Abstract
Aims
Nighttime aircraft noise exposure has been associated with increased risk of hypertension and myocardial infarction, mechanistically linked to sleep disturbance, stress, and endothelial dysfunction. It is unclear, whether the most widely used metric to determine noise exposure, equivalent continuous sound level (Leq), is an adequate indicator of the cardiovascular impact induced by different noise patterns.
Methods and results
In a randomized crossover study, we exposed 70 individuals with established cardiovascular disease or increased cardiovascular risk to two aircraft noise scenarios and one control scenario. Polygraphic recordings, echocardiography, and flow-mediated dilation (FMD) were determined for three study nights. The noise patterns consisted of 60 (Noise60) and 120 (Noise120) noise events, respectively, but with comparable Leq, corresponding to a mean value of 45 dB. Mean value of noise during control nights was 37 dB. During the control night, FMD was 10.02 ± 3.75%, compared to 7.27 ± 3.21% for Noise60 nights and 7.21 ± 3.58% for Noise120 nights (P < 0.001). Sleep quality was impaired after noise exposure in both noise scenario nights (P < 0.001). Serial echocardiographic assessment demonstrated an increase in the E/E′ ratio, a measure of diastolic function, within the three exposure nights, with a ratio of 6.83 ± 2.26 for the control night, 7.21 ± 2.33 for Noise60 and 7.83 ± 3.07 for Noise120 (P = 0.043).
Conclusions
Nighttime exposure to aircraft noise with similar Leq, but different number of noise events, results in a comparable worsening of vascular function. Adverse effects of nighttime aircraft noise exposure on cardiac function (diastolic dysfunction) seemed stronger the higher number of noise events.
Keywords: Environmental health, Aircraft noise exposure, Vascular function, Flow-mediated dilation, Cardiac function, Sleep disturbance
Graphical Abstract
1. Introduction
A growing number of epidemiological studies provide evidence that environmental noise is associated with an increased risk of cardiovascular disease,1,2 and potentially other adverse consequences such as insomnia,3 increased arterial stiffness,4 depression,5 decreased physical activity,6 and obesity.7 Recent data from translational clinical and animal studies8 have provided mechanistic insight on how noise can trigger changes at the molecular level. These studies have indicated that response to noise may not depend on the accumulated sound energy only but also on sound characteristics such as period of the day, intensity, frequency, complexity, and pattern. For example, results from animal studies have suggested that noise exposure during sleep-phase evokes a more pronounced inflammatory and oxidative stress response than wake-phase exposure.9 Also, we previously demonstrated that nighttime aircraft noise exposure was dose-dependently associated with impaired endothelial function in healthy volunteers.10 In this study, exposure to 30 and 60 noise events with equal peak sound pressure levels was associated with a decrease in flow-mediated dilation (FMD) of the brachial artery of similar size for the two noise scenarios. These results were confirmed in a subsequent study performed in patients with prevalent cardiovascular disease,11 showing that the vascular side effects were substantially more pronounced in case cardiovascular disease was already diagnosed. More recently, similar results were observed for train noise exposure-induced endothelial dysfunction (30 and 60 noise events for one night) that was prevented by vitamin C administration and was associated with substantial changes of the plasma proteome centred on redox, pro-thrombotic, and proinflammatory pathways.12 Age and sleep phase or also important determinants of noise-related sleep arousals.13
In most studies, exposure to noise is solely described by equivalent continuous sound pressure levels. However, many aspects of noise, e.g. the frequency of noise events as well as the individual situation and disturbed activities and the resulting annoyance to noise may be crucial factors that have to be taken into account in order to adequately assess the physiological and psychological impact of noise. It is at present completely unknown whether the commonly used average sound pressure level, and here especially the equivalent sound level (Leq(3)),14 adequately represents the increased risk for cardiovascular disease in response to noise or whether other aspects of noise are equally or even more important. For example, it has been demonstrated that sound pressure reduction of nighttime aircraft noise per se does not necessarily lead to reduced annoyance, since the latter rather correlates with the number of noise events.15 Therefore, it has been suggested, that intermittent noise exhibiting similar Leq(3) but with higher event rates may be more annoying and thus may affect cardiovascular health more adversely.
The aim of the present study was to investigate the differential effects of noise exposure scenarios consisting of equal average sound pressure levels but different numbers of noise events and peak sound pressure levels on vascular and myocardial function and the associated annoyance.
2. Methods
We conducted a blinded study of nighttime aircraft noise exposure in volunteers with cardiovascular disease or at increased cardiovascular risk (for design and details see Supplemental material). The study was approved by the state ethics committee for scientific research and registered (DRKS00010670). Informed written consent was obtained from all participants prior to inclusion in the study. The study design and its procedures are in accordance with the principles outlined in the Declaration of Helsinki and recommendations for Good Clinical Practice. Patients were recruited via public announcement. Participants were screened for exclusion criteria via telephone interviews (e.g. asking for heavy traffic noise, sleep disorders, recent medication changes as used in previous similar studies11) and included after an initial visit at the study centre. Patients were exposed in a randomized sequence to one control night and two noise nights in their own homes. The two noise exposures were designed with the same average sound pressure level (Leq(3)) by playback of recorded aircraft noise events at different peak sound pressure levels and different noise event numbers. One noise scenario (Noise60) consisted of 60 total noise events and was identically to the noise pattern used in previous clinical studies,11 with a peak sound pressure levels of 60 dB(A) and a mean sound pressure level of 46 dB(A). The other noise scenario (Noise120) consisted of the same recorded aircraft noise events but played back at a lower peak sound pressure levels but twice as many events (120) during the study night, resulting in an equal average sound pressure level. The intermittency ratio (IR)16 of the Noise60 and the Noise120 scenarios were 95% and 90%, respectively, for representative cases in the field. Study participants presented to the study centre on the morning after each exposure night between 8 and 10 a.m., depending on travel distance and sleep habits. The examinations described below were scheduled at the same time on all visits of an individual participant to minimize circadian variation.
