Abstract
The effect of long-term ageing (up to 700 days) on the mobility, potential bioavailability and bioaccessibility of antimony (Sb) was investigated in two soils (S1: pH 8.2; S2: pH 4.9) spiked with two Sb concentrations (100 and 1000 mg·kg−1). The Sb mobility decreased with ageing as highlighted by sequential extraction, while its residual fraction significantly increased. The concentration of Sb (CDGT), as determined by diffusive gradients in thin films (DGT), showed a reduction in potential contaminant bioavailability during ageing. The DGT analysis also showed that Sb-CDGT after 700 days ageing was significantly higher in S1–1000 compared to S2–1000, suggesting soil pH plays a key role in Sb potential bioavailability. In-vitro tests also revealed that Sb bioaccessibility (and Hazard Quotient) decreased over time. Linear combination fitting of Sb K-edge XANES derivative spectra showed, as a general trend, an increase in Sb(V) sorption to inorganic oxides with ageing as well as Sb(V) bound to organic matter (e.g. up to 27 and 37% respectively for S2–100). The results indicated that ageing can alleviate Sb ecotoxicity in soil and that the effectiveness of such processes can be increased at acidic pH. However, substantial risks due to Sb mobility, potential bioavailability and bioaccessibility remained in contaminated soils even after 700 days ageing.
Keywords: Ageing, Antimony, Potential bioavailability, Bioaccessibility, X-ray absorption near-edge structure (XANES), spectroscopy
Graphical Abstract

1. Introduction
Antimony (Sb) is a potentially toxic element (PTE), considered as a priority pollutant by the United States Environmental Protection Agency and the European Union (CEC, 1998; U.S. EPA, 2009). Antimony concentration in the environment is related to both natural phenomena such as weathering, biological activity, or volcanic activity and anthropogenic inputs (He et al., 2019). In recent years, Sb concentrations in the environment have considerably increased as a result of mining and smelting operations, waste incinerators, coal and petroleum combustion, spent ammunition, polyethylene terephthalate industries, battery factories, and use of pharmaceuticals and pesticides (e.g. Okkenhaug et al., 2016). In this context, knowledge of Sb mobility, potential bioavailability and bioaccessibility in soil is of primary importance for the assessment of ecological risk and potential human health impacts (Diquattro et al., 2020; Fu et al., 2016; Hammel et al., 2000).
Antimony mobility, bioavailability and bioaccessibility in soil depend on its speciation as well as on the pH, redox conditions, amount and type of colloids present and microbial populations (Luo et al., 2014; Wilson et al., 2010). Antimony commonly occurs in the environment as trivalent Sb(III) or pentavalent Sb(V) inorganic species, with antimonite [Sb(OH)3] and antimonate [Sb(OH)6~] the most common Sb(III) and Sb(V) compounds, respectively (Kang et al., 2000). Antimonate is the predominant form present under aerobic and mildly reducing conditions (Filella et al., 2002; Filella et al., 2009; Telford et al., 2008) and its mobility in soil, in the 5.0–8.5 pH range, is generally greater than that of antimonite due to the negative charge of the former species [pKa HSb(OH)6 = 2.55; pKa Sb(OH)3 = 11.8] (Herath et al., 2017; Johnston et al., 2020).
Binding to soil colloid surfaces (e.g., organic matter, Fe, Al, and Mn oxy-hydroxides) by specific and nonspecific sorption reactions, as well as structural incorporation into Fe(III)-oxyhydroxide minerals, can have substantial influence on Sb mobility and bioavailability, eventually leading to contaminant attenuation (Wilson et al., 2010). For instance, it was shown that different humic substance functional groups (e.g., -SH, Ar-OH and -COOH groups) can interact with Sb(III) (Tella and Pokrovski, 2009; Besold et al., 2019). In regards to Sb(V), specific adsorption by humic acids under acid conditions can occur via the establishment of pentagonal or hexagonal rings involving Sb-O-C linkages (Sh et al., 2012; Steely et al., 2007; Tella and Pokrovski, 2012). Moreover, cationic metals within humic acids [e.g, Fe(III), Mg(II), and Ca(II)], can act as bridging elements between the negatively charged humic molecules and the antimonate complex (Diquattro et al., 2018). Finally, stable interactions (e.g., inner sphere complexes) between Sb(V) and Fe and Al oxy-hydroxides have previously been reported (Bagherifam et al., 2014; Essington and Stewart, 2016, 2018), as well as structural Sb(V) incorporation into Fe(III)-oxide (e.g. goethite, ferrihydrite) via heterovalent Sb(V)/Fe(III) substitution (e.g. Karimian et al., 2019; Burton et al., 2020; Hockmann et al., 2020). Overall, the stability of complexes between Sb(V) and soil colloids decreases as the pH raises, due to the increase of electrostatic repulsion phenomena occurring between the Sb(OH)6− oxyanion and the negatively charged surface groups of Fe and Al oxy-hydroxides and organic matter (Xi et al., 2016). Moreover, the strength of Sb binding to soil colloid surfaces is fundamental in regulating contaminant bioaccessibility which is an important parameter for the prediction of exposure and health risks from incidental ingestion of Sb contaminated soils (Ruby et al., 1996). In particular, bioaccessibility generally refers to the fraction of a contaminant that is soluble in the digestive phase and as such potentially available for intestinal absorption (Mele et al., 2015). In-vitro tests, consisting in soil treatment with simulated gastrointestinal tract fluids (at physiological temperature and pH), can be precious in the prediction of such contaminant fraction (i.e. bioaccessible Sb), and therefore in estimating possible health risk due to incidental ingestion of contaminated soils (e.g. Kastury et al., 2019).
Given that the stability of interactions between Sb and soil colloids, and therefore contaminant mobility, bioavailability and bioaccessibility, can change over time, this should be considered in order to gain a greater understanding of ecotoxicological effects of antimony in soil systems and (more in general) in the environment (Violante et al., 2010; Lin et al., 2020a, 2020b). The reduction of PTEs mobility, potential bioavailability and bioaccessibility through specific immobilization mechanisms, which occur as a function of their contact time with soil colloids, is called "ageing” (e.g. Peng et al., 2019; Tang et al., 2006). Several PTEs-fixation reactions such as complexation, surface adsorption, precipitation or diffusion and occlusion into meso- and micropores, can occur in soil with time (Jalali and Khanlari, 2008). For instance, lead (Pb), zinc (Zn), cadmium (Cd) and copper (Cu) tend to become non-exchangeable with an increase in residence time, due to the transformation of weak interactions (e.g. outer sphere complexes) into more stable ones (e.g. inner sphere complexes) (Jalali and Khanlari, 2008; Tang et al., 2006). Similarly, a decrease in water-soluble and exchangeable arsenic (As) and selenium (Se), and an increase in PTEs bound to amorphous Fe/Al oxy-hydroxides, or in residual fractions, have been observed during ageing (e.g. Peng et al., 2019; Tang et al., 2007).
Despite numerous studies over the last decade investigating the effects of ageing on the mobility and bioavailability of many PTEs (e.g. Diagboya et al., 2015; Martinez and McBride, 2001; Yang et al., 2003), limited knowledge is available for Sb. For instance, Sanderson et al. (2014) studied the bioavailability of Pb, Sb, Zn, nickel (Ni), Cu and As in soil after one year ageing, but the study mainly focused on selected ecotoxicological endpoints, i.e. earthworms, plants and microbial activity. Likewise, Zhang et al. (2020) explored Sb toxicity on barley root elongation after 3-months ageing, while Egodawatta et al. (2018) reported higher Sb bioavailability for water spinach when exposed to recently contaminated soil (14 days) compared to historically contaminated (34 years).
In this study we therefore sought to clarify the influence of ageing on the fate and behaviour of Sb in soil by using a combination of well-established and innovative approaches. More specifically, the objective of this work was to investigate the effect of ageing (from 1 day up to 700 days) on the mobility, potential bioavailability, bioaccessibility and speciation of Sb added [as Sb(V)] at two different concentrations (100 and 1000 mg·kg−1) on two distinct soils. In particular, the fate of Sb in soil was studied by sequential extraction to identify labile and relatively immobile Sb pools (Diquattro et al., 2020; Garau et al., 2017; Wenzel et al., 2001). Antimony potential bioavailability was addressed with diffusive gradients in thin-films (DGT) probes (Luo et al., 2010), while Sb bioaccessibility was studied by using simulated gastric and intestinal solutions in in-vitro tests (Ruby et al., 1996). Finally, synchrotron based X-ray absorption near-edge structure (XANES) spectroscopy was used to define Sb speciation in soil (Maher et al., 2018; Wang et al., 2017). The experimental design and the combined use of the above-mentioned techniques were expected to provide a deeper understanding of environmental risk posed by Sb and of possible attenuation mechanisms governed by time.
