Abstract
Background:
CABANA randomized 2204 patients with atrial fibrillation (AF) to catheter ablation or drug therapy. Analysis by intention-to-treat (ITT), showed a non-significant 14% relative reduction in the primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
Objective:
To assess recurrence of atrial fibrillation (AF) in The Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA)
Methods:
We prospectively studied CABANA patients using a proprietary ECG recording monitor for symptom-activated and 24-hour AF auto detection. The AF recurrence endpoint was any post 90-day blanking atrial tachyarrhythmias lasting 30 seconds or longer. Ninety-six-hour Holter monitoring obtained biannually was used to assess AF burden. Patients using the CABANA monitors and provided 90-day post-blanking recordings qualified for this analysis (1240, 56% of CABANA population). Treatment comparisons were performed using a modified- ITT approach.
Results:
Median age of the 1240 patients was 68 years, 34.4% women, and AF was paroxysmal in 43.0%. Over 60 months of follow-up, first recurrence of any symptomatic or asymptomatic AF (hazard ratio (HR) 0.52, 95% confidence interval (CI) 0.45, 0.60, P<0.001) or, first symptomatic-only AF (HR 0.49, 95% CI 0.39, 0.61, P<0.001) were both significantly reduced in the catheter ablation group. Baseline Holter AF burden in both treatment groups was 48%. At 12 months, AF burden in ablation patients averaged 6.3%, and in drug therapy patients, 14.4%. AF burden was significantly less in catheter ablation compared with drug therapy patients across the 5-year follow-up (P <0.001). These findings were not sensitive to the baseline pattern of AF.
Conclusions:
Catheter ablation was effective in reducing recurrence of any AF by 48% and symptomatic AF by 51% as compared to drug therapy over 5 years of follow-up. Furthermore, AF burden was also significantly reduced in catheter ablation patients, regardless of their baseline AF type.
Keywords: Atrial Fibrillation, Paroxysmal Atrial Fibrillation, Persistent Atrial Fibrillation, Long-Standing Persistent Atrial Fibrillation, Anti-Arrhythmic Drug Therapy, Catheter Ablation, Pulmonary Vein Isolation
Condensed Abstract:
This analysis of the CABANA trial examined the benefit of catheter ablation compared to drug therapy to reduce recurrence of atrial fibrillation (AF) in symptomatic patients over 5 years follow-up. The patient population comprised the 1240 patients who used the CABANA specific ECG recording monitor system. Catheter ablation was associated with a significant reduction in recurrence of any AF, (HR 0.52, [95% CI 0.45, 0.60], P<0.001) and symptomatic AF (HR 0.49, 95% CI 0.39, 0.61, P<0.001) compared to drug therapy. AF burden was significantly reduced as assessed by 6-month Holter monitors (P<0.01) regardless of baseline pattern of AF.
Introduction
Over the last twenty years, catheter ablation for atrial fibrillation (AF) has moved from a therapy of last resort for drug-refractory, highly symptomatic patients, to an accepted first line option for patients across the spectrum of AF type and severity. [1,2,3] Part of this evolution can be attributed to the recognition of the central importance of pulmonary vein isolation to the success of AF ablation, along with progress in substantially reducing the most serious procedural risks. As early difficulties with the procedure were overcome with improved equipment and greater experience, advocates speculated that it might be possible to provide permanent cures for at least some AF patients with catheter ablation, particularly if used before irreversible atrial remodeling had taken place. [2,4,5] The difficulties in detecting recurrent AF episodes, particularly when asymptomatic, led to a long period of controversy regarding the actual durability of AF suppression after ablation. [4–12].
The results of the recently reported CABANA trial support the use of catheter ablation as an effective treatment strategy for a broad group of AF patients.[13] CABANA compared the efficacy of catheter ablation to drug therapy (rate or rhythm control) in 2204 patients with symptomatic atrial fibrillation (AF). Eligible patients were 65 years of age or older, or if less than 65 years, were required to have one or more major risk factors for stroke.[13,14] Patients randomized to ablation were required to have pulmonary vein isolation, with ancillary ablation approaches used at the discretion of the treating physician. A total of 1108 patients were randomized to catheter ablation while 1096 patients were randomized to drug therapy. As previously reported, the median age of all enrolled CABANA patients was 68 years, 37.2% were women, 35.4% had heart failure (defined by a baseline NYHA score ≥ 2) and 57.1% had persistent or long-standing persistent AF. Most patients randomized to the drug therapy arm (88.4%) received a rhythm control medication with the intent to maintain normal rhythm [13].
The primary outcome for CABANA was a composite of death, disabling stroke, serious bleeding or cardiac arrest. The median duration of follow up was 48.5 months (25th to 75th percentiles 29.9 to 62.1 months). By intention-to-treat analysis (ITT), the primary results showed a 14% reduction in the primary endpoint (hazard ratio (HR) 0.86, 95% confidence interval (CI) 0.65–1.15; P=0.303 and a 15% reduction in the key secondary endpoint of all-cause mortality (HR 0.85, 95% CI 0.60–1.21; P=0.377). [13].
An important secondary objective of the CABANA trial was to define the long-term risk of AF recurrence in patients treated with catheter ablation compared to drug therapy, both in terms of first recurrence, as well as the overall burden of AF. Initial results reported from the CABANA study showed a significant 48% reduction in recurrence of AF with follow-up to 48 months. [13] In this report, we provide an expanded analysis of the CABANA ECG rhythm data-set. New information regarding the effect of randomized treatment on AF recurrence outcomes is presented with follow-up to 60 months. Additionally, we present data on first recurrence of symptomatic episodes and on AF burden.
Methods
CABANA ECG Core Lab Monitoring Protocol
The CABANA study protocol utilized a CABANA specific proprietary ECG monitoring system intended for all enrolled patients (Medicomp, Inc. Melbourne, Florida). This monitor used interchangeable finger-electrode and 2-channel cables that provide transtelephonic monitor (TTM) symptom-driven two-minute recordings, 24-hour TTM autodetect triggered recordings for atrial tachyarrhythmias, and up to 96-hours of continuous Holter recordings. More details are available in the Appendix.
Patient triggered; symptom-driven recordings were obtained throughout the follow-up period in the trial. Specific symptoms were reported to the site using a standard checklist for all TTM recordings. Autodetect 24-hour loop recordings were obtained once per month in the first year and then quarterly throughout the rest of the trial. Every 6 months, the monitor was programmed to automatically provide up to 96-hours of Holter monitoring for assessment of AF burden. AF burden was defined as the average percentage of time in AF relative to the total analyzable patient Holter recording-time. Systematic collection of symptoms was not obtained for the Holter monitor recordings.
