Abstract
Aims
Atherosclerotic vascular disease has an inflammatory pathogenesis. Heme from intraplaque haemorrhage may drive a protective and pro-resolving macrophage M2-like phenotype, Mhem, via AMPK and activating transcription factor 1 (ATF1). The antidiabetic drug metformin may also activate AMPK-dependent signalling. Hypothesis: Metformin systematically induces atheroprotective genes in macrophages via AMPK and ATF1, thereby suppresses atherogenesis.
Methods and results
Normoglycaemic Ldlr−/− hyperlipidaemic mice were treated with oral metformin, which profoundly suppressed atherosclerotic lesion development (P < 5 × 10−11). Bone marrow transplantation from AMPK-deficient mice demonstrated that metformin-related atheroprotection required haematopoietic AMPK [analysis of variance (ANOVA), P < 0.03]. Metformin at a clinically relevant concentration (10 μM) evoked AMPK-dependent and ATF1-dependent increases in Hmox1, Nr1h2 (Lxrb), Abca1, Apoe, Igf1, and Pdgf, increases in several M2-markers and decreases in Nos2, in murine bone marrow macrophages. Similar effects were seen in human blood-derived macrophages, in which metformin-induced protective genes and M2-like genes, suppressible by si-ATF1-mediated knockdown. Microarray analysis comparing metformin with heme in human macrophages indicated that the transcriptomic effects of metformin were related to those of heme, but not identical. Metformin-induced lesional macrophage expression of p-AMPK, p-ATF1, and downstream M2-like protective effects.
Conclusion
Metformin activates a conserved AMPK-ATF1-M2-like pathway in mouse and human macrophages, and results in highly suppressed atherogenesis in hyperlipidaemic mice via haematopoietic AMPK.
Keywords: Atherosclerosis, Macrophage, Metformin, AMPK, Gene expression, Transcription factor
1. Introduction
Atherosclerotic cardiovascular disease is a major cause of morbidity and mortality in developed nations.1 It is therefore still an area of unmet therapeutic need. Atherosclerosis comprises chronic inflammation of the intima of medium-sized and large arteries, in which the inflammatory cells are primarily macrophages.2 This leads to major adverse cardiovascular events (MACE): ischaemia of the heart, brain, or periphery.1 Inflammation is a major pathological driver of atherosclerosis.
The risk factors for atherosclerosis include hypertension, hyperlipidaemia, diabetes, smoking, and genetics.1 The increasing prevalence and severity of obesity has made Type 2 diabetes mellitus (T2DM) an important cause of cardiovascular disease.1 The current pharmacological first-line treatment of T2DM is metformin, an insulin-sensitizing hypoglycaemic agent that has proven very safe in 50 years of clinical practice. It was once thought that glucose-reduction itself would decrease inflammatory stimulation and atherosclerosis.3,4 Importantly, in several randomized controlled trials, metformin had a possible effect on diabetic macrovascular disease not seen with other oral hypoglycaemic agents.5–9 This raises the possibility of a mechanism additional to glucose-reduction. If correct, it would indicate that metformin might be ‘repurposed’ for direct vascular risk reduction.
Multiple studies have indicated several possible molecular mechanisms of action of metformin. Metformin may act via inhibition of mitochondrial electron transport chain Complex-I,10 or via inhibition of the mitochondrial enzyme GPD2,11 or possibly by modulating glucagon-mediated activation of adenylate cyclase.12 There is clear evidence that metformin activates AMPK.13 However, not all of the effects of metformin are AMPK-mediated. Indeed, in the highly aggressive streptozotocin-diabetes model ApoE−/− model of profound Type I diabetes and hyperlipidaemia, metformin acted via the methylglyoxal pathway to reduce atherosclerosis, and independently of AMPK.14 Importantly, Viollet’s group have recently shown that its key therapeutic effect, reducing blood glucose by acting on the liver, is not AMPK-dependent.13 Therefore, the role of AMPK in metformin responses in vivo is currently uncertain.
Metformin has multiple AMPK-mediated beneficial effects on vascular physiology and normal vessel wall cells [endothelial cells and vascular smooth muscle cells (VSMCs)].15 Metformin has multiple other potential effects, including modulation of macrophage function.16,17 In these studies, it acted effectively as an anti-inflammatory agent.16,17 Our interest stemmed from analysing the endogenous activating transcription factor 1 (ATF1)-dependent homeostatic macrophage phenotype, Mhem.18 This phenotype was driven by intraplaque haemorrhage.19,20 Whilst Mhem is different to M2, it is nevertheless conceptually M2-like in that it is homeostatic.18 We showed that metformin-modulated HO-1, oxidative stress, and lipid handling in human blood-derived macrophages, mediated by AMPK.17 Genetically mediated AMPK activation suppresses macrophage activation.21
We postulated a direct mechanism mediated by plaque macrophage AMPK, similar to the one that we characterized in vitro.17 This was tested in chow-fed Ldlr−/− mice, an in vivo atherosclerosis model with enhanced clinical relevance that we have published.21–26 These develop small early lesions limited to the thoracic aorta and composed predominantly of macrophages.22–26 Metformin profoundly suppressed lesion formation in Ldlr−/− mice dependent on haematopoeitic AMPK. Metformin-induced M2-like macrophage genes, along with multiple other atheroprotective genes via AMPK and ATF1 in humans and mice. This may have therapeutic relevance for the repurposing of metformin by utilizing its so-called pleiotropic effects.27
2. Methods
Methods are detailed in the Supplementary material online. Ldlr−/− mice were studied in experiments that were designed to give complementary information. Chow was used throughout similar to our previous studies,21–26 since high-fat diet is associated with dysglycaemia and would have confounded assessment of glucose and diabetes-associated inflammation. The standard age at analysis was 25 weeks, which on chow produces early atherosclerosis. Lesion assessment, histology, immunohistology, cell culture of mouse bone marrow-derived macrophages (mBMMs), and human monocyte-derived macrophages (hMDMs), and reverse transcription and polymerase chain reaction (RT-qPCR) were all by standard methods (Supplementary material online). Microarrays and bioinformatics followed methods in our previous study.18
In brief, mice were maintained on metformin (Sigma-Aldrich D150959-5G) in drinking water (1 mg⋅mL−1) for the study duration (Supplementary material online). Saccharin was added to disguise the taste. Animals were euthanized, perfusion-fixed, the aortae and aortic roots removed. Aortic roots were cryosectioned and stained with Oil Red O. Aortae were stained with Sudan-IC during perfusion, dissected, opened out and imaged. Immunohistology was carried out on spare aortic root cryosections using immunoperoxidase, immunofluorescence, and immunoalkaline phosphatase protocols for cell signalling molecules and cell lineage markers. Mouse bone marrow-derived macrophages were cultured in L929-conditioned medium and transferred to 24-cell plates for stimulation (Supplementary material online). The macrophages were lysed and RNA purified with a Qiagen RNEasy Mini kit (Supplementary material online). RNA was reverse transcribed and measured with qPCR using a SyBr Green-based mastermix (Supplementary material online).
Human monocyte-derived macrophages were obtained from blood aseptically collected from normal human volunteers (HTA Licence 12275 subcollection VAS_JB-17-076), and monocytes purified using a Ficoll-Hypaque density layer and adherence-purification. Human peripheral blood was obtained with informed consent and favourable ethical opinion from Hammersmith Research Ethics Committee. All procedures conformed to the principles outlined in the Declaration of Helsinki. Monocytes were cultured overnight before stimulation with metformin. Metformin was 10 μmol⋅L−1 final concentration. RNA purification and RT-qPCR was as for the mouse macrophages, but using an RNEasy Micro kit and human-specific primers (Supplementary material online). Metformin-stimulated macrophages were lysed, RNA purified and labelled and hybridized to an Agilent 4x44k microarray system. Genespring, Venn analysis, X2K (https://amp.pharm.mssm.edu/X2K/), PANTHER-GO (http://www.pantherdb.org/), and STRING (https://string-db.org/) (Supplementary material online).
The in vivo experiments had local approval (Local AWERB and UK Home Office PC54F329C and 70/7325) and all procedures conformed to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Euthanasia was by excess CO2 over 7 min with confirmation of death by cervical dislocation. No anaesthetic agents were required.