2. Materials and methods
2.1. Soil physical-chemical analysis and microcosm set up
Different topsoil samples (0–20 cm depth) were collected randomly from two uncultivated fields located in north-western Sardinia (SS), soil 1 (S1): 40°43'32.77''N 8°24'48.6"E; soil 2 (S2): 40°56'15.7”N 8°53'30.4"E. These soils were selected because they were not previously used for agricultural or anthropogenic activities, they were distant from possible contamination sources (i.e. to reduce the possibility of external Sb input), and they exhibited different physico-chemical properties (Table 1). Soil samples were pooled in the laboratory according to their origin (i.e., S1 and S2), sieved to <2 mm and analysed as previously reported (Diquattro et al., 2020). Antimony in S1 and S2 was quantified by Inductively Coupled Plasma Mass Spectrometry (ICP-QQQ-MS) (Agilent 8800) after digestion of soil with aqua regia reverse solution (HNO3/HCl 3:1 ratio) and microwave mineralisation (MARS-6, CEM) using U.S. EPA method 3051A. A standard reference material (NIST-SRM 2711A) was included for quality assurance and quality control. In both soils, Sb was not detected (<0.01 μg·kg−1). Further details on the chemical features of both soils can be found in Diquattro et al. (2020).
Table 1.
Selected physico-chemical characteristics of S1 and S2 soils.
| Physico-chemical parameters | S1 soil | S2 soil |
|---|---|---|
| pH(H2O) | 8.2 ± 0.1 | 4.9 ± 0.2 |
| DOC (mg g−1) | 0.17 ± 0.00 | 0.39 ± 0.02 |
| SOM (%) | 1.75 ± 0.1 | 2.19 ± 0.2 |
| CEC (cmol(+) kg−1) | 20 ± 0.8 | 13 ± 2.5 |
| pHPZC | 5.7 | 2.6 |
| Texture (USDA) | Sandy clay loam | Loamy coarse sand |
| Total metal(loid)s (mg kg−1) | ||
| Fe | 16,350 ± 1061 | 5650 ± 71 |
| Ca | 62,500 ± 2828 | 2426 ± 145 |
| Al | 19,930 ± 1018 | 3924 ± 749 |
| Sb | n.d.a | n.d. |
n.d., not detected (< 0.01 μg·kg−1).
Thirty microcosms (each consisting of 300 g soil) were prepared for each soil type (S1 and S2) in plastic pots (10 cm diameter x 10 cm height). All soils were brought to 60% of their water holding capacity (WHC) with deionised water (Jury et al., 1991). For each soil type, 15 microcosms were spiked with 100 mg·kg−1 Sb(V) (S-100), and another 15 with 1000 mg·kg−1 Sb(V) (S-1000). Sb(V) derived from K[Sb(OH)6] (CAS 12208–13-8; Sigma Aldrich, Saint-Louis, USA) was added as a water solution (K[Sb(OH)6] solubility in water is 20 g L-1). Antimony concentrations added were selected to mimic a medium-low and a high contamination level based on previous studies (Courtin-Nomade et al., 2012; Diquattro et al., 2020; Garau et al., 2017). Before addition to soil, each Sb(V) solution was adjusted to the same pH of the soil using 0.1 M NaOH or HCl solutions. Microcosms were carefully mixed and then left to age at constant temperature (25 °C) and humidity (60% WHC; by weight adjustment) for different times: 1, 7, 31, 91 and 700 days (d). The selected time-points were chosen to evaluate both the short and medium to long-term influence of ageing on Sb mobility, bioavailability, bioaccessibility and speciation. At each time-point, triplicate S1 and S2 microcosms (i.e., S1–100, S1–1000, S2–100 and S2–1000) were analysed as follows. Total concentration of Sb was quantified in spiked microcosms (< 2 mm and < 250 μm particle size fractions) at different time-points (Table 2), by ICP-QQQ-MS as previously described. A standard reference material (NIST-SRM 2711A) was included for quality assurance and quality control.
Table 2.
Concentration of total Sb in <250 μm particle size fractions.
| Time | Sb concentration (mg kg−1) | |
|---|---|---|
| S1–100 | S1–1000 | |
| 1 d | 121.1 ± 2.51 | 1167 ± 19.3 |
| 7d | 159.2 ± 6.86 | 1384 ± 9.64 |
| 31 d | 119.0 ± 5.99 | 1326 ± 24.0 |
| 91 d | 156.3 ± 1.65 | 1321 ± 22.3 |
| 700 d | 139.0 ± 4.25 | 1403 ± 71.9 |
| S2–100 | S2–1000 | |
| 1 d | 234.2 ± 9.13 | 2500 ± 47.2 |
| 7 d | 237.9 ± 7.94 | 2954 ± 438 |
| 31 d | 254.2 ± 1.21 | 2734 ± 250 |
| 91 d | 297.0 ± 30.2 | 2950 ± 268 |
| 700 d | 252.1 ± 5.80 | 2956 ± 287 |
2.2. Sequential extraction of soil Sb
At each time-point, Sb mobility in the different soils was evaluated through the sequential extraction procedure (SEP) proposed by Wenzel et al. (2001) with minor modifications. This method was originally developed for arsenic but has also been widely used for antimony (e.g. Diquattro et al., 2018; Garau et al., 2017; Ngo et al., 2020) because of the similar chemical characteristics of these PTEs (Wilson et al., 2010). Different soil samples (n = 10; 1 g each) were randomly collected from each microcosm and pooled together. Then a representative soil sample (1 g) was collected for Sb sequential extraction. The procedure was repeated 3 times for each microcosm. Triplicate soil samples (1 g) collected from each microcosm at different ageing times (i.e., 1, 7, 31, 91, 700 d) were treated with 25 mL of deionised water and shaken for 2 h at 25 °C to extract water-soluble Sb (Fraction 1, F1). Then, the same soil samples were treated with 25 mL of a 0.05 M (NH4)2SO4 solution and shaken for 4 h at 25 °C to extract non-specifically sorbed Sb (Fraction 2, F2). Finally, the same soil samples were treated with 25 mL of a 0.05 M NH4H2PO4 solution and shaken for 16 h at 25 °C h to extract specifically sorbed Sb (Fraction 3, F3). Then, soil samples were digested with reverse aqua regia (HNO3/HCl 3:1 ratio) and microwave mineralisation (MARS-6, CEM) using U.S. EPA method 3051A, to quantify residual Sb (Fraction 4, F4). After each extraction step, soil suspensions were centrifuged at 1800g for 10 min. Supernatants were then filtered through a 0.45 μm filter and Sb concentrations determined in the liquid phase by ICP-QQQ-MS as previously described. A standard reference material (NIST-SRM 2711A) was included for quality assurance and quality control.
2.3. DGT measurements
At each time-point, Sb potential bioavailability was assessed in different soils through the DGT® technique. This is based on a device (deployed on the soil surface) that accumulates labile soil Sb on a binding gel after its diffusion through a hydrogel, which acts as a diffusion layer (Li et al., 2018). In this study, a titanium oxide (Metsorb) binding gel (DGT Research Ltd., Lancaster, UK) was employed. The exposure area of the binding gel was 3.14 cm2 and its thickness was 0.4 mm. Triplicate soil samples (50 g) collected from each microcosm at different ageing times (i.e., 1, 7, 31, 91,700 d) were brought to 100% of their WHC, then a DGT device was deployed on the soil surface and gentle pressed (to favour the contact between soil and device). DGT probes were left in contact with soils for 24 h at 25 °C. Afterwards, binding gels (of each DGT device) were collected and eluted for 24 h in 2 mL of a 1 M NaOH and 1 M H2O2 solution. Eluent solutions were then 10-fold diluted in 2% ultrapure HNO3 prior to the analysis of Sb content by ICP-QQQ-MS as previously described.
The time-averaged Sb concentration present in the soil solution at the surface of the DGT device (CDGT, expressed as μg·L−1; i.e. a measure of labile or bioavailable soil Sb) was obtained according to the following equation (Eq. (1)) (Davison and Zhang, 1994):
| (1) |
where M is the mass (μg) of element accumulated on the binding gel, Δg is the thickness of the diffusive gel (0.8 mm) plus the thickness of filter membrane (0.1 mm), D is the element diffusion coefficient in the diffusive layer (5.46 x 10-6 cm2 s-1 for Sb; Luo et al., 2010), A is the exposed area of the binding gel (3.14 cm2), and t is the deployment time (s). Moreover, the R value, which reflects the ability of soil to resupply antimony to the device interface, was calculated as the ratio between Sb-DGT (CDGT) to Sb concentration in soil pore water (Csol) (Harper et al., 2000).