All TTM and Holter rhythm recordings received initial external review by the cardiac device monitoring personnel following CABANA standards, with a clinical report provided to the enrolling sites. Additionally, symptom-driven and autodetect rhythms meeting the definition of an endpoint (30 seconds or longer of AF, atrial flutter (AFL) or atrial tachycardia (AT) were transferred to the CABANA ECG Core Lab (Seattle, WA). These were then posted to a secure on-line reading site used by the CABANA ECG Core Lab committee members for final review and adjudication. Each arrhythmia was read by two expert physicians with disagreements settled by a third reviewer. Holter monitor recordings were subjected to a 10% overread by the ECG Core Lab for quality assurance. All atrial tachyarrhythmia endpoints in this analysis, are reported after a 90-day blanking period following initiation of randomly assigned ablation or drug therapy.
Study Patient Population
The study population is described in Figure 1. Of the 2204 enrolled patients, 161 did not have post 90-day blanking data and were excluded from this analysis, (65.8% of whom never received their randomized therapy). Of the remaining patients, 1240 were monitored for recurrent AF, using the specific ECG recording monitor system provided by the CABANA trial, and comprise the study population for this analysis.
Figure 1. Patient Flow in CABANA Recurrent Atrial Fibrillation Analysis.

A) A 90-day blanking period from therapy initiation was used in both randomized treatment groups, during which arrhythmia recurrences were not counted toward the recurrent AF endpoint. 161 patients were excluded as they did not contribute to the post 90-day blanking arrhythmia assessment. B) The CABANA ECG recording monitors were used by the majority of enrollment sites and patients. Patients who were at sites not able to use the CABANA ECG recording monitor, or who declined to use them, were monitored according to their sites’s conventional rhythm recording monitors.
The data from the CABANA study monitors were rigorously and consistently collected, and carefully monitored across both randomized treatment groups. Most of the 126 enrolling sites (108 sites – 86%) were able to use these monitors. Those centers who were unable to use the CABANA study monitors, often due to country-specific regulatory barriers regarding importation of medical diagnostic equipment, used their institution’s conventional ECG recording monitor systems as an alternative. There was also a small number of patients who declined to use the monitor provided by the trial and were similarly monitored using their site’s usual practice. Limited data on those not using the CABANA study monitors can be found in the Appendix.
All sites received approval from their institutional review boards or ethics committee to participate in CABANA. All patients provided written informed consent.
Statistical Analyses
Baseline demographic and clinical characteristics were summarized as medians (25th and 75th percentiles) for continuous variables, and counts (percentages) for categorical variables. Group comparisons with respect to baseline characteristics were performed using Pearson’s chi-square test or Fisher’s exact test for categorical data, and the Wilcoxon rank-sum test for continuous data.
Data completeness of the 24-hr TTM recordings and 96-hr Holter recordings was summarized as medians (25th and 75th percentiles) of recordings obtained within designated time intervals, compared with the number of recordings expected. Patients were counted as eligible for a Holter or TTM recording within a specific time interval if alive and still being followed in the trial at the end of the interval.
Time-to-event comparisons of recurrent arrhythmias, analyzed in the post blanking period (with ‘time-zero’ occurring after 90 days since randomized treatment), were performed between groups as a modified intention-to-treat (mITT) analysis using a covariate adjusted Cox proportional hazards model [15]. The Cox model utilized an adjustment for the following pre-specified baseline covariates: age at enrollment, race (white vs. racial minorities), AF type (paroxysmal, persistent, or longstanding persistent), years since onset of AF, history of congestive heart failure, structural heart disease (present vs. absent), CHA2DS2-VASc score, history of coronary artery disease, and hypertension (present vs. absent). The mITT analysis only included patients with CABANA study monitor data collected greater than 90 days after receiving randomized treatment (in the post-blanking period). Hazard ratios and associated 95% confidence intervals were derived from the Cox model, and p-values were based on the Wald statistics. Cumulative incidence estimates using death as a competing risk were derived using Fine-Gray competing risks methodology [16] and graphically depict freedom from recurrence. This study reports the results for the patients using the CABANA study recorder.
A comparison of treatment groups with respect to percent AF burden from the Holter monitoring recordings was performed using a repeated-measure mixed linear model. Holter monitoring records were also presented graphically (in conjunction with medication and ablation records) in order to summarize subjects on an anti-arrhythmic drug (AAD) at the time of recording, or who were randomized to drug therapy and had received an ablation prior to the recording. Subjects with any Holter monitoring during a designated time-interval were used as a denominator to calculate percentages, and subjects were included in the numerator if no 30-second AF episode was captured during a given time interval. As a result of including patients in the denominator who have non-missing data at a specific timepoint window, but potentially have missing data for other timepoint windows, the number of patients included may increase or decrease from one timepoint to the next.
Whereas time-to-event recurrence analyses were exclusively performed on data captured in the post-blanking period, analyses related to AF burden as well as AAD use among subjects without AF recurrence were started at randomization. Statistical tests are reported without adjustment for multiple comparisons. All statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline Clinical Characteristics
For the 1240 patients in our primary analysis of AF recurrence, the median age was 68 years, 34.4% were women, and 6.0% were African American or belonged to other non-white racial groups (Table 1). The baseline pattern of AF was paroxysmal in 43.0% and persistent or long-standing persistent in 57.0%. The median time from AF onset to enrollment in CABANA was 1 year (25th percentile: 0 years, 75th percentile: 4 years) and the median CHA2DS2-VASc score was 3 (25th percentile: 2, 75th percentile: 4) (Table 1). Baseline demographics and clinical characteristics by randomized therapy were overall comparable.
Table 1.
