shown in Table 1, indicate the capacity of the inhibitors to both enter the cell and bind to full-length ULK1 kinase at intracellular physiological levels of ATP. Compared with the ADP-Glo kinase assay, the IC50 values for compounds were right-shifted in the ULK1 NanoBRET cellular assay. The results in the NanoBRET assay proved to be critical to the selection of compounds for further characterization. Thus, our first-generation chemical probe SBI-0206965 is active in this assay, with a benchmark IC50 value of 785 nM. Remarkably, only five of the new inhibitors (7, 16, 21, 23, and 26) exhibited IC50 values in the sub-micromolar range, with the potencies of the other analogues ranging from 1.0 to >10 μM (Table 1).
The most potent ULK1 inhibitors, as well as SBI-0206965, were next tested for their ability to inhibit ULK2 in vitro and in cells. Thus, ULK2 biochemical inhibition (using the ADP-Glo assay) and cellular target engagement (using the NanoBRET assay) were measured for compounds 1, 7, 15, 16, 21, 23, and 26 (Table 2). As shown in Table 2, in general, the compounds were much less potent against ULK2 compared with ULK1 in both assays, except compound 7, which was similarly potent against ULK1 and ULK2 in the NanoBRET assay, with IC50 values 585 and 703 nM, respectively. As demonstrated with ULK1, compound IC50 values were generally right-shifted in the ULK2 NanoBRET assay relative to the ULK2 ADP-Glo assay. Notably, however, compound 26 was significantly more potent than SBI-0206965 in both ULK2 assays (Table 2).
Table 2.
Inhibitory Activities of Selected Compounds against ULK2
| compound | ULK2 ADP-Glo IC50 (nM) | ULK2 NanoBRET IC50 (nM) |
|---|---|---|
| 1a | 2448 ± 298 | 4578 ± 234 |
| 7 | 720 ± 60 | 703 ± 84 |
| 15 | 1193 ± 88 | 4256 ± 513 |
| 16 | 714 ± 99 | 1647 ± 200 |
| 21 | 695 ± 244 | 1885 ± 141 |
| 23 | 1329 ± 94 | 1687 ± 75 |
| 26a | 476 ± 21 | 1158 ± 116 |
Compounds 1 and 26 are SBI-0206965 and SBP-7455, respectively. Mean ± SD, representative of three independent experiments performed in triplicate (ADP-Glo) or in duplicate (NanoBRET).
The right-shifts in potency for the NanoBRET versus ADP-Glo assays for ULK1 and ULK2 could be due to a combination of effects, including membrane permeability, ATP competition, influence of the activation state and cellular protein interactions. These differences are due to the cellular nature of the NanoBRET assay, which requires compounds to cross the cytoplasmic membrane to interact with the target and to then compete with higher levels of ATP present in the NanoBRET assays, which are performed under cellular levels of ATP as opposed to Km levels of ATP utilized in the ADP-Glo assays.
Since we were keen to identify ULK1/2 inhibitors with improved drug-like properties, we next evaluated aqueous solubility, membrane permeability, and whether compounds could act as a substrate for drug transporters such as Pglycoprotein (PgP). We selected a subset of compounds and determined kinetic solubility in three media: PBS pH 7.4 buffer, fasted simulated intestinal fluid (FaSSIF), or fasted simulated gastric fluid (FaSSGF). The results, shown in Table S4, indicated that several compounds had reasonable solubility (>5 μM) in PBS, increased solubility in FaSSIF, and greatly increased solubility in FaSSGF. We also assessed the bidirectional permeability of the same subset of compounds in Caco-2 cells (Table S5). All the compounds tested, with the exception of 11 and 19, which also have limited solubility in PBS (Table S4), had high levels of permeability (Papp >10 × 10−6 cm/s), and the majority had limited potential as a PgP substrate (efflux ratio < 2).
Given these membrane permeability data, the right-shifts observed in the NanoBRET assays relative to the ADP-Glo assays are more likely due to competition from cellular levels of ATP and not due to differences in membrane permeability. Based on the data shown in Tables 1 and 2 and Tables S4 and S5, compounds 7, 15, 16, 21, 23, and 26 were selected for further characterization.
Evaluation of Downstream Target Engagement by ULK1/2 Inhibitors in Mammalian Cells.
We previously reported a method for quantifying the phosphorylation of VPS34 at Ser249 when ULK1 and VPS34 are overexpressed in HEK293T cells.11 We have established a similar assay to detect the phosphorylation of Beclin1 at Ser15. We therefore performed Western blot analysis to determine whether the new ULK1/2 inhibitors inhibit phosphorylation of Beclin1 or VPS34 (Figure S1 and Figure 2).45
Figure 2.

ULK1/2 inhibitors block phosphorylation of downstream autophagy target proteins. Western blot analysis (upper panels) and quantification (lower panels) of Beclin1 and VPS34 phosphorylation. HEK293T cells were transfected with Myc-tagged kinase inactive (KI) or wildtype (WT) ULK1 and WT Flag-tagged (A) Beclin1 or (B) VPS34 for 24 h. Cells were then treated with DMSO or the indicated ULK1/2 inhibitors at 10 μM for 1 h, lysed, and subjected to Western blotting with the indicated antibodies. Blots were quantified by densitometry. Data are expressed as the ratio of pBeclin1/Beclin1 or pVPS34/VPS34 normalized to DMSO-treated samples. Mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 vs SBI-0206965 by two-sided unpaired test.
For these experiments, Flag-tagged Beclin1 or VPS34 and Myc-tagged ULK1 were overexpressed in HEK293T cells, and the cells were incubated with DMSO or ULK1/2 inhibitors (10 μM, 1 h). Cell lysates were then subjected to Western blot analysis with antibodies to Ser15-phosphorylated and total Beclin1 or Ser249-phosphorylated and total VPS34. Importantly, the ULK1 specificity of the inhibitors was verified by examining cells overexpressing a Myc-tagged kinase-inactive (KI) mutant in place of the WT enzyme. As expected, control (DMSO-treated) cells overexpressing WT ULK1 showed a dramatic increase in Beclin1 phosphorylation compared with untransfected cells or ULK1-KI-expressing cells (Figure 2A). Treatment with SBI-0206965 reduced Beclin1 phosphorylation by ~30% compared with control (DMSO), and a similar level of inhibition was observed for compounds 7, 15, 16, 21, and 23. The most pronounced effect was displayed by compound 26, which inhibited Beclin1 phosphorylation by ~60%. We observed the same pattern of activity for our compounds when phosphorylation of VPS34 Ser249 by ULK1 was measured (Figure 2B). All of the ULK inhibitors greatly reduced VPS34 phosphorylation (by ~70–80%), with compound 26 again having the greatest effect (~90% inhibition) (Figure 2B). These data confirm that our ULK1/2 inhibitors effectively engage their intended targets in the cellular environment and reveal the superior potency of 26, which was selected for further evaluation and characterization.
PK Analysis of Compound 26.
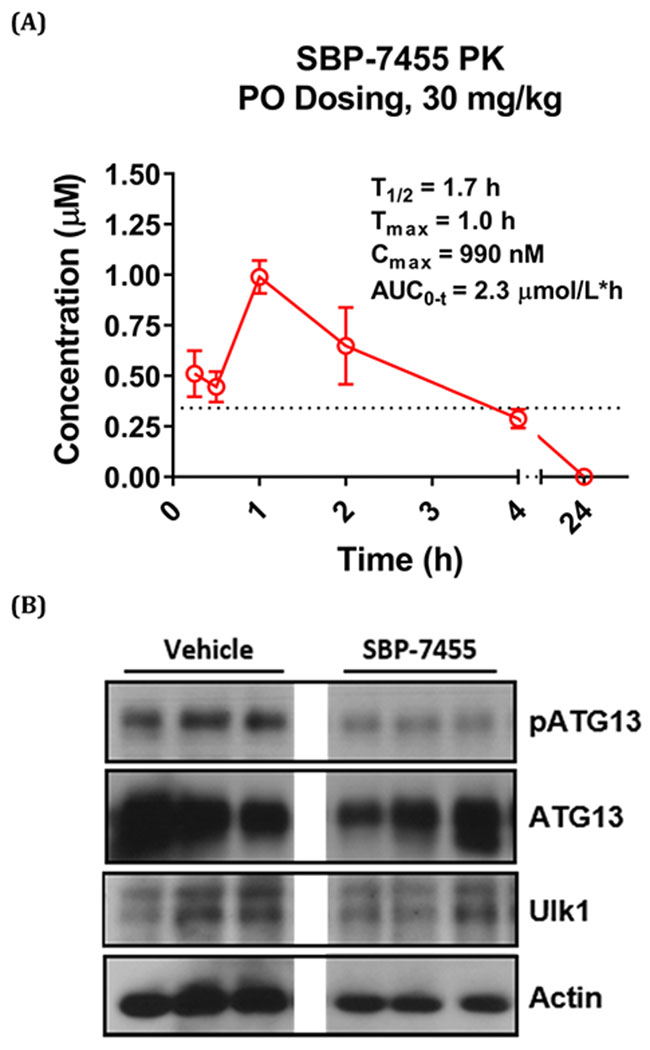
As noted previously, SBI-0206965 has a limited value as an in vivo probe due to poor systemic exposure following ip dosing and no measurable oral bioavailability in mice. To determine whether compound 26 had improved PK properties compared with SBI-0206965, a single dose of 26 (30 mg/kg) was orally administered to mice and its plasma concentration was measured over the following 24 h using LC–MS/MS. This analysis showed that the time to peak plasma concentration (Tmax) for 26 was approximately 1 h and the maximum plasma concentration (Cmax) was 990 nM, which was about 3-fold higher than the IC50 value for 26 (328 nM) against ULK1 in the NanoBRET assay (Figure 3A). The plasma concentration of compound 26 remained above the ULK1 IC50 for almost 4 h after oral dosing. These data indicate that 26 has improved PK properties compared with SBI-0206965, which will enable analysis of its PD properties in vivo.
Figure 3.
PK and PD analyses of compound 26 (SBP-7455). (A) Mice were dosed orally with compound 26 (30 mg/kg in 5% DMSO, 10% Tween 80, and 85% H2O). Plasma samples were taken at the indicated time points, and the concentration of compound was determined by LC—MS/MS analysis. The data were analyzed using PKSolver software. Mean ± SD of N = 3. Dotted line indicates the IC50 of compound 26 in the ULK1 NanoBRET assay. (B) Mice were dosed with compound 26 (10 mg/kg) or vehicle (5% DMSO, 10% Tween 80, and 85% H2O) via oral gavage. Liver samples were harvested and lysed 2 h post dosing, and in vivo target engagement was assessed by Western blot. N = 3. All the lanes were on the same gel; the lanes between vehicle and SBP-7455 were cropped out.
In Vivo Target Engagement of SBP-7455.
As noted above, ATG13 resides with ULK1 in the autophagy preinitiation complex in cells. When autophagy is initiated, it is phosphorylated by ULK1 at Ser318. ATG13 is therefore a reliable PD biomarker for ULK1 activity. To evaluate the PD profile and in vivo target engagement, mice were dosed with compound 26 (10 mg/kg) or vehicle by oral gavage, and liver samples were collected after 2 h. The effects of compound 26 on ULK1 and ATG13 were assessed in the liver lysates. Western blot analysis revealed robust inhibition of pATG13 (Ser318), as well as downregulation of total ATG13 and ULK1 levels by compound 26 (Figure 3B). Based on the PK and PD profiles, our data show that compound 26 is an efficacious tool that can be used to modulate autophagy in vivo.
TNBC Cells Require ULK1 Activity and Autophagy Initiation for Survival and Proliferation.