2.4. In vitro bioaccessibility of soil Sb
At each time-point, Sb bioaccessibility in spiked soils was determined using the Solubility Bio-accessibility Research Consortium in vitro assay (SBRC) which includes a gastric (SBRC-G) and an intestinal phase (SBRC-I) (Mele et al., 2015). The <250 μm (Table 2) soil particle size fraction was employed for analysis, since it represents the fraction that adheres to fingers and is available for incidental ingestion (Mele et al., 2015). Triplicate soil samples (0.4 g) collected (as described for SEP) from microcosms at different ageing times (i.e., 1, 7, 31, 91, 700 d) were treated with 40 mL of a gastric solution (30.03 g·L−1 glycine adjusted to pH 1.5 with 37% HCl) and incubated in agitation (40 rpm on a Ratek rotary suspension mixer) at 37 °C and constant pH (i.e., 1.5). After 1-h incubation, 4 mL of supernatant was collected, filtered through 0.45 μm filters and stored at 4 °C (SBRC-G phase). The remaining suspension was adjusted to pH 7.0 with a 50% NaOH solution, and intestinal solution (3 mL) containing 70 mg of bile bovine (Sigma-Aldrich) and 20 mg of pancreatin (Sigma-Aldrich) was added. The intestinal phase was then incubated at constant pH (i.e., 7.0) as previously mentioned. After 4 h, supernatant aliquots (10 mL) were collected, filtered through 0.45 μm filters and stored at 4 °C (SBRC-I phase). Soluble Sb in SBRC-G and SBRC-I phases was determined by ICP-QQQ-MS (Agilent 8800), while total Sb concentration in soil was quantified after mineralisation (< 250 μm) as previously described. A standard reference material (SRM 2711A) was also included for quality assurance and quality control.
In vitro Sb bioaccessibility values (%) were calculated by dividing the Sb concentration determined in SBRC-G or SBRC-I phases by the total Sb concentration in the soil (< 250 μm) as in the following equation (Eq. (2)):
| (2) |
The health risk assessment for adults due to incidental ingestion of soil particles <250 μm was calculated for S1 and S2 at the different ageing times (i.e., 1, 7, 31,91, 700 d) as described by Oguri et al. (2018). In particular, the daily Sb intake per kg of body weight (DISb, μg·kg−1·d−1) was calculated as in the following equation (Eq. (3)):
| (3) |
where IngR = ingestion rate (0.0001 kg d−1; U.S. EPA,2011), CBA = bioaccessible Sb concentration (mg·kg−1) in each soil after gastrointestinal digestion, BW = body weight (70 kg per adult). Finally, the non-carcinogenic risk was determined by means of Hazard Quotient (HQ) which was calculated as in the following equation (Eq. (4)):
| (4) |
where TDI = tolerable daily intake per kg of body weight (μg·kg−1·d−1); TDISb = 0.4 μg·kg−1·d−1 (U.S. EPA, 1987).
2.5. X-ray absorption spectroscopy
Antimony K-edge X-ray absorption near-edge structure (XANES) spectra were obtained to understand Sb speciation and its interaction with soil components during ageing. XANES spectra were collected at the Materials Research Collaborative Access Team's (MRCAT) beamline 10-BM (Kropf et al., 2010) at the Advanced Photon Source (7 GeV storage ring in top-up mode), Argonne National Laboratory, Argonne, IL (USA). Soil samples, collected at different ageing times (i.e., 1, 31, 700 d), were prepared by freeze drying, grinding with an agate mortar and pestle, and pressing 75mg of the sample in a 7-mm handheld pellet press. Samples from 7 and 91 d were not included in XANES analysis since SEP, DGT and SBRC data from these time-points were similar to those at 31 and 700 d respectively. The sample pellet was then encapsulated with Kapton tape and placed on a sample holder for analysis. The beamline optics and setup parameters for the Sb edge XANES measurements included calibration of the monochromator with a metallic Sb foil (30,491 eV) and the acquisition of metallic foil spectra with each sample scan for spectral calibration verification. Data collection was conducted in both transmission and fluorescence (4-element Vortex Si-drift detector) modes with gas purged ion chambers for I0 (80% Ar/20% N2), [ITransmission (100% Ar), and IReference (100% Ar). At least six scans for each sample were collected and it was evident fluorescence data were of higher quality than transmission. Data analysis was conducted using Athena software (Ravel and Newville, 2005). Multiple scans for each sample were aligned, merged, normalized, and calibration was performed by assigning the first inflection point of the reference Sb metal foil to 30,491 eV. The relative abundance of Sb-bearing solid-phases was examined by linear combination fitting (LCF) of Sb K-edge XANES derivative spectra relative to known Sb reference samples (fitting range of -30 eV to +70 eV relative to the calibration energy). The χ2 is a measure of the mean square sum of misfit at each data point and describes the degree of uncertainty in the fitting process (Ravel and Newville, 2005). Antimony reference standards included Sb2S3, Sb2O3, Sb(V)-tartrate, Sb(V)-citrate and KSb(OH)6 (Sigma Aldrich, used for spike); Sb(V) and Sb(III) sorbed to goethite and Sb(III) sorbed to thiol-functionalised cellulose. Antimony reference standards were selected considering some of the most common Sb mineral phases in soil, or common Sb associations with inorganic or organic soil components (Ji et al., 2017). Reference standards considered for LCF included Sb(V) sorbed to goethite to represent Sb(V) sorption by inorganic oxy-hydroxides (Essington and Stewart, 2018), Sb(V)-citrate to represent Sb(V) bound to organic matter, and KSb(OH)6 to represent labile (e.g. water-soluble and exchangeable) Sb(V) in soil (Essington and Stewart, 2016). This latter interpretation was based on the high similarity of KSb(OH)6 and Sb(OH)6− spectra (e.g.Ji et al., 2017).
2.6. Statistical analysis
All chemical analyses were performed on triplicate soil samples collected from each mesocosm at different ageing times with mean values ± standard errors reported in tables and figures. The Shapiro-Wilk test was performed in order to evaluate the normality of data distribution. For each soil (S1 or S2) and contamination level (100 or 1000 mg·kg−1), the Fisher's LSD test was used to assess the influence of ageing time on Sb mobility, potential bioavailability and bioaccessibility. Moreover, for each ageing time, comparisons between S1 and S2 at the same contamination level were made by using a Student t-test. Differences were considered statistically significant for P < 0.05. Pearson's correlation test was also performed to investigate the relationships between Sb concentrations recovered in the different steps of the SEP procedure, and those detected by DGT (i.e. CDGT) at different ageing times. Correlation values were considered statistically significant for P < 0.05. All statistical analyses were carried out using the Sigma Plot Software (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
3.1. Influence of ageing on Sb mobility in contaminated soils
To investigate the influence of ageing on the mobility of antimony in contaminated soils, different Sb fractions (or pools) characterised by a diverse degree of lability (i.e., solubility) were quantified at different time points. The water-soluble (i.e., highly mobile and therefore environmental relevant) Sb fraction (F1) decreased significantly over time for both soils and at both Sb concentrations (Fig. 1). The highest amounts of soluble Sb were detected at 1 d since spiking (40 and 18% of total Sb for S1–100 and S2–100 and 24 and 26% for S1–1000 and S2–1000 respectively), as previously reported elsewhere for other PTEs (e.g. Pb, Cu, Zn, Cd) (Jalali and Khanlari, 2008; Tang et al., 2006). Overall, such soluble Sb amounts progressively and significantly reduced with ageing in both soils, even if different trends were observed depending on soil type and level of contamination. In particular, significant reductions in water-soluble Sb occurred in all soils (with the exception of S2–100), after 7 d of ageing (Fig. 1). Interestingly, in S2–100, a significant reduction in soluble Sb occurred only at 91 d (Fig. 1). The lowest amounts of soluble Sb were detected at 700 d of ageing even if, within each soil, the differences between 91 and 700 d were limited and, in some cases, not significant (Fig. 1). After 700 d ageing, soluble Sb was significantly reduced, compared to 1 d, by approx. 80% in S1- and S2–100, and by 70 and 95% in S1- and S2–1000 respectively. Taken together, these data indicate that 3 months ageing could be a suitable time for soil to equilibrate with aqueous Sb (i.e. very limited changes occurred after this time) and, consequently, to investigate the environmental fate of the contaminant and its ecotoxicological effects. Moreover, the higher Sb solubility observed in S1 compared with S2, especially after 700 d ageing (6 and 77 mg·kg−1 in S1–100 and – 1000 vs 4 and 13 mg·kg−1 in S2–100 and – 1000), could support previous research findings (Diquattro et al., 2020; Herath et al., 2017) where pH was identified as a key factor influencing Sb mobility. In particular, competition phenomena between hydroxide (OH−) and Sb (OH)6− anions for Sb retention sites and/or repulsion between Sb (OH)6− anions and negatively charged soil surfaces, could explain the higher Sb solubility in the alkaline S1 soil (pH 8.2) compared to the acidic S2 (pH 4.9). On the contrary, Fe/Al oxides and soil organic matter, i.e. other important sinks of soil Sb (Herath et al., 2017), seemed to play a limited or negligible role at influencing Sb mobility in these soils (Fe/Al content was much higher in S1, and SOM content was similar in S1 and S2).
Fig. 1.