Baseline Demographics and Clinical Characteristics By Randomized Treatment Group in 1240 Patients Using the CABANA ECG Recording Monitors Post 90-Day Blanking
| Baseline Characteristics | Drug Group N=629 No. (%)* |
Ablation Group N=611 No. (%)* |
Total N=1240 No. (%)* |
|---|---|---|---|
| Age | |||
| Median (Q1, Q3) | 68 (64, 73) | 68 (64, 73) | 68 (64, 73) |
| < 65 yrs | 183 (29.1%) | 176 (28.8%) | 359 (29.0%) |
| ≥ 65 to <75 yrs | 350 (55.6%) | 342 (56.0%) | 692 (55.8%) |
| ≥ 75 yrs | 96 (15.3%) | 93 (15.2%) | 189 (15.2%) |
| Gender | |||
| Female | 216 (34.3%) | 211 (34.5%) | 427 (34.4%) |
| Male | 413 (65.7%) | 400 (65.5%) | 813 (65.6%) |
| Racea | |||
| White | 592 (94.3%) | 573 (93.8%) | 1165 (94.0%) |
| Black or African American | 19 (3.0%) | 21 (3.4%) | 40 (3.2%) |
| Otherb | 17 (2.7%) | 17 (2.8%) | 34 (2.7%) |
| Minoritya: Hispanic or non-White | 51 (8.1%) | 51 (8.4%) | 102 (8.3%) |
| BMI (kg/m2): Median (Q1, Q3) | 31 (27, 36) | 30 (27, 35) | 31 (27, 35) |
| AF Severity (CCS Class)c | |||
| 0 (least severe) | 43 (6.9%) | 39 (6.4%) | 82 (6.6%) |
| 1 | 77 (12.3%) | 72 (11.8%) | 149 (12.1%) |
| 2 | 195 (31.2%) | 175 (28.7%) | 370 (29.9%) |
| 3 | 260 (41.5%) | 275 (45.1%) | 535 (43.3%) |
| 4 (most severe) | 51 (8.1%) | 49 (8.0%) | 100 (8.1%) |
| Heart function severity (NYHA Class)d | |||
| No CHF or Class I (least severe) | 457 (73.1%) | 446 (73.4%) | 903 (73.2%) |
| Class II or greater (most severe) | 168 (26.9%) | 162 (26.6%) | 330 (26.8%) |
| Medical History | |||
| Hypertension (> 140/90 mmHg) | 500 (79.5%) | 472 (77.3%) | 972 (78.4%) |
| Baseline left ventricle hypertrophy | 157 (36.9%) | 163 (34.3%) | 320 (35.6%) |
| Hypertension or LVH | 512 (87.7%) | 502 (86.7%) | 1014 (87.2%) |
| Diabetes (Glucose ≥126 mg/dl) | 174 (27.7%) | 162 (26.5%) | 336 (27.1%) |
| CVA (prior) | 32 (5.1%) | 33 (5.4%) | 65 (5.2%) |
| Prior CVA or TIA | 57 (9.1%) | 69 (11.3%) | 126 (10.2%) |
| Thromboembolic events (peripheral) | 25 (4.0%) | 25 (4.1%) | 50 (4.0%) |
| Coronary artery disease | 138 (21.9%) | 129 (21.1%) | 267 (21.5%) |
| Congestive heart failure | 94 (14.9%) | 99 (16.2%) | 193 (15.6%) |
| Sleep apnea | 188 (29.9%) | 188 (30.8%) | 376 (30.3%) |
| Family history of atrial fibrillation | 89 (14.2%) | 97 (15.9%) | 186 (15.0%) |
| Left ventricular ejection fraction ≤ 35** | 11 (2.7%) | 23 (5.5%) | 34 (4.1%) |
| Co-morbidities | |||
| CHA2DS2-VASc Scoree | |||
| Median (Q1, Q3) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) |
| 0–1 (lowest risk) | 99 (15.7%) | 104 (17.0%) | 203 (16.4%) |
| 2 | 156 (24.8%) | 149 (24.4%) | 305 (24.6%) |
| 3 | 207 (32.9%) | 171 (28.0%) | 378 (30.5%) |
| 4 | 89 (14.1%) | 99 (16.2%) | 188 (15.2%) |
| ≥5 (9 highest risk) | 78 (12.4%) | 88 (14.4%) | 166 (13.4%) |
| Arrhythmia History | |||
| Years since onset of AF: Median (Q1, Q3) | 1 (0, 3) | 1 (0, 4) | 1 (0, 4) |
| Type of atrial fibrillationf | |||
| Paroxysmal | 273 (43.4%) | 260 (42.6%) | 533 (43.0%) |
| Persistent | 300 (47.7%) | 302 (49.4%) | 602 (48.5%) |
| Longstanding Persistent | 56 (8.9%) | 49 (8.0%) | 105 (8.5%) |
| Prior hospitalization for treatment of AF | 215 (34.2%) | 213 (34.9%) | 428 (34.5%) |
| Prior direct current cardioversion - AF | 252 (40.1%) | 240 (39.3%) | 492 (39.7%) |
| History of atrial flutter | 100 (16.0%) | 91 (15.1%) | 191 (15.5%) |
| Prior ablation for atrial flutter | 34 (5.4%) | 34 (5.6%) | 68 (5.5%) |
| Rhythm Control Therapyg | |||
| 1 Rhythm control drug | 254 (83.0%) | 203 (79.9%) | 457 (81.6%) |
| ≥ 2 Rhythm control drugs | 52 (17.0%) | 51 (20.1%) | 103 (18.4%) |
| Blanking Period Information | |||
| Days from randomization to end of blanking period*** | |||
| Median (Q1, Q3) | 90 (90, 93) | 119 (106, 139) | 101 (90, 121) |
Unless otherwise noted
0.01 < p < 0.05
p < 0.01
Q1 and Q3=quartiles (25th and 75th percentiles), CCS=Canadian Cardiovascular Society, AF=atrial fibrillation, NYHA=New York Heart Association, CHF=congestive heart failure, LVH=left ventricular hypertrophy, CVA=cerebral vascular accident, TIA=transient ischemic attack
Race/Minority was determined by the site investigator in conjunction with the patient based on predefined categories as required by the National Institute of Health (NIH) using NIH-specified categories.
