Abstract
Aims
Prior studies have focused on the role of the kidney and vasculature in salt-induced modulation of blood pressure; however, recent data indicate that sodium accumulates in tissues and can activate immune cells. We sought to examine mechanisms by which salt causes activation of human monocytes both in vivo and in vitro.
Methods and results
To study the effect of salt in human monocytes, monocytes were isolated from volunteers to perform several in vitro experiments. Exposure of human monocytes to elevated Na+ex vivo caused a co-ordinated response involving isolevuglandin (IsoLG)-adduct formation, acquisition of a dendritic cell (DC)-like morphology, expression of activation markers CD83 and CD16, and increased production of pro-inflammatory cytokines tumour necrosis factor-α, interleukin (IL)-6, and IL-1β. High salt also caused a marked change in monocyte gene expression as detected by RNA sequencing and enhanced monocyte migration to the chemokine CC motif chemokine ligand 5. NADPH-oxidase inhibition attenuated monocyte activation and IsoLG-adduct formation. The increase in IsoLG-adducts correlated with risk factors including body mass index, pulse pressure. Monocytes exposed to high salt stimulated IL-17A production from autologous CD4+ and CD8+ T cells. In addition, to evaluate the effect of salt in vivo, monocytes and T cells isolated from humans were adoptively transferred to immunodeficient NSG mice. Salt feeding of humanized mice caused monocyte-dependent activation of human T cells reflected by proliferation and accumulation of T cells in the bone marrow. Moreover, we performed a cross-sectional study in 70 prehypertensive subjects. Blood was collected for flow cytometric analysis and 23Na magnetic resonance imaging was performed for tissue sodium measurements. Monocytes from humans with high skin Na+ exhibited increased IsoLG-adduct accumulation and CD83 expression.
Conclusion
Human monocytes exhibit co-ordinated increases in parameters of activation, conversion to a DC-like phenotype and ability to activate T cells upon both in vitro and in vivo sodium exposure. The ability of monocytes to be activated by sodium is related to in vivo cardiovascular disease risk factors. We therefore propose that in addition to the kidney and vasculature, immune cells like monocytes convey salt-induced cardiovascular risk in humans.
Keywords: Sodium, Monocytes, Dendritic cells, Isolevuglandins, Oxidative stress
Graphical Abstract
1. Introduction
Excessive dietary salt is a major risk factor for hypertension and cardiovascular disease.1,2 The AHA recommends a maximum of 2300 mg of sodium intake per day, however recent estimates indicate that sodium intake is more than twice this in most countries. A meta-analysis by He et al.3 estimated that modest reductions in sodium intake would lower blood pressure and reduce the annual number of new cases of coronary heart disease and stroke by 20%. However, the mechanisms by which sodium contributes to blood pressure elevation and cardiovascular disease is not fully elucidated. A major problem with excess salt consumption is that a substantial portion of the population exhibits salt-sensitivity, defined as a 10 mmHg increase in blood pressure following sodium loading or a similar drop in blood pressure after sodium restriction and diuresis.4Salt-sensitivity is an independent predictor of death and cardiovascular events even in normotensive individuals.5,6
Traditionally studies of how sodium contributes to hypertension have focused on increases extracellular fluid volume, alterations in vascular function, and central stimulation of the sympathetic nervous system.7,8 Recently, it has been observed that sodium accumulates in microdomains of the interstitium in concentrations exceeding those of plasma. In 2009, Machnik et al.9 found that excess dietary salt increases interstitial sodium in the skin without changing plasma concentrations in rodents. Subsequent studies using 23Na magnetic resonance imaging (MRI) demonstrated that sodium accumulates in the skin and skeletal muscle of humans with hypertension and during ageing.10 Such high concentrations of sodium have been shown to polarize immune cells towards an inflammatory phenotype.11–13
These observations regarding tissue sodium have relevance to circulating monocytes as it has now been recognized that these cells can enter and re-emerge from tissues with minimal or no differentiation.14 For more than 50 years, pathologists have described the infiltration of monocyte/macrophages and lymphocytes in the kidneys and vasculature of hypertensive humans and experimental animals.15,16 There is strong evidence that monocytes contribute to both blood pressure elevation and end-organ damage associated with hypertension. Deletion of monocytes markedly reduces experimental hypertension caused by angiotensin II infusion.17 Cells derived from monocytes, including macrophages and dendritic cells (DCs), have also been implicated in hypertension.18–20
We previously found that antigen presenting cells accumulate Isolevuglandin (IsoLG)-protein adducts during angiotensin II and deoxycorticosterone acetate-salt hypertension.20 IsoLGs are highly reactive products of lipid peroxidation that rapidly adduct to lysine on proteins and their accumulation is associated with DC activation.20 We recently established that elevated sodium is a potent stimulus for IsoLG-adduct formation in murine DCs.18 Taken together, these studies in mice suggest that the activation of the immune system might contribute to salt-induced cardiovascular disease. In the present study, we aimed to investigate the effect of high sodium concentration in human monocytes. We isolated monocytes from blood and exposed them to a tissue sodium concentration (190 mM) in culture. Monocytes were evaluated by flow cytometric analysis, RNA sequencing and cytokine measurement in the culture media. To evaluate how a high-salt diet affects human monocytes and consequently T cells, we adaptively transfered human monocytes and T cells to immunodeficient mice fed high salt. Moreover, we evaluated the relationship between sodium tissue concentration as measured by 23Na MRI and monocyte activation markers in a cohort of 70 prehypertensive subjects.
2. Methods
2.1 Human subjects
The cells used in this study were isolated from human volunteers and placed in culture. For analysis of sodium MRI and sodium excretion, we recruited two independent cohorts. All subjects gave written informed consent before enrolling in the study as approved by Institutional Review Board of Vanderbilt University (23Na MRI cohort: IRB #141382) and Research Ethics Committee at the Faculty of Medical Sciences, University of Campinas, Sao Paulo, Brazil (Sodium excretion cohort: IRB#. 188.161/2013). All procedures were performed in accordance with the Declaration of Helsinki.
2.2 Human samples
In experiments to examine responsiveness of monocytes to sodium in vitro (Figures 1–6), we requested sample collection from subjects between 18 and 75 years of age with no history of inflammatory diseases. Physical exam and blood pressure measurements were done at screening. Exclusion criteria were: (i) confirmed or suspected renal, renovascular, or endocrine (primary hyperaldosteronism and pheochromocytoma) causes of secondary hypertension; (ii) diabetes mellitus, type I or II; (iii) concomitant illness requiring corticosteroids or immunosuppressants; (iv) recent (within 3 months) vaccination against any infectious agent; (v) active ongoing malignancy; (vi) severe psychiatric disorders; and (vii) HIV/AIDS. Blood samples were received upon request and monocytes were isolated on the same day. The sample collection was approved by Institutional Review Board of Vanderbilt University (IRB #130979). Clinical characteristics of the donors are presented in Supplementary material online, Tables S5 and S6.
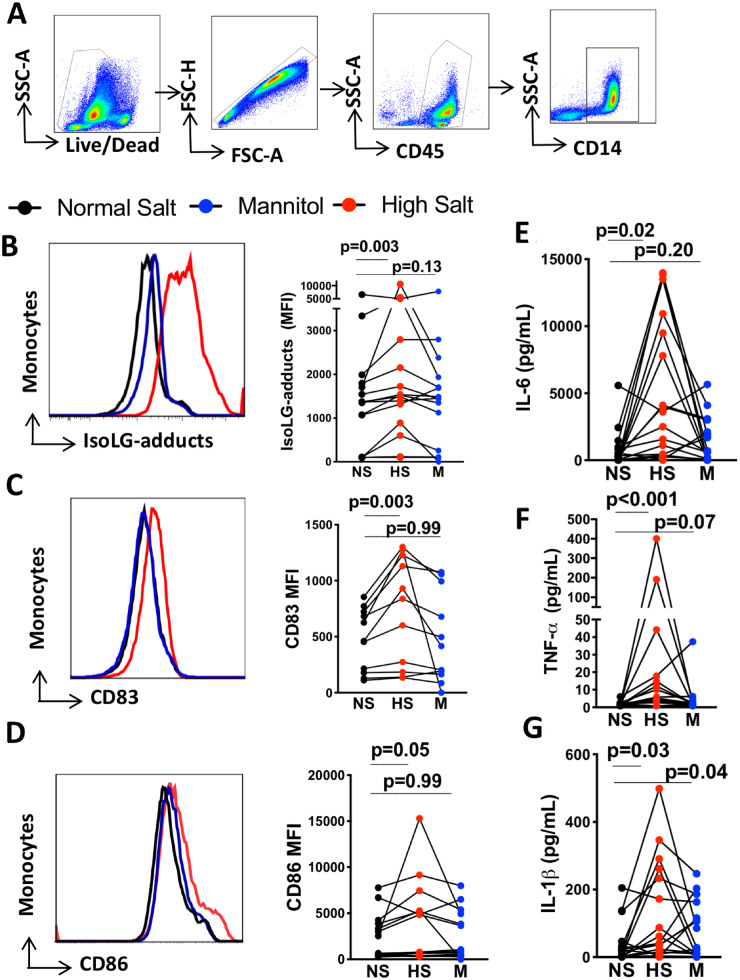
Figure 1.