A growing body of evidence supports a role for autophagy in the survival and proliferation of TNBC cells, suggesting that autophagy inhibition may be an effective strategy for the treatment of TNBC.26,28,46,47 For example, Maycotte and co-workers used short hairpin RNAs (shRNAs) to target the core autophagy genes ATG5, ATG7, and BECN1 in a panel of breast tumor cells.24 Their data showed that TNBC cell lines were sensitive to autophagy inhibition by gene knockdown (KD) of ATG5, ATG7, or BECN1 and pharmacologically with the lysosomotropic autophagy inhibitor CQ. Interestingly, the TNBC cells were dependent on autophagy for survival even in complete media and thus in the absence of stressors known to stimulate autophagy. To examine whether this was true for other core autophagy genes, we performed shRNA-mediated KD of ULK1 or BECN1 in the human TNBC cell lines MDA-MB-468, MDA-MB-231, and BT549. Western blot analysis confirmed that the respective target proteins were effectively and specifically silenced compared with control cells expressing scramble shRNA (Figure S5). Analysis of cell survival and proliferation revealed that ULK1 or BECN1 KD markedly reduced the proliferation of all three cell lines over 8 days, with near-complete abrogation of growth in ULK1-KD and BECN1-KD MDA-MB-468 cells and in ULK1-KD BT549 cells (Figure 4). These data demonstrate that all three TNBC cells require autophagy initiation for survival and growth, with the most pronounced effects observed for MDA-MB-468 and BT549 cells.
Figure 4.

Ablation of ULK1 or BECN1 by shRNA-mediated knockdown reduces the viability of TNBC cells. MDA-MB-468 (A), BT549 (B), and MDA-MB-231 (C) cells transduced with scramble, ULK1-targeting, or BECN1-targeting shRNAs and selected in puromycin for 3 days before analysis. Cell viability was measured with the Promega CellTiter-Glo assay on the indicated days. Mean ± SD of three independent experiments, each performed in quadruplicate. Data are normalized to the viability of scramble shRNA control cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs scramble control by two-sided unpaired t-test.
ULK1/2 Inhibitors Reduce TNBC Cell Viability.
Because our KD results showed that MDA-MB-468 cells were the most dependent on autophagy initiation, we next examined the effects of our ULK1/2 inhibitors in this cell line. MDA-MB-468 cells were incubated for 72 h with varying concentrations of SBI-0206965, compound 26 (SBP-7455), or CQ as a positive control. As shown in Figure 5A, MDA-MB-468 cells were relatively insensitive to CQ (IC50 = 10.6 μM) compared with 26 (IC50 = 0.3 μM) or SBI-0206965 (IC50 = 2.1 μM). These data are consistent with the results of the in vitro (ADP-Glo) and cellular (NanoBRET) ULK1/2 kinase assays, which revealed more potent inhibition by compound 26 compared with SBI-0206965. Mechanistically, the ULK1/2 inhibitors regulate autophagy initiation, whereas the pleiotropic drug CQ inhibits end-stage lysosomal degradation in the autophagy pathway. Accordingly, our data suggest that TNBC cell survival and growth may be more sensitive to inhibition early in the autophagy pathway as opposed to downstream.
Figure 5.

ULK1/2 inhibitors are cytotoxic as single agents against TNBC cells. (A) MDA-MB-468 cells were treated with the indicated concentrations of each ULK1/2 inhibitor or chloroquine (CQ) and incubated for 72 h. Cell viability was then measured using CellTiter-Glo. Compound treatment started at 30 μM for the ULK1/2 inhibitors and 90 μM for CQ with serial dilutions of 1:3. IC50 values were determined using GraphPad Prism 8. The dose—response curves are representative of three independent experiments performed in quadruplicate; mean ± SD. (B) Compound 26 (SBP-7455) promotes apoptosis in TNBC cells under nutrient-deprived conditions. MDA-MB-468 cells were treated with DMSO or compound 26 (10 μM) in normal growth media (RPMI with 10% FBS, upper panel) or starvation media (EBSS, lower panel) for 18 h. The cells were then collected, and apoptosis was analyzed with PE-Annexin V/7-AAD staining by flow cytometry. Numbers indicate the percentage of cells in each gate. Shown is a representative of three independent experiments with similar results.
Given the role of autophagy as a survival mechanism for cancer cells in response to nutrient deprivation, we hypothesized that our new autophagy inhibitor, compound 26, would increase apoptosis in starved cells compared with cells provided with full nutrients. To investigate this, wild-type MDA-MB-468 cells were treated with compound 26 in normal media (RPMI with 10% FBS) or low-glucose, amino acid-free Earle’s balanced salt solution (EBSS) for 18 h. Apoptosis was measured with Annexin V and 7-AAD using flow cytometry, and the results are shown in Figure 5B. In normal media, the same percentages of cells (6.8%) were in late-stage apoptosis regardless of whether they were treated with DMSO or compound 26 (Figure 5B, panels i and ii). Under starvation conditions, however (Figure 5B, panels iii and iv), a much larger percentage of cells (18.4%) treated with compound 26 were in late-stage apoptosis compared with DMSO-treated cells (7.6%). Furthermore, a higher percentages of starved cells (42.9%) underwent early stage apoptosis compared with cells in normal media (28.9%) when treated with compound 26 (Figure 5B, panels ii and iv). The effects of compound 26 were much more pronounced than the percentages of cells (5.6 and 5.7%) in early stage apoptosis for the DMSO controls (Figure 5B, panels i and iii). Taken together, these data are consistent with enhanced cell killing by compound 26 under starved conditions that activate autophagy.
ULK1/2 Inhibitors Block Autophagic Flux in TNBC Cells.
Autophagic flux is the cellular process, whereby autophagosomes fuse with autolysosomes and subsequently degrade their cargo. To confirm that autophagic flux, and therefore autophagy, is inhibited by compound 26, we employed a flow cytometry-based assay using MDA-MB-468 cells expressing a tandem-labeled mCherry-EGFP-LC3 chimeric reporter protein to count the number of autophagosomes and autolysosomes.48 GFP fluorescence, but not mCherry fluorescence, is quenched in the acidic (pH ≤ 5) environment of lysosomes; therefore, inhibition of autophagic flux is reflected by a decrease in the cellular ratio of mCherry to GFP fluorescence (Figure 6A). In the absence of an autophagy stimulus, the MDA-MB-468 cells exhibited low (~55%) or intermediate (~40%) basal levels of autophagy with only ~5% of the cell population in high flux (Figure 6B and Figure S6). After incubation (18 h) in EBSS to starve the cells and induce autophagy, the percentage of cells with high autophagic flux increased to >90%. Treatment of starved cells with 10 μM SBI-0206965 or compound 26 significantly reduced the mCherry:GFP ratio, indicating inhibition of the starvation-induced increase in autophagic flux (by ~10% and ~ 60%, respectively; Figure 6B and Figure S6). Autophagosomes in the cells were visualized by transmission electron microscopy (TEM) (Figure 6C). The amount of autophagosomes under different conditions was consistent with the data from the autophagic flux assay, with the highest number of autophagosomes identified in starved cells (Figure 6C, ii). Again, similar to the autophagic flux assay, compound 26 (Figure 6C, iv) decreased the number of autophagosomes to a greater extent than SBI-0206965 (Figure 6C, iii). These data show that the compounds effectively inhibited autophagy in TNBC cells, with compound 26, a more potent ULK1/2 inhibitor than SBI-0206965, being the most effective.
Figure 6.

ULK1/2 inhibitors reduce autophagic flux. (A) MDA-MB-468 cells expressing mCherry-EGFP-LC3 fusion protein were used to monitor autophagic flux, as measured by the ratio of mCherry:GFP by flow cytometry. (B) Cells were incubated in normal growth media (control), with EBSS (starved) or EBSS plus the ULK1/2 inhibitors (10 μM) for 18 h before autophagic flux was assessed. Three independent experiments were performed; mean ± SD. *P < 0.001 by two-sided unpaired t-test of the high autophagic flux population. (C) Ultrastructural analysis of cells in (B) by TEM to monitor autophagosome accumulation. Red arrows indicate autophagosomes. (i) Representative untreated control cells, (ii) EBSS (starved) cells, (iii) cells treated with SBI-0206965 (10 μM), and (iv) cells treated with compound 26 (10 μM). Images are representative of 15–25 cells for each set of conditions analyzed by TEM.
SBP-7455 Exhibits Synergistic Cytotoxicity with Poly (ADP-ribose) Polymerase (PARP) Inhibitors against TNBC Cells.
PARPs are a family of proteins that maintain genome stability and are responsible for DNA-damage repair in cells.49 Since DNA damage can induce cancer cell death, PARP inhibition, which leads to the accumulation of single-stranded breaks, is a viable strategy to treat cancer.50,51 Several PARP inhibitors, including olaparib, niraparib, rucaparib, and talazoparib, have received regulatory approval for the treatment of cancer.50,51 However, the emergence of resistance has led to interest in combination therapies that can resensitize tumor cells to the effects of PARP inhibitors.52 Recent evidence has emerged that PARP inhibitors activate autophagy in cancer cells, and that this may lead to resistance. For example, inhibition of autophagy with CQ was found to enhance the effects of niraparib in xenograft models of hepatocellular carcinoma, while autophagy inhibition either genetically (ATG5 KD) or with CQ increased the cytotoxicity of talazoparib in models of pediatric chronic myelogenous leukemia (CML).53,54 Because PARP inhibitors have been approved for the treatment of TNBC, we investigated whether combining compound 26 with PARP inhibitors would increase cytotoxicity against TNBC cells. Activity of the PARP inhibitors olaparib, niraparib, and rucaparib was first tested against MDA-MB-468 cells to determine their potencies as single agents (Figure S7). Based on these data, we selected olaparib (IC50 = 10.1 μM) and niraparib (IC50 = 7.6 μM) for further studies. We tested different concentrations of compound 26 with niraparib (Figure S9) and olaparib (Figure 7 and Figure S8) to measure the combined effect of inhibiting both ULK1/2 and PARP. MDA-MB-468 cells were treated with compound 26 (0–15 μM) and either niraparib or olaparib (0–15 μM) in a dose-reponse matrix, and the synergistic effect was analyzed with Combenefit using the Loewe model.55 We observed strong antiproliferative synergy between 26 and both olaparib (Figure 7 and Figure S8) and niraparib (Figure S9), suggesting that autophagy inhibition enhances the cytotoxic effects of PARP inhibitors toward TNBC cells. Specifically, significant synergy was observed with concentrations of 26 at 0.021–15 μM and olaparib at 3.8–15 μM (Figure 7), and with 26 at 0.19–1.7 μM and niraparib at 3.8–7.5 μM (Figure S9). Of note, the synergistic effect was greater with olaparib than with niraparib, with the maximum synergy scores of 34 and 26, respectively. These data support the hypothesis that an ULK1/2 inhibitor in combination with a PARP inhibitor may have therapeutic utility for the treatment of TNBC.
Figure 7.

Compound 26 (SBP-7455) and the PARP inhibitor olaparib synergize to kill MDA-MB-468 cells. (A) MDA-MB-468 cells were treated with 26 (0.25 nM–15 μM) and olaparib (0.47–15 μM) for 72 h, and cell viability was assessed (CellTiter-Glo assay). A synergy graph was generated using the Combenefit Loewe model. Experiments were repeated three times (n = 4) with similar results. (B) Normalized cell viability after incubation with DMSO (vehicle control), olaparib (7.5 μM), compound 26 (0.19 μM), or a combination of both compounds. Mean ± SD. *P < 0.025, **P < 0.01, ***P < 0.001 P vs by two-sided unpaired t-test.
SBP-7455 Reverses Olaparib-Induced Upregulation of Autophagic Flux in TNBC Cells.
To investigate the mechanism by which compound 26 potentiates the cytotoxic effects of PARP inhibitors, we treated MDA-MB-468 cells expressing mCherry-EGFP-LC3 with olaparib (30 μM) and/or 26 (10 μM) for 48 h and then quantified autophagic flux. Olaparib alone was a strong inducer of autophagy, as demonstrated by an approximately 30% increase in autophagic flux compared with control cells (Figure 8 and Figure S10). However, concomitant treatment with compound 26 significantly suppressed the olaparib-induced increase in autophagic flux to a level similar to that observed in untreated control cells (Figure 8 and Figure 10). These data suggest that compound 26 potentiates olaparib activity, at least in part, by abrogating the ability of olaparib to induce autophagy. Of note, the higher autophagic flux levels in untreated MDA-MB-468 cells in Figure 8 compared with Figure 6B can be attributed to the measurement of basal autophagy at different time points, specifically 18 h in Figure 6B versus 48 h in Figure 8.
Figure 8.

Compound 26 (SBP-7455) inhibits the increase in autophagic flux induced by the PARP inhibitor olaparib. MDA-MB-468 cells expressing mCherry-EGFP-LC3 were treated with DMSO control, compound 26 (10 μM), olaparib (30 μM), or a combination of both for 48 h. Two independent experiments were performed; mean ± SD. *P < 0.05, **P < 0.01 for the high autophagic flux population by two-sided unpaired t-test.