Asian, American Indian/Alaskan Indian, Hawaiian or Other Pacific Islander and Multiracial
On a scale of 0 to 4 in which 0 is the least severe and 4 is the most severe symptom of AF
On a scale of I to IV in which I is the least severe and IV is the most severe symptom of heart failure
On a scale of 0 to 9 in which 0 is the lowest risk of stroke and 9 is the highest risk of stroke
Type of AF (paroxysmal = AF episodes lasting ≥1 hour in duration that terminates spontaneously within 7 days or cardioversion is performed within 48h of AF onset; Persistent = AF episode sustained for ≥ 7 days or cardioversion is performed more than 48h after AF onset; Longstanding Persistent = continuous AF of duration >1 year
Current or past use of Rhythm Control Therapy reported at the time of enrollment
Patient Use of the CABANA ECG Recording Monitors
Of the 12 expected times a 24-hour TTM was scheduled to be used in the first year, the median number of per-patient uses of the TTM was 10 (25th percentile: 6, 75th percentile: 11) (Table 2). At five years, the expected number of times the TTMs were to be used was 28, and the median actual per-patient use was 23 (25th percentile: 15, 75th percentile: 26) (Table 2). For Holter monitoring, patients were expected to provide up to 96-hours of Holter recordings every 6 months throughout the entire trial. Of the two timepoints during which patients were anticipated to use their Holter in the first year, the median per-patient use was 2 (25th percentile: 1, 75th percentile: 2). At five years, the expected number of Holter uses was 10, and the median actual per-patient use was 9 (25th percentile: 5, 75th percentile: 10) (Table 2).
Table 2.
Per Patient Use of 24-Hour TTM and 6-month Holter Recordings in 1240 patients Using the CABANA ECG Recording Monitors
| Time Period | Eligible Number of Patients | TTM Recordings Actually Performed Median (Q1, Q3) | Protocol-Specified Number of TTM Recordings |
|---|---|---|---|
| Month 1 – 12 | 1214 | 10 (6, 11) | 12 |
| Month 1 – 24 | 1132 | 13 (8, 15) | 16 |
| Month 1 – 36 | 968 | 16 (9, 19) | 20 |
| Month 1 – 48 | 764 | 19 (12, 23) | 24 |
| Month 1 – 60 | 491 | 23 (15, 26) | 28 |
| Time Period | Eligible Number of Patients | Holter Recordings Actually Performed Median (Q1, Q3) | Protocol-Specified Number of Holter Recordings |
| Month 6 – 12 | 1208 | 2 (1, 2) | 2 |
| Month 6 – 24 | 1129 | 4 (2, 4) | 4 |
| Month 6 – 36 | 965 | 5 (3, 6) | 6 |
| Month 6 – 48 | 760 | 7 (4, 8) | 8 |
| Month 6 – 60 | 496 | 9 (5, 10) | 10 |
Median and interquartile ranges are shown for patient use of 24-hr TTM and for the 6-month Holter monitoring. The assessment is cumulative for each successive year of follow up. TTM=transtelephonic monitoring
Recurrence of Atrial Tachyarrhythmias Post-90-Day Blanking Among 1240 Patients Using the CABANA ECG Recording Monitor by mITT
Cumulative incidence estimates were compared for patients whose first recurrence was noted to be associated with symptoms and for patients with any recurrence of AF (symptomatic or asymptomatic -whichever occurred first). Catheter ablation was associated with a significant reduction in recurrence of symptomatic AF (HR 0.49, 95% CI 0.39–0.61, P<0.001) and any AF (AF: HR 0.52, 95% CI 0.45–0.60, P<0.001) (Central Illustration).
Central Illustration: 1240 Patients Using the CABANA ECG Recording Monitors Post 90-Day Blanking.

* Monitoring for recurrent atrial tachyarrhythmias used a CABANA specific ECG monitor, which included finger-tip symptom-driven TTM monitoring. Additionally, 24 hour continuous TTM loop recordings with AF auto-detection were obtained monthly in the first year of follow-up and then quarterly thereafter. Cumulative incidence of first symptomatic AF episode and cumulative incidence of first symptomatic or asymptomatic AF, is shown as, freedom from recurrence over five years following the 90-day blanking period post randomized therapy (both ablation and drug therapy). Cumulative incidence estimates using death as a competing risk were derived using Fine-Gray competing risks methodology. CI=Confidence interval; TTM= transtelephonic monitoring; symp=symptomatic. *Patients who did not receive randomized therapy, did not use the CABANA ECG recording system, or for any other reason, did not provide post 90-day blanking recordings (Figure 1) are not included in this analysis.
By 12 months, 12.6% of the ablation patients and 27.5% of the drug therapy patients had a recurrence of symptomatic AF and 36.4% of the ablation patients and 59.2% of the drug therapy patients had experienced a first recurrence of any AF. By tallying individual patient records over the full duration of follow-up, 18.4% of the ablation arm and 23.1% of the drug arm patients who had a recurrence experienced a first recurrence that was symptomatic.
Over 5 years of follow up, we identified a significant 48% reduction in time to first recurrence of AF (HR 0.52,[95% CI 0.45–0.60], p<0.001) and a 47% reduction in time to first recurrence of the composite AF, AFL or AT (HR 0.53, [0.46–0.62], p<0.001) (Table 3, Figure 2). AFL and AT did not contribute substantially to the first recurrent atrial arrhythmia.
Table 3.
Cumulative incidence of first recurrent atrial arrhythmias recorded during the CABANA ECG recording monitors post 90-day blanking*”
| Patients with Events, No. | Cumulative Incidence 4-Year Event Rate, % | |||||||
|---|---|---|---|---|---|---|---|---|
| Catheter Ablation (n = 611) |
Drug Therapy (n = 629) |
Catheter Ablation (n = 611) |
Drug Therapy (n = 629) |
Absolute Reduction | Hazard Ratio (95% CI) (Ablation : Drug) |
P-value | ||
| Recurrent AF | 305 | 437 | 52.1 | 70.8 | 18.7 | 0.52 (0.45, 0.60) | <.001 | |
| Recurrent AF, AFL, or AT | 317 | 444 | 53.8 | 71.9 | 18.1 | 0.53 (0.46, 0.62) | <.001 | |
AF=atrial fibrillation; AFL=atrial flutter, AT=atrial tachycardia
Cumulative Incidence 4-Year Event Rate, %” was estimated from a Fine & Gray analysis with death a competing risk event. Patients who did not receive randomized therapy, did not use the CABANA ECG recording system, or for any other reason did not provide post 90-day blanking recordings (see Figure 1) are not included in this analysis.
Figure 2: Freedom From Recurrent Atrial Fibrillation, Atrial Flutter or Atrial Tachycardia by Randomized Therapy in 1240 patients Using the CABANA ECG Recording Monitors Post 90-Day Blanking.

* Figure 2 shows the cumulative incidence of a first occurrence of AF, or the composite of AF, AFL or AT over five years of follow up. Results are shown according to randomized therapy. Cumulative incidence estimates using death as a competing risk were derived using Fine-Gray competing risks methodology. AF=atrial fibrillation; AFL=atrial flutter; AT=atrial tachycardia; CI=Confidence interval; *Patient population shown in Figure 1.