High salt activates and promotes immunogenic IsoLG-adduct formation in human monocytes independently of osmolarity. Human monocytes were isolated by magnetic separation and cultured for 48 h in normal salt media (150 mMol/L NaCl), high-salt media (190 mMol/L NaCl), or normal salt media with added mannitol (80 mMol/L) as an osmotic control for 48 h. (A) Gating strategy to identify monocytes. (B) Representative and group flow cytometric data for monocyte intracellular staining of IsoLG-protein adducts (n = 16). (C) Representative histogram and group data for surface expression of CD83 on monocytes (n = 12). (D) Representative histogram and group data for monocyte surface expression of CD86 (n = 16). (E) Production of cytokines IL-6 (n = 18), (F) TNF-α (n = 18), and (G) IL-1β (n = 18) determined by flow cytometry using bead-based immunoassay on media of monocytes exposed to normal salt, mannitol, and high salt. Monocytes from each individual were exposed to three experimental conditions. The data point joined lines represent paired analyses for each human subject. Friedman nonparametric analysis with a Dunn’s post hoc test was employed in panels B to G. Clinical data of cell donors are shown in Supplementary material online, Table S5.
2.3 23Na MRI cohort
To compare monocyte activation state to in vivo tissue sodium, 70 subjects were recruited as part of Vanderbilt University’s American Heart Association Strategically Focused Research Network (SFRN) protocol. Subjects selected for SFRN protocol were consecutively recruited for the current cohort between 2014 and 2017. Blood collection and 23Na MRI were performed on the same visit day. Clinical and demographic characteristics of these subjects are shown in Supplementary material online, Table S1. Exclusion criteria included; (i) acute cardiovascular events within the previous 6 months; (ii) impaired renal function (estimated glomerular filtration rate of <40 mL/min); (iii) current or recent treatment with systemic glucocorticoid therapy (within 1 month of enrolment); (iv) current use of anti-hypertensive medication; (v) diabetes; (vi) severe obesity [body mass index (BMI) >35]; (vii) inability to understand the nature of the study or to participate in the study; and (viii) claustrophobia preventing the patient from having an MRI. Based on other publications comparing tissue sodium in healthy vs. hypertensive subjects10 and our previous research quantifying IsolG-adduct in animals and humans,18,20 we estimated that salt accumulation or hypertension-related condition to IsolG-adduct formation have a medium to large effect size. An effect size of 0.75 (large) and power of 90% with a significant level at 0.05 in unpaired t-test resulted in a sample size of 78.
2.4 Sodium excretion cohort
To evaluate the relationship between plasma IsoLG-adduct and urinary sodium excretion, an additional 18 subjects were recruited at the Hospital of the University of Campinas. Clinical and demographic characteristics of these subjects are shown in Supplementary material online, Table S2. We excluded subjects with symptomatic ischaemic heart disease, liver disease, impaired renal function, history of stroke, and peripheral vascular disease. Subjects were recruited from outpatient clinic database if they have sodium excretion measurements within 4 months window of blood sample collection.
2.5 Effect of sodium exposure on human monocytes
Heparinized blood (40 mL) was obtained from volunteers and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-gradient. Monocytes were further isolated from these cells by magnetic labelling and negative selection using the Miltenyi monocyte isolation kit (Miltenyi Biotec 130-091-151) and cultured in 12-well plates at 1 × 106/mL density in either control RPMI media (150 mMol/L Na+) or media containing 190 mMol/L Na+. RPMI media 1640 (Gibco) was supplemented with 10% FBS, 1% pen/strep, 1% HEPES, and 2-Mercaptoethanol (0.05 mM). To control for hyperosmolality, other cells were exposed to mannitol (190 mMol/L). To inhibit the NADPH oxidase, 5 μM of GSK2795039 or gp91dstat was added to culture media. For cell culture experiments where a cytokine cocktail was used to convert monocytes to DC, GM-CSF (1000 U/mL) and interleukin (IL)-4 (500 U/mL) was added to culture media. Following culture, attached cells were released using Accutase (Innovative Cell Technologies) according to manufacturer’s instructions.
2.6 Monocyte T-cell co-culture
T-cell stimulatory functions of monocytes were tested in a co-culture system. Monocytes were isolated as previously described and cultured in 96-well U-bottomed plates in either normal or high-salt media. After 48 h, the media was changed to one containing normal sodium concentrations and T cells from the same subjects were added for an additional 72 h. To obtain T cells, subjects were recalled for collection of a fresh blood sample and T cells were isolated by magnetic labelling and negative selection using Miltenyi Pan T cell isolation kit (130-096-535). The ultimate co-culture contained 25 000 monocytes and 250 000 T cells in 250 µL of media.
2.7 T-cell proliferation in humanized mice
Immunodeficient (NSG) male mice were obtained from Jackson Laboratories. All experiments were performed at approximately 9 weeks of age. The mice were fed a high-salt diet (4% NaCl, Teklad TD.92034) or regular chow for 2 weeks. Human monocytes and T cells six male and two female volunteers as were isolated described above. T cells were labelled with Cell Trace Violet (Thermo Fisher). A ratio of 1 monocyte to five T cells (1–2 × 106 monocytes and 5–10 × 106 T cells from the same subject) in 200 μL of sterile physiological buffered saline was injected via the retro-orbital vein in mice anaesthetized with 2% isoflurane. As control, only T cells were injected in some mice that received the high-salt diet. Mice were sacrificed 10 days after adoptive transfer by CO2 inhalation. Spleens were mechanically dissociated, and the cells resuspended in 10 ml of RPMI media and passed through a 40 µm filter. For studies of bone marrow cells, tibias and femurs of mice were flushed using RPMI media. All animal procedures were approved by Vanderbilt University’s Institutional Animal Care and Use Committee, and the mice were housed and cared for in accordance with the Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services.
2.8 Monocyte migration assay
Monocytes were isolated as described above. One million monocytes were placed in the upper chamber of a 24 transwell plate (3-μm pores, Corning Transwell) in which 10 ng/mL of chemokine (C-C motif) ligand 2 (CCL2) (Sigma) or CCL5 (Stemcell) was placed in the lower chamber. During these assays, the monocytes were exposed to either control RPMI media (150 mMol/L Na+) or media containing 190 mMol/L NaCl. After 24 h, monocytes in the lower chamber were quantified by flow cytometry using cell viability dye, CD45 and CD14 markers.
2.9 Flow cytometry
For analysis of monocyte and monocyte-derived cells, we used LIVE/DEAD™ Fixable Violet Dead Cell Stain Kit (Thermo Fisher L34955) or 7AAD to determine the viability of cells and the following antibodies at concentration of 1–2 µg/100 µL: APC-Cy7 anti-CD45 (Biolegend); PerCP-Cy5.5 anti-CD14 (eBioscience), BV510 anti-CD16 (BD Bioscience), PE-Cy7 anti-CD1c (eBioscience); APC anti-CD83 (eBioscience) and PECy7-conjugated anti- CD86 (Biolegend). We used intracellular staining with the single chain antibody D-11 to detect IsoLG protein adducts. The D11 ScFv antibody was labelled with a fluorochrome using the APEXTM Alexa Fluor 488 Antibody Labeling kit (Invitrogen). Cells were then fixed and permeabilized for intracellular detection of IsoLGs using a cell permeabilization kit (Invitrogen).