CONCLUSIONS
We utilized a combination of rational- and structure-based design, based on a high-resolution structure of our first-generation probe SBI-0206965 bound to ULK2, to create a series of novel small molecule ULK1/2 inhibitors. The best compounds potently inhibited ULK1 and ULK2 in vitro and in cells, as measured by ADP-Glo, PTS, and Nano-BRET assays. SBP-7455 (compound 26) was identified as a highly effective inhibitor of phosphorylation of the ULK1 downstream substrates Beclin1 and Vps34 and exhibited promising PK and PD properties when dosed orally in mice. Importantly, inhibition of autophagic flux by shRNA-mediated KD of ULK1 was replicated with the optimized ULK1/2 inhibitor SBP-7455 in MDA-MB-468 TNBC cells.
TNBC therapy is particularly challenging due to its resistance to antihormonal and HER2 targeted therapies. PARP inhibitors are effective in a subset (~20%) of TNBC patients that harbor BRCA mutations;56 however, finding effective ways to sensitize WT BRCA TNBC to anti-cancer therapies is an area of ongoing research. Our results suggest that autophagy inhibition may be an attractive option. In the present study, we found that the autophagy inhibitor SBP-7455 was cytotoxic as a single agent against TNBC cells. In a clinical setting, it is likely that autophagy inhibition will be most effectively deployed as an adjunct to existing therapies. Therefore, we also showed that SBP-7455 and the PARP inhibitor olaparib synergistically kill MDA-MB-468 TNBC cells. Thus, in these cells, PARP inhibition combined with autophagy inhibition is more effective than either tumor cell killing mechanism alone.
Our results from the present study are in alignment with data from other groups on overcoming the protective effects of autophagy in advanced cancers. For example, recent findings in pancreatic ductal adenocarcinoma (PDAC) suggest that inhibition of RAF-MEK-ERK signaling activates a compensatory pro-autophagic LKB1-AMPK-ULK1 axis.57,58 Thus, a growing body of preclinical and clinical evidence suggests that autophagy activation is a common mechanism of drug resistance in many advanced cancers; therefore, combination therapies that block autophagy, such as those described here, could have wide therapeutic applications.59 We focused our efforts on the development of inhibitors of ULK1/2 as initiating kinases in the autophagy signaling pathway. It is likely that autophagy inhibitors acting at later steps in the pathway would have distinct toxicity profiles compared with inhibitors of autophagy initiation. Blockade of lysosomal degradation leads to the accumulation of unprocessed autophagosomes, whereas blockade of the upstream stages causes a buildup of the autophagy cargo; namely, protein aggregates and damaged organelles.30 Chemical probes, such as the one described here, that act at different stages in the autophagy pathway could serve as tools to assess the relative importance of each component of the autophagic machinery from a therapeutic perspective.
EXPERIMENTAL SECTION
General Chemistry Information.
All reactions were performed with oven-dried glassware under a nitrogen atmosphere with magnetic stirring. All solvents and chemicals were purchased from commercial sources and used without further purification unless otherwise stated. Reactions conducted under microwave irradiation were performed in a CEM Discover microwave reactor using 10 mL reaction vessels. Reaction progress was monitored by reverse-phase HPLC and/or thinlayer chromatography (TLC). Chromatographic purification was carried out using prepacked silica or C18 cartridges (from RediSep and Luknova) and eluted using an ISCO companion system. Reversephase purifications were conducted using water and acetonitrile doped with 0.1% formic acid. Purity and characterization of compounds were established by a combination of LC–MS and NMR. HPLC–MS analyses were performed on a Shimadzu 2010EV LC–MS using the following conditions: a Kromasil C18 column (reverse phase, 4.6 mm_50 mm), a linear gradient from 10% acetonitrile and 90% water to 95% acetonitrile and 5% water over 4.5 min, a flow rate of 1 mL/min, and UV photodiode array detection from 200 to 300 nm. High-resolution ESI-TOF mass spectra were acquired from the Scripps Research Institute Center for Metabolomics and Mass Spectrometry (La Jolla, California). Proton (1H) and carbon (13C) NMR spectra were obtained on a JEOL400 spectrometer at 400 and 101 MHz, respectively. Chemical shifts are reported in δ (ppm) and were internally referenced to deuterated solvent signals. The data for 1H NMRs are reported in terms of chemical shift (δ ppm), multiplicity, coupling constant (Hz), and proton integration. The data for 13C NMR are reported in terms of chemical shift (δ ppm) and coupling constant (Hz). The purity of final products submitted for biological testing was determined by analytical HPLC–MS to be ≥95%. For NMR spectrum and LCMS traces of compounds 7, 15, 16, 21, 23, and 26, see the Supporting Information.
2-Chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine.
A solution of 2,4-dichloro-5-(trifluoromethyl)pyrimidine (1.00 g, 4.61 mmol), cyclopropylamine (0.32 mL, 4.61 mmol), and N,N-diisopropylethylamine (0.80 mL, 4.61 mmol) in acetonitrile (15 mL) was microwaved at 70 °C for 10 min in a 38 mL pressure vessel. The reaction mixture was then concentrated in vacuo and purified by automated reverse-phase chromatography (water-acetonitrile eluent). Tan solid (0.349 g, 32% yield). LC–MS (ESI) calcd for C8H8ClF3N3 [M + H]+: 238.04; found 238.30. 1H NMR (400 MHz, DMSO-d6): δ 8.39 (s, 1H), 7.93 (d, J = 3.6 Hz, 1H, 1H), 2.88 (dq, J = 7.2, 3.6 Hz, 1H), 0.79–0.72 (m, 2H), 0.70–0.64 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 162.66, 159.73, 155.42, 123.34 (q, J = 271 Hz), 105.31 (q, J = 32 Hz), 24.72, 6.30.
General Method 1.
A solution of commercially purchased 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (1 equiv) and the appropriate aniline (1 equiv) in acetic acid (2 mL) was microwaved at 110 °C for 10 min and then concentrated in vacuo. The product was purified by automated reverse-phase chromatography (water-acetonitrile eluent).
N2-(Benzo[d][1,3]dioxol-5-yl)-5-(trifluoromethyl)-pyrimidine-2,4-diamine (2).
A solution of 2,4-dichloro-5-(trifluoromethyl)pyrimidine (85 mg, 0.392 mmol), benzo[d][1,3]-dioxol-5-amine (54 mg, 0.394 mmol), and N,N-diisopropylethylamine (0.068 mL) in acetonitrile (3 mL) was microwaved at 100 °C for 10 min. Ammonia (0.22 mL of a 7 M solution in MeOH) and N,N-diisopropylethylamine (0.07 mL) were added, and the reaction mixture was microwaved at 100 °C for 10 min, then concentrated in vacuo. Product was purified by automated reverse-phase chromatography. Brown solid (15 mg, 13%). LC–MS (ESI) calcd for C12H10F3N4O2 [M + H]+: 299.08, found 299.28. 1H NMR (400 MHz, DMSO-d6): δ 9.39 (s, 1H), 8.15 (s, 1H), 7.54 (s, 2H), 7.11 (d, J = 10.5 Hz, 1H), 6.76 (d, J = 8.4 Hz, 1H), 5.93 (s, 2H). 13C NMR (101 MHz, DMSO-d6): δ 161.2, 159.8, 155.1, 147.1, 142.2, 134.8, 125.3 (q, J = 269 Hz), 112.5, 107.9, 102.3, 100.9, 96.5 (q, J = 31 Hz).
N2-(Benzo[d][1,3]dioxol-5-yl)-N4-cyclopropyl-5-(trifluoromethyl)pyrimidine-2,4-diamine (3).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (60 mg, 0.253 mmol) and benzo[d][1,3]dioxol-5-amine (35 mg, 0.253 mmol). Purple solid (56 mg, 66% yield). LC–MS (ESI) calcd for C15H14F3N4O2 [M + H]+: 339.11, found 339.00. 1H NMR (400 MHz, DMSO-d6): δ 9.61 (s, 1H), 8.16 (s, 1H), 7.82 (br. s, 1H), 7.18 (dd, J = 8.5, 2.2 Hz, 1H), 7.15 (br. s, 1H), 6.83 (d, J = 8.6 Hz, 1H), 5.95 (s, 2H), 2.79 (dq, J = 6.2, 3.4 Hz, 1H), 0.78 (td, J = 7.2, 4.9 Hz, 2H), 0.67 (dt, J = 7.3, 4.6 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.6, 159.4, 154.4, 146.5, 142.3, 134.3, 124.96 (q, J = 269 Hz), 111.8, 107.8, 102.3, 100.7, 24.5, 6.7.
N4-Cyclopropyl-N2-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (4).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (50 mg, 0.210 mmol) and 2,2-difluorobenzo[d][1,3]dioxol-5-amine (36 mg, 0.210 mmol). The crude product was purified by automated normal phase chromatography on silica gel (DCM eluent). White solid (35 mg, 44% yield). LC–MS (ESI) calcd for C15H12F5N4O2 [M + H]+: 375.09, found 375.20. 1H NMR (400 MHz, DMSO-d6): δ 9.93 (s, 1H), 8.30 (br. s, 1H), 8.21 (s, 1H), 7.46 (dd, J = 8.8, 2.2 Hz, 1H), 7.30 (d, J = 8.8 Hz, 1H), 2.81 (tq, J = 6.7, 3.1 Hz, 1H), 0.84–0.75 (m, 2H), 0.72–0.66 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.5, 159.4, 154.6, 154.5, 142.6, 137.3, 137.2, 131.4 (t, J = 252 Hz), 124.8 (q, J = 269 Hz) 114.2, 109.7, 101.6, 24.5, 6.7.
N4-Cyclopropyl-N2-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (5).
A solution of 2,4-dichloro-5-(trifluoromethyl)pyrimidine (85 mg, 0.392 mmol), cyclopropanamine (22 mg, 0.385 mmol), and N,N-diisopropylethylamine (0.068 mL) in acetonitrile (3 mL) was microwaved at 100 °C for 10 min, then concentrated in vacuo. 2,3-Dihydrobenzo[b][1,4]dioxin-6-amine (59 mg, 0.390 mmol) was added, and the reaction mixture was processed according to General Method 1. The mixture was microwaved at 120 °C for 10 min. White solid (15 mg, 11%). LC–MS (ESI) calcd for C16H16F3N4O2 [M + H]+: 353.12, found 353.31. 1H NMR (400 MHz, DMSO-d6): δ 11.98 (s, 1H), 9.53 (s, 1H), 8.15 (s, 1H), 7.79 (s, 1H), 7.18 (dd, J = 8.8, 2.6 Hz, 1H), 6.75 (d, J = 8.7 Hz, 1H), 4.23–4.15 (m, 4H), 2.79 (br. s, 1H), 0.82 (dt, J = 6.8, 3.4 Hz, 2H), 0.70–0.65 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.6, 159.5, 154.5, 154.4, 142.9, 138.3, 134.1, 125.0 (q, J = 269 Hz), 116.5, 112.5, 108.4, 64.3, 64.0, 24.6, 6.7. HRMS (ESI-TOF) calcd for C16H16F3N4O2 [M + H]+: 353.1225; found 353.1216.
N4-Cyclopropyl-N2-(3,4-dihydro-2H-benzo[b][1,4] dioxepin-7-yl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (6).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (80 mg, 0.337 mmol) and 3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-amine (56 mg, 0.337 mmol). White solid (103 mg, 84% yield). LC–MS (ESI) calcd for C17H18F3N4O2 [M + H]+: 367.14, found 367.30. 1H NMR (400 MHz, DMSO-d6): δ 9.61 (s, 1H), 8.17 (s, 1H), 7.77 (br. s, 1H), 7.36 (dd, J = 8.8, 2.6 Hz, 1H), 7.16 (br. s, 1H), 6.87 (d, J = 8.6 Hz, 1H), 4.07 (t, J = 5.3 Hz, 2H), 4.03 (t, J = 5.3 Hz, 2H), 2.83–2.76 (m, 1H), 2.05 (q, J = 6.0, 5.4z Hz, 1H), 0.82 (dt, J = 7.1, 3.4 Hz, 2H), 0.67 (td, J = 7.0, 3.5 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.6, 159.4, 154.5, 154.4, 150.9, 145.9, 135.9, 124.9 (q, J = 270 Hz), 121.1, 114.2, 112.6, 70.6, 32.1, 24.6, 6.7. HRMS (ESI-TOF) calcd for C17H18F3N4O2 [M + H]+: 367.1382; found 367.1368.