Statistics were done in SigmaStat (SigmaPlot). Data are shown as scatterplots where informative or as mean ± standard error. All data were tested for normality by Shapiro–Wilk. Normally distributed data in two groups were tested with Student’s t-test, and non-normally distributed data in two groups by Wilcoxon–Mann–Whitney. Normally distributed data in multiple groups were tested by one-way analysis of variance (ANOVA) with Bonferroni’s correction. Non-normally distributed data were tested by ANOVA with Holm–Sidak post-testing. Paired data were tested using the paired versions of t-Student or Wilcoxon.
3. Results
3.1. Metformin reduces atherosclerosis without affecting glucose or lipids
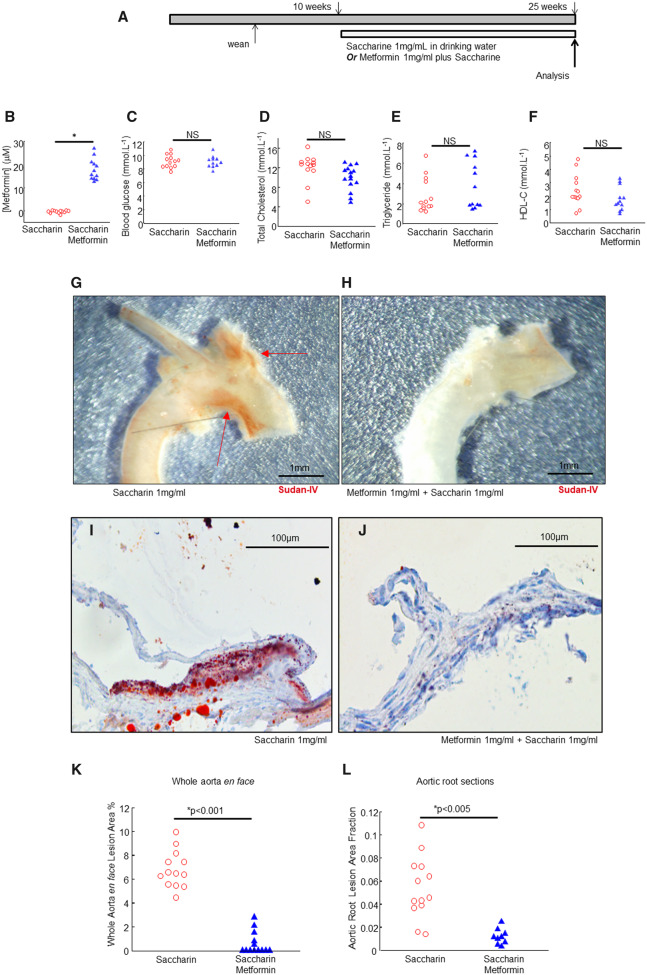
In the first set of experiments, we began Ldlr−/− chow-fed mice on oral metformin at age 10 weeks, culling for analysis at 25 weeks (Figure 1A). The model and timings were based on our previous studies.22–26 Metformin was administered under published conditions at an oral dose that approximated clinical concentrations in humans.28 Metformin was administered in drinking water (1 mg⋅mL−1) with saccharin (1 mg⋅mL−1) to disguise its taste, or matched saccharin-only controls in drinking water (1 mg⋅mL−1).29 Metformin administration yielded detectable metformin in serum but did not detectably modulate body weight, near-terminal random blood glucose, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, or triglycerides (Figure 1B–F and not shown).
Figure 1.
Metformin does not modulate blood glucose or lipids but profoundly reduces early atherosclerotic lesions. (A) Graphical outline of experimental protocol. Ldlr−/− mice were fed a chow diet, with metformin or saccharin in drinking water from 10 weeks and analysed at 25 weeks. (B–F) Serum analytes as indicated, in metformin-treated or control mice as indicated. *P < 0.05, Student’s t-test used where indicated. The glucose measurements were random (non-fasted) on a drop of blood from the tail vein the day before sacrifice, using an Aviva Nano device and strips. (G and H) Representative magnified images of aortic arches, as indicated, scalebars, 1 mm as indicated. (I and J) Representative images of aortic roots, as indicated, stained with Oil-Red O and haematoxylin, scalebars 100 μm as indicated. (K and L) Statistical analysis of lesions in aortic root and aorta, based on imaging in I, J. K, Y-axis, aortic en face, n = 13 mice (saccharin) n = 13 mice (metformin), *significant, indicated P-value, Wilcoxon–Mann–Whitney. (L) Aortic root, n = 13 mice (saccharin) n = 9 mice (metformin) *significant, indicated P-value, Wilcoxon–Mann–Whitney.
Murine atherosclerosis was quantified using standard methods, with lipid stains in the aorta and aortic root (Figure 1G). Consistent with our previous reports,22–26 this very mild model of atherosclerosis (chow-fed Ldlr−/−) had early lesions around the inner arch and major branch-points of the arch with sparing of the descending aorta (Figure 1G and Supplementary material online, Figure SI). Lesion development was profoundly inhibited by metformin administration (Figure 1H). Similarly, the aortic roots had small early lesions comprising collections of foamy macrophages at the valve bases (Figure 1I). Lesion development at this site was also profoundly inhibited by metformin (Figure 1J). The aorta en face lesion area was calculated along the whole aorta and the aortic root lesion area was calculated from sections as before.22–24 Statistically, this effect was consistent when measured at both sites (Figure1K and L).
Next, lesion composition was assessed. The lesions in the saccharin controls corresponded to those expected of this age and diet in Ldlr−/−, being entirely composed of macrophages (CD68+) (Supplementary material online, Figure SIA–D), without significant lesional collagen or VSMCs. The very few lesions that did occur in the treated mice, had a composition similar to those in the untreated. The strength of suppression of lesions precluded further analysis.
3.2. Atherosclerosis-prevention by metformin requires haematopoietic AMPK
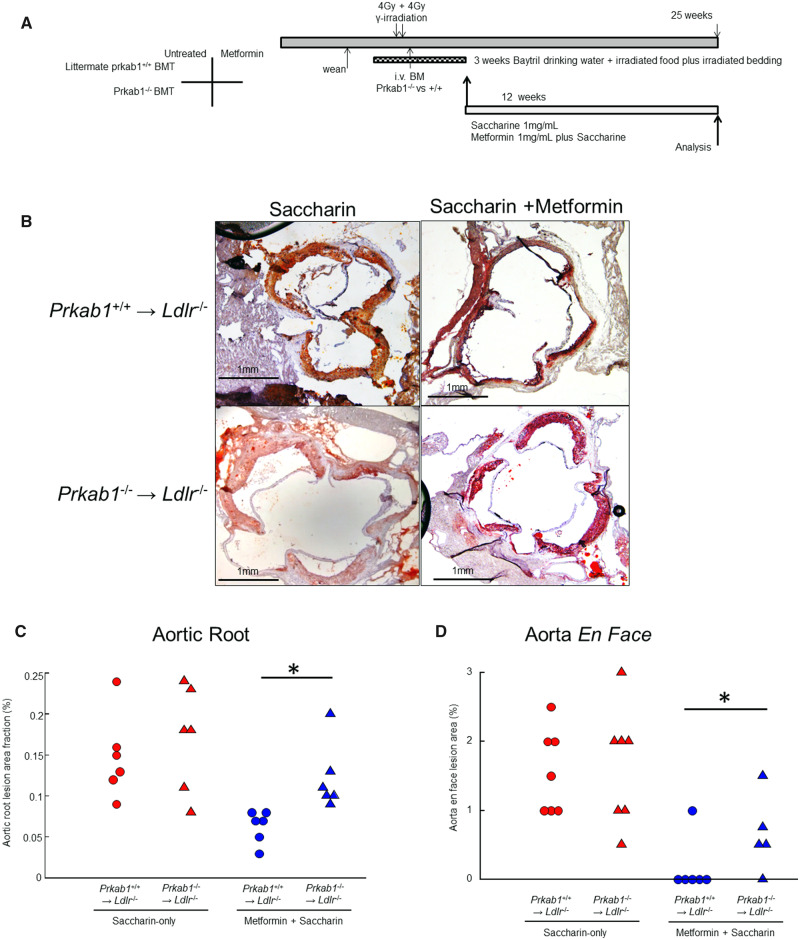
We next asked whether this effect was mediated by haematopoietic AMPK, with a bone marrow transplantation (BMT) approach (Figure 2). This approach is standard in the atherosclerosis literature and is usually interpreted as indicating myeloid specificity and even implied specificity for macrophages.30–34 Recipient Ldlr−/− mice were preconditioned with lethal γ-irradiation, then rescued by adoptive transfer of bone marrow from mice deficient in AMPK (Prkab1−/−) or littermate matched (Prkab1+/+) controls. BMT was performed at age 12 weeks, and metformin treatment from age 14 weeks to age 25 weeks, allowing a prolonged period on metformin after BMT, when there was a cull for analysis as for the first cohort (Figure 2A). Over 90% of lesional cells were negative for immunostainable PRKAB1 in the mice reconstituted with Prkab1−/− marrow (Supplementary material online, Figure SIF). On genotype analysis of peripheral blood by qPCR, there was no difference in reconstitution between saccharin and metformin (Supplementary material online, Figure SIG).