For T-cell analysis, the following antibodies were used: (PerCP-Cy5.5)-conjugated or APC-conjugated anti-CD3 antibody or (BioLegend), PE-Cy7- conjugated anti-CD8 antibody (BioLegend), and APC-H7-conjugated anti-CD4 antibody (BioLegend). Intracellular staining with BV510-conjugated anti-IL-17A (BD Biosciences) and FITC-conjugated IFN-γ (BioLegend) were performed as previously described.21,22 In brief, 1–2 × 106 T cells were suspended in RPMI medium supplemented with 5% FBS and stimulated with 2 µL of BD Leucocyte Activation Cocktail [ionomycin and phorbol myristic acetate along with the golgi inhibitor, brefeldin A] at 37°C for 5 h. Surface staining was performed as described above followed by intracellular staining using FITC-conjugated anti-IFN-γ antibody (eBioscience), BV510-conjugated anti-IL-17A (eBioscience). The cells were then washed and immediately analysed by flow cytometry. For each experiment, we gated on single live cells and used flow minus one controls for each fluorophore to establish the gates. Data analysis was done using either FACS DIVA (Becton Dickinson, Franklin Lakes, NJ) or FlowJo software (Tree Star, Inc.).
2.10 Renal histology
Renal biopsy samples were obtained from banked tissue at the Vanderbilt University Medical Center. Samples from patients with arterionephrosclerosis (n = 10) and controls (n = 9) were studied. Kidney tissue was immersion fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with CD14 (D7A2T), CD68 (D4B9C), and CD11c (D3V1E) (Cell Signaling). The prevalence of cells at the corticomedullary junction expressing these markers were scored as rare/absent, mild or moderate to severe according to the number of cells per field.
2.11 Analysis of cytokine production
Media from monocyte culture were analysed for cytokine release using LEGENDplex cytometric bead array from BioLegend (Human Inflammation Panel #740118) following the manufacturer’s instructions. The panel of analytes included: IL-1β, IFN-α, IFN-γ, tumour necrosis factor (TNF)-α, CCL2 (MCP-1), IL-6, IL-8, IL-10, IL-12 (p70), IL-17A, IL-18, IL-23, and IL-33.
2.12 MRI measurements of tissue Na+ content
Imaging was done on a Philips Achieva 3.0 T MR scanner (Philips Healthcare, Cleveland OH, USA) with a 23Na quadrature knee coil (Rapid Biomedical GmbH, Rimpar, Germany) as reported previously.10,23 We used calibration phantoms (aqueous solutions with increasing NaCl concentrations) as reference standards and scan them together with sections through the subject's calf muscles for quality control. The left lower leg (the widest part of calf region) was scanned with the skin closely in contact with the hard surface of the phantom holder for 3 min and 52 s. All imaging data were processed off-line with custom MATLAB (R2013a) scripts. Na+ quantification was performed by comparing signal intensities between tissue and calibration phantoms on the Na+ image. A linear relationship (Na+ concentration vs. signal intensity) was assessed based on the phantom data, and results from a linear regression are applied to tissue regions to quantify Na+ content. For urine sodium measurement, an ion selective electrode was employed to quantify sodium in samples collected over 24 h.
2.13 Mass spectrometry
Heparin-anticoagulated blood samples were collected after overnight fasting. The blood samples were centrifuged at 2000×g for 10 min and plasma fractions were immediately stored at –80°C until used for IsoLG-adduct by mass spectrometry as previously described.24
2.14 RNA sequencing (RNASeq)
Human monocytes from 11 subjects were isolated and cultured in two experimental conditions: normal salt (150 mM) or high salt (190 mM) for 72 h. Total RNA was isolated using the RNEasy Midi kit (Qiagen, Valencia, CA, USA) per manufacturer’s instruction. The Illumina Tru-seq RNA sample prep kit was employed for targeted analysis of polyadenylated transcripts. Paired-end sequencing was conducted on the Illumina HiSeq 2500. The resulting FASTQ data files for each sample were aligned with TopHat 225 against the human GRCh38 reference genome assembly using the R package. RNAseq data were thoroughly quality controlled (QC) at multiple stages of data processing following the recommendation in ref.26 Raw data and alignment QC were performed using QC3,27 and expression analysis were carried out using the MultiRankSeq method.28 Raw data false discovery rate (FDR < 0.05) was used to correct for multiple hypothesis testing.
2.15 Statistical analyses
Data are presented as mean ± standard error of the mean. Normality of distribution was assessed by the Shapiro–Wilk test. Comparisons of two groups were performed using Student’s t-tests or the Mann–Whitney test if data were not normally distributed. To compare more than two paired groups we used repeated measures analysis of variance (ANOVA) followed by a Tukey’s multiple comparison test. For data not normally distributed, the Friedman tests were used followed by Dunn’s multiple comparison test. Nonparametric data were log transformed for correlation and regression analyses. Correlations were examined using Spearman’s method according to distribution of the data. To understand how tissue sodium affects monocyte activation state, subjects were categorized as low Na+ or high Na+ based the median skin sodium (12.3 mM) for the overall cohort undergoing 23Na MRI. Complete-case analysis was used for 23Na MRI measurement. P-values <0.05 were used to reject the null hypothesis.
3. Results
3.1 High salt induces a pro-inflammatory human monocyte phenotype
Studies in experimental models have estimated that salt feeding can increase interstitial skin sodium concentrations to 190 mMol/L.9 Exposure of human monocytes to this concentration of salt, but not equiosmolar mannitol, increased IsoLG-adduct formation (Figure 1A and B). High salt also increased surface expression of the activation marker CD83 and we found no evidence that an equiosmolar concentration of mannitol increases CD83 (Figure 1C). There was no evidence of an increase in expression of the co-stimulatory ligand CD86 (Figure 1D). We previously showed that experimental hypertension stimulates production of IL-6 and IL-1β by murine DCs.20 In the present study, we found that exposure of human monocytes to elevated Na+ mimics this and also stimulates monocyte production of TNF-α using the Luminex assay (Figure 1E–G). The full panel of analytes is shown in Supplementary material online, Table S3. Since increased osmolarity using mannitol did not significantly activate these cells, we performed all subsequent experiments using normal salt media as control.
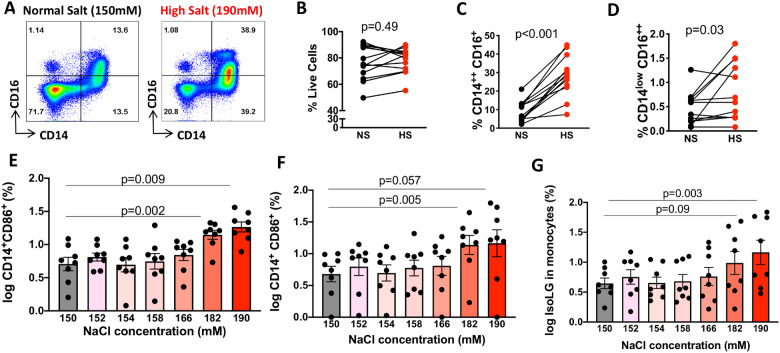
Classical monocytes can transition to an intermediate phenotype characterized by acquisition of CD16 and persistence of the CD14 marker. These CD14++/CD16+ intermediate monocytes can further lose CD14 and become non-classical monocytes (CD14low/CD16+). The CD14++/CD16+ cells comprise about 10% of circulating monocytes and have been found to contribute to inflammation.29 In additional experiments, we found the distribution of classical and intermediate monocytes was altered by exposure to high salt (Figure 2A–E). We found no evidence that high salt affected the percentage of live cells (Figure 2B) but increased the percentage of CD14++CD16− (2 C) CD14++/CD16+ intermediate monocytes (Figure 2D) and the non-classical CD14low/CD16++ population (Figure 2E). Furthermore, we sought to determine the threshold concentration of sodium that affects monocyte phenotype. We found no evidence that concentrations up to 166 mMol/L affect human monocytes, while concentrations of 182 and above increased transformation to an intermediate phenotype (Figure 2F). We found that the sodium concentration of 190 mMol/L was required to significantly increase monocyte surface expression of CD86 (Figure 2G) and production of IsoLG-protein adducts (Figure 2H). Thus, ex vivo exposure of human monocytes to increased extracellular sodium promotes a coordinated increase in IsoLG-protein adduct production, surface expression of activation markers as well as increased cytokine production that is evident in some, but not all subjects. This is accompanied by skewing of the monocytes towards a pro-inflammatory intermediate phenotype.
Figure 2.