2.1 Vascular function
As in previous studies, the primary study outcome was FMD of the brachial artery, which has been shown to be related to aircraft noise exposure in a dose-dependent manner with increasing noise event rate.10 FMD was measured according to standard operating procedures by a single trained technician blinded to noise exposure. We used Brachial Analyzer software (Medical Imaging Applications LLC, IA, USA) for FMD analysis.
2.2 Echocardiography
After each study night participants underwent focused transthoracic echocardiography (TTE) in the echocardiography lab (scheduled for echo between 8 and 10 a.m.). Image acquisition was performed by two experienced (Level III +) physician echocardiographers according to the study protocol, that included standard 2D views, Doppler, tissue Doppler, and 3D acquisitions. Images were stored in pseudonymized way and echocardiographers were blinded to the intervention. Every participant had three TTE exams in the course of the study, amounting to 210 echos per protocol. Measurements were not performed during image acquisition but on later review of stored datasets. Data were analysed with the use of Philips QLAB 10.4 and the corresponding modules cardiac motion quantification (CMQ), cardiac 2D quantification (2DQ), tissue motion annular displacement (TMAD), cardiac 3D quantification (3DQ), and 3DQ Advanced. Examiners remained blinded throughout measurements and results were later matched to noise exposure.
For sample size calculation, we assumed an effect pattern similar to the FLIGHT study, with effects as given in ref.,10 and a correlation of 0.97 for individual values between conditions. At a significance level of 5% and a required power of 80%, an overall sample size of 104 participants was calculated. At interim analysis, we prespecified a P-value <0.001 (Peto limit) for FMD to avoid losing overall statistical power. Randomization was performed with a single block randomization sequence (n = 105) generated at http://www.randomization.com. Randomization and blinding of both participants and study personal was achieved as described.10
2.3 Blood sample analysis
Blood was collected after each study night in the clinics (between 8 and 10 a.m.) and sent to clinical lab for standard analysis. In addition, vials were stored at −80°C. In a subset of 22 patients who were selected according to most pronounced changes in FMD in response to noise, we performed an exploratory analysis for protein changes using a multiplex immunoassay [Olink CARDIOVASCULAR II (v.5003)] panel to evaluate protein biomarkers in Control and Noise120 samples (for methodological description see ref. 12).
2.4 Polygraphy and sound data
Polygraphic data was recorded with SOMNOtouch™ NIBP (SOMNOmedics GmbH, Randersacker, Germany) and analysed using DOMINOlight software provided by the same vendor. The polygraphic sensors were attached in the study centre and participants were instructed on how to ensure optimal measurement results. In addition, participants performed multiple blood pressure measurements with an automated oscillographic blood pressure apparatus to allow calibration. Measurement intervals were defined by the time of noise simulation and correlated with sound pressure levels. Sound pressure levels were recorded with a recording sound level metre during the noise simulation in the participants bedroom as described in our previous studies.11,12
2.5 Questionnaires for sleep quality and annoyance
Questionnaires were identical to those described before.10 In brief, Noise Sensitivity and Chronotype were assessed with NoiSEQ and MEQ, sleep quality primarily with a visual analogue scale in addition to other sleep questionnaires.
2.6 Statistical analysis
Statistical analysis was performed based on a repeated measures general linear model, incorporating the three noise patterns as a fixed factor. Tests were performed with IBM SPSS 24 with a significance level of P = 0.05 in a closed-testing procedure, first evaluating overall differences, then differences between the two non-control patterns and finally a potential priming effect of the two non-control patterns with respect to each other. The linear mixed model included the three noise levels, the sequence, the period, and previous exposure from earlier study nights. The statistical test used for each figure dataset is described in the figure legend.
3. Results
We included 70 participants up until the predetermined interim analysis. Patient characteristics are shown in Table 1. Leq in the control nights was 36.81 ± 8.34 dB(A), in Noise60 nights it was 44.94 ± 7.49 dB(A) and in Noise120 nights it was 45.26 ± 2.78 dB(A). There was a significant difference between control and noise nights, but not between noise nights. Sleep quality was rated worse after noise nights than after the control night. On a visual analogue scale with higher values indicating worse sleep, there was a significant increase (P < 0.001) from 3.96 2.29 (Control) to 6.65 2.45 (Noise60) and 6.75 2.36 (Noise 120) (Figure 1).
Table 1.