Figure 2.
Metformin reduces early development of atherosclerosis via haematopoietic AMPK. (A) Graphical outline of experimental protocol. Mice were culled for analysis at 25 weeks to match the first cohort and had BMT at approximately 12 weeks age. (B) Representative images of Ldlr−/− mice with Prkab+/+ or Prkab1−/− BMT that were fed a chow diet with metformin or saccharin, as indicated, with Oil-Red-O staining and imaging as in Figure 1. Ldlr−/− chow 25 weeks (Prkab1+/+→Ldlr−/−saccharin, n = 6, Prkab1−/−→Ldlr−/−saccharin, n = 6, Prkab1+/+→Ldlr−/−metformin, n = 6, Prkab1−/−→Ldlr−/−metformin, n = 6), scalebars 1 mm as indicated, and quantified in Figure 2C. (C) Statistical analysis of lesion size in aortic roots based on imaging in B (Oil-Red-O staining). *P < 0.03, ANOVA, with Holm–Sidak adjustment, after normality testing (Shapiro–Wilk), n = 6 mice, each point represents a different mouse. (D) Statistical analysis of lesion size in aortae en face (n = 5–7 mice, each point represents a different mouse, Sudan-IV staining, imaging as in Figure 1). *P < 0.05, Student’s t-test for indicated comparison, after normality testing (Shapiro–Wilk).
Neither BMT nor metformin had a significant effect on weight (Supplementary material online, Figure SII). Baseline lesion development at the aortic root was slightly higher than the first cohort, possibly due to irradiation (Figure2B and C). Metformin again profoundly suppressed lesion development although not quite to zero. Importantly, the effect of metformin was lost in mice that had been transplanted from AMPK-deficient donors. The lesions were entirely composed of macrophages (CD68+) (Supplementary material online, Figure SIA–D), without significant lesional collagen or VSMCs (not shown). This was similar to the non-irradiated controls and to the composition in Ldlr−/− on chow at this age that we and others have previously reported.22–26 In aorta en face analysis, again metformin suppressed lesion development, and this effect was also prevented by BMT from Prkab1−/− mice (Figure 2D). Reconstitution was analysed by both peripheral blood qPCR and by immunostaining of the lesions for PRKAB1 protein. We found that atherosclerotic lesions did not contain PRKAB1 in mice reconstituted with Prkab1−/− marrow (Supplementary material online, Figure SIF). Moreover, there was no difference in reconstitution between metformin-treated and control mice (Supplementary material online, Figure SIG). This experiment indicated that the anti-atherosclerotic effects of metformin were mediated by haematopoietic cell AMPK.
3.3. Metformin induces macrophage atheroprotective genes via AMPK and ATF1
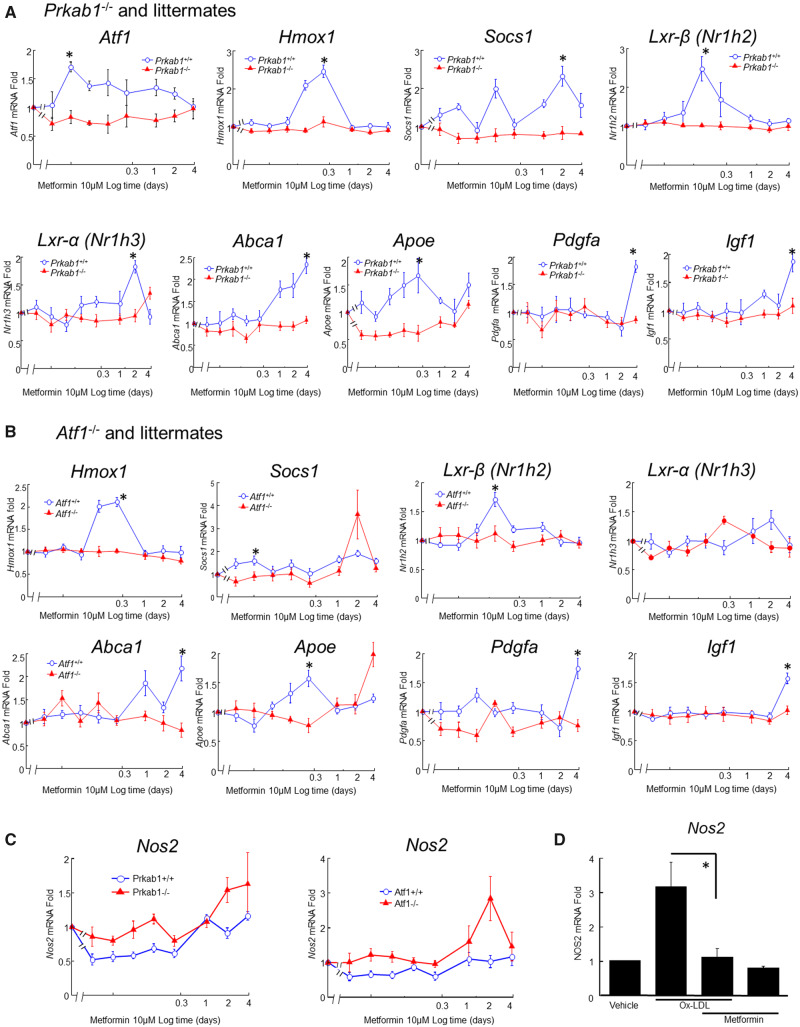
To identify possible AMPK-dependent molecular mechanisms, mBMMs were studied using RT-qPCR to quantify gene activation. Responses were followed over a time-course to identify optimal time points for dynamic responses.18 The gene selection was hypothesis-driven, based on previous work18 and is summarized in Supplementary material online, Figure SIII. Metformin-induced Atf1, Hmox1, Socs1, Lxra, Lxrb, Abca1, Apoe, Pdgf, and Igf1 (Figure 3). These genes were induced in a dynamic pattern, with each gene peaking at different time points (Figure3A and B).
Figure 3.
Metformin induces atheroprotective gene in mBMM dependent on AMPK and ATF1. Genes are as indicated on each sub-graph. X-axes, log time scale after stimulation of mBMM (in days). Y-axes, fold induction from untreated controls (calculated by −ΔΔCt method). Modulated genes, knockouts and littermate controls are as indicated, either Prkab1−/− (A) or Atf1−/− (B). In C, repression of iNOS (Nos2) is shown under baseline conditions. Metformin represses expression of Nos2, and this repression is dependent on both Prkab1 and Atf1. Statistical testing was by ANOVA with Holm–Sidak post hoc correction, after normality testing (Shapiro–Wilk), n = 6–8 different mice per condition. (D) In hMDM, metformin repression of OxLDL-mediated induction of Nos2 is clearer when OxLDL is used to induce NOS2, the repression is clearer. Mean ± SE, *P < 0.05, n = 5 different mice.
Most of the gene induction was lost in mBMM from mice deficient in either Prkab1 or Atf1 (Figure3A and B). That is, both AMPK and ATF1 were required for these responses to metformin. These data therefore indicated that the majority of the atheroprotective effects of metformin on macrophages were mediated by Atf1. This is likely to be due to AMPK activating ATF1, as previously characterized.17 Study of Prkab1−/− x Atf1−/− double-knockouts was precluded by loss during pregnancy (not shown). Nos2 was also assessed, as it has been previously linked to macrophage activation by oxidized low-density lipoproteins (OxLDLs)35,36 and to atherogenesis.37–40 Metformin suppressed Nos2 expression, both basally in mBMM and in hMDM when induced by adding OxLDL (Figure3C and D).