N2-(1H-Benzo[d]imidazol-6-yl)-N4-cyclopropyl-5-(trifluoromethyl)pyrimidine-2,4-diamine (7).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (65 mg, 0.274 mmol) and 1H-benzo[d]imidazol-6-amine (36 mg, 0.274 mmol). The mixture was microwaved at 130 °C for 20 min. White solid (41 mg, 45% yield) LC—MS (ESI) calcd for C15H13F3N6 [M + H]+: 335.13; found 335.00. 1H NMR (400 MHz, DMSO-d6): δ 9.70 (s, 1H), 8.49 (br. s, 1H), 8.19 (s, 1H), 8.12 (s, 1H), 7.48 (s, 2H), 7.13 (br. s, 1H), 2.89 (dq, J = 6.9, 3.3 Hz, 1H), 0.93–0.80 (m, 2H), 0.73–0.64 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.8, 159.5, 154.5, 154.4, 141.6, 136.7, 135.2, 125.0 (q, J = 269 Hz), 115.8, 115.3, 104.5, 24.6, 6.8. HRMS (ESI-TOF) calcd for C15H14F3N6 [M + H]+: 335.1232; found 335.1223.
N4-Cyclopropyl-N2-(1H-indol-5-yl)-5-(trifluoromethyl)-pyrimidine-2,4-diamine (8).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (70 mg, 0.295 mmol) and 1H-indol-5-amine (39 mg, 0.295 mmol). Black solid (72 mg, 73% yield). LC–MS (ESI) calcd for C16H15F3N5 [M + H]+: 334.13, found 334.15. 1H NMR (400 MHz, DMSO-d6): δ 10.92 (s, 1H), 9.47 (s, 1H), 8.25 (s, 1H), 8.15 (s, 1H), 7.43 (d, J = 8.8 Hz, 1H), 7.33–7.24 (m, 2H), 7.02 (s, 1H), 6.32 (t, J = 2.6 Hz, 1H), 2.87 (s, 1H), 0.85–0.76 (m, 2H), 0.74–0.64 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 166.2, 161.0, 159.4, 154.5, 154.4, 132.1, 127.5, 125.6, 125.2 (q, J = 269 Hz), 115.5, 110.8, 100.9, 24.5, 6.7. HRMS (ESI-TOF) calcd for C16H15F3N5 [M + H]+: 334.1280; found 334.1267.
N4-Cyclopropyl-N2-(1H-indazol-5-yl)-5-(trifluoro-methyl)-pyrimidine-2,4-diamine (9).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (80 mg, 0.337 mmol) and 1H-indazol-5-amine (45 mg, 0.337 mmol). The product was isolated by precipitation and filtration with acetone. Pink solid (70 mg, 62% yield). LC–MS (ESI) calcd for C15H14F3N6 [M + H]+: 335.13, found 335.15. 1H NMR (400 MHz, DMSO-d6): δ 11.00 (s, 1H), 8.07 (s, 1H), 7.57 (s, 2H), 2.97–2.88 (m, 1H), 0.89–0.82 (m, 2H), 0.78 (dt, J = 7.8, 3.9 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 172.1, 158.9, 152.6, 145.3, 137.5, 133.5, 133.0, 129.9, 122.8, 122.7 (d, J = 270 Hz), 121.4, 110.6, 25.5, 6.6. HRMS (ESI-TOF) calcd for C15H14F3N6 [M + H]+: 335.1232; found 335.1219.
N2-(1 H-Benzo[d][1,2,3]triazol-6-yl)-N4-cyclopropyl-5-(trifluoromethyl)pyrimidine-2,4-diamine (10).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (90 mg, 0.379 mmol) and 1H-benzo [d][l,2,3]triazol-5-amine (51 mg, 0.379 mmol). The product was isolated by precipitation and filtration with acetone. White solid (97 mg, 76% yield). LC–MS (ESI) calcd for C14H13F3N7 [M + H]+: 336.12, found 336.20. 1H NMR (400 MHz, DMSO-d6): δ 11.27 (br. s, 1H), 8.63 (s, 1H), 8.46 (s, 1H), 7.91 (d, J = 8.8 Hz, 1H), 7.50 (dd, J = 9.0, 1.9 Hz, 1H), 2.93 (dq, J = 7.2, 3.4 Hz, 1H), 0.95 (td, J = 7.2, 4.9 Hz, 2H), 0.75 (dt, J = 7.0, 4.8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 159.1, 154.0, 147.0, 138.0, 136.8, 136.0, 119.4, 116.9, 123.0 (q, J = 270 Hz), 102.8, 98.8 (q, J = 34 Hz), 25.5, 6.7. HRMS (ESI-TOF) calcd for C14H13F3N7 [M + H]+: 336.1185; found 336.1186.
N4-Cyclopropyl-N2-(1H-pyrrolo[2,3-b]pyridin-5-yl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (11).
A solution of 2,4-dichloro-5-(trifluoromethyl)pyrimidine (85 mg, 0.392 mmol), cyclopropanamine (22 mg, 0.385 mmol), and N,N-diisopropylethylamine (0.07 mL) in acetonitrile (3 mL) was microwaved at 100 °C for 10 min. The mixture was concentrated in vacuo and processed according to General Method 1 with the addition of 1H-pyrrolo[2,3-b]pyridin-5-amine (52 mg, 0.391 mmol) in acetic acid (2 mL). The mixture was microwaved at 120 °C for 10 min. Brown solid (13 mg, 10%). LC–MS (ESI) calcd for C15H14F3N6 [M + H]+: 335.12, found 335.25.1H NMR (400 MHz, DMSO-d6): δ 11.48 (s, 1H), 9.67 (s, 1H), 8.61 (s, 1H), 8.51 (s, 1H), 8.19 (s, 1H), 7.42–7.38 (m, 1H), 7.15–7.07 (m, 1H), 6.37 (dd, J = 3.5, 1.8 Hz, 1H), 2.84 (br. s, 1H), 0.79 (td, J = 7.1, 4.7 Hz, 2H), 0.72–0.65 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 161.2, 159.5, 154.6, 154.6, 144.8, 136.8, 130.0, 126.5, 125.1 (q, J = 269 Hz), 119.0, 99.6, 24.5, 6.7.
N4-Cyclobutyl-N2-(1 H-pyrrolo[2,3-b]pyridin-5-yl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (12).
A solution of 2,4-dichloro-5-(trifluoromethyl)pyrimidine (120 mg, 0.553 mmol), cyclobutanamine (49 mg, 0.689 mmol), and N,N-diisopropylethylamine (0.12 mL) in acetonitrile (3 mL) was microwaved at 100 °C for 10 min. 1H-Pyrrolo[2,3-b]pyridin-5-amine (92 mg, 0.691 mmol) and acetic acid (3 mL) were added, and the reaction mixture was processed according to General Method 1 and microwaved at 100 °C for 20 min. Tan solid (50 mg, 42% yield). LC–MS (ESI) calcd for C16H16F3N6 [M + H]+: 349.14, found 349.25.1H NMR (400 MHz, DMSO-d6): δ 11.52 (s, 1H), 9.54 (br. s, 1H), 8.42 (s, 1H), 8.31 (s, 1H), 8.18 (s, 1H), 7.42 (t, J = 3.0 Hz, 1H), 6.94 (d, J = 6.9 Hz, 1H), 6.38 (dd, J = 3.3, 1.9 Hz, 1H), 4.60 (br. s, 1H), 2.29–2.08 (m, 4H), 1.74–1.53 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 161.3, 157.2, 154.9, 154.8, 145.0, 137.3, 129.6, 126.6, 125.0 (q, J = 269 Hz), 119.8, 119.0, 99.5, 45.9, 29.9, 14.8.
N4-Methyl-N2-(1 H-pyrrolo[2,3-b]pyridin-5-yl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (13).
To a solution of 2,4-dichloro-5-(trifluoromethyl)pyrimidine (400 mg, 1.844 mmol) in dichloromethane and tert-butanol (10 mL, 1:1) at −10 °C under nitrogen was added zinc(II) chloride (502 mg, 3.69 mmol) and stirred at −10 to 0 °C. After 1 h, 1H-pyrrolo[2,3-b]pyridin-5-amine (245 mg, 0.844 mmol) and triethylamine (0.28 mL, 2.03 mmol) were added. The reaction mixture was warmed to room temperature. The dichloromethane was removed in vacuo, and the solid precipitate was filtered and washed with water. The crude product (620 mg) was isolated as a 70:30 mix of isomers, with N-(4-chloro-5-(trifluoromethyl)pyrimidin-2-yl)-1H-pyrrolo[2,3-b]pyridin-5-amine as the major isomer. LC–MS (ESI) calcd for C12H8ClF3N5 [M + H]+: 314.04, found 313.80.
A solution of N-(4-chloro-5-(trifluoromethyl)pyrimidin-2-yl)-lH-pyrrolo[2,3-b]pyridin-5-amine (100 mg, 0.319 mmol, containing 30% of the regioisomer) and methanamine (0.19 mL, 0.96 mmol) in DMF (2 mL) was microwaved at 100 °C for 20 min and concentrated in vacuo. The product was purified by automated reverse-phase chromatography. Tan solid (20 mg, 20% yield). LC–MS (ESI) calcd for C13H12F3N6 [M + H]+: 309.11, found 308.95. 1H NMR (400 MHz, DMSO-d6): δ 11.47 (s, 1H), 9.51 (br. s, 1H), 8.43 (s, 1H), 8.31 (s, 1H), 8.14 (s, 1H), 7.43–7.37 (m, 1H), 7.07 (br. s, 1H), 6.38 (dd, J = 5.0, 2.0 Hz, 1H), 2.91 (d, J = 4.2 Hz, 3H). 13C NMR (101 MHz, DMSO-d6): δ 161.6, 158.6, 154.5, 154.4, 145.0, 137.4, 129.8, 126.7, 125.3 (q, J = 269 Hz), 119.2, 99.8, 28.1.
N4-Cyclopropyl-N2-(4-ethyl-3,4-dihydro-2H-pyrido[3,2-b]-[1,4] oxazin-7-yl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (14).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (120 mg, 0.505 mmol) and 4-ethyl-3,4-dihydro-2H-pyrido[3,2-b]-[1,4]oxazin-7-amine (91 mg, 0.505 mmol). Pink solid (76 mg, 40% yield). LC–MS (ESI) calcd for C17H20F3N6O [M + H]+: 381.17, found 381.33. 1H NMR (400 MHz, DMSO-d6): δ 9.45 (s, 1H), 8.13 (s, 1H), 7.99 (s, 1H), 4.18 (t, J = 4.5 Hz, 2H), 3.53 (q, J = 7.0 Hz, 2H), 3.37 (t, J = 4.4 Hz, 2H), 2.82–2.69 (m, 1H), 1.05 (t, J = 7.0 Hz, 3H), 0.77 (dt, J = 6.9, 3.3 Hz, 2H), 0.65 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.8, 159.4, 154.5, 142.5, 138.6, 130.5, 127.7, 125.0 (q, J = 269 Hz), 114.4, 64.3, 44.4, 41.6, 24.4, 11.4, 6.7.
N2-(Benzo[d]oxazol-6-yl)-N4-cyclopropyl-5-(trifluoromethyl)pyrimidine-2,4-diamine (15).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (65 mg, 0.274 mmol) and benzo[d]oxazol-6-amine (37 mg, 0.274 mmol). The mixture was microwaved at 130 °C for 20 min. Brown solid (4 mg, 4% yield). LC–MS (ESI) calcd for C15H13F3N5O [M + H]+: 336.11, found 335.95. 1H NMR (400 MHz, DMSO-d6): δ 10.02 (s, 1H), 8.79 (s, 1H), 8.58 (s, 1H), 8.23 (s, 1H), 7.77–7.55 (m, 2H), 7.28 (s, 1H), 2.87 (dq, J = 6.9, 3.4 Hz, 1H), 0.85 (dt, J = 6.8, 3.3 Hz, 2H), 0.76–0.66 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.7, 159.5, 154.6, 154.5, 153.3, 149.9, 138.6, 134.0, 124.8 (q, J = 269 Hz), 119.4, 116.8, 101.3, 24.6, 6.7. HRMS (ESI-TOF) calcd for C15H13F3N5O [M + H]+: 336.1072; found 336.1064.