Patient characteristics
| N | 70 | Unit |
|---|---|---|
| Age | 62.8 ± 7.06 | years |
| Sex | 56 (80%) | N = male (%) |
| BMI | 26.49 ± 6.74 | kg/m2 |
| Smokers | 10 (14.3%) | % |
| Diabetes mellitus | 11 (15.7%) | % |
| Antihypertensive Medication | 61 (87.1%) | % |
| Framingham score | 24.33 ± 12.63 | No dimension |
| PSQI | 5.50 ± 2.20 | No dimension |
| NoiSeQ | 1.54 ± 0.41 | No dimension |
| LDL | 111.53 ± 43.04 | mg/dL |
| HDL | 50.19 ± 10.85 | mg/dL |
| Triglycerides | 177.44 ± 83.72 | mg/dL |
| HbA1c | 5.88 ± 0.74 | % |
| Creatinine | 0.94 ± 0.19 | mg/dL |
Baseline characteristics and risk factors of the 70 patients included at interim analysis.
BMI, body mass index; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NoiSeQ, Noise Sensitivity Questionnaire; PSQI, Pittsburgh Sleep Quality Index.
Figure 1.

Sleep quality as reported after each study night on a 0–10 visual analogue scale with higher values indicating impaired sleep quality, depicted as scatter plots with mean ± standard deviation. P-values from a repeated measures non-parametric Friedman test with Dunn’s correction for multiple comparisons (normality test failed) for 70 participants.
For FMD, we found a statistically significant difference between noise scenarios (P < 0.001). FMD in the control night was 10.02 ± 3.75%, in the night with the Noise60 scenario FMD was 7.27 ± 3.21% and the Noise120 scenario resulted in an FMD of 7.21 ± 3.58% (Figure 2). Post hoc tests showed a significant difference between the control night and both noise patterns, whereas there was no significant difference between the two noise patterns (Figure 3). We did not observe significant period effects of the study nights, i.e. there was no difference in FMD between the first, second and third night of exposure and therefore no evidence of a ‘first night’ effect. With respect to the exposure sequence, there was a significant trend for a possible carry-over (‘priming’) effect from the night with 60 events to the night with 120 events (P = 0.0413), but this could not be formally tested.
Figure 2.
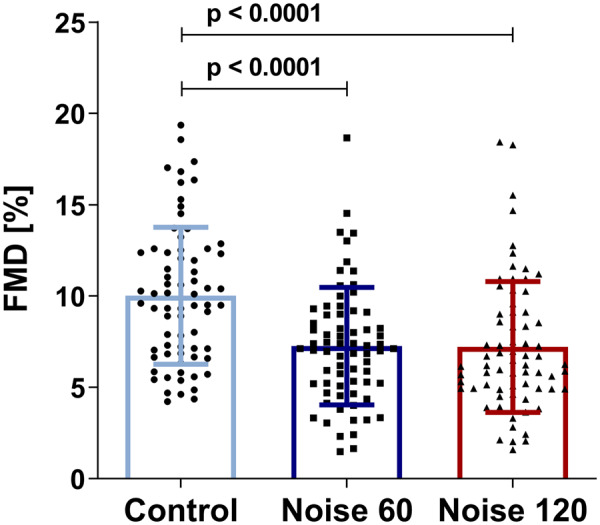
Vascular function as assessed by means of flow-mediated dilation after each study night, depicted as scatter plots with mean ± standard deviation and P-values from a repeated measures non-parametric Friedman test with Dunn’s correction for multiple comparisons (normality test failed) for 70 participants.
Figure 3.

FMD values (%) for the study nights with 60 and 120 noise events on x and y axis for 70 participants.
With regard to laboratory values, we found no significant differences between any of the three scenarios (control, Noise60, and Noise120) for serum markers of inflammation (C-reactive protein, neutrophils, interleukin-6), stress hormone concentration (epinephrine, cortisol), or myocardial injury (hsTroponin). The exploratory protein analysis (Olink Cardiovascular II) yielded statistically significant decreases for plasma concentration of follistatin (FS) (P = 0.016), glyoxalase I (GLO1) (P = 0.044), and angiotensin-converting enzyme 2 (ACE2) (P = 0.032) taken after the Noise120 compared to control nights.
Polygraphic recordings (Table 2) during the study nights were not able to record significant differences between noise and control for heart rate (P = 0.862) or heart rate accelerations (P = 0.894). Similarly, sympathovagal balance which was derived based on electrocardiogram recordings was not different between study nights (P = 0.858). Non-invasive continuous blood pressure recordings found no significant differences for systolic blood pressure during study nights (P = 0.424). Despite significantly altered sleep quality, accelerometer-based movement indices were not affected by noise exposure (P = 0.839).
Table 2.