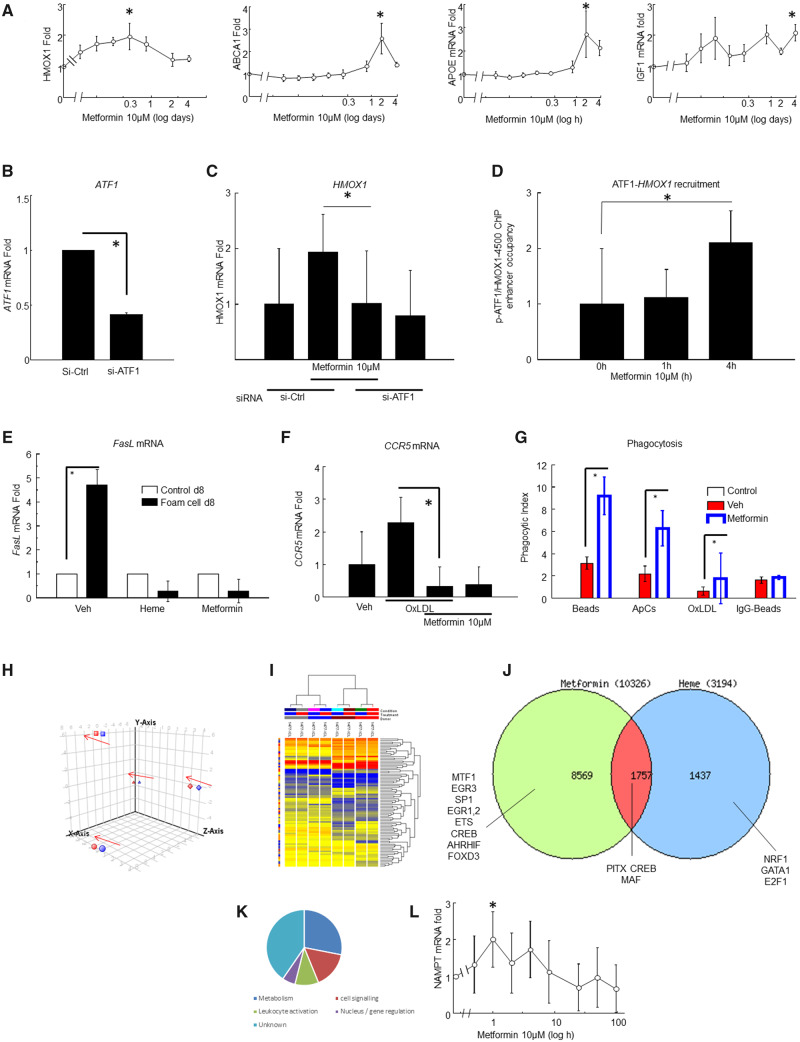
Next, hMDM were assessed (Figure 4 and not shown). Metformin (10 μM) induced ATF1, HMOX1, SOCS1, APOE, ABCA1, APOE, IGF1, ATF1, SOCS1, LXRA, LXRB IGF1, and PDGF (Figure 4A and not shown). Therefore, the mBMM accurately reflected hMDM, indicating species conservation. Metformin-induced HMOX1 was inhibited by si-ATF1 gene knock-down in hMDM (Figure4B and C). A chromatin immunoprecipitation experiment showed that metformin initiated ATF1 binding to the HMOX1 enhancer at approximately −4200 bp from the transcriptional start (Figure 4D). This is the same site that we previously defined as heme-responsive.18 That implies that the metformin-regulated activation is very similar to the heme-regulated activation.
Figure 4.
Metformin induces Mhem-like atheroprotective genes in hMDM. (A) Metformin induces HMOX1, APOE, ABCA1, and IGF1 (n = 8 different normal human donors). Gene induction was measured by RT-qPCR (Methods) by the ΔΔCt method with HPRT as housekeeping control. Equivalence of HPRT, GAPDH, and ACTAB as housekeepers was established. Mean ± SE, *each induction was statistically significant (ANOVA, P < 0.05, with Holm–Sidak correction, after normality testing by Shapiro–Wilk). X-axes, time in days (log scale). (B) hMDM were transfected with si-RNA to knock down ATF1. X-axis, si-RNA transfection of hMDM; Y-axis, ATF1 mRNA levels by RT-qPCR (Methods) by the ΔΔCt method with HPRT as housekeeping control, mean ± SE, *P < 0.05, Student’s t-test, n = 5 different human donors. (C) Metformin induces mRNA for HMOX1 in hMDM in control-transfected macrophages, but not in ATF1-siRNA-transfected macrophages (mean ± SE *Student’s t-test P < 0.05, n = 5 different human donors). (D) Metformin induces binding of p-ATF1 to the HMOX1 enhancer at the CRE site at −4200 bp in hMDM. Chromatin immunoprecipitation was performed as before, HMOX1 enhancer enrichment quantified by qPCR as before and expressed as fold enrichment and normalised to the IgG control (mean ± SE, *Student’s t-test P < 0.05, n = 5 different human donors). (E) FASL expression in hMDM at culture Day 8, measured by RT-qPCR. Y-axis, FASL fold induction measured by the ΔΔCt method (mean ± SE, n = 8 different human donors, *ANOVA P < 0.05, with Holm–Sidak correction and normality testing by Shapiro–Wilk). (F) CCR5 expression is induced in hMDM by loading with OxLDL, and reversed by metformin treatment. Y-axis, CCR5 fold induction by qRT-PCR and ΔΔCt method (*P < 0.05, t-test, n = 4 different human donors). (G) Metformin-enhanced phagocytosis in hMDM. Y-axis, phagocytic index for each phagocytosable particle, defined as number of particles left after washing divided by number of phagocytes (counted by nuclear DAPI stain). Expressed as mean ± SE n = 9, *passes significance, P < 0.002 paired Students’ t-test (beads), P < 0.002 Wilcoxon (ApC), P < 0.03 Wilcoxon (OxLDL). (H) Principal component analysis of microarray analysis of metformin versus vehicle control treatment of cultured hMDM (n = 4 different human donors) with arrows showing a consistent small effect of metformin in the same direction in each of the donors. Each donor is represented by a pair of points. (I) Hierarchical clustering and heat-map of microarray data of hMDM metformin treatment. The donors tended to cluster together. The genes that were significantly modulated by metformin were assessed in greater depth. (J) Venn diagram comparing genes modulated by heme, metformin, or both (intersection) on microarray analysis of over-represented TFBS in each subset of genes indicated likely differences in signalling and transcription factor usage. (K) Classification of top metformin-modulated genes by Gene Ontology from microarray analysis. Many of the genes encoded enzymes in intermediary metabolism, cell signalling, or leucocyte activation. The largest single category was unknown function. (L) Metformin induces NAMPT. NAMPT was one of the few unanticipated metformin-modulated protein-coding genes and was validated by qPCR. Y-axis: fold induction by RT-qPCR and −2ΔΔCt method (mean ± SE *P<0.05, ANOVA, Holm–Sidak adjustment n = 8 different human donors, normality tested by Shapiro–Wilk).
3.4. Metformin inhibits OxLDL-induced expression of pathogenic genes by hMDM
Metformin suppresses hMDM and mBMM foam cell formation via AMPK.17 We assessed the effect of this on gene expression. Genes whose expression was increased in macrophages from human ruptured plaques were identified from the literature.41 Their modulation by OxLDL was validated by qPCR (not shown). The effect of combinations of OxLDL and metformin on gene expression in hMDM was measured (Figure4E and F). OxLDL-induced FASL and CCR5, and this induction was antagonised by metformin (Figure4E and F). Metformin also suppressed macrophage phagocytosis of OxLDL, polystyrene beads and apoptotic cells, which are scavenger receptor ligands, but not IgG-coated beads, which are ligands for Fc-receptors (Figure 4G).
We next asked whether metformin and heme had similar effects across the whole transcriptome. This was done by comparing metformin-modulated gene expression with our published heme dataset, using the same microarray system (Figure 4H–K). This showed significant similarity between heme-induced genes and metformin-induced genes (Figure 4J). Genes that were induced by both heme and metformin had enrichment of transcription factor binding sites (TFBS) for ATF1 [cyclic-AMP response elements (CRE)]. Genes that were induced by metformin but not by heme had enrichment for TFBS for the Forkhead transcription factor family. Heme-specific genes had enrichment of TFBS for NFE2L2 (Nrf2), the oxidative-stress responsive antioxidant transcription factor. GO analysis showed selective enrichment for genes for leucocyte activation, cell signalling and intermediary metabolism (Figure 4J). Many of these genes had been validated by qPCR before performing microarray. Some metformin-induced genes were long non-coding RNA species of unknown function. A novel target enzyme, NAMPT, was validated by qPCR (Figure 4L), Therefore, transcriptomics and qPCR showed that metformin induces an M2-like macrophage phenotype, overlapping with Mhem, in an ATF1-dependent manner.