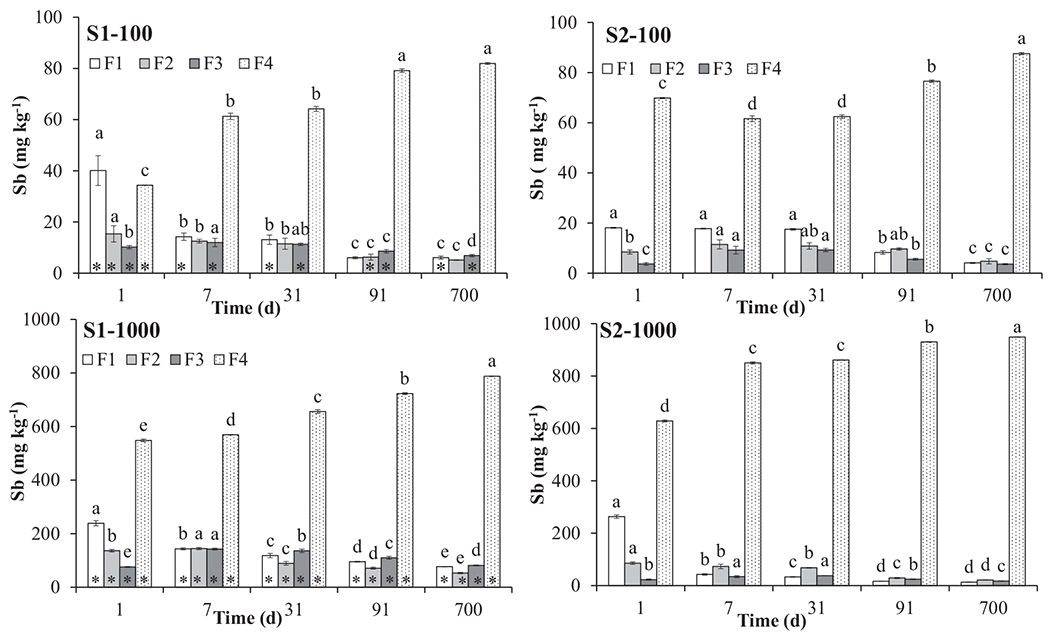
Sequential extraction of antimony from S1 and S2 soils spiked with 100 (S-100) and 1000 (S-1000) mg Sb kg−1 soil at different ageing time. For each Sb fraction (Fx), different letters on top of each bar (e.g. a, b, c, d) denote ageing-dependent statistical differences (Fisher’s LSD, P < 0.05). For each Sb fraction and ageing time, asterisks denote statistical differences between S1–100 and S2–100, and between S1–1000 and S2–1000 soils (Student t-test, P < 0.05). F1, water-soluble Sb; F2, Sb non-specifically sorbed; F3, Sb specifically sorbed; F4, residual Sb.
After 1 d ageing, Sb extracted with (NH4)2SO4 (F2), i.e. the exchangeable pool, was significantly lower than water-soluble Sb in both soils (Fig. 1). Moreover, Sb extracted in F2 was significantly higher in S1–100 and — 1000 (15.4 and 13.7%) compared to S2–100 and — 1000 (8.4 and 8.6%), in agreement with the results of a recent short-term investigation based on the same soils (Fig. 1; Diquattro et al., 2020). Starting from 7 or 31 d, the amounts of exchangeable Sb significantly and progressively decreased (compared to 1 d) in all soils except S2–100 where a reduction was observed only at 700 d ageing (Fig. 1). Overall these data indicate a decrease of weaker (e.g. outer sphere) complexes and a concomitant increase of more specific (e.g. inner sphere) bonding between Sb and soil mineral and organic surfaces over time (Violante et al., 2010; Wenzel et al., 2001). As for the water-soluble F1 fraction, this implied a Sb shift from more mobile (and potentially bioavailable) pools to less mobile (and hardly bioavailable) ones during ageing.
This was partly supported by Sb extracted with NH4H2PO4 (F3), i.e. Sb specifically sorbed by soil colloids (Wenzel et al., 2001). Such Sb pool can be considered as relatively mobile since it can be mobilised following a local pH change and/or a variation in P concentration (Wenzel et al., 2001). Antimony extracted in F3 was generally low at day 1 (i.e., 10.2 and 7.6% of total Sb for S1–100 and — 1000, and 3.7 and 2.3% for S2–100 and — 1000 respectively) which reduced (e.g. S1–100 and S2–1000) or remained constant (e.g. S1–100) during ageing (Fig. 1). The only exception was S1–1000 where Sb concentration slightly (yet significantly) increased with time, i.e. from 76 (at 1 d) to 81 mg·kg−1 soil (at 700 d) (Fig. 1).
Antimony detected in F4, i.e. residual Sb (e.g. associated with amorphous and crystalline Al and Fe oxy-hydroxides and/or precipitated or occluded), significantly increased over time from 34, 70, 55 and 63% at 1 d to 82, 79, 88 and 95% at 700 d ageing in S1- and S2–100, and S1- and S2–1000 respectively (Fig. 1).This was in agreement with previous studies (e.g. Jalali and Khanlari, 2008; Peng et al., 2019) which reported an increase in residual PTEs such as Se, Pb, Cu, Cd, and Zn with ageing. However, the influence of ageing on Sb fractionation has been rarely addressed (e.g. Egodawatta et al., 2018; Zhang et al., 2020).
Taken together, SEP results highlighted a re-distribution of Sb from soluble (i.e., mobile) or weakly bound (easily mobilizable) forms, to less mobile and more stable ones during ageing. From an environmental viewpoint, this implies a time-dependent decrease in (micro)biological-impacting Sb fractions and an increase in less bioavailable forms.
3.2. influence of ageing on Sb bioavailability in contaminated soils
The diffusive gradient in thin film technique (DGT) was used to measure the flux of soluble Sb, which is supplied by diffusion through soil solution, as well as that of labile Sb resupplied by the soil solid phase to the DGT device (Letho, 2016). The diffusion of labile soil Sb (free and complexed soluble ions and weakly bound Sb species) into DGT devices has been previously shown to mimic the continuous uptake of PTEs by plant roots (Zhou et al., 2019). This is because, as pointed out by Zhang and Davison (2000), elemental flux to plant membranes and to DGT can be similar from a quantitative viewpoint (Letho, 2016). For this reason, the use of the DGT technique to estimate PTEs bioavailability to plants and soil (micro)biota has been steadily growing (e.g. Dai et al., 2018; Gu et al., 2017; Wang et al., 2019).
The CDGT measurements showed that bioavailable Sb rapidly decreased at the initial stage of ageing (7–31 d) and reached an equilibrium at 91 d (in agreement with SEP results) in the majority of soils (Fig. 2). At low contamination levels (i.e., 100 mg·kg−1), Sb bioavailability was always higher for soil S2 at all ageing times. However, after 700 d ageing, Sb-CDGT values in the pore water of the two soils were low and similar, i.e. 0.33 and 0.49 μg·L−1 (Fig. 2). This is relevant, as these concentrations are expected to pose limited threats for plants and soil (micro)biota (Geng et al., 2020; Sh et al., 2012). Nonetheless, it should be noted that much higher Sb-CDGT concentrations were detected during the previous time-points and this could have produced substantial perturbations of soil (micro)biological and biochemical features (e.g. Diquattro et al., 2020) whose effects could be still present after 700 d. In soils containing higher Sb concentrations (i.e., 1000 mg·kg−1), Sb bioavailability was significantly higher for soil S1 at all ageing times, with the exception of day 1 (Fig. 2). After 700 d ageing, the Sb-CDGT in S1 was still 4.5-fold higher than in S2, and this supports higher Sb mobility detected by the SEP procedure in soil S1 (Fig. 1). As previously mentioned, this could be attributed to the alkaline pH of soil S1 and/or to the increased formation of stable Sb precipitates (e.g. FeSbO4 and AlSbO4) in the acidic S2 (Dousova et al., 2018; Herath et al., 2017). It should be noted that, at low Sb contamination level (i.e., 100 mg·kg−1), this was not particularly evident as highlighted by both SEP and DGT results (Figs. 1–2). Overall, these results clearly indicate a steady reduction in Sb bioavailability during ageing, which also suggests a parallel increase in stable Sb-soil interactions over time, which was supported by SEP results.
Fig. 2.