Polygraphic and laboratory data
| Parameter | Unit | Control | Noise60 | Noise120 | P-value |
|---|---|---|---|---|---|
| Sleep quality | Dimensionless scale | 3.955 ± 2.294 | 6.654 ± 2.446 | 6.749 ± 2.358 | <0.001 |
| Sleep effectiveness | Dimensionless index | 0.825 ± 0.217 | 0.811 ± 0.209 | 0.844 ± 0.121 | 0.431 |
| RR sys | mmHg | 127.81 ± 18.951 | 129.67 ± 16.714 | 126.71 ± 18.885 | 0.424 |
| Movement index | Dimensionless index | 9.429 ± 20.348 | 8.067 ± 8.526 | 8.526 ± 19.085 | 0.839 |
| HR | b.p.m. | 61.44 ± 8.459 | 61.41 ± 8.064 | 61.13 ± 7.780 | 0.862 |
| HR accel | 11.864 ± 27.583 | 12.324 ± 31.296 | 12.72 ± 33.839 | 0.894 | |
| SVB | Dimensionless index | 18.465 ± 8.498 | 18.143 ± 7.560 | 18.459 ± 8.917 | 0.858 |
| Cortisol | µg/dL | 9.503 ± 3.208 | 9.837 ± 3.348 | 10.019 ± 3.001 | 0.355 |
| Adrenalin | ng/L | 30.887 ± 17.508 | 31.177 ± 16.788 | 33.598 ± 19.919 | 0.399 |
| IL-6 | pg/mL | 3.643 ± 2.580 | 3.747 ± 2.928 | 4.000 ± 4.102 | 0.801 |
| Follistatin | NPX | 12.744 ± 0.322 | 12.615 ± 0.336 | 0.016 | |
| Glyoxalase I | NPX | 4.765 ± 0.851 | 4.535 ± 0.733 | 0.044 | |
| ACE-2 | NPX | 4.827 ± 0.431 | 4.706 ± 0.434 | 0.032 |
Data from questionnaires, polygraphic recordings, and lab values from blood sampling.
P-values are taken from a repeated measures general linear model and not corrected for multiple testing.
ACE-2, angiotensin-converting enzyme 2; HR accel, acceleration in heart rate; HR, heart rate; IL-6, interleukin 6; Movement index, accelerometer based index of changes in body position during the study night; NPX, normalized protein expression is a log2 scale to compare changes in protein levels and should be used for relative quantification; RR sys, systolic blood pressure; SVB, sympathovagal balance (heart rate variability based).
Echocardiographic data (Table 3) did not demonstrate significant changes in left ventricular (LV) size and function after exposure to nighttime aircraft noise. LV ejection fraction remained identical at 55.7% (Control), 55.4% (Noise60) and 55.7%. Also, additional markers of LV function such as mitral annular peak systolic excursion (MAPSE) and global longitudinal strain (GLS), did not demonstrate relevant changes. S′ velocity of the mitral annulus was however significantly lower in Noise60/120 vs. control (P = 0.025). With regard to diastolic function of the left ventricle, the E/E′ ratio was statistically different between study nights with 6.83 2.26 (control), 7.21 2.33(Noise60) and 7.83 3.07 (Noise 120; P = 0.043) (Figure 4), while mitral A velocity was not different (P = 0.556), E velocity (P = 0.005) was changed by noise exposure. No significant changes were seen for left atrial volume. There was no indication for effects on right ventricular function (tricuspid annulus tricuspid excursion, P = 0.702).
Table 3.
Echocardiography
| Parameter | Control | Noise60 | Noise120 | P-value |
|---|---|---|---|---|
| EDV (mL) | 132.45 ± 37.32 | 130.02 ± 35.21 | 132.8 ± 36.25 | 0.620 |
| EF (%) | 55.67 ± 7.16 | 55.44 ± 7.36 | 55.69 ± 7.03 | 0.932 |
| GLS (%) | −18.97 ± 5.68 | −19.60 ± 2.45 | −19.62 ± 2.55 | 0.623 |
| MAPSE (mm) | 11.96 ± 3.32 | 12.73 ± 2.85 | 12.29 ± 2.89 | 0.110 |
| S′ mitral (cm/s) | 9.14 ± 2.07 | 7.99 ± 4.09 | 8.38 ± 1.96 | 0.025 |
| TAPSE (mm) | 21.0 ± 5.09 | 21.25 ± 4.90 | 21.56 ± 3.68 | 0.702 |
| E/E′ ratio | 6.83 ± 2.26 | 7.21 ± 2.33 | 7.83 ± 3.07 | 0.043 |
| E′ (cm/s) | 10.40 ± 2.58 | 9.88 ± 2.75 | 9.86 ± 2.73 | 0.115 |
| E (cm/s) | 67.03 ± 13.71 | 67.03 ± 16.89 | 71.56 ± 17.84 | 0.005 |
| A (cm/s) | 68.48 ± 16.21 | 70.35 ± 17.15 | 68.95 ± 17.91 | 0.556 |
| LA volume (cm3) | 55.37 ± 16.80 | 54.04 ± 18.69 | 54.39 ± 19.39 | 0.839 |
Results of echocardiography conducted after each study night according to study protocol with multiparameter and multimodality echocardiography acquisitions.
P-values are taken from a repeated measures general linear model and not corrected for multiple testing.
A, A-wave velocity; E, transmitral E-wave velocity; E′, tissue Doppler mitral annular early diastolic filling velocity; EDV, end-diastolic volume; EF, ejection fraction; GLS, global longitudinal strain (speckle tracking); LA volume, left atrial volume; MAPSE, mitral annulus systolic excursion; S′ mitral, systolic mitral annulus peak velocity; TAPSE, tricuspid annulus tricuspid excursion.
Figure 4.