3.5. Metformin induces M2-like genes in hMDM via ATF1
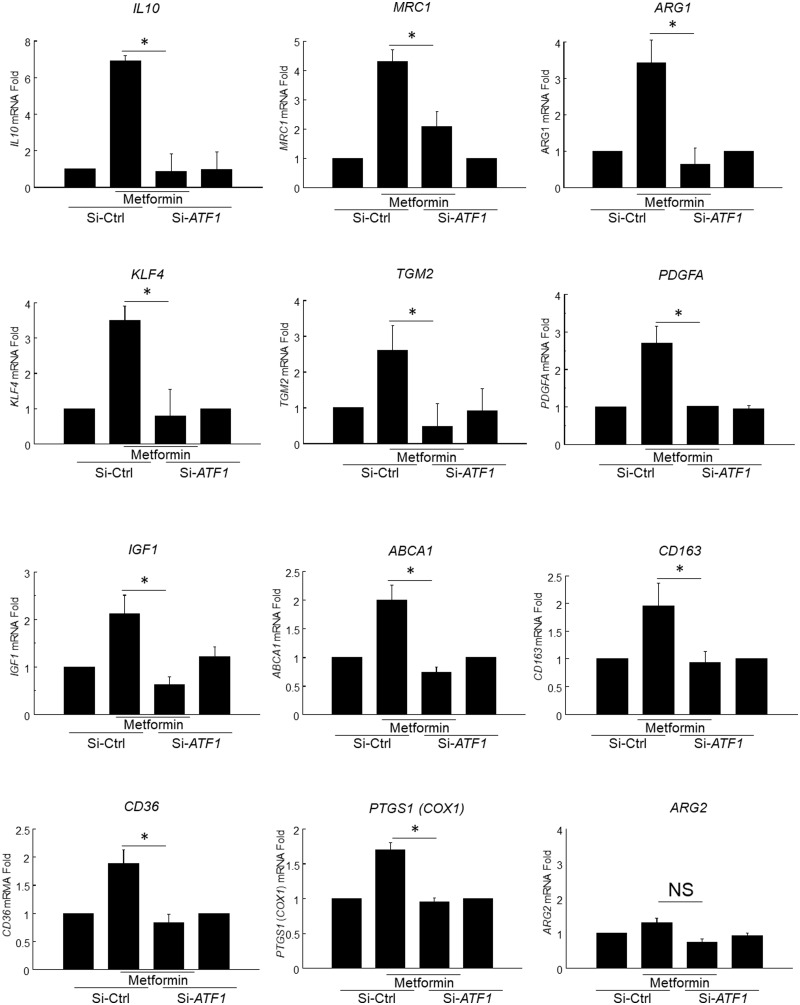
Next, ATF1-dependence of metformin-responses in hMDM was assessed, using si-ATF118 (Figure 5). Metformin induced the signature M2 genes IL10, ARG1, MRC1, CD163, KLF4, TGM. Metformin induced the growth factors PDGF and IGF1, and the lipid-clearance genes CD36 and ABCA1. Induction of each of these was inhibited by si-ATF1, indicating that induction of these genes by metformin was ATF1-dependent. Induction of mouse M2 genes was also Atf1-dependent (Supplementary material online, Figure SIV). The gene modulation was accompanied by increases in the anti-atheroprotective eicosanoids PGI2 and RvD1 and reduction in the pro-atherogenic eicosanoid LTB4 (Supplementary material online, Figures SV and SVI).
Figure 5.
Metformin induces M2-like atheroprotective genes in hMDM. Panel of RT-qPCR experiments in hMDM treated with combinations of ATF1-knockdown and metformin. Genes are arranged by fold induction. Each gene name is in line with HGNC approved nomenclature, as indicated. Each y-axis, fold gene induction from −2ΔΔCt method (mean ± SE *P < 0.05, ANOVA, Holm–Sidak adjustment n = 8 different human donors, normality tested by Shapiro–Wilk. Si-ATF1, pretreatment with ATF1-siRNA as in methods; Si-Ctrl, control si-RNA. Metformin, metformin 10 μM 48 h.
3.6. Metformin activates macrophage AMPK/ATF1/HO-1 signalling and promotes an M2-like macrophage phenotype in the atherosclerotic plaque
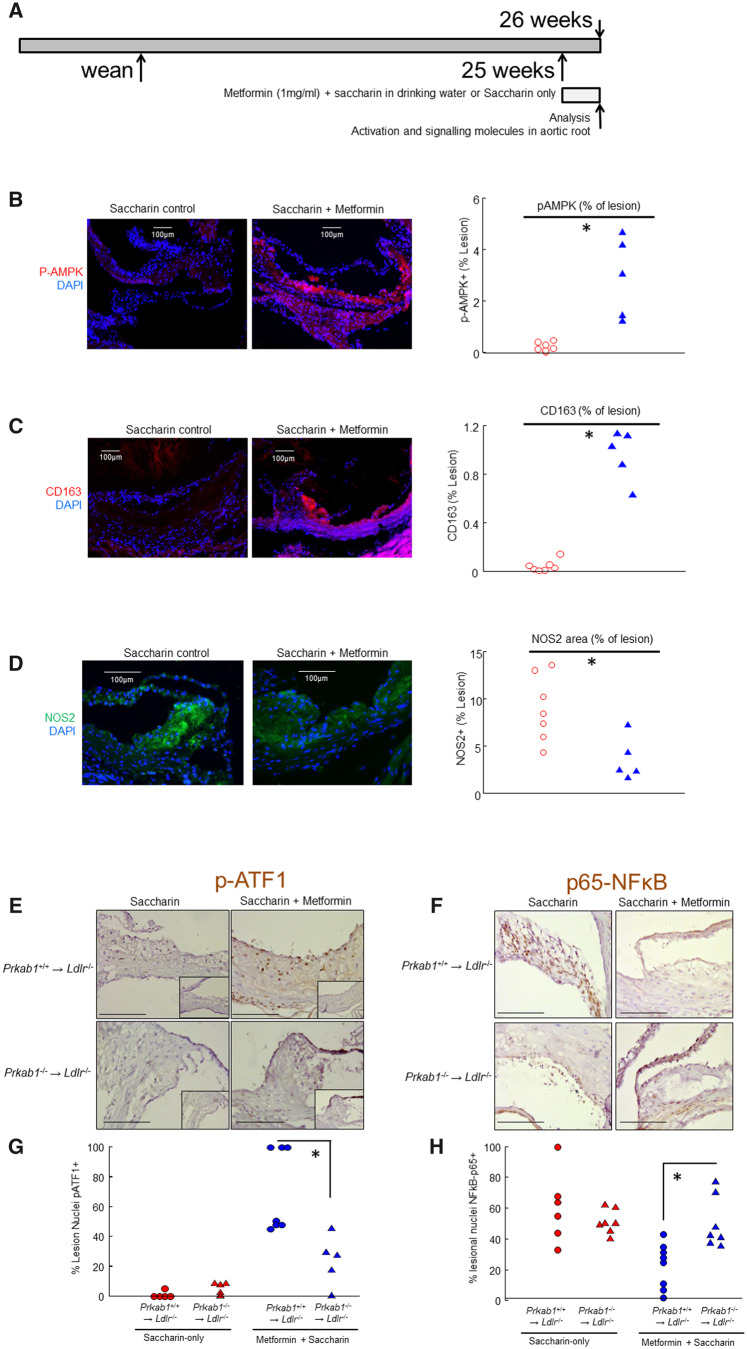
Because metformin had almost completely abrogated lesional macrophage accumulation, a different strategy was adopted to examine lesional macrophage signalling and gene modulation in vivo. Ldlr−/− mice with established lesions on chow were treated with short-term oral metformin, at age 24 weeks old for 1 week (Figure 6A). In this protocol, metformin-induced lesional expression of pAMPK, pATF1, HO-1, CD163, and LXR (Figure 6B, C, and E, Supplementary material online, Figures SVII and SVIII and not shown). Metformin suppressed NOS2 (iNOS) and NF-κBp65 expression and nuclear translocation (Figure 6D, F and not shown). This indicated that metformin co-ordinately activated macrophage resolution properties and countered atherogenic activation.
Figure 6.