Recurrence of Atrial Tachyarrhythmias Post-90-Day Blanking Among 1240 Patients Using the CABANA ECG Recording Monitor by Per-Protocol and As-Treated Analyses
Adjusted per-protocol and as-treated analyses were performed which included the same pre-specified baseline covariates as in the mITT analysis and were conducted using the same statistical approach previously reported in CABANA (13). The per-protocol analysis showed a 52% reduction in first recurrence of AF (HR 0.48, [95% CI 0.42, 0.56], P<0.001) and the as-treated analysis showed a 51% reduction in first recurrence of AF (HR 0.49, [95% CI 0.42, 0.57], P<0.001). Both analyses have shown a similar result as the primary mITT analysis.
Atrial Fibrillation Burden
AF burden was assessed from Holter monitor recordings in patients using the CABANA ECG recording monitor. At baseline, AF was present for almost 50% of the recorded monitoring time in both randomized groups (Figure 3). At subsequent timepoints, the average AF burden of patients randomized to ablation demonstrated a relative reduction of 69% – 88% (33% – 42% absolute), and the average AF burden of patients randomized to drug therapy demonstrated a relative reduction of 48% – 73% (23% – 35% absolute). At 12 months, the AF burden averaged 6.3% of recorded Holter time for the ablation group and 14.4% for the drug therapy group. At 5 years, the corresponding percentages were 14.7% and 20.8%, respectively. While AF burden was reduced in both randomized therapy groups, the treatment difference was greater in ablation treated patients, and significant across the five years of follow-up (P<0.01). AF burden was significantly reduced by catheter ablation regardless of the patients’ baseline AF pattern (paroxysmal, or persistent/long-standing persistent). Although AF burden at baseline was greater in the patients with persistent/long-standing persistent AF, the absolute magnitude of treatment benefit difference was larger for these patients compared to those with paroxysmal AF (Figure 4).
Figure 3. Atrial Fibrillation Burden Post 90-Day Blanking Assessed at Six-Month Intervals in 1240 Patients Using the CABANA ECG Holter Monitors.

* The percentage AF burden is shown according to randomized treatment groups as assessed at each of the 6-month Holter recording time-points. AF=atrial fibrillation. *Patient population shown in Figure 1.
Figure 4: Atrial Fibrillation Burden According to Baseline Pattern of Atrial Fibrillation, 90-Day Blanking Assessed at Six-Month Intervals in 1240 Patients Using the CABANA ECG Holter Monitors.
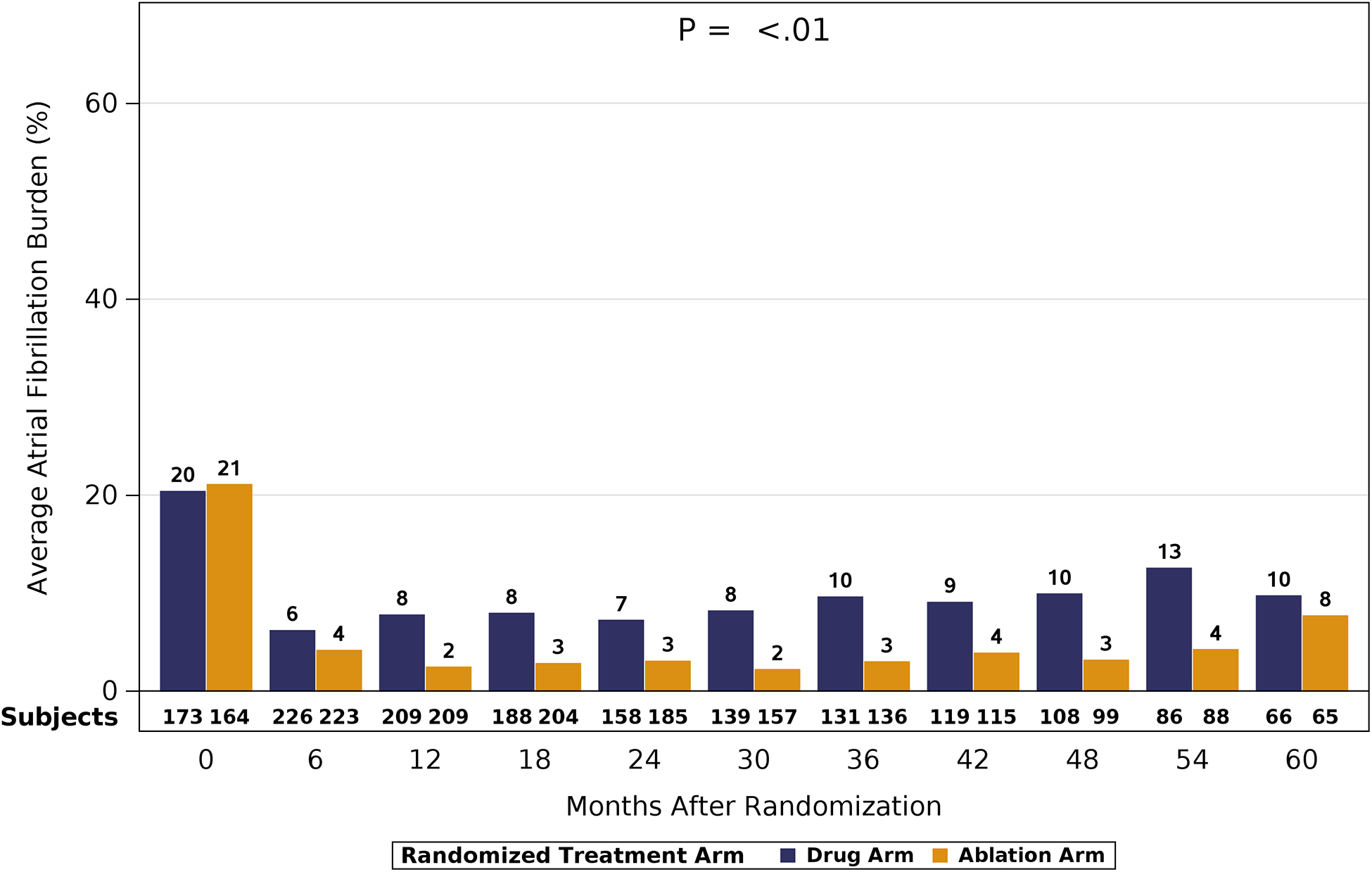

*AF burden is shown according to randomized treatment groups as assessed at each of the 6-month Holter recordings. 4A shows AF burden in patients who were considered paroxsymal AF at baseline and 4B shows AF burden for patients considered to have persistent or long-standing persistent AF at baseline. AF=atrial fibrillation.*Patient population shown in Figure 1.