High salt induces a pro-inflammatory human monocyte phenotype. Human monocytes were magnetically isolated and cultured for 48 h in media containing normal salt (150 mM NaCl) or high salt (190 mM NaCl). (A) Representative flow plots showing differences in subtypes of monocytes cultured in normal or high-salt media (n = 13). (B) Paired comparison of percentages of live cells, (C) intermediate monocytes, and (D) non-classical monocytes. Panels E–G (n = 8) shows the effect of increasing sodium concentrations on conversion to intermediate (CD14++CD16+) monocytes, CD86 expression and IsoLG-adduct formation. Monocytes were cultured in increasing sodium concentrations by adding 2, 4, 8, 16, 32, and 40 mM of NaCl to RPMI media. Paired T-test was employed in B–D. Repeated measures one-way ANOVA with Dunnets post hoc test were employed for E–G. Clinical data of cell donors are shown in Supplementary material online, Table S5.
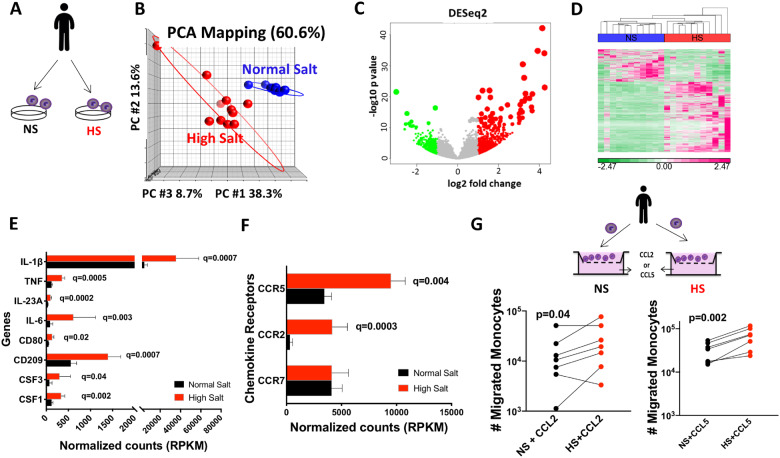
In addition, we employed RNA sequencing on human monocytes from 11 volunteers following ex vivo exposure to either normal or high salt for 72 h (Figure 3A). Principal component analysis revealed that the genes from normal salt-treated monocytes cluster differently from those treated with high salt with the higher variability among the high-salt-treated cells (Figure 3B). The volcano plot in Figure 3C demonstrates the extent to which the genes were up-regulated (red) or down-regulated (green) in response to high-salt exposure. Post-alignment analysis identified 1193 transcripts to be significantly different between normal salt and high-salt-treated monocytes. Hierarchical clustering is shown in Figure 3D.
Figure 3.
Effect of sodium on human monocyte RNA transcriptome and monocytes migration. (A) Human monocytes obtained from 11 subjects were isolated by magnetic isolation and separated for 72-h culture in normal salt (150 mM NaCl) or high salt (190 mM NaCl) media. (B) Principal component analysis (PCA) showing differential clustering of genes in normal salt or high salt treated monocytes. (C) Volcano plot showing the extent to which the genes were up-regulated (red) or down-regulated (green) in response to high-salt treatment. (D) Hierarchical clustering of RNA transcript RPKM (reads per kilobase per million mapped reads) illustrating differences in gene expression in response to normal salt and high-salt treatment. Clustering shown above the heat map were produced using Partek Genomics Suite with average linkage and Euclidian distance measures. Bright pink, bright green, and white represent highest, lowest, and median read values, respectively. Rows represent individual genes, and columns show individual samples. E and F show RPKM for selected chemokine receptors, cytokines and activation markers. (G) Human monocytes (n = 7) were seeded on the upper chamber of a transwell plate and incubated with CCL2 (10 ng/mL) or CCL5 (10 ng/mL) in normal or high-salt media. After 24 h, migrated monocytes were quantified on lower chamber by flow cytometry. Adjusted P-values (q) are shown in E and F using false discovery rate (FDR < 0.05). Clinical data of cell donors are shown in Supplementary material online, Table S5.
FC(edgeR) analysis showed that high-salt exposure increased expression of the inflammatory cytokines IL-1β, TNF-α, and IL-23A, activation markers including CD80 and CD209, and colony stimulating factors CSF 1 and 3 (Figure 3E). We also found that high salt significantly up-regulated mRNA for the chemokine receptors CCR2 and CCR5 (Figure 3F), known to be essential in the migration of monocytes. In additional experiments, we found that high salt exposed monocytes exhibited increased migration towards both CCL2 and CCL5 (Figure 3G). In the absence of the chemokine ligands, migration across the transwell was minimal (data not shown).
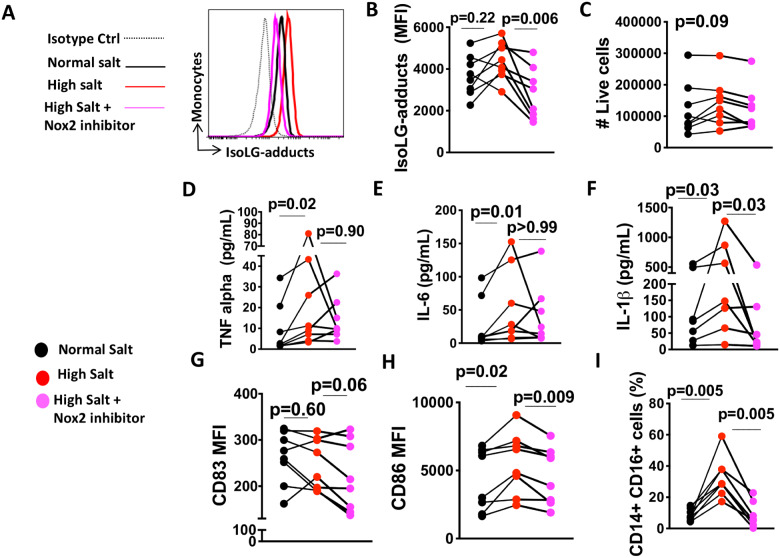
3.2 Sodium-induced activation of human monocytes is NADPH oxidase-dependent
IsoLG protein-adducts have many features of foreign antigens. We previously found that the formation of IsoLG-adducts in murine DCs was mediated by NADPH oxidase.18 Activation of human monocytes was NADPH oxidase-dependent as addition of the Nox2-specific inhibitor GSK2795039 during 48-h incubation with high salt prevented the high salt-induced IsoLG-protein adduct formation (Figure 4A and B), but we found no evidence that high salt affects the number of live cells (Figure 4C). This effect was confirmed using a global NADPH oxidase inhibitor (gp91dstat) shown in Supplementary material online, Figure S1. Gp91dstat significantly decreased the high salt-induced production of IsoLG-protein adducts (Supplementary material online, Figure S1A and C). GSK2795039 also decreased production of the cytokine IL-1β (Figure 4F). We did not find evidence that GSK2795039 has an effect on TNF-α (Figure 4D) or IL-6 (Figure 4E). We also found that Nox2 inhibition decreased the high salt-induced expression of CD83 (Figure 4G) and CD86 (Figure 4H). In addition, inhibition of Nox2 reduced formation of the pro-inflammatory CD14++/CD16+ intermediate monocytes induced by high salt (Figure 4I). These results suggest that activation of the NADPH oxidase and subsequent formation of IsoLG-adducts are likely required for human monocyte activation in response to elevated Na+.
Figure 4.
NADPH oxidase dependence of human monocyte activation by sodium. Isolated monocytes were cultured for 48 h in RPMI media containing 150 mMol/L NaCl, 190 mMol/L NaCl, or 190 mMol/L NaCl plus GSK2795039, a specific inhibitor of Nox2. (A) Representative flow cytometry showing IsoLG-adduct formation in human monocytes. (B) Average data showing IsoLG-adduct formation in human monocytes. Number of live cells (C) in cultured cells (n = 8). Effect of specific inhibition of Nox2 using GSK2795039 (n = 8) on production of cytokines TNF-α (D), IL-6 (E), and IL-1β (F). Effect of Nox2 inhibition (n = 8) on high salt-induced expression of CD83 (G) and CD86 (H). Effect of inhibition of Nox2 on the high salt-induced formation of the pro-inflammatory CD14+/CD16+ intermediate monocytes (I). Data were analysed using RM one-way ANOVA or Friedman test according to data distribution. Clinical data of cell donors are shown in Supplementary material online, Table S5.