Metformin modulates signalling and decreases inflammation in the aortic root. (A) Graphical experimental protocol showing short duration of metformin treatment and increased age of commencement. (B) Representative micrographs for p-AMPK activation and quantification by planimetry. Scalebar = 100 μm. *P < 0.05, Wilcoxon rank sum test. Each point represents a different mouse, n = 6 mice (saccharin) n = 5 mice (metformin). (C) Representative micrographs for CD163 expression and quantification by planimetry. Scalebar = 100 μm. *P < 0.05, Wilcoxon rank sum test. Each point represents a different mouse, n = 7 mice (saccharin) n = 5 mice (metformin). (D) Representative micrographs for iNOS (NOS2) expression and quantification by planimetry. Scalebar = 100 μm. *P < 0.05, Wilcoxon rank sum test. Each point represents a different mouse, n = 7 mice (saccharin) n = 5 mice (metformin). (E) Immunoperoxidase for p-ATF1 in sections of the BMT cohort. Representative micrographs. Scalebar = 100 μm. Transplant status and oral treatment are as indicated. (F) Immunoperoxidase for p65-NFκB, in sections of aortic root of the cohort BMT from AMPK-deficient or littermate donors. Representative micrographs. Scalebar = 100 μm. Transplant status and oral treatment are as indicated. (G) Quantification of p-ATF1 staining of lesions as shown in E, calculated as the number of nuclei immunopositive for p-ATF1 relative to total lesional nuclei. Transplant marrow genotype and treatment are as shown. *Wilcoxon–Mann–Whitney P < 0.003 for indicated comparison, n = 5–7 mice, individual mice are represented by different points as shown. (H) Quantification of p65-NFκB staining of lesions as shown in E, calculated as the number of nuclei immunopositive for p65-NFκB relative to total lesional nuclei. Transplant marrow genotype and treatment are as shown. *Student’s t-test P < 0.005 for indicated comparison, n = 5–7 mice, individual mice are represented by different points as shown.
To assess macrophage colocalization and AMPK-dependence of metformin-induced genes, lesions from the BMT cohort were also immunostained for HO-1 and CD163 and imaged by confocal microscopy. This demonstrated that metformin-induced lesional macrophage p-ATF1, HO-1 and CD163, and suppressed NOS2 and NF-κBp65 expression and nuclear translocation, dependent on AMPK (Figure6E and F, Supplementary material online, Figures SVII and SVIII and not shown).
3.7. Metformin does not modulate circulating monocyte chemokine receptors
We next asked whether metformin was likely to have been effective by modulating peripheral blood monocytes. Since hyperlipidaemia modulates monocyte subsets,42 and CCR2, CCR5, and CX3CR1 chemokine receptors mediate plaque monocyte recruitment,43 these were assessed by flow cytometry (Supplementary material online, Figure SIX). Metformin-treatment did not alter the major monocyte subsets, nor their expression of CCR2, CCR5, and CX3CR1. These data excluded a major role for monocyte-modulation in atherosclerosis prevention by metformin in this model.
3.8. Long-term treatment of established lesions by metformin alters atherosclerotic lesion composition
Since metformin treatment often starts in patients with known disease, its effect on established plaques was examined. Mice were commenced on metformin, using the same oral dosing and analysis as before, but beginning at 25 weeks and lasting for 15 weeks (Supplementary material online, Figure SXA). Under these conditions, metformin did not reduce overall lesion size (Supplementary material online, Figure SXB), but consistently stimulated the formation of a layer of VSMCs between the endothelium and the plaque macrophages (Supplementary material online, Figure SXB and C). These approximated an early fibrous cap, insofar as development in a mouse lesion would allow (Supplementary material online, Figure SXC).
Given that metformin-induced macrophage Igf1 (Supplementary material online, Figure SXD, Figures3A and B, and 4), lesions were next immunostained for p-IGFR1. This is the auto-phosphorylated form of the IGF1 receptor that mediates cell signalling. The immunohistochemistry was validated on formaldehyde-fixed cultured cells (Supplementary material online, Figure SIXE), and then applied to tissue sections from the in vivo experiment. This showed that metformin-induced plaque VSMCs were immunopositive for p-IGF1R (Supplementary material online, Figure SIXF). This indicated that IGF1 was bioactive in the lesions of metformin-treated mice, possibly promoting VSMC survival and growth. Indeed, metformin suppressed macrophage-induced VSMC apoptosis in vitro, reversed by neutralizing anti-IGF antibodies (Supplementary material online, Figure SIXG). Therefore, metformin may have a stabilizing effect in established atherosclerotic lesions.
4. Discussion
Inhibition of diabetic macrovascular disease by metformin had already been suspected.8,44–47 These data demonstrate direct suppression of euglycaemic atherosclerosis. The data also add an in vivo mechanism mediated by haematopoietic AMPK. Moreover, in vivo and in vitro AMP-dependent metformin-mediated gene regulation were correlated and support M2-like gene modulation. The in vitro data show that the gene regulation was mediated by the transcription factor ATF1, which is a novel mechanism. Because metformin is already a first-line oral antidiabetic, these findings are clinically relevant.
Metformin and atorvastatin co-treatment synergistically reduced atherosclerosis in high-fat fed rabbits.44 In fat-fed Apoe−/− mice infused with Angiotensin-II, metformin was hypoglycaemic as well as suppressing atherosclerosis.46 In Apoe−/− mice with infusions of Ang-II that induced aortic aneurysms, metformin suppressed aneurysm formation.45 In Apoe−/− mice fed a high-fat diet, metformin and other AMPK-activators suppressed peripheral monocyte count.47 Thus in each of these studies, other effects of metformin acted as a confounder for assessment of atherogenesis. Our focus on a mild model and on AMPK allowed us to define a clinically relevant specific mechanism. Moreover, AMPK was not required for the hypoglycaemic effects of metformin in vivo.13 That is, the signature drug effect of metformin appears to be AMPK-independent. In contrast, we have positively identified the importance of AMPK (Prkab1) in macrophages in vitro and in haematopoietic cells in atherogenesis in vivo. This adds to the understanding of the pharmacology of metformin.
If these human in vitro and murine in vivo effects have relevance, one might expect some signal in clinical data.27 This is indeed the case, as seen in UKPDS34.7,27 The difficulty of placebo-controlled studies in human diabetic patients complicates interpretation of trials.5–9,27 Since it is not ethical to leave diabetics untreated, the randomized trials must compare metformin with other hypoglycaemic agents. It is therefore difficult to know conclusively whether the apparent benefit of metformin relates to metformin decreasing atherosclerosis or off-target actions of other drugs.5–9 For example, one of the largest and highest quality trials is UKPDS34.7,27 This is a double-blind prospective randomized controlled trial. In it, patients are randomized to aggressive glucose-lowering with either an insulin/sulphonylurea-based regime or a metformin-based regime. Importantly, each regime established equivalent hypoglycaemia.7 Patients were followed up and MACE were counted.7 This is the gold-standard endpoint, comprising myocardial infarction, unstable angina, peripheral arterial occlusion and stroke.7 Metformin significantly reduced MACE, with a progressive significance over a 10–15 years span.7 In one more recent study, metformin and placebo were randomized in a non-diabetic study group of only six patients, and carotid intima: media thickness was measured after 2 years.48 There was no significant difference. This may be due to the relatively early lesions, lack of assessment of plaque vulnerability or clinical events, or to the early timepoint, or to low study numbers. We therefore suggest that we have added significantly to the literature, by providing a novel mechanism for a drug in common clinical usage.
A decade ago, we described a macrophage phenotype that is associated with intraplaque haemorrhage in fatal ruptured human coronary lesions.20 Studying these in greater depth indicated that they had putatively atheroprotective properties.20 Our data then revealed interlinking of iron-regulatory and lipid regulatory gene networks by ATF1.18 Our data then showed the ATF1 was activated by AMPK.17,18 Since metformin may also activate AMPK, this study was an obvious next step.
The mildness of the chow-fed Ldlr−/− model was an asset that probably revealed the effect of metformin. Previous studies included significant confounders such as Ang-II treatment, co-treatment with statins, glucose reduction and monocytosis.44–47 We selected chow-fed Ldlr−/− mice because their relative metabolic normality allowed a better baseline to study directly atheroprotective effects without confounders. In particular, glucose levels were within normal limits for strain, and there was only mild steatohepatitis (not shown). Since the lesions were simple, comprising small collections of macrophages, the model facilitated clear experimental data. Moreover, whilst lesions at this stage do not have intraplaque haemorrhage, we are taking lessons from our previous studies on responses to intraplaque haemorrhage49 in order to repurpose an exogenous protective drug.
