Abstract
To date, managing salinity stress in agriculture relies heavily on development of salt tolerant plant varieties, a time-consuming process particularly challenging for many crops. Plant based biostimulants (PBs) that enhance plant defenses under stress can potentially address this drawback, as they are not crop specific and are easy to apply in the field. Unfortunately, limited knowledge about their modes of action makes it harder to utilize them on a broader scale. Understanding how PBs enhance plant defenses at cellular and molecular levels, is a prerequisite for the development of sustainable management practices utilizing biostimulants to improve crop health. In this study we elucidated the protective mechanism of copper chlorophyllin (Cu-chl), a PB, under salinity stress. Our results indicate that Cu-chl exerts protective effects primarily by decreasing oxidative stress through modulating cellular H2O2 levels. Cu-chl treated plants increased tolerance to oxidative stress imposed by an herbicide, methyl viologen dichloride hydrate as well, suggesting a protective role against various sources of reactive oxygen species (ROS). RNA-Seq analysis of Cu-chl treated Arabidopsis thaliana seedlings subjected to salt stress identified genes involved in ROS detoxification, and cellular growth.
Keywords: biostimulant, copper chlorophyllin, ROS, salinity stress, RNA-Seq
1. Introduction
The beginning of the 21st century is manifested by continual decline of arable land and yield per capita in part due to climate change associated abiotic and biotic stresses [1]. For instance, environmental stresses like salinity, drought, cold, heat and heavy metal can cause more than 50% yield losses [2,3]. Among these stresses, salinity is one of the leading causes of crop yield reduction [4,5]. According to the Food and Agriculture Organization (FAO), salinity affects more than 30% of the irrigated land area worldwide, resulting in a monetary loss of 27.3 billion USD per year [6,7,8]. This is a ubiquitous issue and currently no continent is completely free from soil salinity [9]. It is speculated that soil salinity will increase in future climate change scenarios because of the rise of sea level and temperature, which will inevitably lead to increased evaporation and further salinization [9]. It is estimated that by 2050 50% of arable land will be impacted by salinity [10,11,12].
Salinity stress negatively influences seed germination, plant growth, physiology, yield and it can cause plant death under severe conditions [2]. At the onset of the stress, salt solutes cause accumulation of high concentration of rhizospheric ions (mainly Na+ and Cl−), thus reducing water uptake through the roots [13,14]. This consequently leads to depletion in water potential and osmotic imbalance, while at the same time excessive amounts of salt enter the plant’s transpiration system [8,12,13]. Therefore, salt stress affects plant performance in two ways, either as an inhibitor of water uptake (osmotic effect) or as an accumulator of ions, with subsequent toxic effects [14]. Additional consequences include the closure of stomata, which limits CO2 uptake in leaf tissues and consequently reduces carbon fixation and assimilation [15]. As a result, the photosynthesis rate and carbohydrate production are reduced, negatively impacting plant growth and yield [8]. Another major consequence of salt stress is triggering rapid accumulation of reactive oxygen species (ROS) [16,17,18,19].
ROS comprises of both free radicals (hydroxyl radical: •OH, superoxide radicals: O2•–, perhydroxy radical and alkoxy radicals: RO•) and non-radicals (hydrogen peroxide: H2O2, and singlet oxygen species: 1O2). Plants generate ROS as by-products of normal cellular activity during electron transport and during stress, H2O2 in particular has been established as a signaling molecule that can trigger specific signal transduction pathways [20,21]. Under steady state conditions, the rates of H2O2 production and removal are in balance as endogenous antioxidant defense systems protect cellular homeostasis from its toxic effects [22]. Recent evidence indicates that ROS homeostasis can help in plant vegetative development [21]. For instance, in Arabidopsis H2O2 has been found to accumulate in the elongation zone of the meristem, contributing to cell differentiation [23]. ROS as signaling molecules are involved in regulating seed germination through GA and/or ABA signaling in Arabidopsis [24]. During abiotic stresses, ROS trigger signal transduction pathways in response to those stresses, resulting in environmental adaptation [21]. However, they can also increase dramatically leading to physiological and metabolic changes and to damage in plants [21]. For instance, while pulses of ROS produced by respiratory burst oxidase homologues (RBOHs) in response to stress can propagate ROS signals to prime and acclimate plants to stress [25], accumulation of ROS can disturb the cellular balance [26] and lead to, damage of DNA and proteins, lipid peroxidation and cell death in most extreme cases [27]. To control ROS concentrations in the cell and counteract stress, plants mobilize various antioxidants and ROS degrading enzymes. [22]. The antioxidant systems involve both enzymatic and non-enzymatic H2O2 scavengers. Enzymes, such as catalase (CAT), ascorbate peroxidase (APX), gluthathione S-Transferase (GST), glutathione peroxidase (GPX), glutathione reductase (GR), type III peroxidases and peroxyredoxin (Prx); and non-enzymatic compounds, like ascorbate (AsA), glutathione (GSH), α-tocopherol and flavonoids, are all involved in regulating cellular H2O2 concentrations [28].
The antioxidant mediated defense capacity may vary among plant species and genotypes, and depend on specific types of stresses and their duration [29]. Exogenous application of various chemical compounds (phytohormones, polyamines, melatonin, epigenetic inhibitors, etc.) has been shown to enhance abiotic stress tolerance, including salinity, in many plant species [22]. A relatively new addition to this list is the exogenous application of plant biostimulants (PBs). These are the often modified substances of natural origin or microorganisms that in minute quantities can promote plant growth and development through activation of the plant’s own metabolic and defense mechanisms [30]. When exposed to abiotic stresses, plants pretreated (primed) with these chemical compounds have been shown to have lower ROS accumulation and be more tolerant to oxidative stress. This is a result of plant’s enhanced ROS detoxifying/scavenging capacity which correlates with increased transcript levels of both enzymatic and non-enzymatic components of the antioxidant system [31]. Though PBs have been reported to reduce the adverse effect of stress through the activation of conserved protective pathways, their mode of protection is often poorly characterized [32,33].
Copper chlorophyllin (Cu-chl), a semi synthetic water-soluble chlorophyll derivative, has been shown to serve as oxidative stress reducing agent in mammalian cells through its presumed strong antioxidant activity [34,35,36]. More recently, the protective role of Cu-chl against drought stress in tomato has also been reported, where foliar application of Cu-chl–containing products increased leaf antioxidant enzymes activity, as well as glutathione (GSH) content [37]. However, the molecular pathways through which Cu-chl exerts its activity remain currently not understood. Here, we provide new insights into the biological function of Cu-chl in improving tolerance to high salinity stress in A. thaliana. We use RNA-Seq analysis to investigate the gene regulation underlying Cu-chl’s protective effect. We report that the application of exogenous Cu-chl results in upregulation of several classes of ROS detoxifying genes and genes previously involved in stress protection.
2. Results
2.1. Pretreatment with Cu-chl Reduces H2O2
Salt stress triggers the accumulation of intracellular H2O2, a signature of oxidative imbalance in plant cells [26]. To determine whether Cu-chl reduces oxidative imbalance under salt stress, we examined histochemically (diaminobenzidine (DAB) staining) the levels of H2O2 produced in salt treated A. thaliana seedlings primed with Cu-chl. DAB is oxidized by H2O2 typically in the presence of heme containing peroxidases, in this case horseradish peroxidase, and forms a dark to light brown precipitate inside the cells, whereby the intensity of the precipitate reflects the amounts of H2O2 in cells. A. thaliana seeds were pre-treated with 0, 100 and 200 µM Cu-chl while stratifying at 4 °C for three days. Seeds from each treatment were then grown in 0.5X liquid MS media in duplicates for 10 days. Finally, seedlings were exposed to 150 mM NaCl for three hours and collected for DAB staining.
We found that the pre-treatment of seeds with 200 µM Cu-chl reduced the level of H2O2 under salinity stress compared to untreated seeds as indicated by lighter DAB staining (Figure 1a). We measured quantitatively H2O2 content using Amplex® Red assay, which showed a significant reduction of endogenous H2O2 in Cu-chl pretreated seedlings under salt stress (Figure 1b).
Figure 1.
Copper chlorophyllin, Cu-chl, induced changes in cellular H2O2 accumulation under salt stress. Seedlings were grown in four biological replicates for each treatment under the same growth conditions. Finally, two of them were used for diaminobenzidine (DAB) assay and the other two for the Amplex® Red assay, and the whole experiment was repeated twice: (a) DAB staining of A. thaliana seedlings in the absence (i–iii) of salt and after 3 h salt treatment (iv–vi). Note the lighter DAB staining in seedlings treated with 200 µM Cu-chl. (b) Quantification of H2O2 by Amplex® Red assay. Mean ± SE was calculated from two biological and three technical replicates for each treatment. Asterisk indicates significant difference (p = 0.05) according to two-sample t-test.
2.2. Cu-chl Protects Arabidopsis thaliana Seedlings from Herbicidal Damage by Reducing H2O2
Accumulation of toxic levels of reactive oxygen species in tissues can be caused not only by various abiotic stresses, but also by the application of herbicides [38]. To determine whether Cu-chl has a protective effect against herbicide induced ROS, we used the ROS-generating herbicide, methyl viologen dichloride hydrate (paraquat), to induce oxidative stress in Arabidopsis seedlings [39]. Paraquat accepts electrons from photosystem I and transfers them to molecular oxygen, thereby producing destructive amounts of ROS. When treated with paraquat, Arabidopsis leaves displayed the typical paraquat induced phenotype and bleached under light, remaining small and yellow-brown in color (Figure 2i) [40], and DAB staining revealed higher peroxide accumulation in leaves (Figure 3i). While application of Cu-chl either to seeds or growth media reduced paraquat induced damage to leaf greening (Figure 2ii–ix) and reduced H2O2 accumulation in leaves (Figure 3ii–ix). In addition, pre-treatment and supplement of Cu-chl in the media reduced paraquat induced leaf growth inhibition (Figure 2), suggesting that Cu-chl can improve plant growth under stress.