3.3 High salt drives monocytes to differentiate into a dendritic cell-like phenotype
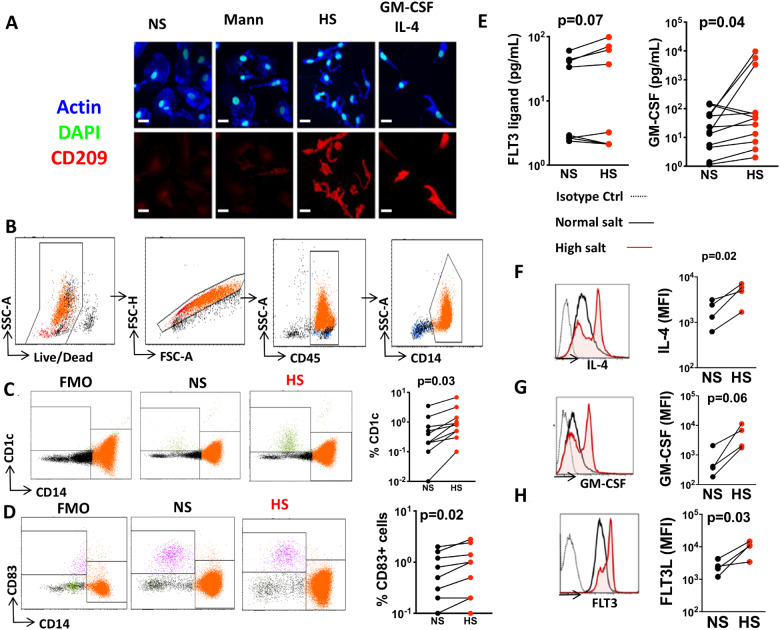
Compared to normal salt and mannitol, the cells exposed to high salt exhibited a marked transformation in morphology and acquired the DC marker CD209. These changes mimicked the effect of exposure to IL4 and GMCSF, which have been commonly used to promote monocyte conversion to DCs (Figure 5A). In additional experiments, we performed flow cytometry on live single CD45+CD14+ monocytes (Figure 5B) and found that compared to normal salt, exposure to excess salt increased surface expression of DC marker CD1c (Figure 5C) and CD83 (Figure 5D). We also examined the ability of the salt-treated monocytes to produce monocyte differentiating growth factors and cytokines including granulocyte-macrophage colony stimulating factor (GM-CSF), IL-4 and fms-like tyrosine kinase 3 (FLT3) ligand. Exposure to excess salt increased release of the FLT3 ligand and GM-CSF in the media using Luminex assay (Figure 5E). We also employed PrimeFlow, which uses branched DNA technology to quantify gene expression in single cells by flow cytometry and found that exposure of monocytes to high salt causes them to produce DC differentiating cytokines IL4, GM-CSF, and FLT3 (Figure 5F–H). These results suggest that human monocytes exposed to high salt produce their own cocktail of monocyte-differentiating cytokines, which likely act in an autocrine fashion to stimulate these cells to convert to a DC-like phenotype.
Figure 5.
High salt drives monocytes to differentiate into a dendritic cell-like phenotype. Monocytes were isolated from the buffy coats of human volunteers and exposed to normal salt (NS), mannitol (Mann), or high salt (HS). (A) Immunofluorescence microscopic image showing morphology and CD209 staining of cells exposed to these conditions and also to a combination of GM-CSF and IL-4 after 6 days in culture (scale bars = 20 µm). (B) Flow cytometry gating strategy to identify monocytes. Flow cytometry representatives and average data (n = 10) showing the effect of high-salt exposure on dendritic cell markers CD1c (C) and CD83 (D). (E) Production of GM-CSF and Flt3 in media using a Luminex based assay (n = 8 and 12). Effect of high salt on IL-4 (F), GM-CSF (G), and FLT3 receptor (H) mRNA expression in monocytes using PrimeFlow (n = 4) (*P < 0.05). Data were analysed using paired t-tests. Clinical data of cell donors are shown in Supplementary material online, Table S5.
3.4 Monocytes exposed to high salt induce T-cell activation
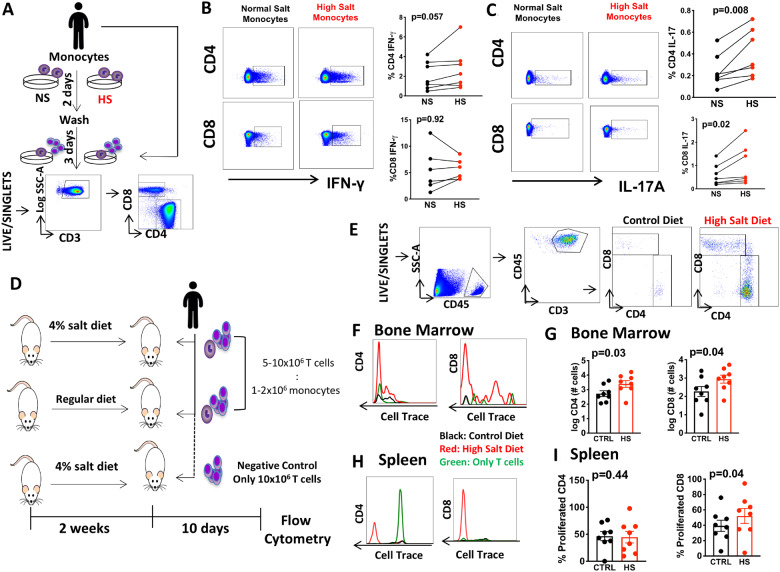
While resting monocytes are not potent antigen presenting cells, they can acquire this property after entering and re-circulating from non-lymphoid tissues.14 To further determine if high salt stimulates the ability of monocytes to activate T cells, we exposed monocytes to normal or high-salt media for 48 h. We recalled the same subjects and autologous T cells were then isolated and co-cultured with these monocytes in the presence of normal salt media (Figure 6A). The ability of monocytes to stimulate CD8 and CD4 T cells was quantified by intracellular staining of IL-17A and IFN-γ. We did not find evidence that high-salt exposure to monocytes affects the production of IFN-γ among CD4 and CD8 T cells (Figure 6B), however, it induced a marked increase in production of IL-17A among both CD4+ T and CD8+ T cells (Figure 6C).
Figure 6.
In vitro and in vivo T-cell activation by monocytes exposed to high salt. (A) Experimental design: monocytes were isolated and exposed to normal (NS) or high salt (HS) for 2 days and T cells were subsequently obtained from the same subjects and cultured for an additional 3 days with monocytes at a ratio 1:10 and analysed by flow cytometry. (B and C) Representatives flow plots and group paired data for intracellular staining of IFN-γ and IL-17 in CD4 and CD8 positive T cells (n = 7). (D) Experimental design to study T-cell activation in vivo. Immunodeficient mice (NSG) received HS diet (4% NaCl) or regular chow for 2 weeks (n = 8). Human monocytes and T cells labelled with Cell Trace Violet (ratio 1:5) from same subject were then adaptively transferred into these animals. Additional HS fed mice were injected with only T cells and no monocytes. (E) Gating strategy to identify human T cells in NSG mice 10 days later. (F and G) Example histograms and group proliferation marker intensity of CD4 and CD8 T cells in the bone marrow. (H and I) Example histograms and group data for CD4 and CD8 T-cell proliferation in the spleen. Data were analysed by paired T tests. Clinical data of cell donors are shown in Supplementary material online, Table S5 (A–C) and 6 (D–I). *Only samples from hypertensive subjects were requested due to likeability to respond to high-salt diet in cells adoptively transfer experiments (D–I).
To determine if monocytes drive T-cell activation in vivo, we fed immunodeficient (NOD-scid IL2Rγ null) mice a regular or high-salt diet for 2 weeks, and adoptively transferred human monocytes together with T cells labelled with the proliferation indicator, cell trace violet (Figure 6D). Ten days after adaptive transfer, flow cytometry was performed on bone marrow and the spleen (Figure 6E). We found that high salt feeding markedly increased proliferation and number of human T cells in the bone marrow (Figure6F and G) and the percentage of proliferated human CD8+ but no evidence was found regarding the percentage of CD4+ T cells in the spleen (Figure6H and I). Importantly, human T-cell proliferation was not observed in mice only received T cells and not monocytes (Figure6F and H in green), showing that monocytes are essential for the activation of T cells in vivo by salt.
3.5 Monocyte IsoLG-adduct formation by elevated Na+ is associated with cardiovascular risk factors
Cardiovascular risk factors including increased BMI, cholesterol, glucose, and pulse pressure (PP) are associated with salt sensitivity.30 We sought to determine if these risk factors predispose to the ex vivo response of monocytes to salt. We compared the monocytes response to salt in vitro, denoted by the difference of IsoLG content in high salt minus the IsoLG content in normal salt in Figure 1B, to the individual’s cardiovascular risk factors. Strikingly, monocyte IsoLG-adduct formation in response to salt was positively correlated with higher BMI and pulse pressures, a marker of arterial stiffness (Table 1 and Supplementary material online, Figure S4). These associations suggest that monocytes exhibit salt-sensitivity depending on in vivo cardiovascular risk factors.