The BMT experiment was important in two respects. First, it showed that metformin directly and profoundly suppresses the development of atherosclerosis, by a direct effect on haematopoietic cells. Secondly, it demonstrated a requirement for haematopoietic AMPK for the metformin effects in vivo. These data may explain the apparent unique effect of metformin as an antidiabetic drug that prevents MACE.
The human and mouse time-course qPCR data for metformin-induced gene expression were closely matched, indicating that mice are a representative model, adding to the validity of the in vivo experiments. This point was important, since the representativeness of murine inflammatory models is questioned.50,51 When human metformin responses were assessed transcriptomically in comparison with heme, there was significant overlap. This indicated that metformin does indeed partially reproduce the protective response to intraplaque haemorrhage. This common geneset had significant enrichment for CRE (ATF1 sites), also validating our views of the signalling. There were also many non-overlapping genes, indicating that responses to heme and metformin are distinct. Metformin-specific genes had increased numbers of Forkhead TFBS, which is consistent with the literature.52 In passing, the microarrays detected OCT1 (SLC22A1), a metformin transporter, explaining its effectiveness in macrophages.18 One of the most highly induced genes, NAMPT (visfatin) has been implicated in mitochondrial metabolism, cell survival, atherosclerosis susceptibility and lipid export,53 as well as renal preservation in vivo54 and will be a future point of study.
There are parallels in the molecular signalling and gene expression caused by heme and metformin. This is not an argument that the pharmacological effect of metformin is due to the pathophysiological event of intraplaque haemorrhage. Rather, the argument is that some protective effects of intraplaque haemorrhage may be reproduced by metformin. Engaging these mechanisms in advance of actual intraplaque haemorrhage may therefore be preventive.
The number of AMPK/ATF1 coordinated effector mechanisms prevented a full mechanistic dissection of each. However, control by AMPK, ATF1 and usually both, was shown. The number of mechanisms involved may contribute to the relative strength of overall in vivo effect. Others have shown the importance of each in atherosclerosis: IL-10,55 HO-1,56 CD163,57 ABCA1,58 LTB4 and RvD1,59 PGI2,60 and M2-like macrophages.61
5. Conclusions
Metformin directly protects against atherosclerosis, independently of normalization of lipids or glucose. It does so via haematopoietic AMPK in vivo. This contrasts with the hypoglycaemic effect, which is AMPK-independent.13In vitro metformin effects were also mediated by ATF1 and a macrophage M2-like gene expression. Targets included the antioxidant gene HMOX-1; lipid homeostasis genes such as ABCA1; M2-like genes such as IL10 and CD163; growth factors and anti-atherosclerotic eicosanoids. Our data therefore support further experimental medicine studies of metformin in non-diabetic patients at cardiovascular risk.
Data availability
The microarray datasets were uploaded to GEO (https://www.ncbi.nlm.nih.gov/geo/) and will be available after their curation. Further data underlying this article are available in the article and in its online supplementary material. Any remaining data underlying this article will be shared on reasonable request to the corresponding author.
Translational perspective
The work shows that oral antidiabetic drug metformin may suppress atherosclerotic lesion development via haematopoietic AMPK at clinically relevant concentrations, rather than via a hypoglycaemic effect. Activating transcription factor 1 may mediate induction of key atheroprotective genes by metformin. This suggests a mechanism for some of the effects of metformin. It has strong implications for a possible role as an atheroprotective agent beyond the context of diabetes. The data support a clinical trial of metformin in non-diabetic patients at high risk of atherosclerosis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
The work was supported by the British Heart Foundation (BHF SCRF03 FS/13/12/30037 to J.J.B., also PG/15/57/31580, PG/17/71/33242). Some of the imaging was carried out with instruments in FILM facility, Imperial College London. The Facility for Imaging by Light Microscopy (FILM) at Imperial College London is part supported by funding from the Wellcome Trust (104931/Z/14/Z) and Biotechnology and Biological Sciences Research Council (BBSRC) (BB/L015129/1). This work was supported by the NIHR Imperial Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Supplementary Material
Time for primary review: 26 days
References
- 1. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van, Dis I, Verschuren WMM.. [2016 European guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts. Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation]. G Ital Cardiol (Rome) 2017;18:547–612. [DOI] [PubMed] [Google Scholar]
- 2. Libby P, Hansson GK.. Taming immune and inflammatory responses to treat atherosclerosis. J Am Coll Cardiol 2018;71:173–176. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt AM. Recent highlights of ATVB: diabetes mellitus. Arterioscler Thromb Vasc Biol 2014;34:954–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan SF, Ramasamy R, Schmidt AM.. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Rev Endocrinol 2008;4:285–293. [DOI] [PubMed] [Google Scholar]
- 5. Kooy A, de Jager J, Lehert P, Bets D, Wulffele MG, Donker AJ, Stehouwer CD.. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616–625. [DOI] [PubMed] [Google Scholar]
- 6. Hong J, Zhang Y, Lai S, Lv A, Su Q, Dong Y, Zhou Z, Tang W, Zhao J, Cui L, Zou D, Wang D, Li H, Liu C, Wu G, Shen J, Zhu D, Wang W, Shen W, Ning G; on behalf of the SPREAD-DIMCAD Investigators. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013;36:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–865. [PubMed] [Google Scholar]
- 8. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA.. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 9. Lo C, Jun M, Badve SV, Pilmore H, White SL, Hawley C, Cass A, Perkovic V, Zoungas S.. Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients. Cochrane Database Syst Rev 2017;2:CD009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Owen MR, Doran E, Halestrap AP.. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- 11. Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, Shulman GI.. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014;510:542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ.. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013;494:256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B.. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 2010;120:2355–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu A, Li K, Xu L, Si M, Teng G, Li G, Xue J, Liang S, Song W.. Metformin delays the development of atherosclerosis in type 1 diabetes mellitus via the methylglyoxal pathway. Diabetes Ther 2020;11:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B.. Metformin: from mechanisms of action to therapies. Cell Metab 2014;20:953–966. [DOI] [PubMed] [Google Scholar]
- 16. Driver C, Bamitale KDS, Kazi A, Olla M, Nyane AN, Owira PMO.. Cardioprotective effects of metformin. J Cardiovasc Pharmacol 2018;72:121–127. [DOI] [PubMed] [Google Scholar]
- 17. Wan X, Huo Y, Johns M, Piper E, Mason JC, Carling D, Haskard DO, Boyle JJ.. 5'-AMP-activated protein kinase-activating transcription factor 1 cascade modulates human monocyte-derived macrophages to atheroprotective functions in response to heme or metformin. Arterioscler Thromb Vasc Biol 2013;33:2470–2480. [DOI] [PubMed] [Google Scholar]
- 18. Boyle JJ, Johns M, Kampfer T, Nguyen AT, Game L, Schaer DJ, Mason JC, Haskard DO.. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res 2012;110:20–33. [DOI] [PubMed] [Google Scholar]
- 19. Boyle JJ, Johns M, Lo J, Chiodini A, Ambrose N, Evans PC, Mason JC, Haskard DO.. Heme induces heme oxygenase 1 via Nrf2: role in the homeostatic macrophage response to intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2011;31:2685–2691. [DOI] [PubMed] [Google Scholar]
- 20. Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO.. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am.J.Pathol 2009;174:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sag D, Carling D, Stout RD, Suttles J.. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol 2008;181:8633–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, Haskard DO.. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol 2007;170:416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yun S, Leung VW, Botto M, Boyle JJ, Haskard DO.. Brief report: accelerated atherosclerosis in low-density lipoprotein receptor-deficient mice lacking the membrane-bound complement regulator CD59. Arterioscler Thromb Vasc Biol 2008;28:1714–1716. [DOI] [PubMed] [Google Scholar]