N4-Cyclopropyl-N2-(quinolin-6-yl)-5-(trifluoromethyl)-pyrimidine-2,4-diamine (16).
The title compound was prepared according to General Method b using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (60 mg, 0.253 mmol), copper (1.6 mg, 0.025 mmol) and quinolin-6-amine (36 mg, 0.253 mmol). The reaction mixture was microwaved at 120 °C for 10 min. The product was isolated by precipitation with acetone and further purified by automated reverse-phase chromatography of the filtrate. White solid (7 mg, 8% yield). LC—MS (ESI) calcd for C17H15F3N5 [M + H]+: 346.13 found 345.95. 1H NMR (400 MHz, DMSO-d6): δ 10.09 (s, 1H), 8.76 (d, J = 2.4 Hz, 1H), 8.72 (dd, J = 4.1, 1.7 Hz, 1H), 8.27 (s, 1H), 8.13 (dd, J = 8.3, 1.6 Hz, 1H), 8.04 (dd, J = 9.1, 2.4 Hz, 1H), 7.93 (d, J = 9.1 Hz, 1H), 7.43 (dd, J = 8.3, 4.2 Hz, 1H), 7.29 (s, 1H), 2.99 (dq, J = 7.1, 3.4 Hz, 1H), 0.88 (td, J = 7.1, 4.8 Hz, 2H), 0.78–0.69 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.8, 159.5, 154.6 (q, J = 5 Hz), 148.2, 144.2, 138.3, 135.1, 129.0, 128.53, 124.8 (q, J = 270 Hz), 124.1, 121.7, 113.9, 40.2, 39.9, 39.7, 39.5, 39.3, 39.1, 38.9, 24.6, 6.8. HRMS (ESI-TOF) calcd for C17H15F3N5 [M + H]+: 346.1280; found 346.1284.
N4-Cyclopropyl-N2-(quinolin-3-yl)-5-(trifluoromethyl)-pyrimidine-2,4-diamine (17).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifiuoromethyl)pyrimidin-4-amine (60 mg, 0.253 mmol) and quinolin-3-amine (36 mg, 0.253 mmol). The mixture was microwaved at 120 °C for 20 min. The product was isolated by precipitation with acetone and further purified by automated reverse-phase chromatography of the filtrate. White solid (8 mg, 9% yield). LC—MS (ESI) calcd for C17H15F3N5 [M + H]+: 346.13 found 345.80. 1H NMR (400 MHz, DMSO-d6): δ 10.20 (s, 1H), 9.12 (d, J = 2.7 Hz, 1H), 9.06 (br. s, 1H), 8.29 (s, 1H), 7.93 (dd, J = 8.5, 1.5 Hz, 1H), 7.79 (dd, J = 7.8, 1.7 Hz, 1H), 7.62–7.49 (m, 2H), 7.35 (br. s, 1H), 2.96 (tq, J = 7.1, 3.7 Hz, 1H), 0.88 (td, J = 7.0, 4.8 Hz, 2H), 0.74 (dt, J = 6.9, 4.9 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.9, 159.5, 154.6, 145.3, 143.4, 134.1, 128.6, 128.1, 127.3, 127.0, 127.0, 124.7 (q, J = 270 Hz), 120.7, 24.6, 6.8. HRMS (ESI-TOF) calcd for C17H15F3N5 [M + H]+: 346.1280; found 346.1281.
N4-Cyclopropyl-N2-(quinoxalin-6-yl)-5-(trifluoromethyl)-pyrimidine-2,4-diamine (18).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (60 mg, 0.253 mmol) and quinoxalin-6-amine (37 mg, 0.253 mmol). The mixture was microwaved at 120 °C for 20 min. White solid (17 mg, 19% yield). LC–MS (ESI) calcd for C16H14F3N6 [M + H]+: 347.13, found 347.10. 1H NMR (400 MHz, DMSO-d6): δ 10.31 (s, 1H), 9.15 (s, 1H), 8.83 (d, J = 1.8 Hz, 1H), 8.74 (d, J = 1.9 Hz, 1H), 8.31 (s, 1H), 8.09 (dd, J = 9.2, 2.3 Hz, 1H), 7.99 (d, J = 9.1 Hz, 1H), 7.45 (s, 1H), 2.91 (dq, J = 7.1, 3.2 Hz, 1H), 1.03 (dt, J = 7.2, 3.6 Hz, 2H), 0.74 (td, J = 7.1, 3.6 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.7, 159.6, 154.7, 154.6, 145.8, 143.7, 143.1, 141.6, 138.6, 129.0, 124.7 (q, J = 273 Hz), 124.5, 114.5, 24.8, 6.8. HRMS (ESI-TOF) calcd for C16H14F3N6 [M + H]+: 347.1232; found 347.1216.
6-((4-(Cyclopropylamino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-3,4-dihydronaphthalen-1(2H)-one (19).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (60 mg, 0.253 mmol) and6-amino-3,4-dihydro-naphthalen-1(2H)-one (41 mg, 0.253 mmol). The mixture was microwaved at 110 °C for 20 min and then concentrated in vacuo. Ethyl acetate-dichloromethane was added, and the precipitate filtered to obtain the desired product. Tan solid (61 mg, 67% yield). LC–MS (ESI) calcd for C18H18F3N4O [M + H]+: 363.15, found 363.15. 1H NMR (400 MHz, DMSO-d6): δ 11.10 (s, 1H), 8.47 (s, 1H), 8.27 (br. s, 1H), 7.92 (br. s, 1H), 7.85 (d, J = 8.7 Hz, 1H), 7.71 (dd, J = 8.7, 2.2 Hz, 1H), 2.95 (tq, J = 7.2, 3.6 Hz, 1H), 2.90 (t, J = 6.0 Hz, 2H), 2.54 (t, J = 6.4 Hz, 2H), 2.02 (p, J = 6.3 Hz, 2H), 0.92–0.80 (m, 2H), 0.84–0.72 (m, 2H). 13C NMR(101 MHz, DMSO-d6): δ 196.2, 172.1, 159.2, 155.1, 148.5, 145.9, 142.8, 127.6, 127.4, 123.2 (q, J = 270 Hz), 118.4, 117.8, 99.01 (q, J = 34 Hz), 38.0, 29.8, 25.3, 22.9, 7.1. HRMS (ESI-TOF) calcd for C18H18F3N4O [M + H]+: 363.1433; found 363.1439.
N4-Cyclopropyl-N2-(pyridazin-4-yl)-5-(trifluoromethyl)-pyrimidine-2,4-diamine (20).
Glassware and the stir bar were flame dried, and nitrogen was bubbled through reagents and solvents prior to use. 2-Chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (70 mg, 0.295 mmol), pyridazin-4-amine (28 mg, 0.295 mmol), 9,9-dimethyl-(9H-xanthene-4,5-diyl)bis(diphenyl)phosphane (17 mg, 0.029 mmol), diacetoxypalladium (3.3 mg, 0.015 mmol), and cesium carbonate (120 mg, 0.589 mmol) were mixed in 1,4-dioxane (1 mL). The mixture was microwaved at 140 °C for 20 min, filtered through celite with methanol, and concentrated in vacuo. The crude product was purified by automated reverse-phase chromatography. White solid (5 mg, 6% yield). LC–MS (ESI) calcd for C12H12F3N6 [M + H]+: 297.11, found 296.75. 1H NMR (400 MHz, DMSO-d6): δ 10.39 (s, 1H), 9.51 (d, J = 2.1 Hz, 1H), 8.96 (d, J = 5.9 Hz, 1H), 8.35–8.28 (m, 2H), 7.54 (d, J = 2.4 Hz, 1H), 2.87 (dq, J = 7.2, 3.7 Hz, 1H), 0.86 (td, J = 7.0, 4.8 Hz, 2H), 0.70 (dt, J = 7.2, 4.6 Hz, 2H).
N4-Cyclopropyl-N2-(5-methoxypyridin-3-yl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (21).
Glassware and the stir bar were flame dried, and nitrogen was bubbled through reagents and solvents prior to use. 2-Chloro-N-cyclopropyl-5-(trifluoromethyl)-pyrimidin-4-amine (65 mg, 0.274 mmol), 5-methoxypyridin-3-amine (34 mg, 0.274 mmol), (9,9-dimethyl-9H-xanthene-4,5-diyl)bis-(diphenylphosphane) (16 mg, 0.027 mmol), diacetoxypalladium (3.1 mg, 0.014 mmol), and cesium carbonate (134 mg, 0.410 mmol) were mixed in 1,4-dioxane (1 mL). The mixture was microwaved at 140 °C for 20 min, filtered through celite with methanol, and concentrated in vacuo. The crude product was purified by automated reverse-phase chromatography. White solid (9 mg, 10% yield). LC–MS (ESI) calcd for C14H15F3N5O [M + H]+: 326.13, found 325.90. 1H NMR (400 MHz, DMSO-d6): δ 9.85 (s, 1H),8.56 (d, J = 2.1 Hz, 1H), 8.20 (s, 1H), 8.6, (t, J = 2.3 Hz, 1H), 7.88 (d, J = 2.5 Hz, 1H), 7.25 (br. s, 1H), 3.76 (s, 3H), 2.82 (dq, J = 7.2, 3.4 Hz, 1H), 0.76 (td, J = 7.2, 4.7 Hz, 2H), 0.65 (dt, J = 7.1, 4.8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.8, 159.4, 155.3, 154.7, 154.6, 137.7, 133.7, 129.2, 124.7 (q, J = 270 Hz), 111.4, 55.5, 24.6, 6.6.
2-((4-(Cyclopropylamino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)phenol (22).
The title compound was prepared according to General Method 1 using 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (60 mg, 0.253 mmol) and 2-aminophenol (28 mg, 0.253 mmol). The mixture was microwaved at 110 °C for 20 min and then concentrated in vacuo. Ethyl acetate was added, and the precipitate filtered to obtain the desired product. Brown solid (57 mg, 73% yield). LC–MS (ESI) calcd for C14H14F3N4O [M + H]+: 31z1.11, found 311.15. 1H NMR (400 MHz, DMSO-d6): δ 10.07 (s, 1H), 8.26 (s, 1H), 8.17 (d, J = 11.6 Hz, 2H), 7.20 (s, 1H), 6.90–6.84 (m, 2H), 6.84–6.74 (m, 1H), 2.84 (dq, J = 7.0, 3.5 Hz, 1H), 0.77 (td, J = 7.1, 4.6, Hz, 2H), 0.73–0.62 (m, 2H).13C NMR (101 MHz, DMSO-d6): δ 160.6, 159.5, 154.6, 154.5, 146.8, 127.6, 124.8 (q, J = 270 Hz), 123.0, 120.6, 119.1, 115.2, 24.5, 6.6.
N2-(4-Aminophenyl)-N4-cyclopropyl-5-(trifluoro-methyl)-pyrimidine-2,4-diamine (23).
A solution of 2-chloro-N-cyclopropyl-5-(trifluoromethyl)pyrimidin-4-amine (60 mg, 0.253 mmol) and benzene-1,4-diamine (55 mg, 0.505 mmol) in butan-l-ol (1 mL) was microwaved at 120 °C for 20 min and concentrated in vacuo. Acetone was added, and the mixture was filtered. The filtrate was concentrated to give the desired product. Brown solid (92 mg, 98% yield). LC—MS (ESI) calcd for C14H15F3N5 [M + H]+: 310.13, found 310.00. 1H NMR (400 MHz, DMSO-d6): δ 9.24 (s, 1H), 8.09 (s, 1H), 7.49 (br. s, 1H), 6.95 (s, 1H), 6.49 (d, J = 8.5 Hz, 2H), 4.75 (br. s, 2H), 2.85–2.77 (m, 1H), 0.78–0.68 (m, 2H), 0.67–0.61 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.8, 159.4, 154.4 (q, J = 4.6 Hz), 143.8, 129.4, 125.2 (q, J = 269 Hz), 121.1, 113.8, 24.4, 6.6.
N-(4-((4-(Cyclopropylamino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)phenyl)cyclopropanecarbox-amide (24).