E/E′ ratio after each study night, depicted as scatter plots with mean ± standard deviation and P-values from a repeated measures non-parametric Friedman test with Dunn’s correction for multiple comparisons (normality test failed) for 63 participants (7 excluded values as not all repeated measures for Control, Noise60, and Noise120 nights were available).
4. Discussion
The results of the present studies demonstrate that nighttime exposure to aircraft noise with similar Leq, but with a striking different number of noise events, results in a comparable worsening of vascular function. In addition, nighttime exposure to 120 noise events resulted in a deterioration of the E/E′ ratio, reflecting diastolic dysfunction of the heart. The exploratory protein analysis (Olink Cardiovascular II) yielded a statistically significant decrease for plasma concentration of FS, a suppressor of the transforming growth factor-β superfamily including regulation of the paracrine hormone activin, pointing towards dysregulated fibrosis.17 Also, the expression of glyoxalase I (GLO1) was decreased in response to noise, indicating impaired detoxification of methylglyoxal and other reactive aldehydes and accordingly pointing to an altered defense against metabolic stress.18 Finally, noise reduced the levels of ACE2, an enzyme attached to the outer cell membranes of cells in the lungs, arteries, heart, kidney, and intestines. ACE2 lowers blood pressure by removal of the vasoconstrictor angiotensin II and accordingly its down-regulation by noise will indirectly increase the blood pressure.19
Almost all studies of noise and health estimate noise based on metrics of sound pressure levels. Average sound pressure levels such as Leq have been used for decades to measure loudness. Associations between equivalent sound pressure levels and cardiovascular disease have been demonstrated repeatedly.20 However, this sound pressure levels have largely been developed on a theoretical basis with little validation in human studies. It was therefore a question of scientific as well as political relevance whether established average sound pressure levels reflect cardiovascular effects of noise and annoyance in an experimental setting. The results of the current study confirm that Leq can be used to estimate the impact of noise on exposed individuals in terms of vascular function and also annoyance levels. In our study scenario, there was no difference in noise health effects, regardless whether repetitive noise events were either louder (Noise60) or more frequent (Noise120), although there was a certain dose–response relationship for number of noise events and cardiac dysfunction (E/E′ ratio). Sleep quality and FMD were significantly different between control nights without noise and both noise patterns, but no differences could be found between the two noise patterns, which essentially had the same effects. It thus seems that average sound pressure levels such as Leq adequately describe noise effects (during night) in a setting were individual noise events only differ in terms of loudness and number, indicating that sound pressure levels are relevant for biological effects and can remain a basic comparator for noise exposure on population-based studies. However, we have to stress that the IRs were also basically identical between both noise scenarios.16,21 Sound pressure levels remains the most fundamental measure for quantifying exposure and can be supplemented by modifiers such as IR.
Equivalent continuous sound pressure guidance levels have been developed in parallel both in the USA and Germany with the intention to describe and regulate aircraft noise levels for military and civilian aircraft noise exposure. In Germany, the Leq(3) was proposed in a scientific treatment commissioned by the ministry of health.14 Subsequently, the Leq(3) was quickly adopted in other countries and has been widely used ever since. Nevertheless, for the purpose of more detailed noise characterization in interventional studies Leq(3) should be supplemented by additional noise metrics as e.g. the IR.
In conclusion, we are able to demonstrate in this study that equivalent continuous sound pressure levels not only correlate with sleep quality but also with vascular function in patients with cardiovascular disease. However, it is important to note, that our results are only applicable to comparisons of a single noise modality. It has been demonstrated that road, train, and aircraft noise elicit different levels of annoyance22 and should therefore not be compared solely on the basis of Leq(3) values. The IR may be helpful to characterize the noise, however for aircraft noise the value in terms of annoyance reaction appears to be less pronounced than for other noise sources. In general, an IR around 50% appears to have the biggest effect on annoyance.
Despite unambiguous effects of noise exposure on sleep quality and vascular function in this and previous studies, polygraphic recordings were essentially unable to detect any differences, despite our best efforts to obtain high-quality recordings. While polygraphic recordings in a field setting without the possibility to make intra-night corrections to the signal may be affected by artefacts and lost signals, this has to be considered a limitation of the method rather than our study. In addition, values averaged over the night may not be sensitive enough and additional noise-event correlations may be needed to detect differences. In conclusion, polygraphic data on macrostructure of sleep appears insensitive to these changes23,24 and results from studies based on actigraphic data should therefore be interpreted with caution.25
For the first time, we evaluated cardiac function after noise exposure based on comprehensive echocardiography. While standard 2D-based measures of systolic function were stable (ejection fraction, MAPSE, GLS), we found statistical differences for Doppler-based values with a significant increase in E/E′ ratio in the Noise120 group, the most widely used measure of cardiac diastolic function and filling pressures.26 It has been demonstrated that diastolic function can be affected early for example by ischaemia,27 even before systolic function. While 2D-based measures of systolic function like Simpson based ejection fraction or speckle tracking based GLS may be either to insensitive or to variable, simple tissue Doppler may be most accurate. It is sensitive due to high temporal resolution and can be reliably and accurately measured.