Table 1.
Monocyte IsoLG stimulation by salt is associated with cardiovascular risk factors
| Spearman’s correlation | log ΔIsoLG (HS-NS) |
||
|---|---|---|---|
| n | r | P | |
| PP (mmHg) | 16 | 0.50 | 0.04 |
| BMI (kg/m2) | 16 | 0.56 | 0.03 |
| TC (mg/dL) | 14 | 0.52 | 0.05 |
| Glucose (mg/dL) | 15 | 0.43 | 0.10 |
Clinical characteristics from subjects used in Figure 1B were correlated with the formation of IsoLG-adducts in response to salt.
The scatterplots are shown in Supplementary material online, Figure S4.
BMI, body mass index; HS, high salt; IsoLG, isolevuglandins; LDL, low-density lipoprotein cholesterol; NS, normal salt; PP, pulse pressure; TC, total cholesterol.
3.6 Sites of activation of human monocytes by high sodium
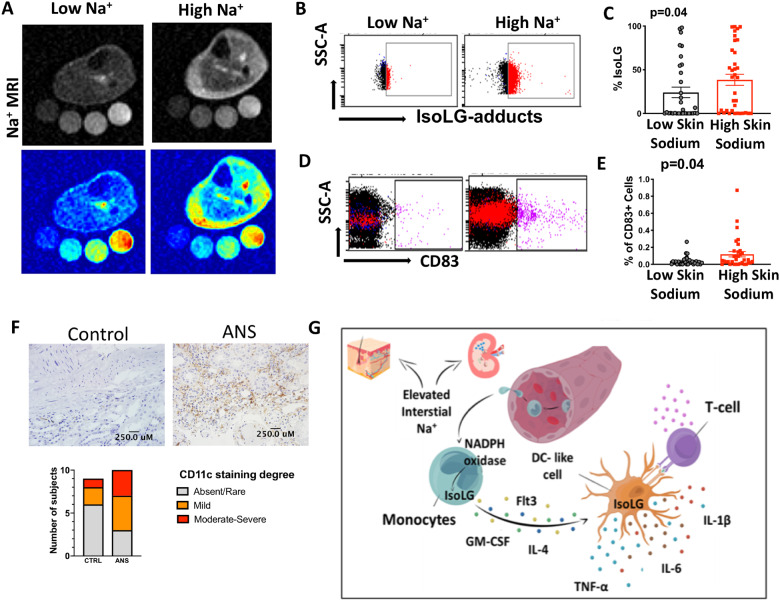
In additional experiments, we sought to determine sites where human myeloid cells, including monocytes, macrophages, and DCs encounter a high sodium environment. Salt feeding increases sodium content in the skin of experimental animals.9 Moreover, skin sodium as detected by 23Na MRI is increased in humans with ageing, hypertension and heart failure.31,32 We therefore sought to determine if skin Na+ concentrations are associated with IsoLG-protein-adduct formation and activation of human monocytes. We recruited 70 subjects and quantified skin sodium using 23Na MRI as previously described.23,33 We examined freshly isolated PBMCs obtained on the same day using flow cytometry. Representative 23Na MRI images of a subject with a low and high Na+ concentration in the interstitium are shown in Figure 7A. We found that subjects with a higher Na+ concentration in the skin had increased formation of IsoLG-adducts in their monocytes and a higher percentage of cells expressing CD83 (Figure 7B–E). In contrast to skin Na+, we found no evidence that monocyte CD83 expression and IsoLG-protein adduct content were different when subjects were partitioned by muscle sodium (Supplementary material online, Figure S2). However, the lack of difference may be due to small sample size when the patients were divided. In an additional cohort of 18 subjects (sodium excretion cohort), we found that increased 24-h urine Na+ correlated with increased plasma IsoLG-protein adducts as measured by mass spectrometry (Supplementary material online, Figure S3). Subjects with 24-h urine Na+ concentrations greater than 200 mEq, equivalent to an intake of >4.8 g of sodium/day, had increased plasma IsoLG-protein adducts (Supplementary material online, Figure S3).
Figure 7.
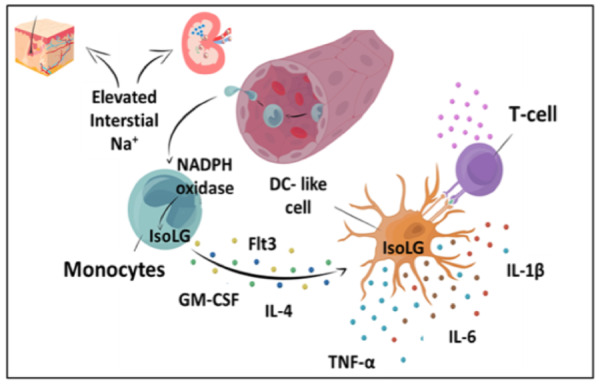
Sites of activation of human monocytes by high sodium. (A) Representative 23Na magnetic resonance image (23Na MRI) of the lower leg of subject with low Na+ content vs. high Na+ content in the skin. (B and C) Representative flow plots and quantification for monocyte intracellular staining of isolevuglandin-protein adducts in subjects with low (n = 33) or high skin sodium (n = 37). (D and E) Representative flow plots and quantification of surface CD83 expression. (F) Representative CD11c staining in the corticomedullary junction of a control patient and an patient with arterionephrosclerosis (ANS) (scale bars = 250 µm). (G) Pathway whereby monocytes migrate to a high sodium environment and acquire a DC-like phenotype and accumulate immunogenic isolevuglandins. Exposure of monocytes to high salt promotes formation of inflammatory cytokines (IL-1β, IL-6, and TNF-α), up-regulates activation markers (CD86 and CD83) and arms these cells to promote T-cell activation and proliferation. Mann–Whitney tests were performed in C and E. Data were obtained from subjects in sodium MRI cohort.
Another possible site of monocyte activation by salt is the kidney, where sodium concentrations progressively increase from the corticomedullary junction to levels exceeding 1000 mMol/L at the medullary papilla. We therefore examined sections of human kidneys for antigen presenting cells, including macrophages, DCs, and monocytes (Figure 7F). We observed each of these cell types in the renal medulla, ranging from the corticomedullary junction to deeper portions of the medulla. Importantly, patients with arterionephrosclerosis, the injury pattern of hypertension-associated kidney disease, had a greater proportion of moderate to severe infiltration of CD11c+ than control samples. These data support the concept that monocytes exist in sites of high-salt environment and support our in vitro findings showing that elevated Na+ drives monocyte activation. Demographic, clinical characteristics, and histology scores are shown in Supplementary material online, Table S4. While our main goal for the kidney histological analysis was to show that antigen presenting cells including monocytes are present in the cortical medullary junction and therefore are exposed to high sodium concentrations in vivo, our sample size is underpowered to detect statistical significance.
4. Discussion
When circulating monocytes extravasate into tissues, they can differentiate into macrophages, DCs or re-emerge as activated monocytes with enhanced capacity for antigen presentation (Figure 7G).34 Increasing evidence suggest that sodium can accumulate in tissues in a variety of pathophysiological states including ageing and hypertension. Our current findings suggest that high sodium in these interstitial microdomains can activate human monocytes to an antigen presenting, DC-like phenotype. This is reflected by activation of the NADPH oxidase, production of IsoLG-adducts, increased production of cytokines and the ability to stimulate T cells. Moreover, the ability of monocytes to respond ex vivo to high salt correlated with known cardiovascular risk factors, including BMI and pulse pressure which are known to be associated with salt sensitivity. In addition, we found that monocytes obtained from subjects with higher tissue sodium exhibited increased IsoLG-adducts and increased surface expression of CD83. Likewise, plasma levels of IsoLGs correlated with 24-h sodium excretion, an estimate of salt consumption. We also demonstrated that high-salt feeding of humanized mice caused striking proliferation of human T cells only when monocytes were present. Finally, human monocytes and monocyte-derived cells including DCs and macrophages are found in the kidney, including the corticomedullary junction and medulla, where sodium concentrations are markedly increased compared to the plasma. Taken together, these results suggest a relationship between sodium intake, tissue sodium levels, and monocyte activation.