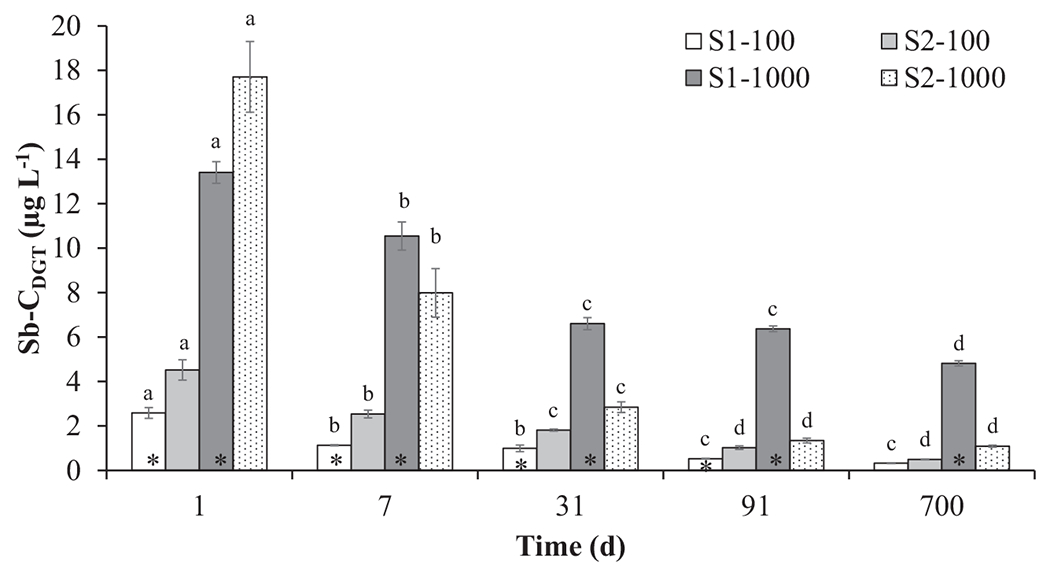
Antimony concentrations as obtained by DGT (Sb-CDGT) in S1 and S2 soils spiked with 100 (S-100) and 1000 (S-1000) mg Sb kg−1 soil at different ageing time. For each soil, different letters on top of each bar (e.g. a, b, c, d) denote ageing-dependent statistical differences (Fisher’s LSD, P < 0.05). For each ageing time, asterisks denote statistical differences between S1–100 and S2–100, and between S1–1000 and S2–1000 soils (Student t-test, P < 0.05).
The resupply of Sb from the solid phase to the soil pore water, i.e. R value (CDGT / Csol), was low in both soils for all ageing times (i.e., R<0.12 and < 0.05 for S-100 and S-1000 soils; Table 3) indicating that diffusion was the main process supplying Sb to the DGT device (Ma et al., 2020; Letho, 2016; Peng et al., 2019). Since the selected Sb fractions extracted by SEP are often correlated with the most labile (and potentially bioavailable) fractions in soil, i.e. those readily taken up by plants and soil microorganisms (e.g. Castaldi et al., 2018; Diquattro et al., 2018, 2020; Garau et al., 2017), the correlation between Sb-CDGT and that recovered in F1-F4 was tested (Table 4). Significant positive correlations were observed between F1 (0.786–0.959; P < 0.01), and F1 + F2 (0.683–0.979; P < 0.01), and Sb-CDGT while weaker relationships were recorded for F2 (Table 4). No significant relationship between Sb-CDGT and F3 was found, whereas significant negative correlations were observed between F4 (i.e., the less mobile and less bioavailable Sb) and Sb-CDGT (Table 4).
Table 3.
Concentration of Sb in soil solution (Csol) and R values in soils during ageing.
| Ageing time (d) | Csol(μg·L−1) |
R value (R = CDGT/Csol) |
||||||
|---|---|---|---|---|---|---|---|---|
| S1–100 | S2–100 | S1–1000 | S2–1000 | S1–100 | S2–100 | S1–1000 | S2–1000 | |
| 1 | 88.88 | 38.06 | 535.6 | 596.6 | 0.049 | 0.119 | 0.045 | 0.050 |
| 7 | 28.83 | 37.44 | 300.3 | 91.2 | 0.039 | 0.068 | 0.035 | 0.049 |
| 31 | 27.64 | 39.11 | 260.3 | 72.2 | 0.036 | 0.046 | 0.033 | 0.039 |
| 91 | 13.33 | 16.73 | 212.3 | 35.3 | 0.039 | 0.061 | 0.030 | 0.038 |
| 700 | 15.76 | 8.84 | 173.0 | 26.6 | 0.021 | 0.056 | 0.028 | 0.041 |
Table 4.
Pearson correlation coefficients (r) between the Sb fractions detected by SEP and those quantified by DGT (CDGT) in S1 and S2 soils‡.
| S1–100 |
S1–1000 |
S2–100 |
S2–1000 |
|
|---|---|---|---|---|
| CDGT | CDGT | CDGT | CDGT | |
| F1 | 0.959** | 0.946** | 0.786** | 0.947** |
| F2 | 0.853** | 0.911** | 0.297NS | 0.780** |
| F1 + F2 | 0.967** | 0.979** | 0.683** | 0.966** |
| F3 | 0.425NS | −0.106NS | −0.041NS | −0.032NS |
| F4 | −0.958** | −0.921** | −0.537* | −0.963** |
NS not significant (P > 0.05).
Statistically significant at P < 0.05.
Statistically significant at P < 0.01.
Taken together, these data highlight a good agreement between DGT and SEP results and confirmed the suitability of both approaches for the assessment of Sb mobility and potential bioavailability in soil. Overall, Sb-CDGT values showed that ageing had a marked influence on the potential bioavailability of Sb. This latter steadily decreased with time due to the conversion of Sb labile fractions into non-labile ones as indicated by SEP results.
3.3. influence of ageing on Sb bioaccessibility
To investigate the influence of ageing on potential exposure for humans and animals related to incidental ingestion of Sb-contaminated soil, gastric and intestinal phase Sb bioaccessibility was determined at different ageing times using the in vitro SBRC assay. Antimony bioaccessibility varied over time, and different trends were observed depending on soil type and Sb concentration (Fig. 3). When assessed using SBRC-G, Sb bioaccessibility significantly decreased with increasing ageing times with the exception of S1–100 when no variation in Sb bioaccessibility was recorded (Fig. 3). After 700 d ageing, Sb bioaccessibility in the SBRC-G phase was ~30, 26,39 and 9% (of total Sb present in the <250 μm soil fractions) in S1- and S2–100, and S1- and S2–1000 respectively.
Fig. 3.

In vitro bioaccessibility of Sb in S1 and S2 soils spiked with 100 (S-100) and 1000 (S-1000) mg Sb kg−1 soil at different ageing time. For each soil, different letters on top of each bar (e.g. a, b, c, d) denote ageing-dependent statistical differences (Fisher’s LSD, P < 0.05). For each ageing time, asterisks denote statistical differences between S1–100 and S2–100, and between S1–1000 and S2–1000 soils (Student t-test, P < 0.05). SBRC-G = bioaccessible Sb in the gastric phase; SBRC-I = bio-accessible Sb in the intestinal phase.
Considering that elemental absorption takes place in the intestinal compartment (Hamel et al., 1998), SBRC-I data are of particular interest. Antimony bioaccessibility in the SBRC-I phase quickly declined with ageing in the majority of soils (Fig. 3). In particular, significant reductions in bioaccessible Sb were recorded after 7 d for S2–1000 and after 31 d for S2–100 and S1–1000. However, it should be noted that after one day, Sb bioaccessibility in the SBRC-I phase was 46% (of total Sb present in the <250 μm soil fraction; Table 2) in S1–100 and between 61 and 72% in the remaining soils (Fig. 3), that means an approx. 55–1800 mg Sb·kg−1 soil. Nonetheless, ageing had a clear influence on Sb bioaccessibility (SBRC-I) which declined, compared to day 1, by a minimum of 15% in S1–100, up to 60% in S2–1000 (Fig. 3). Again, this supports a time-dependent increase of stable bonds between Sb and soil components (e.g. Fe oxy-hydroxides and organic matter), able to resist dissolution processes which take place during digestion (Denys et al., 2008). However, this was more pronounced for S2 soils where Sb binding to Fe/Al oxy-hydroxides and organic matter was likely favoured by the lower pH. Indeed, the acidic pH of the gastric phase commonly promotes the solubilisation of mineral phases and the release of bonded PTEs, while the higher concentration of OH− in the intestinal phase can prevent anion sorption/adsorption [e.g. Sb(OH)6−] by soil colloids which favours the release of further Sb into solution (Udovic and McBride, 2012).
Even if these results indicate a decreased Sb bioaccessibility over time, nonetheless, absolute Sb concentrations recorded in the SRBC-I phase at 700 d (i.e., 43 and 511 mg·kg−1 for S1–100 and S1–1000 respectively, and 90 and 367 mg·kg−1 for S2–100 and S2–1000) highlighted substantial potential health risks in both contaminated soils (Filella et al., 2002). To further investigate this aspect, the daily Sb intake per kg of body weight (due to incidental ingestion of S1 and S2 fine soil particles) was determined, at the different ageing times, by employing the biaccessible concentrations of Sb after gastrointestinal digestion. Subsequently, the respective Hazard Quotient values were calculated (Table S1). Significant risks for human health are indicated by HQ> 1, while values <1 indicate no risk (e.g. Oguri et al., 2018). Starting from 7 d, HQ values progressively decreased in all soils with ageing, and no probable risk for adults was highlighted for S1- and S2–100 soils (HQ in the 0.24–0.56 range; Table S1). On the contrary, significant risks were highlighted for S1- and S2–1000 soils at all ageing times (most HQ values >2). Even after 700 d ageing HQ values were 1.82 and 1.31 for S1- and S2–1000, respectively indicating that approx. 2 years ageing was not sufficient to eliminate health risks in these soils.