Figure 2.
Paraquat induced phenotypes in Arabidopsis seedlings in the presence and absence of Cu-chl. Note bleaching on leaves, and stunted leaf growth (i) in the absence of Cu-chl. Cu-chl pre-treatment only (ii,iii), and pre-treatment and supplement (iv–ix) in the media showed improved growth and less bleaching of Arabidopsis seedlings.
Figure 3.
DAB staining of 2-week-old Arabidopsis seedlings pre-treated with Cu-chl and incubated with 100 nM paraquat for 3 h. Cu-chl was applied as seed treatment (concentrations indicated on the left) and in the growing media (concentrations indicated on the top right corner of each image) followed by paraquat treatment. Note the stronger DAB staining in leaves in the absence of Cu-chl treatment (i).
2.3. RNA-Seq Reveals the Molecular Mechanism of Cu-chl
To investigate the H2O2 regulatory mechanism induced by Cu-chl, we conducted RNA-Seq under salinity stress with (Cu-chl NaCl in the following) and without treatment (NaCl in the following) of 1 mM Cu-chl. RNA-Seq data resulted in high quality reads across all three biological replicates in each treatment (Cu-chl NaCl, NaCl). Sequencing data and the statistics of their genomic alignment, considering average value of the replicates for each treatment is summarized in Table S1. For each of the treatments, the overall alignment percentage was reliable (more than 98%). We also assessed the variation of each gene between replicates by dispersion plot, which showed that data were clustered around the curve (red line, ideal fitted line for differential gene expression (DGE), with the dispersion decreasing with increasing mean expression levels (Figure 4a). This indicates that the data are a good fit for the DGE analysis [41]. Moreover, we estimated the variation among the replicates and throughout the treatments by principal component analysis (PCA) plot (Figure 4b). It showed strong grouping of replicates for NaCl, whereas data points for Cu-chl NaCl were dispersed on the plane. This implies that the expression of genes among the replicates of NaCl treatment is more convergent than Cu-chl NaCl. However, we considered all of them in the downstream analysis which makes it more conservative.
Figure 4.
Distribution of samples and variation among the replicates and throughout the treatments: (a) dispersion/variation of each gene among the replicates. Black dot and blue circle designate, respectively, the mean of normalized counts and variation of a gene. Strongly clustered data points around the red line suggests that data are well distributed and fit for differential gene expression (DGE) analysis (b) principal component analysis (PCA) plot of relative distribution of biological replicates and the treatments. PCA1 and PCA2, respectively, denote the highest and second highest variation of samples among the treatments. All three replicates for NaCl showed strong grouping, where Cu-chl NaCls were dispersed on the plane. However, note the low percent of variation with PCA1, which indicates, there were no extreme outliers throughout the samples.
Differentially expressed genes along with significantly up and downregulated genes (padj < 0.05 and log2FoldChange ≥ 1.0, indicated in red color) were determined using DESeq2 [41], and are shown as Volcano plot (Figure 5a). We found 879 genes were upregulated and 192 genes were downregulated in the Cu-chl NaCl compared to NaCl treatment (Figure 5a). Gene annotation for the significant genes was then carried out using DAVID bioinformatics resources v6.8 [42]. Top gene classes based on their molecular functions are shown as bubble plot, where color and size of the bubble, respectively indicate the False Discovery rate (FDR) and number of genes belong to each class (Figure 5b). Since many of them had higher FDR values (>0.05), we have manually checked each class and removed false positive associations.
Figure 5.
Differentially expressed genes and their corresponding molecular functions. (a) Volcano plot of significantly up and downregulated genes. X-axis and y-axis denote the log2FoldChange and -log10 of padj values, respectively; where log2FoldChange ≥ 1.0 and padj < 0.05 were considered as significant and indicated in red color. (b) Gene enrichment analysis of the significant genes. Color and bubble indicate the false discovery rate (FDR) and number of genes belong to each class, respectively.
Within the enriched cohort we found two predominant gene classes associated with H2O2 detoxification: 34 peroxidases and 16 glutathione S-transferases (GSTs), enriched with much lower FDR < 0.05 (Figure 5b, Table 1). Eleven of the 16 GST genes and all 34 peroxidases were more upregulated in the Cu-chl NaCl than NaCl. Among the 34 peroxidases, 26 were found to belong to the class III peroxidase super family (Table 1). Among the GSTs upregulated in Cu-chl NaCl, we found members of the phi (GSTF), tau (GSTU) and lambda (GSTL) classes, while members of the minor GST classes, dehydroascorbate reductase (DHARs), and tetrachlorohydroquinone dehalogenase were not affected by Cu-chl (Table 1). In addition, we found 5 Rboh genes associated with H2O2 signaling and priming, were more upregulated in Cu-chl NaCl (Table 1) [43]. Interestingly, expression of other classes of antioxidant enzymes involved in H2O2 degradation/scavenging was not affected by treatment with Cu-chl (Table S2).
Table 1.
List of genes involved in H2O2 detoxification or signaling were more upregulated in Cu-chl NaCl compared to NaCl. AtPrx, arabidopsis thaliana Class III peroxidase; Trx, thioredoxin superfamily protein; Rboh, respiratory burst oxidase homolog/riboflavin synthase-like superfamily protein; Dox, alpha dioxygenase Tpx, thioredoxin-dependent peroxidase; GSTU, glutathione S-transferase class tau; GSTL, glutathione S-transferase lambda; GSTF, glutathione S-transferase class phi.
| Function | Gene ID | Gene Name | log2Fold Change | Previously Reported | References |
|---|---|---|---|---|---|
| H2O2 detoxification | Peroxidases | ||||
| Class III peroxidases | |||||
| AT1G05260 | AtPrx3 | 1.93 | Cold inducible tolerance, Stamen abscission | [44,45] | |
| AT1G14550 | AtPrx5 | 3.50 | |||
| AT1G30870 | AtPrx7 | 5.33 | TNT treatment | [46] | |
| AT1G49570 | AtPrx10 | 3.90 | |||
| AT1G68850 | AtPrx11 | 1.88 | Cuticle metabolism regulation in response to abiotic stress | [47] | |
| AT2G18980 | AtPrx16 | 2.75 | |||
| AT2G37130 | AtPrx21 | 1.43 | Stamen abscission, aluminum stress | [45,48] | |
| AT2G38380 | AtPrx22 | 2.02 | potassium deficiency | [49] | |
| AT2G38390 | AtPrx23 | 2.52 | |||
| AT2G39040 | AtPrx24 | 3.42 | |||
| AT3G01190 | AtPrx27 | 4.11 | Aluminum stress, TNT treatment | [46,48] | |
| AT3G03670 | AtPrx28 | 2.87 | |||
| AT3G21770 | AtPrx30 | 1.42 | Cell elongation, Stamen abscission, Monolignin polymerization | [45,50,51] | |
| AT3G32980 | AtPrx32 | 1.69 | Cell elongation | [50] | |
| AT4G26010 | AtPrx44 | 1.65 | |||
| AT4G30170 | AtPrx45 | 2.35 | Cell elongation, aluminum stress, TNT treatment, Stamen abscission | [45,48,50,52] | |
| AT4G37520 | AtPrx50 | 1.34 | Low oxygen response, phosphate starvation, Stamen abscission | [45,53,54] | |
| AT5G06730 | AtPrx54 | 2.07 | |||
| AT5G14130 | AtPrx55 | 2.82 | |||
| AT5G15180 | AtPrx56 | 1.42 | Aluminum stress | [48] | |
| AT5G17820 | AtPrx57 | 4.38 | Arsenic stress, TNT treatment, cell elongation | [46,50,55] | |
| AT5G19890 | AtPrx59 | 4.22 | Aluminum stress, Mechanical stimulus | [48,51] | |
| AT5G24070 | AtPrx61 | 3.46 | |||
| AT5G64100 | AtPrx69 | 2.67 | Phosphate starvation, sulphur deficiency | [52,53] | |
| AT5G66390 | AtPrx72 | 1.33 | Cell elongation | [50] | |
| AT5G67400 | AtPrx73 | 2.56 | Aluminum stress | [48] | |
| H2O2 detoxification and signaling | Other peroxidases | ||||
| AT1G60740 | Trx | 4.66 | |||
| AT5G07390 | RbohA | 2.94 | Lateral root emergence, salinity and cold stress | [54,55] | |
| AT1G09090 | RbohB | 3.26 | Nitrogen fixation, lateral root emergence | [56,57] | |
| AT5G51060 | RbohC | 1.99 | Lateral root emergence, salinity and cold stress | [58,59] | |
| AT4G25090 | RbohG | 2.96 | Lateral root emergence | [60] | |
| AT4G11230 | RbohI | 1.12 | Drought stress | [61] | |
| AT3G01420 | Dox1 | 2.93 | |||
| AT1G65970 | Tpx2 | 1.27 | |||
| H2O2 detoxification | Glutathione S-transferase | ||||
| AT2G29490 | GSTU1 | 2.79 | Herbicide treatment, phytoremediation, oxidative stress response (SO2), salinity, drought and cold stress | [62,63,64,65,66] | |
| AT2G29480 | GSTU2 | 2.63 | Herbicide treatment, salinity and drought stress | [65,67] | |
| AT2G29470 | GSTU3 | 2.64 | Oxidative stress response (SO2) | [64] | |
| AT2G29460 | GSTU4 | 1.76 | Oxidative stress response (SO2), salinity | [64,68] | |
| AT2G29420 | GSTU7 | 1.37 | Seed germination, ABA response and osmotic stress | [69] | |
| AT3G09270 | GSTU8 | 1.40 | Cadmium treatment | [70] | |
| AT1G69920 | GSTU12 | 1.68 | Salinity stress | [71] | |
| AT1G27140 | GSTU14 | 4.32 | |||
| AT1G78340 | GSTU22 | 2.89 | |||
| AT1G17170 | GSTU24 | 1.61 | TNT treatment, herbicide treatment, phytoremediation, oxidative stress response (SO2) | [62,63,64] | |
| AT5G02780 | GSTL1 | 1.27 | Increased tolerance to salinity stress | [72] | |
We also identified 33 transcription factors (TFs) that are reported to be involved in abiotic stress regulation. These include 9 MYBs, 5 basic helix loop helix superfamily proteins (bHLH), 7 WRKYs, 3 NAC domain containing transcription factors, 7 Zinc finger proteins, and 2 Heat shock proteins (Table 2). We also checked the differential expression of these peroxidases, glutathione S-transferases and TFs upon Cu-chl application under control conditions (without salt stress). We found that these genes were also induced by Cu-chl under control conditions, however less than when Cu-chl was applied with salt stress (Tables S3 and S4).