We observed substantial variability in the response of human monocytes to sodium exposure, suggesting that they exhibit differences in salt sensitivity. Prior studies have focused on the role of the kidney, vasculature and sympathetic nervous system as major determinants of salt sensitivity. As an example, studies in humans have shown that both salt-sensitive and resistant subjects expand circulating volume and increase their cardiac output upon sodium loading equally, however the salt-resistant subjects display a decrease in systemic vascular resistance, while the salt sensitive do not, implicating vascular dysfunction.7 Salt feeding also increases sodium concentration in the cerebral spinal fluid and sympathetic outflow in rats, suggesting a neurogenic response to sodium.35 Alterations in the renal pressure natriuresis relationship are uniformly present in hypertension.36 Our current observations suggest that immune cells like monocytes exhibit different degrees of salt sensitivity. It is possible that these varying cells and organs are related. For example, activated monocytes can accumulate in vessels and promote vascular dysfunction.37 Likewise, bone marrow-derived cells can enter the brain and contribute to neuroinflammation in hypertension.38 Thus, the variable response of human monocytes to salt might contribute to clinical salt sensitivity by altering vascular and central neural function.
While our present study clearly shows that exposure of monocytes to high sodium promotes their activation, the precise sites in vivo where such cells encounter elevated sodium remain uncertain. Plasma sodium levels are not significantly changed by high sodium intake, however increasing evidence suggest that sodium can be concentrated in peripheral tissues including the skin and skeletal muscle. Skin sodium storage is increased in experimental animals by salt feeding and in humans by age and in the presence of hypertension. In keeping with this, we observed a significant relationship between skin sodium as estimated by 23Na MRI and monocyte surface levels of the activation marker CD83 and the intracellular levels of IsoLG adducts. The relationship between these markers and muscle sodium was less impressive, in keeping with the concept that the skin might represent a unique site for sodium mediated immune cell activation.39
Another site where myeloid cells encounter high sodium levels is the kidney, where interstitial sodium concentrations can exceed >1000 mOsM/L. We observed CD14+, CD68+, and CD11c+ cells in histological sections of human kidneys, with a trend for these to be increased in those with arterionephrosclerosis. Interestingly, these were particularly prevalent at the corticomedullary junction, where interstitial sodium concentrations approximate those used in our in vitro study. In this context, a previous study found enrichment of CD14+ cells in the renal medulla. These authors showed that the migration of monocytes to explanted medullary samples was higher than to cortical samples, suggesting that a high sodium environment orchestrates endogenous renal cells to attract immune cells. The same authors showed that medullary CD14+ cells have higher expression of CCR2, consistent with our in vitro findings that salt stimulate this chemokine receptor (Figure 3).40 In preliminary experiments, we found that high-salt feeding of NSG mice increased mRNA expression of the chemokine ligand CCL2, vascular cell adhesion molecule 1 (VCAM-1), and Intercellular Adhesion Molecule 1 (ICAM-1) in the kidney, which could enhance migration of myeloid cells. Endothelial dysfunction, which commonly occurs in hypertension, also favours transmigration of leucocytes to interstitial sites. Thus, excessive salt consumption and the endothelial dysfunction that occurs in hypertension may exacerbate the transmigration of these cells to high sodium environments, where they become activated and induce T-cell proliferation and production of inflammatory cytokines.
Our data also indicate that reactive oxygen species (ROS) play an important role in activation of human monocytes in response to high salt. We found that the Nox2 inhibitor GSK2795039 inhibited formation of IsoLG adducts and reduced monocyte production of TNF-α, IL-1β and the formation of intermediate monocytes in response to salt exposure. IsoLGs are highly reactive products of lipid peroxidation that rapidly adduct to lysine on proteins and their accumulation is associated with DC activation.20 Our current data are in keeping with a recent study in which we found that elevated Na+ is a potent stimulus for IsoLG-adduct formation in murine DCs.18 Na+ enters DCs through amiloride sensitive transporters. Intracellular Na+ is exchanged for calcium (Ca2+) via the Na+/Ca2+ exchanger. Ca2+ activates protein kinase C which in turn phosphorylates the NADPH oxidase subunit p47phox. This leads to activation of the NADPH oxidase, increased superoxide and IsoLG-adduct formation.18 Our data with GSK2795039 suggest that a similar pathway likely exists in human monocytes.
Our understanding of how high sodium microenvironments govern the responses observed in human monocytes is incomplete; however, exposure to high salt increased endogenous expression and production of GM-CSF, IL-4, and Flt3. These factors are often used to stimulate production of DCs from both monocytes and bone marrow-derived cells, although recent data suggest such cells generated ex vivo by GM-CSF (and IL-4) are different from DCs formed in vivo.41 The receptor tyrosine kinase Flt3 is, however, thought to be required for DC differentiation in vivo.42,43 Deletion of Ftl3 ligand or treatment with Flt3 tyrosine kinase inhibitors reduce DC formation.44 Interestingly, ROS have roles both upstream and downstream of Flt3 signalling.45,46 Likewise, GM-CSF increases production of ROS by peritoneal macrophages.47 Thus, there is likely interplay between ROS formation in response to high salt and signalling molecules like Flt3 and GM-CSF that modulate monocyte phenotype and function.
In in vitro studies, we observed that high-salt exposure caused a coordinated acquisition of the surface markers CD86 and CD83 and the production of IsoLG-protein adducts. We also observed an increase in CD1c and CD209, which are expressed in DCs. Despite these observations we do not conclude that high-salt exposure causes transformation of monocytes to DCs. Recently, Villani et al.48 used single-cell RNA sequencing to characterize circulating DCs in humans and identified discriminative mRNAs characteristic of subclasses of these cells. Our RNA sequencing experiments did not identify an increase in these unique mRNAs, however we found high expression of several of mRNAs in human monocytes including HLA-DPA1, S100A9, and FCGR3A, which are expressed in the DC clusters DC1, DC3, and DC4 identified by Villani et al.48 We did however observe that salt caused a coordinated increase in surface levels and gene expression of proteins that enhance the ability of monocytes to act as antigen presenting cells and promote local inflammation. These include the co-stimulatory proteins CD86, CD80, the cytokines IL-1β, IL23A, IL-6, and TNF-α. It has recently been shown that monocytes can enter tissues and re-emerge in an activated state without transformation to macrophages or DCs. These cells have enhanced ability to drive T-cell proliferation and the enhanced production IL-1β and IL-6 skew T cells to produce IL-17A. To investigate how salt affects the ability of human monocytes to modulate T-cell proliferation in vivo, we injected monocytes and T cells from human volunteers into immune-deficient (NSG) mice and found that high salt feeding dramatically enhanced proliferation of human T cells only when monocytes were present (Figure 6).
The present study has several important limitations. Our in vitro experiments involved placement of monocytes in culture for up to 72 h, which can lead to changes in phenotype and function. Our results from sodium MRI and sodium excretion cohorts are associative rather than causal. We cannot conclude that the variability in sodium response of monocytes mediates salt-sensitivity in vivo. Future studies using protocols to study salt sensitivity in humans would provide additional insight into how monocyte phenotypes correspond to salt sensitivity of blood pressure.
In conclusion, our results provide a novel mechanism by which excess dietary sodium promotes inflammation, known to contribute to hypertension and cardiovascular diseases. The ability of monocytes to be activated by elevated sodium is exacerbated in subjects with cardiovascular risk factors including increased BMI and pulse pressure. These risk factors are associated with endothelial dysfunction, which in turn can promote monocyte translocation to interstitial sites like those of the skin and kidney where they encounter elevated sodium. These cells can propagate inflammation by producing cytokines and promoting T-cell activation and proliferation. These studies provide insight into how elevated dietary sodium contributes to inflammation, hypertension, and end-organ damage.
Data availability
The datasets used and/or analysed during the current study are available in Figshare: https://figshare.com/s/d810937dc537eeb361a5.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
N.R.B., J.D.F., W.C., K.R.M., M.A., A.A., R.L., J.B.V., A.P., L.C., L.X., and J.V.B. and A.K. performed the experiments. N.R.B., F.E., C.L.L., and S.Z. performed statistical analysis. N.R.B., D.G.H., and A.K. conceived the research programme, designed experiments, and wrote the manuscript. A.K., F.E., and D.G.H. edited and approved the manuscript. N.R.B., D.G.H., and A.K. obtained funding for the manuscript. The authors thank Larissa Dale for the illustration in Figure 7G.
Conflict of interest: none declared.
Funding
This work was supported by the American Heart Association (16POST2909000, 18POST34020009 and SFRN204200), and National Institutes of Health (K01HL130497, K01HL121045, R35HL140016, and Program Project P01HL129941).