The increase in E/E′ values can be interpreted as an impairment of diastolic function due to frequent noise exposure. Cardiac function is sensitive to outside stressors and noise affects not only vascular function but also cardiac function.28 Changes in vascular and cardiac function are often caused by similar vegetative or neuro-hormonal mechanisms. In addition, heart and vessels are part of the same system, were changes to one component also affect the other parts of the cardiovascular system. Diastolic function is very sensitive and can be affected before relevant changes in systolic function.29 Associations between diastolic function and endothelial function have been demonstrated previously.30 Changes in mitral inflow have prognostic relevance in certain groups.31 Our findings demonstrate effects of noise exposure on cardiac haemodynamics in addition to previously shown vascular effects.
An exploratory proteomics analysis in a subset of patients was conducted with the intent to produce hypothesis generating findings and facilitate the detection of novel pathways involved in noise-induced cardiovascular damage. We found significant reductions for three biomarkers after noise exposure. Glyoxalase I is involved in detoxification of highly toxic methylglyoxal, a byproduct of physiological biochemistry. It has been associated with oxidative stress at the endothelial level32 and also with cardiac function after myocardial infarction.33 ACE2 is expressed in vascular endothelial cells and is an important regulator of heart function. Follistatin is an autocrine glycoprotein involved in inflammatory and fibrotic response. The involvement of these proteins in noise induced changes appears possible with regard to their known functions, however, this has to be replicated in other data sets.
4.1 Limitations
Despite exclusion of participants living in areas with heavy traffic noise based on municipal noise maps, the actual recorded sound pressure levels demonstrated significant background noise in participants homes (even during control nights). There are various noise sources in the field like noise from appliances, neighbours, traffic, and the patient itself that pollute the noise simulation. The achieved Leq(3) was comparable in both groups at 45 dB(A) and therefore roughly 1 dB(A) lower than intended. In general, this may dilute some effects and differences may be more difficult to demonstrate. While we tried to control the study conditions by protocol and participant instruction, many unmeasured environmental and nutritional factors may influence FMD results, such as salt consumption.
For echocardiographic assessment of cardiac function, the variability between exams and examiners may contribute to loss of small effects. Echocardiographic parameters of LV function do not always directly correlate with clinical outcomes.34 For some variables, incomplete values limit the results, most importantly for epinephrine were the central laboratory terminated the routine assay midway through the study. However, in the available data, contrary to previous results,35 we observed no corresponding changes with FMD. The early termination of the trial and thus the reduced sample size limits the statistical power to detect significant differences between the two noise patterns. However, even on the basis of the predetermined sample size of 104 participants, it seemed unlikely to achieve a statistical significant difference for the primary study parameters between the two noise patterns based on the observed data distribution. Accordingly, we did not expect to gain additional mechanistic insights by continuing the study, requiring early termination to comply with the ethical guidelines. In general, short-term exposure can only produce circumferential evidence with regard to long-term health outcomes. Termination of the study at the interim analysis may limit the studies power to detect secondary effects (e.g. priming).
5. Conclusion
In patients with and without cardiovascular risk factors nighttime noise exposure resulted in a deterioration of subjective sleep quality, vascular function, and for the first time demonstrated impairment in cardiac diastolic function. In summary, a randomized exposure to two noise patterns with different noise event rates but similar equivalent continuous sound pressure levels did not demonstrate significant differences between noise patterns with respect to the above effects.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
All authors were involved in drafting the manuscript, approved it for publication and confirm its accuracy. In addition, individual authors were involved as follows in the study: F.P.S.: design, analysis, and interpretation; J.H.: acquisition and analysis; B.S.: acquisition and analysis; M.A.O.: design and acquisition; L.L.: design and acquisition; O.H.: analysis and interpretation; G.S.: design and acquisition; T.G.: analysis and interpretation; M.S.: interpretation; A.D.: analysis and interpretation; T.M.: design and interpretation.
Supplementary Material
Acknowledgements
We thank Hannelore Seiler for her essential part as a study nurse. We also thank the Gutenberg Health study team (Wild, Prochaska, and others) for valuable input. For statistical support, we thank Irene Schmidtmann from IMBEI Mainz. This manuscript contains results from the doctoral thesis of L.L. and G.S. T.M. is PI of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine-Main, Mainz, Germany.
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Funding
This study was supported by a grant from the German Heart Foundation and by the Foundation Heart of Mainz.
Translational perspective
With the present field study, we tested whether 60 vs. 120 simulated aircraft noise events with an equal average sound pressure level (Leq) have comparable adverse effects on endothelial and diastolic function of the heart of subjects with established cardiovascular disease or at increased cardiovascular risk. The results demonstrated that two different nighttime noise patterns with similar Leq, despite different number of noise events, results in a comparable worsening of vascular and cardiac diastolic function. These results may explain at least in part the increased incidence of coronary heart disease and heart failure being observed in response to nighttime aircraft noise.