Table 2.
List of transcription factors (TFs) involved in abiotic stresses regulation and signaling that were more upregulated in Cu-chl NaCl compared to NaCl.
| Gene ID | Gene Name | log2Fold Change | Previously Reported | References |
|---|---|---|---|---|
| MYB containing domain | ||||
| AT5G49620 | MYB 78 | 4.44 | Abiotic and biotic stress | [73] |
| AT1G74080 | MYB122 | 3.18 | Dehydration stress | [74] |
| AT1G79180 | MYB63 | 2.52 | Dehydration stress | [74] |
| AT5G54230 | MYB49 | 2.39 | Cadmium accumulation | [75] |
| AT1G09540 | MYB61 | 2.17 | Stomatal aperture | [76] |
| AT5G65790 | MYB68 | 1.85 | High temperature | [77] |
| AT1G48000 | MYB112 | 1.75 | Salinity and high light stress | [78] |
| AT4G34990 | MYB32 | 1.35 | Salinity stress | [79] |
| AT3G49690 | MYB84 | 1.27 | High temperature | [77] |
| Basic helix-loop-helix DNA binding superfamily protein | ||||
| AT4G21340 | bHLH | 4.04 | Response to phytotoxicity | [80] |
| AT1G02340 | bHLH | 2.57 | Dark induced senescence | [81] |
| AT4G29930 | bHLH | 1.67 | Dehydration stress | [74] |
| AT1G10585 | bHLH | 1.46 | Dehydration stress | [74] |
| AT5G51780 | bHLH | 1.11 | Salinity stress | [82] |
| WRKY DNA binding protein | ||||
| AT1G68150 | AtWRKY09 | 3.97 | Abiotic stresses | [83] |
| AT5G15130 | AtWRKY72 | 2.90 | Abscisic acid signal | [84] |
| AT4G22070 | AtWRKY31 | 2.70 | Root growth, pathogen attack | [85] |
| AT5G13080 | AtWRKY75 | 2.65 | Leaf senescence | [86] |
| AT1G69810 | AtWRKY36 | 1.61 | UV responsive | [87] |
| AT1G30650 | AtWRKY14 | 1.31 | Abiotic stresses | [88] |
| AT3G01970 | AtWRKY45 | 1.16 | Dehydration stress tolerance | [89] |
| NAC containing domain | ||||
| AT3G18400 | ANAC058 | 1.91 | ABA mediated germination | [90] |
| AT1G01010 | ANAC001 | 1.67 | Dehydration stress | [74] |
| AT3G29035 | ANAC003 | 1.34 | Leaf senescence | [91] |
| Zinc finger protein | ||||
| AT1G67030 | AtZFP67 | 3.98 | ABA repressor | [92] |
| AT5G22890 | AtSTOP2 (C2HC ZFP) | 3.56 | Aluminum and low pH | [93] |
| AT5G57520 | AtZFP2 | 3.51 | Salinity stress | [82] |
| AT1G10480 | AtZFP5 | 3.31 | Phosphate and potassium deficiency | [94] |
| AT1G68360 | AtGIS3 (C2HC ZFP) | 1.59 | Cold stress | [95] |
| AT2G28200 | C2H2 ZFP | 1.12 | Dehydration stress | [74] |
| AT2G19810 | AtOZF1(CCCH ZFP) | 1.08 | Hydrogen peroxide, abscisic acid and salinity responsive | [96] |
| Heat shock family protein | ||||
| AT3G51910 | AtHSFA7A 2 | 2.12 | Heat shock response | [97] |
| AT2G26150 | AtHSFA2 | 1.94 | Heat shock response | [97] |
2.4. RNA-Seq Validation by Real-Time RT-PCR (qPCR)
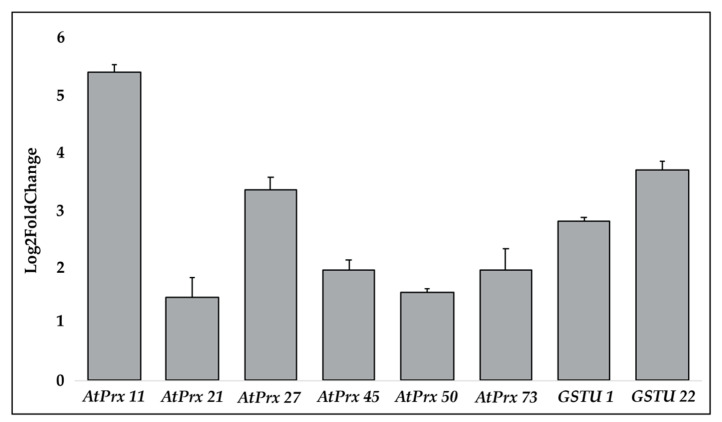
To confirm the data obtained by RNA-Seq, the expression of 6 class III peroxidases (AtPrx 11, AtPrx 21, AtPrx 27, AtPrx 45, AtPrx 50, and AtPrx 73) and 2 glutathione S-transferases (GSTU1, GSTU22) were carried out by qPCR (primer sequences are in Table S5). qPCR results confirmed the data obtained by RNA-Seq, and showed all 6 class III peroxidases, and the two glutathione S-transferases were more upregulated in Cu-chl NaCl than NaCl treatment (Figure 6).
Figure 6.
qPCR of class III peroxidase and glutathione S-transferase genes under salinity stress with and without Cu-chl treatment. Log2FoldChange (treated vs not treated) ≥1.0 was considered as significant upregulation, and all of them showed similar level of differential expression as RNA-Seq data. Log2FoldChange ± SE shown here are from three technical replicates of a biological sample.
2.5. Effect of Cu-chl on Arabidopsis thaliana Growth
To determine whether Cu-chl has beneficial effects on plant growth, we grew A. thaliana seeds in 0.5X liquid MS media supplemented with 100 and 200 µM Cu-chl and without Cu-chl for 14 days and measured the shoot length and fresh shoot weight. Both shoot length (Figure 7a) and weight (Figure 7b) were significantly higher with 100 and 200 µM Cu-chl treatment than in the control, suggesting that Cu-chl can improve plant growth.
Figure 7.
Effect of Cu-chl on growth and plant biomass: (a) shoot length of A. thaliana seedlings (n = 20, values are mean ± SE). (b) Fresh shoot weight (groups of four seedlings, n = 5) of the 20 seedlings measured in (a). Four randomly chosen seedlings were weighed together, different letters indicate significant difference between treatments according to Fisher’s least significant difference (LSD) test at p = 0.05.
3. Discussion
To withstand salt stress, plants utilize various protective physiological, cellular and molecular mechanisms many of them geared towards reducing cellular concentrations of reactive oxygen species (ROS) (mainly hydrogen peroxide, H2O2) [29]. Exogenously applied biostimulants have been reported to be able to protect plants against stresses [30]. Our results indicate that pretreatment of Arabidopsis seedlings with Cu-chl can reduce cellular oxidative stress in salt or herbicide treated plants, primarily by decreasing cellular oxidative stress through modulating H2O2 levels. Although the precise molecular pathways through which Cu-chl maintains oxidative balance during stress are not known, genome wide analysis of RNA-seq data of salt stressed plants treated with Cu-chl suggests that Cu-chl may do that through upregulation of H2O2 detoxifying cellular pathways involving primarily class III peroxidases and Gluthathione S-transferases (Figure 8).
Figure 8.
Mechanism of Cu-chl mediated oxidative stress regulation under salinity stress. Application of Cu-chl induces the expression of class III peroxidases, Glutathione S-transferase, Rbohs and TFs under salinity stress. Upon stimulation, class III peroxidases and glutathione S-transferases activate the peroxidative cycle and reduce H2O2. On the other hand, through Rbohs, Cu-chl activates the hydroxylic cycle, thereby producing •OH and O2•– from H2O2 to help in cell growth. Solid and dashed green pointed lines, respectively, indicate the genes and mechanisms found in our RNA-Seq data and in previous studies.