Translational perspective
Immune cells like monocytes convey salt-sensitivity to humans and this is associated with cardiovascular risk factors. Our research provides a new mechanism by which a high-salt diet may induce inflammation and hypertension and support the current recommendations of a low sodium consumption, especially in subjects with elevated cardiovascular risk. Our data indicate that scavenging isolevuglandins or targeting monocytes may be therapeutically beneficial in salt-sensitive hypertension.
Supplementary Material
Time for primary review: 26 days
References
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J.. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Murray CJ, Lopez AD.. Measuring the global burden of disease. N Engl J Med 2013;369:448–457. [DOI] [PubMed] [Google Scholar]
- 3. He FJLJ, Macgregor GA.. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2013;4:CD004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS.. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 1986;8:II127–II134. [DOI] [PubMed] [Google Scholar]
- 5. Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G.. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997;350:1734–1737. [DOI] [PubMed] [Google Scholar]
- 6. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M.. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001;37:429–432. [DOI] [PubMed] [Google Scholar]
- 7. Laffer CL, Scott RC, Titze JM, Luft FC, Elijovich F.. Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension 2016;68:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stocker SD, Monahan KD, Browning KN.. Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Curr Hypertens Rep 2013;15:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J.. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 2009;15:545–552. [DOI] [PubMed] [Google Scholar]
- 10. Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J.. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 2013;61:635–640. [DOI] [PubMed] [Google Scholar]
- 11. Zhang WC, Zheng XJ, Du LJ, Sun JY, Shen ZX, Shi C, Sun S, Zhang Z, Chen XQ, Qin M, Liu X, Tao J, Jia L, Fan HY, Zhou B, Yu Y, Ying H, Hui L, Liu X, Yi X, Liu X, Zhang L, Duan SZ.. High salt primes a specific activation state of macrophages, M(Na). Cell Res 2015;25:893–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jorg S, Kissel J, Manzel A, Kleinewietfeld M, Haghikia A, Gold R, Muller DN, Linker RA.. High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol 2016;279:212–222. [DOI] [PubMed] [Google Scholar]
- 13. Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA.. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013;496:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DWH, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ.. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 2013;39:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olsen F. Inflammatory cellular reaction in hypertensive vascular disease in man. Acta Pathol Microbiol Scand A 2009;80A:253–256. [PubMed] [Google Scholar]
- 16. Heptinstall RH. Renal biopsies in hypertension. Br Heart J 1954;16:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T.. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011;124:1370–1381. [DOI] [PubMed] [Google Scholar]
- 18. Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A.. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep 2017;21:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirabo A. A new paradigm of sodium regulation in inflammation and hypertension. Am J Physiol Regul Integr Comp Physiol 2017;313:R706–R710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirabo A, Fontana V, de Faria APC, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen S-C, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, Harrison DG.. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 2014;124:4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG.. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res 2016;118:1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA.. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma-/- and interleukin-17A-/- mice. Hypertension 2015;65:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schofl C, Renz W, Santoro D, Niendorf T, Muller DN, Neininger M, Cavallaro A, Eckardt KU, Schmieder RE, Luft FC, Uder M, Titze J. ( 23)Na magnetic resonance imaging of tissue sodium. Hypertension 2012;59:167–172. [DOI] [PubMed] [Google Scholar]
- 24. Davies SS, Amarnath V, Brame CJ, Boutaud O, Roberts LJ 2nd. Measurement of chronic oxidative and inflammatory stress by quantification of isoketal/levuglandin gamma-ketoaldehyde protein adducts using liquid chromatography tandem mass spectrometry. Nat Protoc 2007;2:2079–2091. [DOI] [PubMed] [Google Scholar]
- 25. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL.. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 2013;14:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheng Q, Vickers K, Zhao S, Wang J, Samuels DC, Koues O, Shyr Y, Guo Y.. Multi-perspective quality control of Illumina RNA sequencing data analysis. Brief Funct Genomics 2017;16:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo Y, Zhao S, Sheng Q, Ye F, Li J, Lehmann B, Pietenpol J, Samuels DC, Shyr Y.. Multi-perspective quality control of Illumina exome sequencing data using QC3. Genomics 2014;103:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo Y, Zhao S, Ye F, Sheng Q, Shyr Y.. MultiRankSeq: multiperspective approach for RNAseq differential expression analysis and quality control. Biomed Res Int 2014;2014:248090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Passlick B, Flieger D, Ziegler-Heitbrock HW.. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989;74:2527–2534. [PubMed] [Google Scholar]
- 30. Guzik TJ, Touyz RM.. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017;70:660–667. [DOI] [PubMed] [Google Scholar]
- 31. Hammon M, Grossmann S, Linz P, Kopp C, Dahlmann A, Garlichs C, Janka R, Cavallaro A, Luft FC, Uder M, Titze J.. 23Na magnetic resonance imaging of the lower leg of acute heart failure patients during diuretic treatment. PLoS One 2015;10:e0141336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kopp C, Linz P, Maier C, Wabel P, Hammon M, Nagel AM, Rosenhauer D, Horn S, Uder M, Luft FC, Titze J, Dahlmann A.. Elevated tissue sodium deposition in patients with type 2 diabetes on hemodialysis detected by (23)Na magnetic resonance imaging. Kidney Int 2018;93:1191–1197. [DOI] [PubMed] [Google Scholar]
- 33. Linz P, Santoro D, Renz W, Rieger J, Ruehle A, Ruff J, Deimling M, Rakova N, Muller DN, Luft FC, Titze J, Niendorf T.. Skin sodium measured with 23Na MRI at 7.0 T. NMR Biomed 2014;28:54–62. [DOI] [PubMed] [Google Scholar]
- 34. Ingersoll MA, Platt AM, Potteaux S, Randolph GJ.. Monocyte trafficking in acute and chronic inflammation. Trends Immunol 2011;32:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gomes PM, Sa RWM, Aguiar GL, Paes MHS, Alzamora AC, Lima WG, de Oliveira LB, Stocker SD, Antunes VR, Cardoso LM.. Chronic high-sodium diet intake after weaning lead to neurogenic hypertension in adult Wistar rats. Sci Rep 2017;7:5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guyton AC. Abnormal renal function and autoregulation in essential hypertension. Hypertension 1991;18:III49–III53. [DOI] [PubMed] [Google Scholar]
- 37. Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ.. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol 2003;23:776–782. [DOI] [PubMed] [Google Scholar]
- 38. Santisteban MM, Ahmari N, Marulanda Carvajal J, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J.. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res 2015;117:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muller DN, Wilck N, Haase S, Kleinewietfeld M, Linker RA.. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat Rev Immunol 2019;19:243–254. [DOI] [PubMed] [Google Scholar]
- 40. Berry MR, Mathews RJ, Ferdinand JR, Jing C, Loudon KW, Wlodek E, Dennison TW, Kuper C, Neuhofer W, Clatworthy MR.. Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell 2017;170:860–874.e19. [DOI] [PubMed] [Google Scholar]
- 41. Alcantara-Hernandez M, Leylek R, Wagar LE, Engleman EG, Keler T, Marinkovich MP, Davis MM, Nolan GP, Idoyaga J.. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity 2017;47:1037–1050.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim SW, Choi SM, Choo YS, Kim IK, Song BW, Kim HS.. Flt3 ligand induces monocyte proliferation and enhances the function of monocyte-derived dendritic cells in vitro. J Cell Physiol 2015;230:1740–1749. [DOI] [PubMed] [Google Scholar]
- 43. Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M.. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol 2008;9:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ.. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000;95:3489–3497. [PubMed] [Google Scholar]
- 45. Woolley JF, Naughton R, Stanicka J, Gough DR, Bhatt L, Dickinson BC, Chang CJ, Cotter TG.. H2O2 production downstream of FLT3 is mediated by p22phox in the endoplasmic reticulum and is required for STAT5 signalling. PLoS One 2012;7:e34050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalwa H, Sartoretto JL, Martinelli R, Romero N, Steinhorn BS, Tao M, Ozaki CK, Carman CV, Michel T.. Central role for hydrogen peroxide in P2Y1 ADP receptor-mediated cellular responses in vascular endothelium. Proc Natl Acad Sci USA 2014;111:3383–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ding AH, Nathan CF, Stuehr DJ.. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol 1988;141:2407–2412. [PubMed] [Google Scholar]
- 48.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, Jardine L, Dixon D, Stephenson E, Nilsson E, Grundberg I, McDonald D, Filby A, Li W, De Jager PL, Rozenblatt-Rosen O, Lane AA, Haniffa M, Regev A, Hacohen N. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017;356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available in Figshare: https://figshare.com/s/d810937dc537eeb361a5.