Use of Antiarrhythmic Drugs
Use of antiarrhythmic drugs (AAD) in CABANA patients randomized to ablation was at the discretion of the managing physician, although discontinuation was encouraged. We assessed the use of AADs over the 5 years of follow-up in patients who did not have 30 seconds or more of AF identified on the 6-month Holter recordings (Figure 5). AAD use was assessed by matching dates from medication logs with dates of Holter acquisition. At 12 months post randomization, AAD usage was only 20% for patients randomized to ablation who were free of Holter-determined AF (≥30 seconds). Over the 5 years of follow up, AAD use in the ablation randomized patients remained low (10–20%).
Figure 5: Use of Antiarrhythmic Drugs Among Subjects Without AF Recurrence Post 90-Day Blanking Assessed at Six-Month Intervals in 1240 Patients Using the CABANA ECG Holter Monitors.

* The percentage of patients without an AF episode lasting 30 seconds or more in duration captured at any time during the scheduled 6-month Holter recordings is shown in this graph. Results are displayed according to randomized therapy. The insets within the bars shows the proportion of patients taking or not taking an antiarrythmic drug at the time of Holter acquisition. The cross hatching within the randomized drug therapy arm shows the percentage of patients who were not taking an antiarrhythmic drug and had crossed over from drug therapy to ablation therapy prior to Holter acquistion at that 6 month timepoint. AF=atrial fibrillation; AAD=antiarrhythmic drug. *Patient population shown in Figure 1.
In patients randomized to drug therapy, 57% of the patients were taking an AAD at 12 months. Over the 5 years of follow up, AAD use tapered to 33% in these patients. The decreasing use of AADs over time in the drug randomized patients appears to have been largely impacted by patient cross-overs to ablation therapy.
Discussion
Our study has several important findings. The first key finding is that randomization to catheter ablation was associated with a significant ~ 50% reduction in first recurrence of AF, AFL or AT regardless of whether the first recurrent event was symptomatic or asymptomatic. The second key finding was that the occurrence of AFL or AT as the initial recurrent post treatment arrhythmia contributed little to first recurrent atrial tachyarrhythmias. The third key finding is that AF burden, measured as the average of proportions of time subjects were in AF during the Holter-monitor, was significantly less for the ablation treated patients compared to drug treated patients over the entire 5 years of follow-up. The findings were consistent, regardless of baseline AF type (paroxysmal or persistent/long-standing persistent). The absolute reduction in AF burdenwas greater for those patients with baseline persistent or longstanding persistent AF.
This analysis examines the durability of AF control using two rhythm monitoring methods to identify AF recurrence. [13,14] The first, reflects the definition of AF recurrence as stated in the 2012 HRS/EHRA/ECAS/ACC/AHA/ APHRS/STS/ESC expert consensus statement on catheter and surgical ablation of atrial fibrillation:[2] which defines recurrence as an episode of continuous AF lasting for 30 seconds or longer. The second method used was the average percentage AF burden as determined from six-month Holter recordings, which provided an overall assessment of AF burden in the patients analyzed. To achieve a robust assessment of recurrent atrial tachyarrhythmias, CABANA used a combination of ECG rhythm recording methods. The frequency of monitoring sought to balance patient acceptance, with monitoring intensity adequate to capture symptomatic and asymptomatic atrial arrhythmia events.
CABANA has several important differences compared to earlier and smaller trials of ablation therapy. [5–12] CABANA patients with either treatment-naïve or undertreated AF, could be enrolled, regardless of whether the patient had paroxysmal or persistent/long-standing persistent AF. These factors, in addition to the longer follow-up and larger number of patients enrolled, allowed for a robust analysis of the effect of AF catheter ablation to prevent recurrent AF in a broad population of AF patients.
Although CABANA enrolled patients with symptomatic AF, we observed that much of the first recurrence of AF was asymptomatic, consistent with prior population studies, more recent studies of patients with previously unknown AF detected on implantable pacemakers, and post-AF catheter ablation studies. [17–23] Throughout the study, we observed that only ~18% of first recurrent AF was symptomatic in the catheter ablation group and ~23% in the drug treatment group.
An interesting finding from our study was the small contribution of atrial flutter or atrial tachycardia as the first recurrent atrial arrhythmia type, regardless of randomization. These highly symptomatic rhythms often require repeat ablation, and often are seen in patients with . persistent or long-standing persistent AF; patients particularly challenging for AF management [9, 11, 24–26]. CABANA stipulated that pulmonary vein isolation be performed in all ablation patients; additional ablation (19%) was at the discretion of the investigator. It is unknown whether these or other aspects of the CABANA trial led to the observed low incidence of initial organized atrial arrhythmias.
AF burden is a conceptually appealing alternative endpoint measure for identification of discrete time-based episodes of AF (e.g., 30 seconds). With this approach, even very brief episodes of AF (symptomatic or asymptomatic) may be detected, and the total exposure to AF quantified. In CABANA, we evaluated the total average AF burden within the randomized treatment groups measured at 6-month intervals. Overall Holter-HHdetected AF burden showed a 69% – 88% relative reduction (33% – 42% absolute) in patients randomized to catheter ablation compared with a 48% – 73% relative reduction (23% – 35% absolute) in patients randomized to drug therapy at all time-points sampled across the five years of follow-up. Significant reductions in AF burden were observed for patients with paroxysmal AF as well as, persistent/long-standing persistent AF. Perhaps not surprising, was that the greatest absolute decrease in burden was seen in the patients with persistent/longstanding persistent AF (who spend a greater proportion of time in AF), although the absolute burden of AF was lower in the PAF patient groups (Figure 4). The ability of catheter ablation to significantly reduce time spent in AF, may improve the patient’s quality of life, and other outcome measures not assessed in this study.