Class III peroxidases have been shown to scavenge H2O2 in vivo leading to increased ROS/H2O2 detoxification and osmotic adjustment, thereby improving tolerance to abiotic stresses. For instance, overexpression of AtPrx 3 has been shown to increase tolerance under dehydration and salt stress, while its suppression gave dehydration and salt-sensitive phenotypes in A. thaliana [44]. In another study, AtPrx 11 was found to be part of the complex network controlling cell expansion and cuticle deposition in response to osmotic stress, ABA and salt treatment [47]. AtPrx 7 also has been reported to be involved in controlling the H2O2 concentration at the germination stage [98]. Functions of these peroxidases have, been investigated in other crop plants as well. In a study on rice, OsPrx 24, a class III peroxidase, was found to be regulated by a transcription factor complex and to function as a ROS scavenger to enhance tolerance against drought and salt stress [99]. Jin et al. showed overexpression of the class III peroxidase GsPRX 9 conferred soybean salt tolerance through mediation of the ROS regulatory network [100]. In transgenic Tobacco, heterologous expression of two class III peroxidases, CrPrxs from Catharanthus roseus exhibited increased tolerance to H2O2 treatment and improved germination rate under drought, salt and cold stress [101].
GSTs from all major classes have also been shown to be involved in the detoxification of toxic substances and attenuation of oxidative stresses [102]. In Arabidopsis, expression of two GSTs was found elevated in response to aluminum, cold, heat and metal stress, suggesting a common induction mechanism in response to the oxidative stresses [103]. Overexpression of a tobacco GST with glutathione peroxidase activity in transgenic tobacco showed increased glutathione-dependent peroxide scavenging that leads to reduced oxidative damage and enhanced tolerance to abiotic stresses [104]. They also found that cold or salt-stress treatments had less inhibitory effects on the growth of GST overexpressed transgenic lines. In barley leaves, a senescence-induced tau class GST has been suggested as an antioxidant, protecting senescing cells from ROS damages, and as an inducer that involves in secondary metabolism [105]. These studies support our data that upon Cu-chl treatment, class III peroxidases and GSTs act on reducing H2O2 under salinity stress. In addition, we found members of several transcription factor families, such as MYB, WRKY, NAC, bHLH, etc. that are known to play key roles in ROS signaling pathways in response to stresses, resulting in salt tolerance in Arabidopsis, rice and wheat [106].
This study exhibited that pretreatment of Arabidopsis seeds with Cu-chl was sufficient to reduce oxidative stress in salt or herbicide treated seedlings, suggesting treated plants have enhanced defensive capacity against salt stress. One possible scenario is that Cu-chl can activate defense signaling networks culminating in ROS scavenging through activation of genes involved in ROS signaling. We found 5 NADPH/respiratory burst oxidases (Rbohs) upregulated more in Cu-chl treated plants under stress. Rbohs are reported to be involved in ROS signaling, for example in barley, they have been marked as a hallmark of salt tolerant genotype, as their expression was increased significantly in a salt-tolerant mutant compared to the control after exposure to salt stress [106]. In Arabidopsis, overexpression of RbohI is reported to significantly improve the drought tolerance [61]. In another study on Brassica campestris, expression of RbohA and RbohD was found to be induced by different abiotic stressors like low temperature, salt and dehydration [55]. Rbohs are also reported to assist in class III peroxidases mediated induction of cellular processes like seed germination, cell elongation, lignification, wound healing, and plant senescence [107,108]. The O2•– released during the oxidative cycle by Rboh can convert peroxidase into compound III, an oxygenated intermediate state of peroxidase. This compound consequently can catalyze the production of •OH from H2O2 in the cell wall and induce cell elongation [109]. We found enhanced seedlings growth by pre-treatment and supplement of Cu-chl in the media under both control (Figure 7) and stress conditions (Figure 2), which further supports this Cu-chl induced growth stimulatory pathways by class III peroxidases and Rbohs. Taken together, we propose a mechanism in which Cu-chl reduces H2O2 under salinity stress where class III peroxidases and GSTs function as the central molecules (Figure 8). Upon induction by Cu-chl, class III peroxidases and GSTs act by reducing the local concentration of H2O2 (peroxidative), and in association with Rbohs, class III peroxidases may favor plant growth by generating oxygen radicals (hydroxylic cycle) [110]. Cu-chl also stimulates TFs that can induce downstream salt responsive genes to alleviate stress.
In a paper published by Zhang et al. on tomato, Cu-chl was shown to alleviate oxidative stress caused by water deficit [37]. The availability of extensive genomic resources on Arabidopsis makes it the ideal host to investigate the functions and molecular mechanisms underpinning the role of Cu-chl in the mediation of plant defense under stress, but results obtained from Arabidopsis sometimes cannot be translated to crops. However, our data show that Cu-chl induced plant defense through upregulation of class III peroxidases, glutathione S-transferases and abiotic stress responsive transcription factors, which are highly conserved in plants. Further experiments should be done to see if the described mechanism is shared across plant species, and if the results seen in this study can be translated for field applications to other crops.
4. Materials and Methods
4.1. Seed Sterilization and Stratification
A. thaliana seeds (Columbia-0) were surface sterilized in 70% ethanol for 30 sec followed by 40% sodium hypochlorite for five min and rinsed four times in sterile water. For seeds that needed pre-treatment, Cu-chl was added at different concentrations (see paragraphs below) to 1 mL of sterile water directly to the seeds in an Eppendorf tube. Tubes containing seeds pre-treated or un-treated were then wrapped with aluminum foil and kept in the dark at 4 °C for three days.
4.2. H2O2 Accumulation Measurement via DAB (3,3′-Diaminobenzidin) and Amplex® Red Assay under Salt Stress
After pre-treatment with 100 or 200 μM Cu-chl and three days incubation at 4 °C, four groups of 20 seeds per treatment (0, 100 and 200 μM Cu-chl) were placed in 10 mL of 0.5X liquid MS media in 12 separate Petri plates and grown in a shaking incubator at 21 °C and 20 rpm, maintaining 12 h light cycle for 10 days. Seedlings were then transferred to 10 mL of fresh 0.5 × liquid MS media, treated with 150 mM NaCl for three hours, washed with sterile distilled water and collected for DAB staining and Amplex® Red assay. To qualitatively measure the level of H2O2 in the tissue, DAB assay was conducted using the SIGMAFASTTM DAB with Metal Enhancer kit (Sigma Aldrich, St. louis, MO, USA) following the user manual. In brief, four tablets from each of DAB/Cobalt and urea hydrogen peroxide were dissolved in 20 mL ultrapure MiliQ water (MilliporeSigma™ Milli-Q™ Ultrapure Water Systems, Thermo Fisher scientific, Waltham, MA, USA) by vortexing and poured in a 50 mL beaker. Seedlings from each treatment (two biological replicates of 20 seedlings/treatment) were dipped into the DAB solution and vacuum infiltrated for two minutes. After that, seedlings were placed in sterile distilled water in Petri plates and incubated in the dark overnight, when DAB is oxidized by H2O2 and forms dark-brown color. To observe color intensity, chlorophyll was removed by incubating the seedlings in 3:1 v:v, 90% ethanol:acetic acid at 70℃ for 10 min.
Quantification of H2O2 was carried out by using the Amplex® Red hydrogen peroxide/peroxidase assay kit (Thermo Fisher Scientific, Waltham, MA, USA) on the two remaining biological replicates per each treatment. Standard curve was generated using H2O2 supplied in the kit (R2 = 0.98). Fifty mg of seedlings from each of the 6 plates (two out of the four biological replicates per each of the three treatments) were ground and diluted in 50 mM sodium phosphate buffer (pH = 7.4). Fluorescence intensity of three technical replicates for each of the six plates was measured by setting excitation at 560 nm and emission at 590 nm using SpectraMax® i3x Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA, USA). Finally, concentrations of H2O2 in the samples were calculated using the standard curve. Both DAB and Amplex® Red assays were repeated twice.
4.3. H2O2 Accumulation Measurement under Herbicide Stress
Protective role of Cu-chl against herbicidal damage was determined by using a ROS-generating herbicide, methyl viologen dichloride hydrate (paraquat) (Sigma Aldrich, St. louis, MO, USA). After 0, 100 and 200 μM Cu-chl pre-treatment, followed by stratification at 4 °C, 12 petri plates of 20 seeds/plate for each treatment (0, 100 and 200 μM Cu-ch, total 36 plates) were grown in 10 mL of 0.5 × liquid MS media. Plates from each pre-treatment group were separated in three groups of four plates each. Four plates from each pre-treatment (12 plates) were grouped and supplemented with 0, 100 or 200 μM Cu-chl, so that each pre-treatment group was supplemented with all three post-treatments. After two weeks (growth conditions as in 4.2), 100 nM paraquat was applied in each Petri plate. Seedlings from two petri plates for each treatment were then collected for DAB assay three hours post paraquat application and the remaining two plates were left for 10 days to observe bleaching of the leaves.