Time for primary review: 13 days
References
- 1. Münzel T, Herzog J, Schmidt FP, Sørensen M.. Environmental stressors and cardiovascular disease: the evidence is growing. Eur Heart J 2017;38:2297–2299. [DOI] [PubMed] [Google Scholar]
- 2. Seidler A, Wagner M, Schubert M, Dröge P, Römer K, Pons-Kühnemann J, Swart E, Zeeb H, Hegewald J.. Aircraft, road and railway traffic noise as risk factors for heart failure and hypertensive heart disease—a case-control study based on secondary data. Int J Hyg Environ Health 2016;219:749–758. [DOI] [PubMed] [Google Scholar]
- 3. Kwak KM, Ju YS, Kwon YJ, Chung YK, Kim BK, Kim H, Youn K.. The effect of aircraft noise on sleep disturbance among the residents near a civilian airport: a cross-sectional study. Ann Occup Environ Med 2016;28:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foraster M, Eze IC, Schaffner E, Vienneau D, Héritier H, Endes S, Rudzik F, Thiesse L, Pieren R, Schindler C, Schmidt-Trucksäss A, Brink M, Cajochen C, Marc Wunderli J, Röösli M, Probst-Hensch N.. Exposure to road, railway, and aircraft noise and arterial stiffness in the SAPALDIA study: annual average noise levels and temporal noise characteristics. Environ Health Perspect 2017;125:097004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beutel ME, Junger C, Klein EM, Wild P, Lackner K, Blettner M, Binder H, Michal M, Wiltink J, Brahler E, Munzel T.. Noise annoyance is associated with depression and anxiety in the general population—the contribution of aircraft noise. PLoS One 2016;11:e0155357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foraster M, Eze IC, Vienneau D, Brink M, Cajochen C, Caviezel S, Heritier H, Schaffner E, Schindler C, Wanner M, Wunderli JM, Roosli M, Probst-Hensch N.. Long-term transportation noise annoyance is associated with subsequent lower levels of physical activity. Environ Int 2016;91:341–349. [DOI] [PubMed] [Google Scholar]
- 7. Christensen JS, Raaschou-Nielsen O, Tjønneland A, Nordsborg RB, Jensen SS, Sørensen TIA, Sørensen M.. Long-term exposure to residential traffic noise and changes in body weight and waist circumference: a cohort study. Environ Res 2015;143:154–161. [DOI] [PubMed] [Google Scholar]
- 8. Münzel T, Daiber A, Steven S, Tran LP, Ullmann E, Kossmann S, Schmidt FP, Oelze M, Xia N, Li H, Pinto A, Wild P, Pies K, Schmidt ER, Rapp S, Kröller-Schön S.. Effects of noise on vascular function, oxidative stress, and inflammation: mechanistic insight from studies in mice. Eur Heart J 2017;38:2838–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kröller-Schön S, Daiber A, Steven S, Oelze M, Frenis K, Kalinovic S, Heimann A, Schmidt FP, Pinto A, Kvandova M, Vujacic-Mirski K, Filippou K, Dudek M, Bosmann M, Klein M, Bopp T, Hahad O, Wild PS, Frauenknecht K, Methner A, Schmidt ER, Rapp S, Mollnau H, Münzel T.. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur Heart J 2018;39:3528–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt FP, Basner M, Kröger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A, Münzel T.. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J 2013;34:3508–3514a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M, Binder H, Gori T, Münzel T.. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin Res Cardiol 2015;104:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herzog J, Schmidt FP, Hahad O, Mahmoudpour SH, Mangold AK, Garcia Andreo P, Prochaska J, Koeck T, Wild PS, Sørensen M, Daiber A, Münzel T.. Acute exposure to nocturnal train noise induces endothelial dysfunction and pro-thromboinflammatory changes of the plasma proteome in healthy subjects. Basic Res Cardiol 2019;114:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rudzik F, Thiesse L, Pieren R, Héritier H, Eze IC, Foraster M, Vienneau D, Brink M, Wunderli JM, Probst-Hensch N, Röösli M, Fulda S, Cajochen C.. Ultradian modulation of cortical arousals during sleep: effects of age and exposure to nighttime transportation noise. Sleep 2020;43:zsz324. doi: 10.1093/sleep/zsz324. [DOI] [PubMed] [Google Scholar]
- 14. Burck W, Grutzmacher M, Meister FJ, Muller EA, Matschat K.. Aircraft Noise: Expert Recommendations Submitted Under Commission from the German Federal Ministry for Public Health. Gottingen; 1965.