Few prior catheter ablation randomized trials have reported on AF burden. [6,27,28] The MANTRA-PAF study, identified AF burden as the primary outcome measure in 294 AAD-naïve paroxysmal. [6] Cumulative and per-visit AF burden was assessed from quarterly 96-hour Holter-monitors, and a significant difference between randomized treatment was not observed. (P=0.10). The CASTLE-AF trial reported on AF burden (assessed by ICD arrhythmia logs) in 363 NYHA Class II-IV HF (EF <36%) patients who previously failed AF drug therapy. [27] Most patients had persistent/long-standing persistent AF (~70%). The average AF burden ranged from ~ 50–65% in drug treated patients compared with 20–30% of ablation treated patients (CASTLE-AF Supplement) [27]. In the recently reported CIRCA-DOSE study, Andrade and colleagues used implantable loop recorders in all 346 drug-refractory paroxysmal AF patients randomized in three arms to ablation, using contact force-guided RF, 4-minute cryoballoon freeze times, or 2-minute freeze times. [28] Median AF burden at baseline, was ~ 2% which became nearly zero at 12 months. In our present paper, we report average AF burden, which may better reflect treatment effect at the cohort level.
More work is needed to understand the relationship between burden of AF and adverse clinical outcomes from AF. For instance, several studies have now shown a temporal relationship of subclinical AF burden to thromboembolic events using implantable pacemakers or implantable cardioverter defibrillator (ICD) logs. [18–20] These findings have highlighted the need to better understand the value of using metrics other than time to first recurrence for AF detection, and importantly, consideration of what, AF burden and/or duration of AF is clinically important.
Limitations
Several caveats should be considered in the interpretation of out findings. First, 14% of the enrolling sites were unable to use the designated CABANA recoding system due to country level regulatory restrictions. These sites were instructed to use their standard recording technologies. Despite differences in absolute rates of AF recurrence based on which system was employed, the relative treatment benefit was the same regardless of the recording system used (Supplemental Table 3). Thus, we demonstrated that time to first recurrent AF was significantly improved with catheter ablation for all patients randomized to catheter ablation in CABANA. Second, time to first recurrence of AF has important limitations as a measure of treatment durability in AF due to the highly variable nature of the disease. To complement these measures, we also estimated AF burden as an average composite of the per-patient percent AF at 6-month intervals. These results showed treatment benefit very consistent with the time to recurrence estimates.
Conclusions
These results from the CABANA trial demonstrate a substantial and clinically important benefit of catheter ablation over drug therapy in reducing recurrent symptomatic and asymptomatic atrial fibrillation over 5 years of follow-up.
Supplementary Material
Perspectives.
Clinical Competency in Patient Care and Procedural Skills:
Catheter-based ablation is associated with greater reduction of symptomatic and asymptomatic atrial tachyarrhythmias, including atrial fibrillation, atrial flutter, and atrial tachycardia, than antiarrhythmic drug therapy over 5 years follow-up.
Translational Outlook:
Further studies are needed to clarify the relationship of recurrent atrial tachyarrhythmias to various clinical outcomes, including quality of life.
Acknowledgements:
CABANA ECG Core Lab Adjudication Committee
Chair: Jeanne E. Poole, M.D., University of Washington, Seattle WA
Nazem Akoum, M.D., University of Washington, Seattle WA
Pierre Aoukar, M.D., Kaiser Permanente, San Diego, CA
Ulrika Birgersdotter-Green, M.D., UCSD, San Diego, CA
Joseph Blatt, M.D., Kaiser Permanente, San Diego, CA
Yong Mei Cha, M.D., Mayo Clinic, Rochester, MN
Mina Chung, M.D., Cleveland Clinic, OH
Marye Gleva, M.D., Washington University at St. Louis, MO
Taya Glotzer, M.D., Hackensack University Medical Center, New Jersey
Charles Henrickson, M.D., Oregon Health Sciences University, Portland, OR
Jack Kron, M.D., Oregon Health Sciences University, Portland, OR
Vikas Kuriachan, M.D., University of Calgary, Alberta, Canada
Siva Mulpuru, M.D., Mayo Clinic, Rochester, MN
Peter Noseworthy, M.D., Mayo Clinic, Rochester, MN
Kris Patton, M.D., University of Washington Medical Center, Seattle, WA
Jordan Prutkin, M.D., University of Washington Medical Center, Seattle, WA
Ravi Ranjan, M.D., University of Utah, Salt Lake City, UT
Robert Rho, M.D., Virginia Mason Medical Center, Seattle WA
Andrea Russo, M.D., Cooperstown, New Jersey
Eric Stecker, M.D., Oregon Health Sciences University, Portland, OR
Wendy Tzou, M.D., University of Colorado, CO
Laura Vitali Serdoz, M.D., Klinikum Coburg, Coburg, Germany
Principal Contact: Medicomp, Inc., Melbourne, Florida: Mauri Wilson, RN
Funded by: NIH: (U01HL89709, U01HL089786, U01HL089907 and U01HL089645), St Jude Medical Drug Foundation and Corporation, Biosense Webster Inc, Medtronic Inc, and Boston Scientific Corporation
Disclosures
Jeanne E. Poole - Reported receiving research funding from ATriCure, Biotronik, Medtronic, and Kestra, Inc. outside the submitted work; serving on the advisory board with compensation for Boston Scientific; speaking with honorarium from Boston Scientific, Medtronic, and MediaSphere Medical, LLC.; and serving on data and safety monitoring board on study funded by EBR Systems
Tristram D. Bahnson - Grants from the NIH/NHLBI and Mayo Clinic during the conduct of the study and grants from St Jude Medical Inc, Abbott Medical, Medtronic Inc, Biosense Webster Inc, Johnson & Johnson, the NIH, and Boston Scientific Corp; and consulting fees from Cardiofocus Inc and Ventrix outside the submitted work. Dr Bahnson has patents pending for a catheter for intracardiac imaging and intracardiac electrogram signal analysis.
Kristi H. Monahan - Grants from the NIH/NHLBI, St Jude Foundation and Corporation, Biosense Webster Inc, Medtronic Inc, and Boston Scientific Corp during the conduct of the study; consulting without compensation from Biosense Webster Inc; and receiving personal fees from Thermedical outside the submitted work.
George Johnson - None
Hoss Rostami - None
Adam P. Silverstein - None
Hussein R. Al-Khalidi - Grants from the NIH/NHLBI and Mayo Clinic during the conduct of the study.