4.4. Cu-chl and Salt Stress Application for RNA-Seq and qPCR
After stratification at 4 °C, 15 seeds/petri plate without any pre-treatment were grown in a shaking incubator maintaining the same growth conditions as mentioned above. Seedlings were then transferred to 10 mL of fresh 0.5 × liquid MS media containing either 100 μL DMSO (mock treatment) or 1 mM Cu-chl in DMSO. Seedlings were incubated for 24 h followed by two hours salt treatment with 100 mM NaCl. Cu-chl and NaCl concentrations, and duration of the treatment were different than the ones used in the assays above and were optimized based on preliminary experiments to reach maximum level of gene expression. Fifty mg of seedlings per plate and three biological replicates for each treatment (Control without any Cu-chl and NaCl, Cu-chl, NaCl and Cu-chl NaCl) were harvested for RNA extraction using Quick RNA Plant Miniprep kit (Zymo Research Corporation, Irvine, CA, USA).
4.5. Effect of Cu-chl on Seedling Growth
After pre-treatment with 100 and 200 μM Cu-chl, A. thaliana seedlings were grown for two weeks maintaining the same growth conditions as above. Twenty seedlings from each of the treatments (0, 100 and 200 μM Cu-chl) were randomly selected to measure shoot length using Image J and fresh shoot weight.
4.6. RNA Sequencing and Analysis
A uniquely barcoded library was made from each sample using the Illumina TruSeq Stranded mRNA Library kit (Illumina, Inc., San Diego, CA, USA) at the Genomic Core Facility, Penn State. An approximately equimolar pool of the libraries was made and sequenced on a NextSeq 550 high output system (Illumina, San Diego, CA, USA), which was set for 75 nt single read sequences. This provided about 30 million reads per sample. Sequences were analyzed using a series of bioinformatics tools in Bash and R background. In brief, raw reads for all of the replicates in each treatment were checked and preprocessed by Fastqc [111]. After checking the quality, reference genome (A. thaliana, TAIR 10) was used, and sequences were aligned and mapped against the genome by Hisat2 [112]. The aligned files were assembled and merged into one file by StringTie2 [113] and raw reads per sample were counted by featureCounts [114]. Finally, differential gene expression (DGE) analysis was carried out using the DESeq2 package in Bioconductor library [41] and differentially expressed genes throughout the treatments were functionally annotated by using DAVID bioinformatics resources v6.8 [42].
4.7. cDNA Synthesis and qPCR
Total RNA was extracted using Quick-RNA Plant Kit (Zymo Research, Irvine, CA, USA) the manufacturer’s instructions. 400 ng of total RNA was used for cDNA synthesis using the High-Capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the user manual.
qPCR was done using ssoAdvanced universal SYBR green super mix (Bio-Rad, Hercules, CA, USA) in BIO-RAD CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad) following the manufacturer’s instructions. The reaction was set up in technical triplicates using Elongation factor 1 α as the internal control. Thermo cycling conditions were 95°C for 30 sec, followed by 39 cycles of 95 °C for 5 s, 60 °C for 30 s. A dissociation protocol with a gradient from 65 °C to 95 °C (5.0 secs and 0.5 °C ramp/cycle) was used for generating melting curves. Primers used for qPCR are listed in Table S5. Three biological replicates and three technical replicates were used for analysis. Finally, a software provided by Bio-Rad was used to analyze differential gene expression (DGE) using 2-ΔΔCT method [115] and statistical analysis was done by two samples t-test using Minitab19 [116].
Acknowledgments
We would like to thank Crosley Kudla-Williams for helping with the experiments and Alessandra Castro for help with literature searches.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/10/4/625/s1 Table S1. Summary of sequencing data and the statistics of their genomic mapping, Table S2. Genes that are part of the classical antioxidant system, and predominantly involved in H2O2 detoxification were not differentially expressed [117]. Table S3. Comparative differential expression of Peroxidases and Glutathione S-transferases with and without salt stress (control) upon Cu-chl application. Table S4. Comparative differential expression of abiotic stress responsive Transcription factors with and without salt stress (control) upon Cu-chl application. Table S5. Sequences of primers used in the study.
Author Contributions
Conceptualization, W.C., M.F., M.T.I., C.R., J.L.; methodology, M.T.I., W.C., C.R.; validation, M.T.I.; investigation, M.T.I., C.R., W.C.; data curation, M.T.I., C.R., W.C.; writing—original draft preparation, M.T.I.; writing—review and editing, M.T.I.; C.R., W.C.; supervision, C.R., W.C., W.U.; funding acquisition, C.R., W.U. The authors declare no competing interests. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Suncor Energy Inc., the USDA National Institute of Food and Agriculture and Hatch Appropriations under Project No. PEN04652 Accession No. 1016243 and Project No. PEN04662 Accession No. 1016817.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Den Herder G., Van Isterdael G., Beeckman T., De Smet I. The Roots of a New Green Revolution. Trends Plant Sci. 2010;15:600–607. doi: 10.1016/j.tplants.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan S., Tuteja N. Cold, Salinity and Drought Stresses: An Overview. Arch. Biochem. Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Von Braun J. The World Food Situtation: New Driving Forces and Required Actions. International Food Policy Research Institute; Washington, DC, USA: 2007. [Google Scholar]
- 4.Fahad S., Hussain S., Matloob A., Khan F.A., Khaliq A., Saud S., Hassan S., Shan D., Khan F., Ullah N., et al. Phytohormones and Plant Responses to Salinity Stress: A Review. Plant Growth Regul. 2015;75:391–404. doi: 10.1007/s10725-014-0013-y. [DOI] [Google Scholar]
- 5.Shabala S. Learning from Halophytes: Physiological Basis and Strategies to Improve Abiotic Stress Tolerance in Crops. Ann. Bot. 2013;112:1209–1221. doi: 10.1093/aob/mct205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qadir M., Quillérou E., Nangia V., Murtaza G., Singh M., Thomas R.J., Drechsel P., Noble A.D. Economics of Salt-Induced Land Degradation and Restoration. Nat. Resour. Forum. 2014;38:282–295. doi: 10.1111/1477-8947.12054. [DOI] [Google Scholar]
- 8.Riyazuddin R., Verma R., Singh K., Nisha N., Keisham M., Bhati K.K., Kim S.T., Gupta R. Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules. 2020;10:959. doi: 10.3390/biom10060959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahid S.A., Zaman M., Heng L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques. Springer; Berlin/Heidelberg, Germany: 2018. Soil salinity: Historical perspectives and a world overview of the problem; pp. 43–53. [Google Scholar]
- 10.Jamil A., Riaz S., Ashraf M., Foolad M.R. Gene Expression Profiling of Plants under Salt Stress. Crit. Rev. Plant Sci. 2011;30:435–458. doi: 10.1080/07352689.2011.605739. [DOI] [Google Scholar]
- 11.Pitman M.G., Läuchli A. Salinity: Environment-Plants-Molecules. Springer; Berlin/Heidelberg, Germany: 2002. Global impact of salinity and agricultural ecosystems; pp. 3–20. [Google Scholar]
- 12.Ashraf M. Biotechnological Approach of Improving Plant Salt Tolerance Using Antioxidants as Markers. Biotechnol. Adv. 2009;27:84–93. doi: 10.1016/j.biotechadv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Carillo P., Cirillo C., De Micco V., Arena C., De Pascale S., Rouphael Y. Morpho-Anatomical, Physiological and Biochemical Adaptive Responses to Saline Water of Bougainvillea Spectabilis Willd. Trained to Different Canopy Shapes. Agric. Water Manag. 2019;212:12–22. doi: 10.1016/j.agwat.2018.08.037. [DOI] [Google Scholar]
- 14.Dell’Aversana E., Hessini K., Ferchichi S., Fusco G.M., Woodrow P., Ciarmiello L.F., Abdelly C., Carillo P. Salinity Duration Differently Modulates Physiological Parameters and Metabolites Profile in Roots of Two Contrasting Barley Genotypes. Plants. 2021;10:307. doi: 10.3390/plants10020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fricke W. Rapid and Tissue-Specific Accumulation of Solutes in the Growth Zone of Barley Leaves in Response to Salinity. Planta. 2004;219:515–525. doi: 10.1007/s00425-004-1263-0. [DOI] [PubMed] [Google Scholar]