3.4. influence of ageing on Sb speciation
Biogeochemical redox processes strongly influence Sb speciation, its interactions with soil colloids (and hence mobility and stability) as well as its toxicity. In this sense, spectroscopic techniques such as X-ray absorption spectroscopy (XAS, including XANES) have been useful for the identification of metal and metalloid speciation and their complexes at surfaces of Al, Fe or Mn oxy-hydroxides, silicate clays and soil organic matter (Violante et al., 2010).
Derivative XANES spectra of Sb reference materials considered for LCF and of S1 and S2 soils are shown in Figs. S1 and S2 respectively. LCF results for S1 and S2 samples (Table 5, Fig. S2) show the presence of labile Sb(V) (i.e. KSb(OH)6 spectra) at varying degrees. Importantly, Sb(V) was the only redox state identified in XANES data. Labile Sb(V) concentration was higher for S1 samples than S2. Again, this could be due to the influence of pH (8.2 vs 4.9, respectively) given the similar SOM content of S1 and S2 (1.75 and 2.19%; note that differences in SOM quality could have contributed to the observed results), and the higher Fe and Al content in the former soil (~ 3 and 5-fold respectively compared to S2). A similar influence of pH on Sb sorption by ferrihydrite and soil was recently reported by Garau et al. (2019) and Veerbeck et al. (2020) respectively. As previously discussed, the higher pH of S1 likely limited the formation of stable inner sphere complexes which on the contrary was favoured at the low pH conditions of S2. Consistently, higher Sb-organic interactions (Sb(V)-citrate) and Sb sorbed to inorganic oxides (Sb (V)-goethite LCF reference) were recorded in S2 relative to S1 over time (Table 5). The initial concentration of Sb in each soil influenced final speciation results. S1–100 at 700 d resulted in Sb speciation components of 57% KSb(OH)6 (which is indicative of labile Sb(OH) 6−), 18% organic bound Sb(V), and 24% sorbed to inorganic oxides; whereas S1–1000 at 700 d observed 81% KSb(OH)6, 14% organic bound Sb(V), and 5% sorbed to inorganic oxides. A similar trend was noted for the S2 concentration series where the KSb(OH)6 fraction was larger for the higher concentrated spiked sample. Taken together these data support the view that ageing decreased the presence of labile Sb(V) in all soils while favouring the formation of more stable associations with Fe and/Al oxides (as supported by SEP results) and organic matter in soil. As previously mentioned, this can have positive environmental and, above all, human health implications as highlighted by the reduced HQ values recorded at 700 d ageing.
Table 5.
Linear Combination Fitting (LCF) – XANES analysis of Sb speciation.
| Sb speciation distribution (%) | ||||
|---|---|---|---|---|
| KSb(OH)6 | Sb(V)-Citrate | Sb(V)-Goethite | χ2 | |
| S1–100 1 d | 88 | 12 | 0 | 0.0059 |
| S1–100 31 d | 63 | 19 | 18 | 0.0242 |
| S1–100 700 d | 57 | 18 | 24 | 0.0063 |
| S2–100 1 d | 55 | 32 | 13 | 0.0088 |
| S2–100 31 d | 43 | 36 | 21 | 0.0150 |
| S2–100 700 d | 36 | 37 | 27 | 0.0091 |
| S1–10001 d | 88 | 12 | 0 | 0.0022 |
| S1–1000 31 d | 85 | 12 | 3 | 0.0021 |
| S1–1000 700 d | 81 | 14 | 5 | 0.0034 |
| S2–10001d | 62 | 25 | 13 | 0.0037 |
| S2–1000 31 d | 59 | 23 | 18 | 0.0014 |
| S2–1000 700 d | 58 | 21 | 21 | 0.0543 |
Finally, it should be mentioned that XANES spectroscopy is often not sensitive enough to distinguish if elements are sorbed by Fe or Al oxyhydroxides in a complex soil matrix; however, spectra obtained in this study are unique enough to distinguish them from organic complexes. This also applies to KSb(OH)6 spectra which in this study, as well as in others (e.g. Scheinost et al., 2006; Mitsunobu et al., 2010), were distinguished by those of Sb sorbed to inorganic oxides. However, this may not always the case and introduces a certain degree of uncertainty in our interpretation.
4. Conclusions
Ageing had a clear influence on Sb mobility, bioavailability and bioaccessibility in contaminated soils. Labile (i.e., more soluble) Sb fractions progressively reduced during ageing indicating a decrease of weaker (e.g. outer-sphere) complexes and a concomitant increase of more specific (e.g. inner-sphere) bonding between Sb and the soil components over time. This was accompanied by a parallel decrease of bioavailable and bioaccessible Sb which implied: i) a time-dependent increase of stable bonding between Sb and soil components and ii) a reduced ecotoxicological Sb impact on soil (micro)biota and humans with ageing. XANES LCF results indicated that Sb(V) was the only redox state present in soils during the almost 2-years experiment and that soil organic matter and Fe/Al oxy-hydroxides were the main sink of Sb during ageing. In this regard, soil pH appeared as a key parameter influencing the fate of Sb in our soils, i.e. Sb mobility, bioavailability and bioaccessibility were overall higher in the alkaline S1 soil compared to the acidic S2. Consistently, Sb fixation by soil organic matter and Fe/Al oxy-hydroxides was quantitatively less in S1 compared with S2 at all time-points considered. Nevertheless, bioaccessible Sb concentrations recorded in the intestinal phase at 700 d highlight substantial health risks in the most contaminated soils (i.e. S1- and S2–1000).
This study provides evidence on the relevance of soil pH, organic matter and Fe/Al oxy-hydroxides in natural attenuation processes through which Sb progressively reduces its environmental impact during ageing. Such natural attenuation process seems more effective (and faster) in more acidic soils where a higher degree of stable bonding between Sb(V) with organic matter and Fe/Al minerals is expected to reduce Sb(V) ecotoxicity, bioaccessibility and health risks. This will have a positive impact on the abundance and activity of soil (micro)biota which in turn will further contribute to Sb(V) fixation and speed up its natural attenuation. The relevance of such living components in natural attenuation processes taking place during ageing of Sb(V)-contaminated soil was not considered here and certainly deserves more study.
Supplementary Material
HIGHLIGHTS.
Ageing decreased antimony (Sb) mobility and bioavailability in contaminated soils.
In vitro gastric and intestinal Sb bioaccessibility decreased with ageing.
Sb(V) binding to inorganic oxides and organic matter increased with ageing.
High ecotoxicological risks remained in contaminated soils.
Acknowledgements
The financial support of the University of Sassari (Fondo di Ateneo per la Ricerca 2019) is gratefully acknowledged.
MRCAT operations are supported by the Department of Energy and the MRCAT member institutions. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357. Although EPA contributed to this article, the research presented was not performed by or funded by EPA and was not subject to EPA's quality system requirements. Consequently, the views, interpretations, and conclusions expressed in this article are solely those of the authors and do not necessarily reflect or represent EPA's views or policies.