A solution of N2-(4-aminophenyl)-N4-cyclopropyl-5-(trifluoromethyl)-pyrimidine-2,4-diamine (20 mg, 0.065 mmol), cyclopropanecarbonyl chloride (6.2 μL, 0.068 mmol), and triethylamine (0.012 mL, 0.084 mmol) in DMF (1 mL) was microwaved at 60 °C for 10 min and concentrated in vacuo. The crude product was purified by automated reverse phase chromatography. White solid (3 mg, 12% yield). LC—MS (ESI) calcd for C18H19F3N5O [M + H]+: 378.16, found 378.00. 1H NMR (400 MHz, DMSO-d6): δ 10.06 (s, 1H), 9.61 (br. s, 1H), 8.17 (s, 1H), 7.80 (d, J = 8.6 Hz, 2H), 7.48 (d, J = 8.9 Hz, 2H), 7.11 (br. s, 1H), 2.84 (td, J = 7.2, 3.6 Hz, 1H), 1.80–1.69 (m, 1H), 0.84–0.72 (m, 6H), 0.71–0.60 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 171.1, 160.7, 159.4, 154.5, 154.5, 135.5, 133.7, 124.97 (q, J = 269 Hz), 119.6, 119.3, 24.5, 14.0, 6.9, 6.6. HRMS (ESI-TOF) calcd for C18H19F3N5O [M + H]+: 378.1542; found 378.1530.
N-(Cyclopropanecarbonyl)-N-(4-((4-(cyclopropyl amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)phenyl)cyclopropanecarboxamide (25).
A solution of N2-(4-aminophenyl)-N4-cyclopropyl-5-(trifluoromethyl)pyrimidine-2,4-diamine (43 mg, 0.139 mmol), cyclopropanecarbonyl chloride (16 mg, 0.153 mmol) and N-ethyl-N-isopropyl propan-2-amine (0.03 mL, 0.167 mmol) in DMF (1 mL) was microwaved at 60 °C for 10 min. Additional cyclopropanecarbonyl chloride (16 mg, 0.153 mmol) was added and microwaved at 60 °C for an additional 10 min. The crude product was purified by automated reverse-phase chromatography. White solid (10 mg, 16% yield). LC—MS (ESI) calcd for C22H23F3N5O2 [M + H]+: 446.18, found 446.35. 1H NMR (400 MHz, DMSO-d6): δ 10.25 (s, 1H), 8.36 (s, 1H), 7.79 (d, J = 8.6 Hz, 1H), 7.57 (d, J = 8.7 Hz, 2H), 7.47 (d, J = 9.1 Hz, 1H), 7.07 (d, J = 8.7 Hz, 2H), 2.76 (dq, J = 7.1, 3.3 Hz, 1H), 2.45–2.35 (m, 1H), 1.82–1.68 (m, 1H), 1.10–0.82 (m, 4H), 0.82–0.73 (m, 4H), 0.70–0.47 (m, 4H).
N4-Cyclopropyl-N2-(3,4-dimethoxyphenyl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (26, SBP-7455).
A solution of 4-dichloro-5-(trifluoromethyl)pyrimidine (503 mg, 2.32 mmol) and zinc chloride (379 mg, 2.78 mmol) in 1,2-dichloroethane and tert-butanol (1:1, 20 mL) was set to stir at 0 °C. After 30 min, a solution of 3,4-dimethoxyaniline (355 mg, 2.32 mmol) and triethylamine (0.40 mL, 2.78 mmol) in 1,2-dichloroethane and tert-butanol (1:1, 2 mL) was added dropwise to the reaction mixture. The reaction mixture was stirred at room temperature for 4 h then concentrated in vacuo. The crude product was triturated with methanol, filtered, and dried to yield 4-chloro-N-(3,4-dimethoxyphenyl)-5-(trifluoromethyl)-pyrimidin-2-amine. White solid (702 mg, 91% yield) used crude in the next step. LC—MS (ESI) calcd for C13H12ClF3N3O2 [M + H]+: 334.06 found 334.00. 1H NMR (400 MHz, DMSO-d6): δ 10.49 (s, 1H), 8.74 (s, 1H), 7.35 (d, J = 2.6 Hz, 1H), 7.19 (d, J = 7.8 Hz, 1H), 6.93 (d, J = 8.7, Hz, 1H), 3.74 (s, 3H), 3.74 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 160.6, 158.0, 148.6, 145.5, 131.6, 123.1 (q, J = 270 Hz), 112.9, 112.0, 106.1, 55.7, 55.5.
A solution of 4-chloro-N-(3,4-dimethoxyphenyl)-5-(trifluoromethyl)pyrimidin-2-amine (500 mg, 1.50 mmol), cyclopropanamine (125 μL, 1.80 mmol) and N,N-diisopropyl ethylamine (0.31 mL, 1.80 mmol) in DMF (2 mL) was microwaved at 120 °C for 10 min. The crude product was concentrated in vacuo and purified by automated reverse-phase chromatography. White solid (291 mg, 55% yield). LC—MS (ESI) calcd for C16H18F3N4O2 [M + H]+: 355.14 found 355.50. 1H NMR (400 MHz, DMSO-d6): δ 9.46 (s, 1H), 8.16 (s, 1H), 7.55 (br. s, 1H), 7.42 (d, J = 2.4 Hz, 1H), 7.05 (br. s, 1H), 6.88 (d, J = 8.8 Hz, 1H), 3.71 (s, 3H), 3.70 (s, 3H), 2.91 (dq, J = 6.9, 3.3 Hz, 1H), 0.77 (td, J = 7.2, 4.9 Hz, 2H), 0.67 (dt, J = 7.3, 4.4 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 160.8, 159.4, 154.5, 154.4, 148.5, 144.2, 133.8, 125.0 (q, J = 269 Hz), 112.2, 111.4, 105.2, 55.8, 55.6, 24.5, 6.6. HRMS (ESI-TOF) calcd for C16H18F3N4O2 [M + H]+: 355.1382; found 355.1388.
Crystallization and Structure Determination.
ULK2 kinase domain was purified and crystallized as previously described.34 Data collection was performed at an SLS X06SA, and data were processed and scaled with XDS and aimless, respectively.60,61 Molecular replacement was performed using a Phaser and the published ULK2 structure (PDB ID: 6QAV).62 The structure was rebuilt in COOT and refined using REFMAC.63,64 Data collection and refinement statistics are summarized in Table S1.
In Silico Docking.
Fully continuously flexible small-molecule ligand docking to the ULK2 protein, represented by grid interaction potentials, was performed using the ICM docking algorithm [i] as implemented in the ICM-Pro program (v3.8, Molsoft, LLC.). The coordinates of the three-dimensional structure of ULK2 (PDB ID: 6YID) reported here were converted into an ICM object, charges were assigned, orientations of sidechain amides were corrected, and hydrogen atoms added and their positions optimized by energy minimization using the MMFF force field. The docking receptor site was defined as an 8 A radius around the SBI-0206965 ligand. An energy-minimized three-dimensional molecular model of the compound was generated and docking performed using the implemented routine in ICM.
ULK1/2 ADP-Glo Assay.
The kinase reaction was performed in 5 μL total volume containing 25 μM ATP (Sigma-Aldrich #A7699). For the ULK1 ADP-Glo assay, the reaction employed 2 μg/mL recombinant human ULK1 protein (1-649, SignalChem #U01-11G) and 80 μg/mL myelin basic protein (MBP, Sigma-Aldrich #M1891). The ULK2 assay included 4 μg/mL recombinant human ULK2 protein (1-478, SignalChem #U02-11G) and 80 μg/mL MBP. IC50 values were determined in 16-dose assay mode with threefold serial dilutions starting at 30 μM compound, performed in triplicate. Staurosporine, a nonselective protein kinase inhibitor, was used as a positive control.
ULK1/2 NanoBRET Assay.
Human embryonic kidney cells (HEK293T) were transfected with a NanoLuc-ULK1 Fusion Vector (Promega #NV2211) or a NanoLuc-ULK2 Fusion Vector (Promega #NV2221) using the jetPRIME transfection reagent (Polyplus Transfection #114-15) for 24 h. Cells were trypsinized and resuspended in Opti-MEM I (1X) Reduced Serum Medium (Gibco, #11058–021) and plated at approximately 7 × 103 cells/34 μL/well total volume in nonbinding surface 384 well plates (Corning #3574). Complete NanoBRET 20× Tracer K-5 reagent, prepared according to the manufacturer’s directions, was added at 2 μL/well, and the plate was mixed on an orbital shaker for 15 s at 700 rpm. Compounds were serially diluted at 10× the final concentration in an assay medium (Opti-MEM I, Reduced Serum Medium) and added to the cells at 4 μL/well, and the assay plate was mixed for 15 s at 700 rpm. The assay plate was incubated for 2 h in a 37 °C incubator with 5% CO2 and then equilibrated to rt for 15 min. 3X Complete Substrate plus Inhibitor Solution was prepared according to the manufacturer’s directions, with Extracellular NanoLuc Inhibitor (60 μM), and added to the cells at a final working concentration of 20 μM/well. The assay plate and incubated at rt for 2–3 min, and the signals at the donor (450 nm) and acceptor (610 nm) emission wavelengths were measured using a Spark multimode microplate reader (Tecan).
Thermal Shift Assay.
Recombinant kinase domains of ULK1 and ULK2 (2 μM) were mixed with each ULK1/2 inhibitor (10 μM), and the assay was performed as previously described.34
pBeclin1 Assay.
HEK293T cells were incubated in 6-well dishes at 6 × 105 cells/well in normal growth media (DMEM containing 10% fetal bovine serum [FBS] and 1% penicilin/streptomycin) for 24 h and then transfected with WT or KI Myc-tagged ULK1 plus WT Flag-tagged Beclin1 using a jetPRIME transfection reagent (Polyplus Transfection #114-15). At 24 h posttransfection, the cells were incubated in fresh media containing DMSO or ULK1/2 inhibitor (10 μM) for 1 h. The cells were lysed in RIPA buffer (Sigma #R0278) containing protease inhibitors (Roche #5892970001) and phosphatase inhibitors (Roche #4906837001). The lysates were immunoblotted with a Beclin1 (pSer15) antibody (Abbiotec #254515) or glycer-aldehyde 3-phosphate dehydrogenase (GAPDH; 14C10 Cell Signaling Technology [CST] #2118S) and then stripped with Restore Western Blot Stripping Buffer (Thermo Scientific #21059) and incubated with Beclin1 antibody (CST #3738S) to probe total Beclin1. Densitometry analysis was performed with Image Lab 5.2.1 (Bio-Rad).
pVPS34 Assay.
HEK293T cells (6 × 105) cells were incubated for 24 h as described for pBeclin1 and then transfected with WT or KI Myc-tagged ULK1 plus WT Flag-tagged Vps34 (WT Vps34) using a standard Lipofectamine 2000 transfection protocol (Invitrogen #11668500). At 24 h posttransfection, the cells were incubated in fresh media containing DMSO or ULK1/2 inhibitor (10 μM) for 1 h, and the cells were lysed with cell lysis buffer (CST #9803). The cell lysates were immunoblotted with anti-Myc tag (CST #2278), anti-FLAG tag polyclonal (Sigma #F7425), and anti-pVPS34 (CST #13857) antibodies. Bands were quantified as described for pBeclin1.
PK and PD Studies.
All animal care and handling was in accordance with the NIH guidelines for the Care and Use of Laboratory Animals, and the procedures were approved by the Sanford Burnham Prebys Medical Discovery Institute Institutional Animal Care and Use Committee. Female C57BL/6J mice (8 weeks of age) were purchased from the Jackson Laboratory, housed in pathogen-free conditions on a 12 h light/dark cycle, and given free access to food and water. Mice were orally administered compound 26 (30 mg/kg) formulated in vehicle (5% DMSO, 10% Tween-80, and 85% distilled and deionized H20). At the indicated times of postdosing, blood samples were collected retroorbitally and plasma was separated by centrifugation. Plasma samples were extracted with acetonitrile: water (4:1) with 0.1% formic acid-containing indomethacin as an internal standard. Samples were centrifuged, and supernatants were diluted with acetonitrile: water and analyzed by LC—MS/MS on a Shimadzu Nexera X2 HPLC coupled to an AB Sciex 6500 QTRAP. For the PD studies, mice were orally administered compound 26 (10 mg/kg) in a vehicle (5% DMSO, 10% Tween-80, and 85% distilled and deionized H2O) or vehicle alone. The mice were sacrificed 2 h post-dose by cervical dislocation, and liver samples flash frozen in liquid nitrogen then homogenized on ice in CST lysis buffer. The protein lysates were equilibrated for protein levels using a BCA protein assay kit (Pierce) and resolved on 8% gels. The lysates were immunoblotted with pATG13 S318 (Rockland #600-401-C49S), ATG13 (CST #13273), ULK1 (CST #8054), and β-actin (Sigma A5441) antibodies to evaluate on-target inhibition in the livers.