- 16.Fichman Y., Miller G., Mittler R. Whole-Plant Live Imaging of Reactive Oxygen Species. Mol. Plant. 2019;12:1203–1210. doi: 10.1016/j.molp.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Inze D. Oxidative Stress in Plants. CRC Press; Boca Raton, FL, USA: 2001. [Google Scholar]
- 18.Li J.-Y., Jiang A.-L., Zhang W. Salt Stress-Induced Programmed Cell Death in Rice Root Tip Cells. J. Integr. Plant Biol. 2007;49:481–486. doi: 10.1111/j.1744-7909.2007.00445.x. [DOI] [Google Scholar]
- 19.Gill S.S., Tuteja N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Veal E.A., Day A.M., Morgan B.A. Hydrogen Peroxide Sensing and Signaling. Mol. Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Huang H., Ullah F., Zhou D.-X., Yi M., Zhao Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019:10. doi: 10.3389/fpls.2019.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decros G., Baldet P., Beauvoit B., Stevens R., Flandin A., Colombié S., Gibon Y., Pétriacq P. Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Front. Plant Sci. 2019;10:1091. doi: 10.3389/fpls.2019.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukagoshi H., Busch W., Benfey P.N. Transcriptional Regulation of ROS Controls Transition from Proliferation to Differentiation in the Root. Cell. 2010;143:606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Baek D., Cha J.-Y., Kang S., Park B., Lee H.-J., Hong H., Chun H.J., Kim D.H., Kim M.C., Lee S.Y., et al. The Arabidopsis a Zinc Finger Domain Protein ARS1 Is Essential for Seed Germination and ROS Homeostasis in Response to ABA and Oxidative Stress. Front. Plant Sci. 2015:6. doi: 10.3389/fpls.2015.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollist H., Zandalinas S.I., Sengupta S., Nuhkat M., Kangasjärvi J., Mittler R. Rapid Responses to Abiotic Stress: Priming the Landscape for the Signal Transduction Network. Trends Plant Sci. 2019;24:25–37. doi: 10.1016/j.tplants.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Neill S.J., Desikan R., Clarke A., Hurst R.D., Hancock J.T. Hydrogen Peroxide and Nitric Oxide as Signalling Molecules in Plants. J. Exp. Bot. 2002;53:1237–1247. doi: 10.1093/jexbot/53.372.1237. [DOI] [PubMed] [Google Scholar]
- 27.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. Oxford University Press; Oxford, England: 2015. [Google Scholar]
- 28.Noctor G., Foyer C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 29.Hasanuzzaman M., Bhuyan M.H.M., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J. Al, Fujita M., Fotopoulos V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants. 2020;9:681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Jardin P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015;196:3–14. doi: 10.1016/j.scienta.2015.09.021. [DOI] [Google Scholar]
- 31.Li L., Wang Y., Shen W. Roles of Hydrogen Sulfide and Nitric Oxide in the Alleviation of Cadmium-Induced Oxidative Damage in Alfalfa Seedling Roots. Biometals. 2012;25:617–631. doi: 10.1007/s10534-012-9551-9. [DOI] [PubMed] [Google Scholar]
- 32.Rouphael Y., Colla G. Biostimulants in Agriculture. Front. Plant Sci. 2020;11:40. doi: 10.3389/fpls.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvo P., Nelson L., Kloepper J.W. Agricultural Uses of Plant Biostimulants. Plant Soil. 2014;383:3–41. doi: 10.1007/s11104-014-2131-8. [DOI] [Google Scholar]
- 34.Ong T., Whong W.-Z., Stewart J., Brockman H.E. Chlorophyllin: A Potent Antimutagen against Environmental and Dietary Complex Mixtures. Mutat. Res. Lett. 1986;173:111–115. doi: 10.1016/0165-7992(86)90086-2. [DOI] [PubMed] [Google Scholar]
- 35.Sato M., Fujimoto I., Sakai T., Aimoto T., Kimura R., Murata T. Effect of Sodium Copper Chlorophyllin on Lipid Peroxidation. IX.: On the Antioxidative Components in Commercial Preparations of Sodium Copper Chlorohyllin. Chem. Pharm. Bull. 1986;34:2428–2434. doi: 10.1248/cpb.34.2428. [DOI] [Google Scholar]
- 36.Kamat J.P., Boloor K.K., Devasagayam T.P.A. Chlorophyllin as an Effective Antioxidant against Membrane Damage in Vitro and Ex Vivo. Biochim. Et Biophys. Acta (Bba)-Mol. Cell Biol. Lipids. 2000;1487:113–127. doi: 10.1016/S1388-1981(00)00088-3. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X., Goatley M., Conner J., Wilkins M., Teshler I., Liu J., Fefer M., Ckurshumova W. Copper Chlorophyllin Impacts on Growth and Drought Stress Tolerance of Tomato Plants. HortScience. 2019;54:2195–2201. doi: 10.21273/HORTSCI14434-19. [DOI] [Google Scholar]
- 38.Song N.H., Le Yin X., Chen G.F., Yang H. Biological Responses of Wheat (Triticum Aestivum) Plants to the Herbicide Chlorotoluron in Soils. Chemosphere. 2007;68:1779–1787. doi: 10.1016/j.chemosphere.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Scarpeci T.E., Valle E.M. Rearrangement of Carbon Metabolism in Arabidopsis Thaliana Subjected to Oxidative Stress Condition: An Emergency Survival Strategy. Plant Growth Regul. 2008;54:133–142. doi: 10.1007/s10725-007-9236-5. [DOI] [Google Scholar]
- 40.Chen R., Sun S., Wang C., Li Y., Liang Y., An F., Li C., Dong H., Yang X., Zhang J., et al. The Arabidopsis PARAQUAT RESISTANT2 Gene Encodes an S-Nitrosoglutathione Reductase That Is a Key Regulator of Cell Death. Cell Res. 2009;19:1377–1387. doi: 10.1038/cr.2009.117. [DOI] [PubMed] [Google Scholar]
- 41.Love M.I., Huber W., Anders S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman B.T., Lempicki R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009;4:44. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 43.Chapman J.M., Muhlemann J.K., Gayomba S.R., Muday G.K. RBOH-Dependent ROS Synthesis and ROS Scavenging by Plant Specialized Metabolites to Modulate Plant Development and Stress Responses. Chem. Res. Toxicol. 2019;32:370–396. doi: 10.1021/acs.chemrestox.9b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Llorente F., López-Cobollo R.M., Catalá R., Mart\’\inez-Zapater J.M., Salinas J. A Novel Cold-Inducible Gene from Arabidopsis, RCI3, Encodes a Peroxidase That Constitutes a Component for Stress Tolerance. Plant J. 2002;32:13–24. doi: 10.1046/j.1365-313X.2002.01398.x. [DOI] [PubMed] [Google Scholar]
- 45.Cai S., Lashbrook C.C. Stamen Abscission Zone Transcriptome Profiling Reveals New Candidates for Abscission Control: Enhanced Retention of Floral Organs in Transgenic Plants Overexpressing Arabidopsis ZINC FINGER PROTEIN2. Plant Physiol. 2008;146:1305–1321. doi: 10.1104/pp.107.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mentewab A., Cardoza V., Stewart C.N., Jr. Genomic Analysis of the Response of Arabidopsis Thaliana to Trinitrotoluene as Revealed by CDNA Microarrays. Plant Sci. 2005;168:1409–1424. doi: 10.1016/j.plantsci.2004.10.021. [DOI] [Google Scholar]
- 47.Cominelli E., Sala T., Calvi D., Gusmaroli G., Tonelli C. Over-Expression of the Arabidopsis AtMYB41 Gene Alters Cell Expansion and Leaf Surface Permeability. Plant J. 2008;53:53–64. doi: 10.1111/j.1365-313X.2007.03310.x. [DOI] [PubMed] [Google Scholar]
- 48.Kumari M., Taylor G.J., Deyholos M.K. Transcriptomic Responses to Aluminum Stress in Roots of Arabidopsis Thaliana. Mol. Genet. Genom. 2008;279:339. doi: 10.1007/s00438-007-0316-z. [DOI] [PubMed] [Google Scholar]
- 49.Kang J.G., Pyo Y.J., Cho J.W., Cho M.H. Comparative Proteome Analysis of Differentially Expressed Proteins Induced by K+ Deficiency in Arabidopsis Thaliana. Proteomics. 2004;4:3549–3559. doi: 10.1002/pmic.200400898. [DOI] [PubMed] [Google Scholar]
- 50.Irshad M., Canut H., Borderies G., Pont-Lezica R., Jamet E. A New Picture of Cell Wall Protein Dynamics in Elongating Cells of Arabidopsis Thaliana: Confirmed Actors and Newcomers. Bmc Plant Biol. 2008;8:94. doi: 10.1186/1471-2229-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moseyko N., Zhu T., Chang H.-S., Wang X., Feldman L.J. Transcription Profiling of the Early Gravitropic Response in Arabidopsis Using High-Density Oligonucleotide Probe Microarrays. Plant Physiol. 2002;130:720–728. doi: 10.1104/pp.009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammond J.P., Bennett M.J., Bowen H.C., Broadley M.R., Eastwood D.C., May S.T., Rahn C., Swarup R., Woolaway K.E., White P.J. Changes in Gene Expression in Arabidopsis Shoots during Phosphate Starvation and the Potential for Developing Smart Plants. Plant Physiol. 2003;132:578–596. doi: 10.1104/pp.103.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikiforova V., Freitag J., Kempa S., Adamik M., Hesse H., Hoefgen R. Transcriptome Analysis of Sulfur Depletion in Arabidopsis Thaliana: Interlacing of Biosynthetic Pathways Provides Response Specificity. Plant J. 2003;33:633–650. doi: 10.1046/j.1365-313X.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 54.Arthikala M.-K., Quinto C. RbohA Coordinates Lateral Root Emergence in Common Bean. Commun. Integr. Biol. 2018;11:1–5. doi: 10.1080/19420889.2018.1467188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang T., Lai J., Li P., Sun W., Diao Z., Wang J., Zheng S. Expression Analysis of RbohA and RbohD Genes in Brassica. Campestris under Different Treatments. Chin. J. Ecol. 2019;38:173. [Google Scholar]