The Future Industries Institute, University of South Australia is acknowledged for supporting this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.145354.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bagherifam S, Lakzian A, Fotovat A, Khorasani R, Komarneni S, 2014. In situ stabilization of As and Sb with naturally occurring Mn, Al and Fe oxides in a calcareous soil: bioaccessibility, bioavailability and speciation studies.J. Hazard. Mater 273, 247–252. [DOI] [PubMed] [Google Scholar]
- Besold J, Kumar N, Scheinost AC, Lezama Pacheco J, Fendorf S, Planer-Friedrich B, 2019. Antimonite complexation with thiol and carboxyl/phenol groups of peat organic matter. Environ. Sci. Technol 53, 5005–5015. [DOI] [PubMed] [Google Scholar]
- Burton ED, Hockmann K, Karimian N, 2020. Antimony sorption to goethite: effects of Fe(II)-catalyzed recrystallization. ACS Earth Space Chem. 4, 476–487. 10.1021/acsearthspacechem.0c00013. [DOI] [Google Scholar]
- Castaldi P, Diquattro S, Lauro GP, Marceddu S, Garau G, 2018. Water treatment residuals as a resource for the recovery of soil and water polluted with Sb(V): sorption and desorption trials at different pH values. Water Air Soil Pollut. 229 (6), 174. [Google Scholar]
- CEC, 1998. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption (OJ L 330 05.12.1998 p. 32). In: Sands P, Galizzi P (Eds.), Documents in European Community Environmental Law. Cambridge University Press, Cambridge, pp. 865–878. [Google Scholar]
- Courtin-Nomade A, Rakotoarisoa O, Bril H, Grybos M, Forestier L, Foucher F, Kunz M, 2012. Weathering of Sb-rich mining and smelting residues: insight in solid speciation and soil bacteria toxicity. Chem. Erde-Geochem 72, 29–39. [Google Scholar]
- Dai Y, Nasir M, Zhang Y, Gao J, Lv Y, Lv J, 2018. Comparison of DGT with traditional extraction methods for assessing arsenic bioavailability to Brassica chinensis in different soils. Chemosphere 191, 183–189. [DOI] [PubMed] [Google Scholar]
- Davison W, Zhang H, 1994. In situ speciation measurements of trace components in natural waters using thin-flm gels. Nature 367, 546–548. [Google Scholar]
- Denys S, Tack K, Caboche J, Delalain P, 2008. Bioaccessibility, solid phase distribution, and speciation of Sb in soils and in digestive fluids. Chemosphere 74, 711–716. [DOI] [PubMed] [Google Scholar]
- Diagboya PN, Olu-Owolabi BI, Adebowale KO, 2015. Effects of aging, soil organic matter, and iron oxides on the relative retention of lead, cadmium, and copper on soils. Environ. Sci. Pollut. Res 22, 10331–10339. [DOI] [PubMed] [Google Scholar]
- Diquattro S, Garau G, Lauro GP, Silvetti M, Deiana S, Castaldi P, 2018. Municipal solid waste compost as a novel sorbent for antimony(V): adsorption and release trials at acidic pH. Environ. Sci. Pollut. Res 25, 5603–5615. [DOI] [PubMed] [Google Scholar]
- Diquattro S, Garau G, Mangia NP, Drigo B, Lombi E, Vasileiadis S, Castaldi P, 2020. Mobility and potential bioavailability of antimony in contaminated soils: short-term impact on microbial community and soil biochemical functioning. Ecotoxicol. Environ. Safe 196, 110576. [DOI] [PubMed] [Google Scholar]
- Dousova B, Lhotka M, Filip J, Kolousek D, 2018. Removal of arsenate and antimonate by acid-treated Fe-rich clays. J. Hazard. Mater 357, 440–448. [DOI] [PubMed] [Google Scholar]
- Egodawatta LP, Macoustra GK, Ngo LK, Jolley DF, 2018. As and Sb are more labile and toxic to water spinach (Ipomoea aquatica) in recently contaminated soils than historically co-contaminated soils. Environ. Sci. Proc. Imp 20, 833–844. [DOI] [PubMed] [Google Scholar]
- Essington ME, Stewart MA, 2016. Adsorption of antimonate by gibbsite: reversibility and the competitive effects of phosphate and sulfate. Soil Sci. Soc. Am. J 80, 1197–1207. [Google Scholar]
- Essington ME, Stewart MA, 2018. Adsorption of antimonate, sulfate, and phosphate by goethite: reversibility and competitive effects. Soil Sci. Soc. Am. J 82, 803–814. [Google Scholar]
- Filella M, Belzile N, Chen YW, 2002. Antimony in the environment: a review focused on natural waters I. Occurence. Earth-Sci. Rev 57, 125–176. [Google Scholar]
- Filella M, Williams PA, Belzile N, 2009. Antimony in the environment: Knowns and unknowns. Environ. Chem 6, 95–105. [Google Scholar]
- Fu Z, Zhang G, Li H, Chen J, Liu F, Wu Q, 2016. Influence of reducing conditions on the release of antimony and arsenic from a tailings sediment. J. Soils Sediments 16, 2471–2481. 10.1007/s11368-016-1484-4. [DOI] [Google Scholar]
- Garau G, Silvetti M, Vasileiadis S, Donner E, Diquattro S, Deiana S, Lombi E, Castaldi P, 2017. Use of municipal solid wastes for chemical and microbiological recovery of soils contaminated with metal(loid)s. Soil Biol. Biochem 111, 25–35. [Google Scholar]
- Garau G, Lauro GP, Diquattro S, Garau M, Castaldi P, 2019. Sb(V) adsorption and desorption onto ferrihydrite: influence of pH and competing organic and inorganic anions. Environ. Sci. Pollut. Res 26, 27268–27280. [DOI] [PubMed] [Google Scholar]
- Geng L, Yang Z, Xu Z, 2020. Effects of antimony contamination in soil on the nutrient composition of three green leafy vegetables. Journal Soil. Sediment 20, 2217–2224. [Google Scholar]
- Gu X, Liu Z, Wang X, Luo J, Zhang H, Davison W, Ma LQ, Xue Y, 2017. Coupling biological assays with diffusive gradients in thin-films technique to study the biological responses of Eisenia fetida to cadmium in soil. J. Hazard. Mater 339, 340–346. [DOI] [PubMed] [Google Scholar]
- Hamel SC, Buckley B, Lioy PJ, 1998. Bioaccessibility of metals in soils for different liquid to solid ratios in synthetic gastric fluid. Environ. Sci. Technol 32, 358–362. [Google Scholar]
- Hammel W, Debus R, Steubing L, 2000. Mobility of antimony in soil and its availability to plants. Chemosphere 41, 1791–1798. [DOI] [PubMed] [Google Scholar]
- Harper MP, Davison W, Tych W, 2000. DIFS - a modelling and simulation tool for DGT induced trace metal remobilisation in sediments and soils. Environ. Model. Softw 15, 55–66. [Google Scholar]
- He M, Wang N, Long X, Zhang C, Ma C, Zhong Q, Wang A, Wang Y, Pervaiz A, Shan J, 2019. Antimony speciation in the environment: recent advances in understanding the biogeochemical processes and ecological effects. J. Environ. Sci. (China) 75, 14–39. [DOI] [PubMed] [Google Scholar]
- Herath I, Vithanage M, Bundschuh J, 2017. Antimony as a global dilemma: geochemistry, mobility, fate and transport. Environ. Pollut 223, 545–559. [DOI] [PubMed] [Google Scholar]
- Hockmann K, Planer-Friedrich B, Johnston SG, Peiffer S, Burton ED, 2020. Antimony mobility in sulfidic systems: coupling with sulfide-induced iron oxide transformations. Geochim. Cosmochim. Acta 282, 276–296. [Google Scholar]
- Jalali M, Khanlari ZV, 2008. Effect of aging process on the fractionation of heavy metals in some calcareous soils of Iran. Geoderma 143, 26–40. [Google Scholar]
- Ji Y, Sarret G, Schulin R, Tandy S, 2017. Fate and chemical speciation of antimony (Sb) during uptake, translocation and storage by rye grass using XANES spectroscopy. Environ. Pollut 231, 1322–1329. [DOI] [PubMed] [Google Scholar]
- Johnston SG, Bennett WW, Doriean N, Hockmann K, Karimian N, Burton ED, 2020. Antimony and arsenic speciation, redox-cycling and contrasting mobility in a mining-impacted river system. Sci. Total Environ 710, 136354. [DOI] [PubMed] [Google Scholar]
- Jury W, Gardner WR, Gardner WH, 1991. Soil Physics 5th ed. John Wiley & Sons, New York. [Google Scholar]
- Kang M, Kawasaki M, Tamada S, Kamei T, Magara Y, 2000. Effect of pH on the removal of arsenic and antimony using reverse osmosis membranes. Desalination 131, 293–298. [Google Scholar]
- Karimian N, Burton ED, Johnston SJ, Hockmann K, Choppala G, 2019. Humic acid impacts antimony partitioning and speciation during iron(II)-induced ferrihydrite transformation. Sci. Total Environ 683, 399–410. [DOI] [PubMed] [Google Scholar]
- Kastury F, Smith E, Doelsch E, Lombi E, Donnelley M, Cmielewski PL, Parsons DW, Scheckel KG, Paterson D, De Jonge MD, Herde C, Juhasz AL, 2019. In vitro, in vivo, and spectroscopic assessment of lead exposure reduction via ingestion and inhalation pathways using phosphate and iron amendments. Environ. Sci. Technol 53, 10329–10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf AJ, Katsoudas J, Chattopadhyay S, Shibata T, Lang EA, Zyryanov VN, Ravel B, McIvor K, Kemner KM, Scheckel KG, Bare SR, Terry J, Kelly SD, Bunker BA, Segre CU, 2010. The new MRCAT (sector 10) bending magnet beamline at the advanced photon source. AIP Conference Proceedings 1234, 299–302. [Google Scholar]
- Letho NJ, 2016. Principles and application in soils and sediments. In: Davison W (Ed.), Diffusive Gradients in Thin-Films for Environmental Measurements. Cambridge University Press, Padstow (United Kingdom), pp. 146–173. [Google Scholar]