- 24. Leung VW, Yun S, Botto M, Mason JC, Malik TH, Song W, Paixao-Cavalcante D, Pickering MC, Boyle JJ, Haskard DO.. Decay-accelerating factor suppresses complement C3 activation and retards atherosclerosis in low-density lipoprotein receptor-deficient mice. Am.J.Pathol 2009;175:1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO.. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2009;120:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malik TH, Cortini A, Carassiti D, Boyle JJ, Haskard DO, Botto M.. The alternative pathway is critical for pathogenic complement activation in endotoxin- and diet-induced atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2010;122:1948–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jenkins AJ, Welsh P, Petrie JR.. Metformin, lipids and atherosclerosis prevention. Curr Opin Lipidol 2018;29:346–353. [DOI] [PubMed] [Google Scholar]
- 28. Chandel NS, Avizonis D, Reczek CR, Weinberg SE, Menz S, Neuhaus R, Christian S, Haegebarth A, Algire C, Pollak M.. Are metformin doses used in murine cancer models clinically relevant? Cell Metab 2016;23:569–570. [DOI] [PubMed] [Google Scholar]
- 29. Whittington HJ, Hall AR, McLaughlin CP, Hausenloy DJ, Yellon DM, Mocanu MM.. Chronic metformin associated cardioprotection against infarction: not just a glucose lowering phenomenon. Cardiovasc Drugs Ther 2013;27:5–16. [DOI] [PubMed] [Google Scholar]
- 30. Sreeramkumar V, Hidalgo A.. Bone marrow transplantation in mice to study the role of hematopoietic cells in atherosclerosis. Methods Mol Biol 2015;1339:323–332. [DOI] [PubMed] [Google Scholar]
- 31. Gong FH, Cheng WL, Wang H, Gao M, Qin JJ, Zhang Y, Li X, Zhu X, Xia H, She ZG.. Reduced atherosclerosis lesion size, inflammatory response in miR-150 knockout mice via macrophage effects. J Lipid Res 2018;59:658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merched AJ, Williams E, Chan L.. Macrophage-specific p53 expression plays a crucial role in atherosclerosis development and plaque remodeling. Arterioscler Thromb Vasc Biol 2003;23:1608–1614. [DOI] [PubMed] [Google Scholar]
- 33. Aparicio-Vergara M, Shiri-Sverdlov R, Koonen DP, Hofker MH.. Bone marrow transplantation as an established approach for understanding the role of macrophages in atherosclerosis and the metabolic syndrome. Curr Opin Lipidol 2012;23:111–121. [DOI] [PubMed] [Google Scholar]
- 34. Rong S, Cao Q, Liu M, Seo J, Jia L, Boudyguina E, Gebre AK, Colvin PL, Smith TL, Murphy RC, Mishra N, Parks JS.. Macrophage 12/15 lipoxygenase expression increases plasma and hepatic lipid levels and exacerbates atherosclerosis. J Lipid Res 2012;53:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Groeneweg M, Kanters E, Vergouwe MN, Duerink H, Kraal G, Hofker MH, de Winther MP.. Lipopolysaccharide-induced gene expression in murine macrophages is enhanced by prior exposure to oxLDL. J Lipid Res 2006;47:2259–2267. [DOI] [PubMed] [Google Scholar]
- 36. Persson K, Sauma L, Safholm A, Xu L, Li W, Yuan XM.. LDL and UV-oxidized LDL induce upregulation of iNOS and NO in unstimulated J774 macrophages and HUVEC. APMIS 2009;117:1–9. [DOI] [PubMed] [Google Scholar]
- 37. Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL.. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 2001;104:448–454. [DOI] [PubMed] [Google Scholar]
- 38. Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL.. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation 2001;103:3099–3104. [DOI] [PubMed] [Google Scholar]
- 39. Mallat Z, Heymes C, Ohan J, Faggin E, Leseche G, Tedgui A.. Expression of interleukin-10 in advanced human atherosclerotic plaques: relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol 1999;19:611–616. [DOI] [PubMed] [Google Scholar]
- 40. Cromheeke KM, Kockx MM, De Meyer GR, Bosmans JM, Bult H, Beelaerts WJ, Vrints CJ, Herman AG.. Inducible nitric oxide synthase colocalizes with signs of lipid oxidation/peroxidation in human atherosclerotic plaques. Cardiovasc.Res 1999;43:744–754. [DOI] [PubMed] [Google Scholar]
- 41. Lee K, Santibanez-Koref M, Polvikoski T, Birchall D, Mendelow AD, Keavney B.. Increased expression of fatty acid binding protein 4 and leptin in resident macrophages characterises atherosclerotic plaque rupture. Atherosclerosis 2013;226:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mallat Z. ATVB in focus. Monocyte subsets and their relevance to cardiovascular diseases. Arterioscler Thromb Vasc Biol 2009;29:1411–1411. [DOI] [PubMed] [Google Scholar]
- 43. Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van, Rooijen N, Lira SA, Habenicht AJ, Randolph GJ.. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 2007;117:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luo F, Guo Y, Ruan GY, Long JK, Zheng XL, Xia Q, Zhao SP, Peng DQ, Fang ZF, Li XP.. Combined use of metformin and atorvastatin attenuates atherosclerosis in rabbits fed a high-cholesterol diet. Sci Rep 2017;7:2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S.. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes 2015;64:2028–2041. [DOI] [PubMed] [Google Scholar]
- 46. Forouzandeh F, Salazar G, Patrushev N, Xiong S, Hilenski L, Fei B, Alexander RW.. Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis. J Am Heart Assoc 2014;3:e001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang J, Ma A, Zhao M, Zhu H.. AMPK activation reduces the number of atheromata macrophages in ApoE deficient mice. Atherosclerosis 2017;258:97–107. [DOI] [PubMed] [Google Scholar]
- 48. Preiss D, Lloyd SM, Ford I, McMurray JJ, Holman RR, Welsh P, Fisher M, Packard CJ, Sattar N.. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:116–124. [DOI] [PubMed] [Google Scholar]
- 49. Boyle JJ. Heme and haemoglobin direct macrophage Mhem phenotype and counter foam cell formation in areas of intraplaque haemorrhage. Curr Opin Lipidol 2012;23:453–461. [DOI] [PubMed] [Google Scholar]
- 50. Tompkins RG, Warren HS, Mindrinos MN, Xiao W, Davis RW.. Reply to Osterburg et al.: To study human inflammatory diseases in humans. Proc Natl Acad Sci USA 2013;110:E3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Osterburg AR, Hexley P, Supp DM, Robinson CT, Noel G, Ogle C, Boyce ST, Aronow BJ, Babcock GF.. Concerns over interspecies transcriptional comparisons in mice and humans after trauma. Proc Natl Acad Sci USA 2013;110:E3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGee SL, Hargreaves M.. AMPK-mediated regulation of transcription in skeletal muscle. Clin Sci (Lond) 2010;118:507–518. [DOI] [PubMed] [Google Scholar]
- 53. Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W.. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 2015;11:535–546. [DOI] [PubMed] [Google Scholar]
- 54. Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, Parikh SM.. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 2016;531:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A.. Protective role of interleukin-10 in atherosclerosis. Circ Res 1999;85:e17–e24. [DOI] [PubMed] [Google Scholar]
- 56. Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, Lau AK, Stocker R.. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med 2006;203:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guo L, Akahori H, Harari E, Smith SL, Polavarapu R, Karmali V, Otsuka F, Gannon RL, Braumann RE, Dickinson MH, Gupta A, Jenkins AL, Lipinski MJ, Kim J, Chhour P, de Vries PS, Jinnouchi H, Kutys R, Mori H, Kutyna MD, Torii S, Sakamoto A, Choi CU, Cheng Q, Grove ML, Sawan MA, Zhang Y, Cao Y, Kolodgie FD, Cormode DP, Arking DE, Boerwinkle E, Morrison AC, Erdmann J, Sotoodehnia N, Virmani R, Finn AV.. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest 2018;128:1106–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N.. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab 2008;7:365–375. [DOI] [PubMed] [Google Scholar]
- 59. Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O.. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ Res 2016;119:1030–1038. [DOI] [PubMed] [Google Scholar]
- 60. Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, Katagiri H, Majima M, Yokode M, Kita T, Narumiya S.. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest 2004;114:784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J, Gusarova V, Gromada J, Weinstock A, Moore KJ, Loke P, Fisher EA.. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest 2017;127:2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microarray datasets were uploaded to GEO (https://www.ncbi.nlm.nih.gov/geo/) and will be available after their curation. Further data underlying this article are available in the article and in its online supplementary material. Any remaining data underlying this article will be shared on reasonable request to the corresponding author.
Translational perspective
The work shows that oral antidiabetic drug metformin may suppress atherosclerotic lesion development via haematopoietic AMPK at clinically relevant concentrations, rather than via a hypoglycaemic effect. Activating transcription factor 1 may mediate induction of key atheroprotective genes by metformin. This suggests a mechanism for some of the effects of metformin. It has strong implications for a possible role as an atheroprotective agent beyond the context of diabetes. The data support a clinical trial of metformin in non-diabetic patients at high risk of atherosclerosis.