- 56.Arthikala M.-K., Sánchez-López R., Nava N., Santana O., Cárdenas L., Quinto C. RbohB, a Phaseolus Vulgaris NADPH Oxidase Gene, Enhances Symbiosome Number, Bacteroid Size, and Nitrogen Fixation in Nodules and Impairs Mycorrhizal Colonization. New Phytol. 2014;202:886–900. doi: 10.1111/nph.12714. [DOI] [PubMed] [Google Scholar]
- 57.Montiel J., Arthikala M.-K., Quinto C. Phaseolus Vulgaris RbohB Functions in Lateral Root Development. Plant Signal. Behav. 2013;8:e22694. doi: 10.4161/psb.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X., Köster P., Schlücking K., Balcerowicz D., Hashimoto K., Kuchitsu K., Vissenberg K., Kudla J. CBL1-CIPK26-Mediated Phosphorylation Enhances Activity of the NADPH Oxidase RBOHC, but Is Dispensable for Root Hair Growth. FEBS Lett. 2018;592:2582–2593. doi: 10.1002/1873-3468.13187. [DOI] [PubMed] [Google Scholar]
- 59.Zhang T.G., Li Q.L., Diao Z.H., Li P., Wang J., Zheng S. Effects of Salt and Drought Stresses on Antioxidant System and RbohC and RbohF Genes Expression in Brassica Campestris. Ying Yong Sheng Tai Xue Bao= J. Appl. Ecol. 2019;30:969–978. doi: 10.13287/j.1001-9332.201903.017. [DOI] [PubMed] [Google Scholar]
- 60.Orman-Ligeza B., Parizot B., De Rycke R., Fernandez A., Himschoot E., Van Breusegem F., Bennett M.J., Périlleux C., Beeckman T., Draye X. RBOH-Mediated ROS Production Facilitates Lateral Root Emergence in Arabidopsis. Development. 2016;143:3328–3339. doi: 10.1242/dev.136465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He H., Yan J., Yu X., Liang Y., Fang L., Scheller H.V., Zhang A. The NADPH-Oxidase AtRbohI Plays a Positive Role in Drought-Stress Response in Arabidopsis Thaliana. Biochem. Biophys. Res. Commun. 2017;491:834–839. doi: 10.1016/j.bbrc.2017.05.131. [DOI] [PubMed] [Google Scholar]
- 62.Brentner L.B., Mukherji S.T., Merchie K.M., Yoon J.M., Schnoor J.L., Van Aken B. Expression of Glutathione S-Transferases in Poplar Trees (Populus Trichocarpa) Exposed to 2, 4, 6-Trinitrotoluene (TNT) Chemosphere. 2008;73:657–662. doi: 10.1016/j.chemosphere.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 63.Mezzari M.P., Walters K., Jelínkova M., Shih M.-C., Just C.L., Schnoor J.L. Gene Expression and Microscopic Analysis of Arabidopsis Exposed to Chloroacetanilide Herbicides and Explosive Compounds. A Phytoremediation Approach. Plant Physiol. 2005;138:858–869. doi: 10.1104/pp.104.056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L., Yi H. Differential Expression of Arabidopsis Defense-Related Genes in Response to Sulfur Dioxide. Chemosphere. 2012;87:718–724. doi: 10.1016/j.chemosphere.2011.12.064. [DOI] [PubMed] [Google Scholar]
- 65.Cicero L.L., Madesis P., Tsaftaris A., Piero A.R. Lo Tobacco Plants Over-Expressing the Sweet Orange Tau Glutathione Transferases (CsGSTUs) Acquire Tolerance to the Diphenyl Ether Herbicide Fluorodifen and to Salt and Drought Stresses. Phytochemistry. 2015;116:69–77. doi: 10.1016/j.phytochem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Yang G., Xu Z., Peng S., Sun Y., Jia C., Zhai M. In Planta Characterization of a Tau Class Glutathione S-Transferase Gene from Juglans Regia (JrGSTTau1) Involved in Chilling Tolerance. Plant Cell Rep. 2016;35:681–692. doi: 10.1007/s00299-015-1912-8. [DOI] [PubMed] [Google Scholar]
- 67.Dixon D.P., McEwen A.G., Lapthorn A.J., Edwards R. Forced Evolution of a Herbicide Detoxifying Glutathione Transferase. J. Biol. Chem. 2003;278:23930–23935. doi: 10.1074/jbc.M303620200. [DOI] [PubMed] [Google Scholar]
- 68.Sharma R., Sahoo A., Devendran R., Jain M. Over-Expression of a Rice Tau Class Glutathione s-Transferase Gene Improves Tolerance to Salinity and Oxidative Stresses in Arabidopsis. PLoS ONE. 2014;9:e92900. doi: 10.1371/journal.pone.0092900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu J., Zhang N., Liu Z., Liu S., Liu C., Lin J., Yang H., Li S., Yukawa Y. The AtGSTU7 Gene Influences Glutathione-Dependent Seed Germination under ABA and Osmotic Stress in Arabidopsis. Biochem. Biophys. Res. Commun. 2020;528:538–544. doi: 10.1016/j.bbrc.2020.05.153. [DOI] [PubMed] [Google Scholar]
- 70.Sarry J.-E., Kuhn L., Ducruix C., Lafaye A., Junot C., Hugouvieux V., Jourdain A., Bastien O., Fievet J.B., Vailhen D., et al. The Early Responses of Arabidopsis Thaliana Cells to Cadmium Exposure Explored by Protein and Metabolite Profiling Analyses. Proteomics. 2006;6:2180–2198. doi: 10.1002/pmic.200500543. [DOI] [PubMed] [Google Scholar]
- 71.Srivastava A.K., Ramaswamy N.K., Suprasanna P., D’souza S.F. Genome-Wide Analysis of Thiourea-Modulated Salinity Stress-Responsive Transcripts in Seeds of Brassica Juncea: Identification of Signalling and Effector Components of Stress Tolerance. Ann. Bot. 2010;106:663–674. doi: 10.1093/aob/mcq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan C., Lam H.-M. A Putative Lambda Class Glutathione S-Transferase Enhances Plant Survival under Salinity Stress. Plant Cell Physiol. 2014;55:570–579. doi: 10.1093/pcp/pct201. [DOI] [PubMed] [Google Scholar]
- 73.Mengiste T., Chen X., Salmeron J., Dietrich R. The Botrytis Susceptible1 Gene Encodes an R2R3MYB Transcription Factor Protein That Is Required for Biotic and Abiotic Stress Responses in Arabidopsis. Plant Cell. 2003;15:2551–2565. doi: 10.1105/tpc.014167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ding Y., Liu N., Virlouvet L., Riethoven J.-J., Fromm M., Avramova Z. Four Distinct Types of Dehydration Stress Memory Genes in Arabidopsis Thaliana. BMC Plant Biol. 2013;13:229. doi: 10.1186/1471-2229-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang P., Wang R., Ju Q., Li W., Tran L.-S.P., Xu J. The R2R3-MYB Transcription Factor MYB49 Regulates Cadmium Accumulation. Plant Physiol. 2019;180:529–542. doi: 10.1104/pp.18.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang Y.-K., Dubos C., Dodd I.C., Holroyd G.H., Hetherington A.M., Campbell M.M. AtMYB61, an R2R3-MYB Transcription Factor Controlling Stomatal Aperture in Arabidopsis Thaliana. Curr. Biol. 2005;15:1201–1206. doi: 10.1016/j.cub.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 77.Feng C., Andreasson E., Maslak A., Mock H.P., Mattsson O., Mundy J. Arabidopsis MYB68 in Development and Responses to Environmental Cues. Plant Sci. 2004;167:1099–1107. doi: 10.1016/j.plantsci.2004.06.014. [DOI] [Google Scholar]
- 78.Lotkowska M.E., Tohge T., Fernie A.R., Xue G.-P., Balazadeh S., Mueller-Roeber B. The Arabidopsis Transcription Factor MYB112 Promotes Anthocyanin Formation during Salinity and under High Light Stress. Plant Physiol. 2015;169:1862–1880. doi: 10.1104/pp.15.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu S., Yang R., Liu M., Zhang S., Yan K., Yang G., Huang J., Zheng C., Wu C. AtPLATZ2 Negatively Regulates Salt Tolerance in Arabidopsis Seedlings by Directly Suppressing the Expression of the CBL4/SOS3 and CBL10/SCaBP8 Genes. J. Exp. Bot. 2020;71:5589–5602. doi: 10.1093/jxb/eraa259. [DOI] [PubMed] [Google Scholar]
- 80.Horiuchi J., Badri D.V., Kimball B.A., Negre F., Dudareva N., Paschke M.W., Vivanco J.M. The Floral Volatile, Methyl Benzoate, from Snapdragon (Antirrhinum Majus) Triggers Phytotoxic Effects in Arabidopsis Thaliana. Planta. 2007;226:1–10. doi: 10.1007/s00425-006-0464-0. [DOI] [PubMed] [Google Scholar]
- 81.Ueda H., Ito T., Inoue R., Masuda Y., Nagashima Y., Kozuka T., Kusaba M. Genetic Interaction Among Phytochrome, Ethylene and Abscisic Acid Signaling During Dark-Induced Senescence in Arabidopsis Thaliana. Front. Plant Sci. 2020;11:564. doi: 10.3389/fpls.2020.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zang D., Wang J., Zhang X., Liu Z., Wang Y. Arabidopsis Heat Shock Transcription Factor HSFA7b Positively Mediates Salt Stress Tolerance by Binding to an E-Box-like Motif to Regulate Gene Expression. J. Exp. Bot. 2019;70:5355–5374. doi: 10.1093/jxb/erz261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen L., Song Y., Li S., Zhang L., Zou C., Yu D. The Role of WRKY Transcription Factors in Plant Abiotic Stresses. Biochim. Et Biophys. Acta (Bba)-Gene Regul. Mech. 2012;1819:120–128. doi: 10.1016/j.bbagrm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Song Y., Chen L., Zhang L., Yu D. Overexpression of OsWRKY72 Gene Interferes in the Abscisic Acid Signal and Auxin Transport Pathway of Arabidopsis. J. Biosci. 2010;35:459–471. doi: 10.1007/s12038-010-0051-1. [DOI] [PubMed] [Google Scholar]