- Li C, Ding S, Yang L, Wang Y, Ren M, Chen M, Fan X, Lichtfouse E, 2018. Diffusive gradients in thin films: devices, materials and applications. Environ. Chem. Lett 17, 801–831. [Google Scholar]
- Lin X, He F, Sun Z, Hou H, Zhao L, 2020a. Influences of soil properties and long-time aging on phytotoxicity of antimony to barley root elongation. Environ. Pollut 262, 114330. [DOI] [PubMed] [Google Scholar]
- Lin X, Sun Z, Ma J, Hou H, Zhao L, 2020b. Effects of soil properties and long aging time on the toxicity of exogenous antimony to soil-dwelling springtail Folsomia Candida. Chemosphere 241, 125100. [DOI] [PubMed] [Google Scholar]
- Luo J, Zhang H, Santner J, Davison W, 2010. Performance characteristics of diffusive gradients in thin films equipped with a binding gel layer containing precipitated ferrihydrite for measuring arsenic(V), selenium(VI), vanadium(V), and antimony(V). Anal. Chem 82, 8903–8909. [DOI] [PubMed] [Google Scholar]
- Luo J, Bai Y, Liang J, Qu J, 2014. Metagenomic approach reveals variation of microbes with arsenic and antimony metabolism genes from highly contaminated soil. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Li C, Yang L, Ding S, Zhang M, Zhang Y, Zhao T, 2020. Evaluating the mobility and labile of As and Sb using diffusive gradients in thin-films (DGT) in the sediments of Nansi Lake. China. Sci. Total Environ 713, 136569. [DOI] [PubMed] [Google Scholar]
- Maher WA, Krikowa F, Foster SD, Ellwood MJ, Bennett WW, 2018. Antimony measurements in environmental matrices: seven considerations. J. Anal. At. Spectrom 33, 706–712. [Google Scholar]
- Martínez CE, McBride MB, 2001. Cd, Cu, Pb, and Zn coprecipitates in Fe oxide formed at different pH: aging effects on metal solubility and extractability by citrate. Environ. Toxicol. Chem 20, 122–126. [PubMed] [Google Scholar]
- Mele E, Donner E, Juhasz AL, Brunetti G, Smith E, Betts AR, Castaldi P, Deiana S, Scheckel KG, Lombi E, 2015. In situ fixation of metal(loid)s in contaminated soils: a comparison of conventional, opportunistic, and engineered soil amendments. Environ. Sci. Technol 49, 13501–13509. [DOI] [PubMed] [Google Scholar]
- Mitsunobu S, Takahashi Y, Terada Y, Sakata M,2010. Antimony(V) incorporation into synthetic ferrihydrite, goethite, and natural iron oxyhydroxides. Environ. Sci. Technol 44, 3712–3718. [DOI] [PubMed] [Google Scholar]
- Ngo LK, Price H, Bennet WW, Teasdale PR, Jolley DF, 2020. DGT and selective extractions reveal differences in arsenic and antimony uptake by the white icicle radish (Raphanus sativus). Environ. Pollut 259, 113815. [DOI] [PubMed] [Google Scholar]
- Oguri T, Suzuki G, Matsukami H, Uchida N, Tue NM, Tuyen LH, Viet PH, Takahashi S, Tanabe S, Takigami H, 2018. Exposure assessment of heavy metals in an e-waste processing area in northern Vietnam. Sci. Total Environ 621, 1115–1123. [DOI] [PubMed] [Google Scholar]
- Okkenhaug G, Grasshorn Gebhardt KA, Amstaetter K, Lassen Bue H, Herzel H, Mariussen E, Rossebø Almås Å, Cornelissen G, Breedveld GD, Rasmussen G, Mulder J, 2016. Antimony (Sb) and lead (Pb) in contaminated shooting range soils: Sb and Pb mobility and immobilization by iron based sorbents, a field study. J. Hazard. Mater 307, 336–343. [DOI] [PubMed] [Google Scholar]
- Peng Q, Li J, Wang D, Wei TJ, Chen CEL, Liang DL, 2019. Effects of ageing on bioavailability of selenium in soils assessed by diffusive gradients in thin-films and sequential extraction. Plant Soil 436, 159–171. [Google Scholar]
- Ravel B, Newville M, 2005. Athena, artemis. HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat 12, 537–541. [DOI] [PubMed] [Google Scholar]
- Ruby MV, Davis A, Schoof R, Eberle S, Sellstone CM, 1996. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ. Sci. Technol 30, 422–430. [Google Scholar]
- Sanderson P, Naidu R, Bolan N, 2014. Ecotoxicity of chemically stabilised metal(loid)s in shooting range soils. Ecotoxicol. Environ. Safe 100, 201–208. [DOI] [PubMed] [Google Scholar]
- Scheinost AC, Rossberg A, Vantelon D, Xifra I, Kretzschmar R, Leuz A-K, Funke H, Johnson CA, 2006. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Cosmochim. Acta 13, 3299–3312. [Google Scholar]
- Sh T, Liu CQ, Wang L, 2012. Antimony coordination to humic acid: nuclear magnetic resonance and X-ray absorption fine structure spectroscopy study. Microchem. J 103, 68–73. [Google Scholar]
- Steely S, Amarasiriwardena D, Xing B, 2007. An investigation of inorganic antimony species and antimony associated with soil humic acid molar mass fractions in contaminated soils. Environ. Pollut 148, 590–598. [DOI] [PubMed] [Google Scholar]
- Tang XY, Zhu YG, Cui YS, Duan J, Tang L, 2006. The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China. Environ. Int 32, 682–689. [DOI] [PubMed] [Google Scholar]
- Tang XY, Zhu Y-G, Shan X-Q, McLaren R, Duan J, 2007. The ageing effect on the bioaccessibility and fractionation of arsenic in soils from China. Chemosphere 66, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Telford K, Maher W, Krikowa F, Foster S, 2008. Measurement of total antimony and antimony species in mine contaminated soils by ICPMS and HPLC-ICPMS. J. Environ. Monit 10, 136–140. [DOI] [PubMed] [Google Scholar]
- Tella M, Pokrovski GS, 2009. Antimony(III) complexing with O-bearing organic ligands in aqueous solution: an X-ray absorption fine structure spectroscopy and solubility study. Geochim. Cosmochim. Acta 73, 268–290. [Google Scholar]
- Tella M, Pokrovski GS, 2012. Stability and structure of pentavalent antimony complexes with aqueous organic ligands. Chem. Geol 292–293, 57–68. [Google Scholar]
- U.S. EPA, 1987. Antimony (CASRN 7440-36-09). https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0006_summary.pdf, Accessed date: 07 January 2021.
- U.S. EPA, 2009. National Primary Drinking Water Regulations. [Google Scholar]
- U.S. EPA, 2011. Exposure Factors Handbook. 2011 edition. US Environmental Protection Agency, Washington: http://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=522996, Accessed date: 07 January 2021. [Google Scholar]
- Udovic M, McBride MB, 2012. Influence of compost addition on lead and arsenic bioavailability in reclaimed orchard soil assessed using Porcellio scaber bioaccumulation test. J. Hazard. Mater 205–206, 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerbeck M, Thiry Y, Smolders E, 2020. Antimonate sorption in soils increases with ageing. Eur.J. Soil Sci 71, 55–59. [Google Scholar]
- Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M, 2010. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr 10, 268–292. [Google Scholar]
- Wang P, Lombi E, Sun S, Scheckel KG, Malysheva A, McKenna BA, Menzies NW, Zhao FJ, Kopittke PM, 2017. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano 4, 448–460. [PMC free article] [PubMed] [Google Scholar]
- Wang M, Cui Z, Xue M, Peng Q, Zhou F, Wang D, Dinh QT, Liu Y, Liang D, 2019. Assessing the uptake of selenium from naturally enriched soils by maize (Zea mays L.) using diffusive gradients in thin-films technique (DGT) and traditional extractions. Sci. Total Environ 689, 1–9. [DOI] [PubMed] [Google Scholar]
- Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC, 2001. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 436, 309–323. [Google Scholar]
- Wilson SC, Lockwood PV, Ashley PM, Tighe M, 2010. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: a critical review. Environ. Pollut 158, 1169–1181. [DOI] [PubMed] [Google Scholar]
- Xi J, He M, Kong L, 2016. Adsorption of antimony on kaolinite as a function of time, pH. HA and competitive anions. Environ. Earth Sci 75, 1–7. [Google Scholar]
- Yang J-K, Barnett MO, Jardine PM, Brooks SC, 2003. Factors controlling the bioaccessibility of arsenic(V) and lead(II) in soil. Soil Sediment Contam. 12 (2), 165–179. [Google Scholar]
- Zhang H, Davison W, 2000. Direct in situ measurements of labile inorganic and organically bound metal species in synthetic solutions and natural waters using diffusive gradients in thin films. Anal. Chem 72, 4447. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wu TL, Ata-Ul-Karim ST, Ge YY, Cui X, Zhou DM, Wang YJ, 2020. Influence of soil properties and aging on antimony toxicity for barley root elongation. B. Environ. Contam. Tox 104, 714–720. [DOI] [PubMed] [Google Scholar]
- Zhou JW, Wu LH, Zhou T, Li Z, Sun XY, Luo YM, Christie P, 2019. Comparing chemical extraction and a piecewise function with diffusive gradients in thin films for accurate estimation of soil zinc bioavailability to Sedum plumbizincicola. Eur. J. Soil Sci 70, 1141–1152. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