Yves Rosenberg - None
Daniel B Mark - Grants from the NIH/ NHLBI and Mayo Clinic during the conduct of the study and grants from Merck, Oxygen Therapeutics, Bristol-Myers Squibb, AstraZeneca, the University of Calgary, Eli Lilly & Company, AGA Medical, St Jude Medical, and Tufts University and personal fees from CeleCor outside the submitted work.
Kerry L. Lee - Grants from the NIH/NHLBI, Mayo Clinic, St Jude Medical Foundation and Corporation, Biosense Webster Inc, Medtronic Inc, and Boston Scientific Corp and serving on data and safety monitoring boards on studies funded by AstraZeneca, Medtronic Inc, Merck, Amgen, and the Cardiovascular Research Foundation during the conduct of the study.
Douglas L Packer - Grants from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI), St Jude Medical Corporation and Foundation, Biosense Webster Inc, Medtronic Inc, and Boston Scientific Corp during the conduct of the study and receiving grants from Abbott, Biosense Webster Inc, Boston Scientific Corp, CardioFocus, Medtronic Inc, St Jude Medical, CardioInsight, the NIH, Siemens, Thermedical, Endosense, Robertson Foundation, and Hansen Medical; serving on the advisory board without compensation for Abbott, Biosense Webster Inc, Boston Scientific Corp, CardioFocus, Medtronic Inc, St Jude Medical, Spectrum Dynamics, Siemens, Thermedical, Johnson & Johnson, and SigNum Preemptive Healthcare Inc; speaking with honorarium from Biotronik and MediaSphere Medical LLC; and receiving royalties from Wiley & Sons, Oxford, and St Jude Medical. Dr Packer and Mayo Clinic jointly have equity in a privately held company, External Beam Ablation Medical Devices, outside the submitted work. In addition, Dr Packer has mapping technologies with royalties paid.
Abbreviations:
- AAD
antiarrhythmic drug
- AF
atrial fibrillation
- AFL
atrial flutter
- AT
atrial tachycardia
- CI
confidence interval
- ECG
electrocardiogram
- HF
heart failure
- HR
hazard ratio
- IR
interquartile
- ITT
intention to treat
- mITT
modified intention to treat
- NYHA
New York Heart Association
- TTM
transtelephonic monitoring
Footnotes
Publisher's Disclaimer: Disclaimer: The content of this article does not necessarily represent the views of the National Heart, Lung, and Blood Institute or the Department of Health and Human Services
Clinicaltrials.gov Identifier: NCT00911508
References
- 1.Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997;95(3):572–6. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Kuck K, Cappato R et al. 2012 HRS/EHRA/ECAS/ACC/AHA/ APHRS/STS/ESC expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendation for patient selection, procedural techniques, patient management, and follow up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm 2012;9:632–6. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017. October;14(10):e275–e444. doi: 10.1016/j.hrthm.2017.05.012. Epub 2017 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293(21):2634–40. [DOI] [PubMed] [Google Scholar]
- 5.Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006;48(11):2340–7. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen CJ, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. The MANTRA-PAF Trial. N Engl J Med. 2012;367:1587–95 [DOI] [PubMed] [Google Scholar]
- 7.Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311(7):692–700. [DOI] [PubMed] [Google Scholar]
- 8.Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J. 2006;27(2):216–21. [DOI] [PubMed] [Google Scholar]
- 9.Mont L, Bisbal F, Hernández-Madrid A, et al. for the SARA investigators. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J. 2014;35(8):501–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packer DL, Kowal RC, Wheelan KR, et al. , STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713–23. [DOI] [PubMed] [Google Scholar]
- 11.Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–41. [DOI] [PubMed] [Google Scholar]
- 12.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303(4):333–40. [DOI] [PubMed] [Google Scholar]
- 13.Packer D, Mark D, Robb R et al. for the CABANA Investigators. Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation (CABANA) trial. JAMA. 2019;321(13):1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Packer D, Mark D, Robb R et al. for the CABANA Investigators. Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation (CABANA) trial: Study rationale and design. Am Hrt J 2018;199:192–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox D Regression models and life-tables (with discussion) JR Stat Soc B. 1972;34:187–220 [Google Scholar]
- 16.Fine JP, and Gray RJ. “A Proportional Hazards Model for the Subdistribution of a Competing Risk.” Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 17.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study JAMA, 271 (1994), pp. 840–844 [PubMed] [Google Scholar]
- 18.Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 19.Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G, et al. ; Italian AT500 Registry Investigators. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–20 [DOI] [PubMed] [Google Scholar]
- 20.Healey JS, Connolly SJ, Gold MR, et al. , for the ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–29 [DOI] [PubMed] [Google Scholar]
- 21.Hindricks G, Piorkowski C, Tanner H, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation. 2005;112(3):307–13. [DOI] [PubMed] [Google Scholar]
- 22.Pontoppidan J, Nielsen JC, Poulsen SH, et al. Symptomatic and asymptomatic atrial fibrillation after pulmonary vein ablation and the impact on quality of life. Pacing Clin Electrophysiol. 2009. June;32(6):717–26. [DOI] [PubMed] [Google Scholar]
- 23.Verma A, Champagne J, Sapp J et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study JAMA Intern Med. 2013. January 28;173(2):149–56. [DOI] [PubMed] [Google Scholar]
- 24.Kobza R, Hindricks G, Tanner H, et al. Late recurrent arrhythmias after ablation of atrial fibrillation: incidence, mechanisms, and treatment. Heart Rhythm. 2004;6:676–83. [DOI] [PubMed] [Google Scholar]
- 25.Prabhu S, Voskoboinik A, McLellan AJA, et al. Biatrial Electrical and Structural Atrial Changes in Heart Failure Electroanatomic Mapping in Persistent Atrial Fibrillation in Humans. JACC Clin Electrophysiol. 2018;4(1):87–96 [DOI] [PubMed] [Google Scholar]
- 26.Boriani G, Laroche C, Diemberger I, et al. ‘Real-world’ management and outcomes of patients with paroxysmal vs. non-paroxysmal atrial fibrillation in Europe:the EUR Observational Research Programme–Atrial Fibrillation (EORP-AF) General Pilot Registry in Europe: the EURObservational Research Programme–Atrial Fibrillation (EORP-AF) General Pilot Registry. Europace 2016;18:648–57 [DOI] [PubMed] [Google Scholar]
- 27.Marrouche NF, Brachmann J, Andresen D, et al. , for the CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 28.Andrade JG, Champagne J, Dubuc M et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring. Circulation 2019;140:1779–1788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