- 15. Quehl J, Müller U, Mendolia F.. Short-term annoyance from nocturnal aircraft noise exposure: results of the NORAH and STRAIN sleep studies. Int Arch Occup Environ Health 2017;90:765–778. [DOI] [PubMed] [Google Scholar]
- 16. Wunderli JM, Pieren R, Habermacher M, Vienneau D, Cajochen C, Probst-Hensch N, Röösli M, Brink M.. Intermittency ratio: a metric reflecting short-term temporal variations of transportation noise exposure. J Expo Sci Environ Epidemiol 2016;26:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Kretser DM, O‘Hehir RE, Hardy CL, Hedger MP.. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol 2012;359:101–106. [DOI] [PubMed] [Google Scholar]
- 18. Mey JT, Haus JM.. Dicarbonyl stress and glyoxalase-1 in skeletal muscle: implications for insulin resistance and type 2 diabetes. Front Cardiovasc Med 2018;5:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin S, Pan H, Wu H, Ren D, Lu J.. Role of the ACE2− Ang− (1− 7)− as axis in blood pressure regulation and its potential as an antihypertensive in functional foods (review). Mol Med Rep 2017;16:4403–4412. [DOI] [PubMed] [Google Scholar]
- 20. Dimakopoulou K, Koutentakis K, Papageorgiou I, Kasdagli MI, Haralabidis AS, Sourtzi P, Samoli E, Houthuijs D, Swart W, Hansell AL, Katsouyanni K.. Is aircraft noise exposure associated with cardiovascular disease and hypertension? Results from a cohort study in Athens, Greece. Occup Environ Med 2017;74:830–837. [DOI] [PubMed] [Google Scholar]
- 21. Brink M, Schäffer B, Vienneau D, Pieren R, Foraster M, Eze IC, Rudzik F, Thiesse L, Cajochen C, Probst-Hensch N, Röösli M, Wunderli JM.. Self-reported sleep disturbance from road, rail and aircraft noise: exposure-response relationships and effect modifiers in the SiRENE study. Int J Environ Res Public Health 2019;16:4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brink M, Schäffer B, Vienneau D, Foraster M, Pieren R, Eze IC, Cajochen C, Probst-Hensch N, Röösli M, Wunderli JM.. A survey on exposure-response relationships for road, rail, and aircraft noise annoyance: differences between continuous and intermittent noise. Environ Int 2019;125:277–290. [DOI] [PubMed] [Google Scholar]
- 23. Thiesse L, Rudzik F, Spiegel K, Leproult R, Pieren R, Wunderli JM, Foraster M, Héritier H, Eze IC, Meyer M, Vienneau D, Brink M, Probst-Hensch N, Röösli M, Cajochen C.. Adverse impact of nocturnal transportation noise on glucose regulation in healthy young adults: effect of different noise scenarios. Environ Int 2018;121:1011–1023. [DOI] [PubMed] [Google Scholar]
- 24. Thiesse L, Rudzik F, Kraemer JF, Spiegel K, Leproult R, Wessel N, Pieren R, Héritier H, Eze IC, Foraster M, Garbazza C, Vienneau D, Brink M, Wunderli JM, Probst-Hensch N, Röösli M, Cajochen C.. Transportation noise impairs cardiovascular function without altering sleep: the importance of autonomic arousals. Environ Res 2020;182:109086. [DOI] [PubMed] [Google Scholar]
- 25. Basner M, Asch DA, Shea JA, Bellini LM, Carlin M, Ecker AJ, Malone SK, Desai SV, Sternberg AL, Tonascia J, Shade DM, Katz JT, Bates DW, Even-Shoshan O, Silber JH, Small DS, Volpp KG, Mott CG, Coats S, Mollicone DJ, Dinges DF.. Sleep and alertness in a duty-hour flexibility trial in internal medicine. N Engl J Med 2019;380:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD.. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 27. Mansour MJ, Aljaroudi W, Mroueh A, Hamoui O, Honeine W, Khoury N, Nassif JA, Chammas E.. Stress-induced worsening of left ventricular diastolic function as a marker of myocardial ischemia. J Cardiovasc Echography 2017;27:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Münzel T, Sørensen M, Schmidt F, Schmidt E, Steven S, Kröller-Schön S, Daiber A.. The adverse effects of environmental noise exposure on oxidative stress and cardiovascular risk. Antioxid Redox Signal 2018;28:873–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsougos E, Panou F, Paraskevaidis I, Dagres N, Karatzas D, Kremastinos DT.. Exercise-induced changes in E/E‘ ratio in patients with suspected coronary artery disease. Coron Artery Dis 2008;19:405–411. [DOI] [PubMed] [Google Scholar]
- 30. Chin CW, Chin CY, Ng MX, Le TT, Huang FQ, Fong KY, Thumboo J, Tan RS.. Endothelial function is associated with myocardial diastolic function in women with systemic lupus erythematosus. Rheumatol Int 2014;34:1281–1285. [DOI] [PubMed] [Google Scholar]
- 31. Traversi E, Pozzoli M, Cioffi G, Capomolla S, Forni G, Sanarico M, Tavazzi L.. Mitral flow velocity changes after 6 months of optimized therapy provide important hemodynamic and prognostic information in patients with chronic heart failure. Am Heart J 1996;132:809–819. [DOI] [PubMed] [Google Scholar]
- 32. Wang Z, Zhang J, Chen L, Li J, Zhang H, Guo X.. Glycine suppresses AGE/RAGE signaling pathway and subsequent oxidative stress by restoring Glo1 function in the aorta of diabetic rats and in HUVECs. Oxid Med Cell Longev 2019;2019:4628962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blackburn NJR, Vulesevic B, McNeill B, Cimenci CE, Ahmadi A, Gonzalez-Gomez M, Ostojic A, Zhong Z, Brownlee M, Beisswenger PJ, Milne RW, Suuronen EJ.. Methylglyoxal-derived advanced glycation end products contribute to negative cardiac remodeling and dysfunction post-myocardial infarction. Basic Res Cardiol 2017;112:57. [DOI] [PubMed] [Google Scholar]
- 34. Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, Düngen HD, Tschöpe C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B, Aldo-Dhf I.. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013;309:781–791. [DOI] [PubMed] [Google Scholar]
- 35. Kaplon RE, Walker AE, Seals DR.. Plasma norepinephrine is an independent predictor of vascular endothelial function with aging in healthy women. J Appl Physiol 2011;111:1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.