Lentiviral Preparation and Viral Infection of Cells.
Lentiviruses containing shRNA-encoding pLKO.1 vectors were prepared by the Sanford Burnham Prebys Viral Vector Core Facility following a previously described protocol.65 The ULK1 and BECN1 target sequences are listed in Table S3. For the viral infection, MDA-MB-468 cells were plated at 1 × 105/well of 6-well plates and incubated for 24 h. The viruses were mixed with 8 μg/mL Polybrene (Santa Cruz Biotechnology #sc-134220) and added to the cells. Three days later, infected cells were selected by the addition of 1 μg/mL puromycin (Gibco #A11138-03) for an additional 3 days, harvested by trypsinization, and then dispensed into 96-well plates for experiments.
Apoptosis Assay.
MDA-MB-468 cells were treated with compound 26 or DMSO for 18 h after which cells the were trypsinized and washed twice with cold PBS. The cells were then stained using a PE Annexin V Apoptosis Detection Kit I (BD Pharmingen #559763). Specifically, the cells were resuspended in 1 × binding buffer at a concentration of 1 × 106 cells/mL, and 100 μL of the solution was transferred to a5 mL culture tube. PE Annexin V (5 μL) and 7-AAD (5 μL) were added to the tube with cells. The mix was incubated for 15 min at room temperature in the dark after gentle vortexing. Next, 1× binding buffer (400 μL) was added to each tube, and flow cytometry to detect PE-Annexin V and 7-AAD was performed within 1 h using a LSRFortessa 14-color (BD Biosciences) to analyze apoptosis. Events of 10,000 were collected for each sample.
mCherry-GFP-LC3 FACS Assay.
MDA-MB-468 cells stably expressing the mCherry-GFP-LC3 fusion protein were plated in 6-well plates at a density of 6 × 105 cells/well in growth media DMEM/F12 + GlutaMAX-1 (Gibco) supplemented with 10% FBS (Gibco) and 1× Anti-Anti (Gibco) and allowed to grow overnight. Cells were treated, trypsinized, and then resuspended in PBS, and analytical cytometry was performed in the Sanford Burnham Prebys flow cytometry core using an LSRFortessa 14-color (BD Biosciences) with a 488 nm and 610 nM laser. For flow cytometry, WT MDA-MB-468 cells were used as the unstained control, and MDA-MB-468 cells expressing either mCherry or GFP were used for compensation. Events of 10,000 were collected for each sample.
TEM Imaging of Autophagy Vacuoles.
Cells were seeded on 10 cm acrylic culture dishes (Corning) until 70% confluence, fixed in a cold solution containing4% formaldehyde (EMS), 2.5% glutaraldehyde (EMS), 100 mM cacocylate buffer (Sigma) for 1 min using a microwave oven (at 150 watts, Ted Pella), and additional 40 min at 4 °C. The samples were washed in the same buffer (3 × 15 min), post-fixed with 1% osmium tetroxide (EMS) and 1.2% potassium ferrocyanide in cacodylate buffer (100 mM) for 40 min, washed with buffer (3 × 15 min), stained with 1% uranyl acetate (EMS) for 40 min, and washed with Milliq water (3 × 15 min). The cells were scraped off the plates, transferred to 1.5 mL eppendorfs, and pelleted for 15 min at 15,000 rpm (Eppendorf Centrifuge model 5415R).The cells were dehydrated in an acetone series until 100% (3×) and embedded in an Epon resin. Ultrathin sections of 50 nm were obtained with a diamond knife (Diatome), collected on 300 mesh copper grids, and post-stained with UranyLess (EMS) for 5 min. Sections were observed in a Zeiss Libra 120 operated at 80 kV, and high-resolution images (4K × 4K) obtained with a CCD camera Gatan US4000.
Statistics.
The 50% inhibitory concentrations (IC50 values) were determined by analyzing the log of the concentration-response curves by nonlinear regression analysis using Prism 8 (GraphPad).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Andrew Thorburn (U.C. Denver) for providing the MDA-MB-468 cells expressing mCherry-GFP-LC3, Dr. Do-Hyung Kim (University of Minnesota) for sharing the ULK1 shRNA construct, the Viral Vector Core Facility at Sanford Burnham Prebys Medical Discovery Institute (SBP) for generating lentiviruses, the Flow Cytometry Core at SBP for help generating the data in Figure 5B, and the Waitt Advanced Biophotonics Core at the Salk Institute for Biological Studies for taking the TEM images. The authors thank the staff at Swiss Light Source for their assistance with data collection, which was also supported by the funding from the European Union’s Horizon 2020 research and innovation program under grant agreement number 730872, project CALIPSOplus.
Funding
This work was supported by the Epstein Family Foundation, a Sanford Burnham Prebys National Cancer Institute Cancer Center Support Grant P30 CA030199, a Larry L. Hillblom Foundation Grant 2019-A-005-NET, and a 2019 Pancreatic Cancer Action Network Translational Research Grant 19-65-COSF to NDPC. NAB was supported by the National Cancer Institute of the National Institutes of Health under award number T32CA211036. S.K. and A.C. are grateful for support from the SGC, a registered charity (no. 1097737) that receives funds from AbbVie, Bayer Pharma AG, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genome Canada through Ontario Genomics Institute [OGI-196], EU/EFPIA/OICR/McGill/KTH/Diamond, Innovative Medicines Initiative 2 Joint Undertaking (EUbOPEN grant 875510), Janssen, Merck KGaA (a.k.a. EMD in Canada and US), Merck & Co (aka MSD outside Canada and US), Pfizer, São Paulo Research Foundation-FAPESP, Takeda, and Wellcome. S.K. and A.C. are also grateful for funding by the DKTK and FCI cancer network as well as the DFG funded SFB1177 (selective autophagy). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS USED
- ULK
Unc-51-like autophagy activating kinase
- TNBC
triple-negative breast cancer
- PARP
poly (ADP-ribose) polymerase
- SAR
structure—activity relationship
- PTS
protein thermal shift
- GFP
green fluorescent protein
- LC3
MAP1LC3B
- TEM
transmission electron microscope
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c00873.
Overview of the autophagy pathway, structures of ULK1/2 inhibitors: SBI-0206965 and Shokat compound, data collection and refinement statistics for the ULK2 structure, representative dose—response curves of compounds in the ADP-Glo assay, representative dose—response curves of compounds in the NanoBRET assay, protein thermal shift data of selected compounds, shRNA target sequences for knockdown studies, Western blot analysis of shRNA-mediated ULK1 and BECN1 knockdown in three TNBC cell lines, data showing the downregulation of starvation-induced autophagic flux in MDA-MB-468 cells by ULK inhibitors, data showing that PARP inhibitors kill MDA-MB-468 cells with low potency, data showing that SBP-7455 and olaparib synergize to inhibit the viability of MDA-MB-468 cells, data showing that SBP-7455 and niraparib synergize to inhibit the viability of MDA-MB-468 cells, data showing that induction of autophagic flux in MDA-MB-468 cells by olaparib is inhibited by concomitant treatment with SBP-7455, kinetic solubility of selected compounds, bidirectional permeability of selected compounds in Caco-2 cells, NMR and LC—MS spectra of selected compounds, and molecular formula strings (PDF).
Accession Codes
X-ray crystal structure of SBI-0206965 in complex with ULK2, PDB ID: 6YID. Authors will release the atomic coordinates and experimental data upon article publication.
The authors declare no competing financial interest.
Contributor Information
Huiyu Ren, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States.
Nicole A. Bakas, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States
Mitchell Vamos, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States.
Apirat Chaikuad, Structural Genomics Consortium, Buchmann Institute for Molecular Life Sciences and Institute of Pharmaceutical Chemistry, Goethe-University Frankfurt, Frankfurt 60438, Germany.
Allison S. Limpert, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States
Carina D. Wimer, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States
Sonja N. Brun, Molecular and Cell Biology Laboratory, The Salk Institute for Biological Studies, La Jolla, California 92037, United States
Lester J. Lambert, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States
Lutz Tautz, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States.
Maria Celeridad, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States.
Douglas J. Sheffler, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States
Stefan Knapp, Structural Genomics Consortium, Buchmann Institute for Molecular Life Sciences and Institute of Pharmaceutical Chemistry, Goethe-University Frankfurt, Frankfurt 60438, Germany.
Reuben J. Shaw, Molecular and Cell Biology Laboratory, The Salk Institute for Biological Studies, La Jolla, California 92037, United States
Nicholas D. P. Cosford, Cancer Molecules & Structures Program, NCI-Designated Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California 92037, United States.
REFERENCES
- (1).Bento CF; Renna M; Ghislat G; Puri C; Ashkenazi A; Vicinanza M; Menzies FM; Rubinsztein DC Mammalian autophagy: how does it work? Annu. Rev. Biochem 2016, 85, 685–713. [DOI] [PubMed] [Google Scholar]
- (2).Kimmelman AC The dynamic nature of autophagy in cancer. Genes Dev. 2011, 25, 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Yang Z; Klionsky DJ Eaten alive: a history of macroautophagy. Nat. Cell Biol 2010, 12, 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Amaravadi RK; Yu D; Lum JJ; Bui T; Christophorou MA; Evan GI; Thomas-Tikhonenko A; Thompson CB Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Invest 2007, 117, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hosokawa N; Sasaki T; Iemura S.-i.; Natsume T; Hara T; Mizushima N Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy 2009, 5, 973–979. [DOI] [PubMed] [Google Scholar]
- (6).Ganley IG; Lam DH; Wang J; Ding X; Chen S; Jiang X ULK1· ATG13 FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem 2009, 284, 12297–12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Laplante M; Sabatini DM mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Galluzzi L; Pietrocola F; Levine B; Kroemer G Metabolic control of autophagy. Cell 2014, 159, 1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Papinski D; Schuschnig M; Reiter W; Wilhelm L; Barnes CA; Maiolica A; Hansmann I; Pfaffenwimmer T; Kijanska M; Stoffel I; Lee SS; Brezovich A; Lou JH; Turk BE; Aebersold R; Ammerer G; Peter M; Kraft C Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Mol. Cell 2014, 53, 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Papinski D; Kraft C Regulation of autophagy by signaling through the Atg1/ULK1 complex. J. Mol. Biol 2016, 428, 1725–1741. [DOI] [PubMed] [Google Scholar]
- (11).Egan DF; Chun MGH; Vamos M; Zou H; Rong J; Miller CJ; Lou HJ; Raveendra-Panickar D; Yang C-C; Sheffler DJ; Teriete P; Asara JM; Turk BE; Cosford NDP; Shaw RJ Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol. Cell 2015, 59, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Alsaadi RM; Losier TT; Tian W; Jackson A; Guo Z; Rubinsztein DC; Russell RC ULK1-mediated phosphorylation of ATG16L1 promotes xenophagy, but destabilizes the ATG16L1 Crohn’s mutant. EMBO Rep. 2019, 20, No. e46885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Saha S; Panigrahi DP; Patil S; Bhutia SK Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother 2018, 104,485–495. [DOI] [PubMed] [Google Scholar]
- (14).Galluzzi L; Bravo-San Pedro JM; Levine B; Green DR; Kroemer G Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Discovery 2017, 16, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Rao S; Tortola L; Perlot T; Wirnsberger G; Novatchkova M; Nitsch R; Sykacek P; Frank L; Schramek D; Komnenovic V; Sigl V; Aumayr K; Schmauss G; Fellner N; Handschuh S; Glösmann M; Pasierbek P; Schlederer M; Resch GP; Ma Y; Yang H; Popper H; Kenner L; Kroemer G; Penninger JM A dual role for autophagy in a murine model of lung cancer. Nat. Commun 2014, 5, 3056. [DOI] [PubMed] [Google Scholar]
- (16).Rosenfeldt MT; O’Prey J; Morton JP; Nixon C; MacKay G; Mrowinska A; Au A; Rai TS; Zheng L; Ridgway R; Adams PD; Anderson KI; Gottlieb E; Sansom OW; Ryan KM p53 status determines the role of autophagy in pancreatic tumour development. Nature 2013, 504, 296. [DOI] [PubMed] [Google Scholar]
- (17).White E The role for autophagy in cancer. J. Clin. Invest. 2015, 125, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Guo JY; Xia B; White E Autophagy-mediated tumor promotion. Cell 2013, 155, 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).O’Donovan TR; O’Sullivan GC; McKenna SL Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy 2011, 7, 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chen S; Rehman SK; Zhang W; Wen A; Yao L; Zhang J Autophagy is a therapeutic target in anticancer drug resistance. Biochim. Biophys. Acta, (BBA)-Rev. Cancer 2010, 1806, 220–229. [DOI] [PubMed] [Google Scholar]
- (21).Sui X; Chen R; Wang Z; Huang Z; Kong N; Zhang M; Han W; Lou F; Yang J; Zhang Q; Wang X; He C; Pan H Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell death dis. 2013, 4, No. e838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Holohan C; Van Schaeybroeck S; Longley DB; Johnston PG Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 2013, 13,714. [DOI] [PubMed] [Google Scholar]
- (23).Limpert AS; Lambert LJ; Bakas NA; Bata N; Brun SN; Shaw RJ; Cosford NDP Autophagy in cancer: regulation by small molecules. Trends Pharmacol. Sci. 2018, 39, 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Maycotte P; Gearheart CM; Barnard R; Aryal S; Levy JMM; Fosmire SP; Hansen RJ; Morgan MJ; Porter CC; Gustafson DL; Thorburn A STAT3-mediated autophagy dependence identifies subtypes of breast cancer where autophagy inhibition can be efficacious. Cancer Res. 2014, 74, 2579–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yao H; He G; Yan S; Chen C; Song L; Rosol TJ; Deng X Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget 2017, 8, 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Prat A; Perou CM Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011, 5, 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).O’Reilly EA; Gubbins L; Sharma S; Tully R; Guang MHZ; Weiner-Gorzel K; McCaffrey J; Harrison M; Furlong F; Kell M; McCann A The fate of chemoresistance in triple negative breast cancer (TNBC). BBA clin. 2015, 3, 257–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Chittaranjan S; Bortnik S; Dragowska WH; Xu J; Abeysundara N; Leung A; Go NE; DeVorkin L; Weppler SA; Gelmon K; Yapp DT; Bally MB; Gorski SM Autophagy inhibition augments the anticancer effects of epirubicin treatment in anthracycline-sensitive and-resistant triple-negative breast cancer. Clin. Cancer Res. 2014, 20, 3159–3173. [DOI] [PubMed] [Google Scholar]
- (29).Vogel RI; Coughlin K; Scotti A; Iizuka Y; Anchoori R; Roden RBS; Marastoni M; Bazzaro M Simultaneous inhibition of deubiquitinating enzymes (DUBs) and autophagy synergistically kills breast cancer cells. Oncotarget 2015, 6, 4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Amaravadi RK; Kimmelman AC; Debnath J Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer discovery 2019, 9, 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hwang DY; Eom J-I; Jang JE; Jeung H-K; Chung H; Kim JS; Cheong J-W; Min YH ULK1 inhibition as a targeted therapeutic strategy for FLT3-ITD-mutated acute myeloid leukemia. J. Exp. Clin. Cancer Res. 2020, 39, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Qiu L; Zhou G; Cao S Targeted inhibition of ULK1 enhances daunorubicin sensitivity in acute myeloid leukemia. Life Sci. 2020, 243, 117234. [DOI] [PubMed] [Google Scholar]
- (33).Tang F; Hu P; Yang Z; Xue C; Gong J; Sun S; Shi L; Zhang S; Li Z; Yang C; Zhang C; Xie C SBI0206965, a novel inhibitor of Ulk1, suppresses non-small cell lung cancer cell growth by modulating both autophagy and apoptosis pathways. Oncol. Rep. 2017, 37, 3449–3458. [DOI] [PubMed] [Google Scholar]
- (34).Chaikuad A; Koschade SE; Stolz A; Zivkovic K; Pohl C; Shaid S; Ren H; Lambert LJ; Cosford NDP; Brandts CH; Knapp S Conservation of structure, function and inhibitor binding in UNC-51-like kinase 1 and 2 (ULK1/2). Biochem. J. 2019, 476, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Roskoski R Jr. Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacol. Res. 2016, 103, 26–48. [DOI] [PubMed] [Google Scholar]
- (36).de Oliveira PSL; Ferraz FAN; Pena DA; Pramio DT; Morais FA; Schechtman D Revisiting protein kinase—substrate interactions: Toward therapeutic development. Sci. Signaling 2016, 9, re3–re3. [DOI] [PubMed] [Google Scholar]
- (37).Dar AC; Shokat KM The evolution of protein kinase inhibitors from antagonists to agonists of cellular signaling. Annu. Rev. Biochem. 2011, 80, 769–795. [DOI] [PubMed] [Google Scholar]
- (38).Lazarus MB; Novotny CJ; Shokat KM Structure of the human autophagy initiating kinase ULK1 in complex with potent inhibitors. ACS Chem. Biol. 2015, 10, 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Abagyan R; Totrov M; Kuznetsov D ICM—a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J. Comput. Chem. 1994, 15, 488–506. [Google Scholar]
- (40).Zegzouti H; Zdanovskaia M; Hsiao K; Goueli SA ADP-Glo: A Bioluminescent and homogeneous ADP monitoring assay for kinases. Assay Drug Dev. Technol. 2009, 7, 560–572. [DOI] [PubMed] [Google Scholar]
- (41).Robers MB; Dart ML; Woodroofe CC; Zimprich CA; Kirkland TA; Machleidt T; Kupcho KR; Levin S; Hartnett JR; Zimmerman K; Zimmerman K; Niles AL; Ohana RF; Daniels DL; Slater M; Wood MG; Cong M; Cheng Y-Q; Wood KV Target engagement and drug residence time can be observed in living cells with BRET. Nat. Commun. 2015, 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Vasta JD; Corona CR; Wilkinson J; Zimprich CA; Hartnett JR; Ingold MR; Zimmerman K; Machleidt T; Kirkland TA; Huwiler KG; Ohana RF; Slater M; Otto P; Cong M; Wells CI; Berger B-T; Hanke T; Ding CGK; Drewry DH; Huber KVM; Willson TM; Knapp S; Müller S; Meisenheimer PL; Fan F; Wood KV; Robers MB Quantitative, wide-spectrum kinase profiling in live cells for assessing the effect of cellular ATP on target engagement. Cell Chem. Biol. 2018, 25, 206–214.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Robers MB; Friedman-Ohana R; Huber KVM; Kilpatrick L; Vasta JD; Berger B-T; Chaudhry C; Hill S; Müller S; Knapp S; Wood KV Quantifying target occupancy of small molecules within living cells. Annu. Rev. Biochem. 2020, 89, 557–581. [DOI] [PubMed] [Google Scholar]
- (44).Niesen FH; Berglund H; Vedadi M The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc 2007, 2, 2212. [DOI] [PubMed] [Google Scholar]
- (45).Russell RC; Tian Y; Yuan H; Park HW; Chang Y-Y; Kim J; Kim H; Neufeld TP; Dillin A; Guan K-L ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol 2013, 15, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Lefort S; Joffre C; Kieffer Y; Givel A-M; Bourachot B; Zago G; Bieche I; Dubois T; Meseure D; Vincent-Salomon A; Camonis J; Mechta-Grigoriou F Inhibition of autophagy as a new means of improving chemotherapy efficiency in high-LC3B triple-negative breast cancers. Autophagy 2014, 10, 2122–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Liang DH; Choi DS; Ensor JE; Kaipparettu BA; Bass BL; Chang JC The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett. 2016, 376, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Gump JM; Thorburn A Sorting cells for basal and induced autophagic flux by quantitative ratiometric flow cytometry. Autophagy 2014, 10, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Bai P Biology of poly (ADP-ribose) polymerases: the factotums of cell maintenance. Mol. Cell 2015, 58, 947–958. [DOI] [PubMed] [Google Scholar]
- (50).Krishnakumar R; Kraus WL The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol. Cell 2010, 39, 8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Rouleau M; Patel A; Hendzel MJ; Kaufmann SH; Poirier GG PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer 2010, 10, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Dréan A; Lord CJ; Ashworth A PARP inhibitor combination therapy. Crit. Rev. Oncol. Hematol 2016, 108, 73–85. [DOI] [PubMed] [Google Scholar]
- (53).Zai W; Chen W; Han Y; Wu Z; Fan J; Zhang X; Luan J; Tang S; Jin X; Fu X; Gao H; Ju D; Liu H Targeting PARP and autophagy evoked synergistic lethality in hepatocellular carcinoma. Carcinogenesis 2019, 41, 345–357. [DOI] [PubMed] [Google Scholar]
- (54).Liu Y; Song H; Song H; Feng X; Zhou C; Huo Z Targeting autophagy potentiates the anti-tumor effect of PARP inhibitor in pediatric chronic myeloid leukemia. AMB Express 2019, 9, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Di Veroli GY; Fornari C; Wang D; Mollard S; Bramhall JL; Richards FM; Jodrell DI Combenefit: an interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Papadimitriou M; Mountzios G; Papadimitriou CA The role of PARP inhibition in triple-negative breast cancer: unraveling the wide spectrum of synthetic lethality. Cancer Treat. Rev 2018, 67, 34–44. [DOI] [PubMed] [Google Scholar]
- (57).Kinsey CG; Camolotto SA; Boespflug AM; Guillen KP; Foth M; Truong A; Schuman SS; Shea JE; Seipp MT; Yap JT; Burrell LD; Lum DH; Whisenant JR; Gilcrease GW III; Cavalieri CC; Rehbein KM; Cutler SL; Affolter KE; Welm AL; Welm BE; Scaife CL; Snyder EL; McMahon M; Tomar G; Papke B; Hobbs GA; Yan L; Hayes TK; Diehl JN; Goode GD; Chaika NV; Wang Y; Zhang G-F; Witkiewicz AK; Knudsen ES; Petricoin EF III; Singh PK; Macdonald JM; Tran NL; Lyssiotis CA; Ying H; Kimmelman AC; Cox AD; Der CJ Protective autophagy elicited by RAF→ MEK→ ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med 2019, 25, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Bryant KL; Stalnecker CA; Zeitouni D; Klomp JE; Peng S; Tikunov AP; Gunda V; Pierobon M; Waters AM; George SD; Tomar G; Papke B; Hobbs GA; Yan L; Hayes TK; Diehl JN; Goode GD; Chaika NV; Wang Y; Zhang G-F; Witkiewicz AK; Knudsen ES; Petricoin EF III; Singh PK; Macdonald JM; Tran NL; Lyssiotis CA; Ying H; Kimmelman AC; Cox AD; De CJ Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med 2019, 25, 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Rebecca VW; Amaravadi RK Emerging strategies to effectively target autophagy in cancer. Oncogene 2016, 35, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Kabsch W. Xds. Acta Crystallogr., Sect. D: Biol. Crystallogr 2010, 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Evans PR; Murshudov GN How good are my data and what is the resolution? Acta Crystallogr., Sect. D: Biol. Crystallogr 2013, 69, 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).McCoy AJ Acknowledging errors: advanced molecular replacement with Phaser. In Protein Crystallography; Springer: Humana Press: NewYork, NY, 2017; pp. 421–453. [DOI] [PubMed] [Google Scholar]
- (63).Emsley P Tools for ligand validation in Coot. Acta Crystallogr., Sect. D: Struct. Biol. 2017, 73, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Skubák P; Murshudov GN; Pannu NS Direct incorporation of experimental phase information in model refinement. Acta Crystallogr. Sect. D: Biol. Crystallogr. 2004, 60, 2196–2201. [DOI] [PubMed] [Google Scholar]
- (65).Cunningham TJ; Michael SY; McKeithan WL; Spiering S; Carrette F; Huang C-T; Bushway PJ; Tierney M; Albini S; Giacca M; Mano M; Puri PL; Sacco A; Ruiz-Lozano P; Riou J-F; Umbhauer M; Duester G; Mercola M; Colas AR Id genes are essential for early heart formation. Genes Dev. 2017, 31, 1325–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.