- 85.Zhang J., Peng Y., Guo Z. Constitutive Expression of Pathogen-Inducible OsWRKY31 Enhances Disease Resistance and Affects Root Growth and Auxin Response in Transgenic Rice Plants. Cell Res. 2008;18:508–521. doi: 10.1038/cr.2007.104. [DOI] [PubMed] [Google Scholar]
- 86.Guo P., Li Z., Huang P., Li B., Fang S., Chu J., Guo H. A Tripartite Amplification Loop Involving the Transcription Factor WRKY75, Salicylic Acid, and Reactive Oxygen Species Accelerates Leaf Senescence. Plant Cell. 2017;29:2854–2870. doi: 10.1105/tpc.17.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Y., Liang T., Zhang L., Shao K., Gu X., Shang R., Shi N., Li X., Zhang P., Liu H. UVR8 Interacts with WRKY36 to Regulate HY5 Transcription and Hypocotyl Elongation in Arabidopsis. Nat. Plants. 2018;4:98–107. doi: 10.1038/s41477-017-0099-0. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L., Qin L.-N., Zeng Z.-R., Wu C.-Z., Gong Y.-Y., Liu L.-H., Cao F.-Q. Molecular Identification of a Root Apical Cell-Specific and Stress-Responsive Enhancer from an Arabidopsis Enhancer Trap Line. Plant Methods. 2019;15:1–11. doi: 10.1186/s13007-019-0393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiu Y., Yu D. Over-Expression of the Stress-Induced OsWRKY45 Enhances Disease Resistance and Drought Tolerance in Arabidopsis. Environ. Exp. Bot. 2009;65:35–47. doi: 10.1016/j.envexpbot.2008.07.002. [DOI] [Google Scholar]
- 90.Coego A., Brizuela E., Castillejo P., Ru\’\iz S., Koncz C., del Pozo J.C., Pineiro M., Jarillo J.A., Paz-Ares J., León J., et al. The TRANSPLANTA Collection of A Rabidopsis Lines: A Resource for Functional Analysis of Transcription Factors Based on Their Conditional Overexpression. Plant J. 2014;77:944–953. doi: 10.1111/tpj.12443. [DOI] [PubMed] [Google Scholar]
- 91.Kim H.J., Hong S.H., Kim Y.W., Lee I.H., Jun J.H., Phee B.-K., Rupak T., Jeong H., Lee Y., Hong B.S., et al. Gene Regulatory Cascade of Senescence-Associated NAC Transcription Factors Activated by Ethylene-Insensitive2-Mediated Leaf Senescence Signalling in Arabidopsis. J. Exp. Bot. 2014;65:4023–4036. doi: 10.1093/jxb/eru112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joseph M.P., Papdi C., Kozma-Bognár L., Nagy I., López-Carbonell M., Rigó G., Koncz C., Szabados L. The Arabidopsis ZINC FINGER PROTEIN3 Interferes with Abscisic Acid and Light Signaling in Seed Germination and Plant Development. Plant Physiol. 2014;165:1203–1220. doi: 10.1104/pp.113.234294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kobayashi Y., Ohyama Y., Kobayashi Y., Ito H., Iuchi S., Fujita M., Zhao C.-R., Tanveer T., Ganesan M., Kobayashi M., et al. STOP2 Activates Transcription of Several Genes for Al-and Low PH-Tolerance That Are Regulated by STOP1 in Arabidopsis. Mol. Plant. 2014;7:311–322. doi: 10.1093/mp/sst116. [DOI] [PubMed] [Google Scholar]
- 94.Huang L., Jiang Q., Wu J., An L., Zhou Z., Wong C., Wu M., Yu H., Gan Y. Zinc Finger Protein 5 (ZFP5) Associates with Ethylene Signaling to Regulate the Phosphate and Potassium Deficiency-Induced Root Hair Development in Arabidopsis. Plant Mol. Biol. 2020;102:143–158. doi: 10.1007/s11103-019-00937-4. [DOI] [PubMed] [Google Scholar]
- 95.Vergnolle C., Vaultier M.-N., Taconnat L., Renou J.-P., Kader J.-C., Zachowski A., Ruelland E. The Cold-Induced Early Activation of Phospholipase C and D Pathways Determines the Response of Two Distinct Clusters of Genes in Arabidopsis Cell Suspensions. Plant Physiol. 2005;139:1217–1233. doi: 10.1104/pp.105.068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang P., Chung M.-S., Ju H.-W., Na H.-S., Lee D.J., Cheong H.-S., Kim C.S. Physiological Characterization of the Arabidopsis Thaliana Oxidation-Related Zinc Finger 1, a Plasma Membrane Protein Involved in Oxidative Stress. J. Plant Res. 2011;124:699–705. doi: 10.1007/s10265-010-0397-3. [DOI] [PubMed] [Google Scholar]
- 97.Lin K.-F., Tsai M.-Y., Lu C.-A., Wu S.-J., Yeh C.-H. The Roles of Arabidopsis HSFA2, HSFA4a, and HSFA7a in the Heat Shock Response and Cytosolic Protein Response. Bot. Stud. 2018;59:15. doi: 10.1186/s40529-018-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lariguet P., Ranocha P., De Meyer M., Barbier O., Penel C., Dunand C. Identification of a Hydrogen Peroxide Signalling Pathway in the Control of Light-Dependent Germination in Arabidopsis. Planta. 2013;238:381–395. doi: 10.1007/s00425-013-1901-5. [DOI] [PubMed] [Google Scholar]
- 99.Cui L.-G., Shan J.-X., Shi M., Gao J.-P., Lin H.-X. DCA1 Acts as a Transcriptional Co-Activator of DST and Contributes to Drought and Salt Tolerance in Rice. Plos Genet. 2015;11:e1005617. doi: 10.1371/journal.pgen.1005617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin T., Sun Y., Zhao R., Shan Z., Gai J., Li Y. Overexpression of Peroxidase Gene GsPRX9 Confers Salt Tolerance in Soybean. Int. J. Mol. Sci. 2019;20:3745. doi: 10.3390/ijms20153745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar S., Jaggi M., Sinha A.K. Ectopic Overexpression of Vacuolar and Apoplastic Catharanthus Roseus Peroxidases Confers Differential Tolerance to Salt and Dehydration Stress in Transgenic Tobacco. Protoplasma. 2012;249:423–432. doi: 10.1007/s00709-011-0294-1. [DOI] [PubMed] [Google Scholar]
- 102.Gullner G., Komives T., Király L., Schröder P. Glutathione S-Transferase Enzymes in Plant-Pathogen Interactions. Front. Plant Sci. 2018;9:1836. doi: 10.3389/fpls.2018.01836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ezaki B., Suzuki M., Motoda H., Kawamura M., Nakashima S., Matsumoto H. Mechanism of Gene Expression of Arabidopsis Glutathione S-Transferase, AtGST1, and AtGST11 in Response to Aluminum Stress. Plant Physiol. 2004;134:1672–1682. doi: 10.1104/pp.103.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roxas V.P., Lodhi S.A., Garrett D.K., Mahan J.R., Allen R.D. Stress Tolerance in Transgenic Tobacco Seedlings That Overexpress Glutathione S-Transferase/Glutathione Peroxidase. Plant Cell Physiol. 2000;41:1229–1234. doi: 10.1093/pcp/pcd051. [DOI] [PubMed] [Google Scholar]
- 105.Kunieda T., Fujiwara T., Amano T., Shioi Y. Molecular Cloning and Characterization of a Senescence-Induced Tau-Class Glutathione S-Transferase from Barley Leaves. Plant Cell Physiol. 2005;46:1540–1548. doi: 10.1093/pcp/pci167. [DOI] [PubMed] [Google Scholar]
- 106.Yousefirad S., Soltanloo H., Ramezanpour S.S., Zaynali Nezhad K., Shariati V. The RNA-Seq Transcriptomic Analysis Reveals Genes Mediating Salt Tolerance through Rapid Triggering of Ion Transporters in a Mutant Barley. PLoS ONE. 2020;15:e0229513. doi: 10.1371/journal.pone.0229513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Passardi F., Cosio C., Penel C., Dunand C. Peroxidases Have More Functions than a Swiss Army Knife. Plant Cell Rep. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- 108.Yan J., Su P., Li W., Xiao G., Zhao Y., Ma X., Wang H., Nevo E., Kong L. Genome-Wide and Evolutionary Analysis of the Class III Peroxidase Gene Family in Wheat and Aegilops Tauschii Reveals That Some Members Are Involved in Stress Responses. Bmc Genom. 2019;20:666. doi: 10.1186/s12864-019-6006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liszkay A., Kenk B., Schopfer P. Evidence for the Involvement of Cell Wall Peroxidase in the Generation of Hydroxyl Radicals Mediating Extension Growth. Planta. 2003;217:658–667. doi: 10.1007/s00425-003-1028-1. [DOI] [PubMed] [Google Scholar]
- 110.Passardi F., Penel C., Dunand C. Performing the Paradoxical: How Plant Peroxidases Modify the Cell Wall. Trends Plant Sci. 2004;9:534–540. doi: 10.1016/j.tplants.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data 2010. [(accessed on 1 February 2021)]; Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 112.Kim D., Paggi J.M., Park C., Bennett C., Salzberg S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kovaka S., Zimin A.V., Pertea G.M., Razaghi R., Salzberg S.L., Pertea M. Transcriptome Assembly from Long-Read RNA-Seq Alignments with StringTie2. Genome Biol. 2019;20:1–13. doi: 10.1186/s13059-019-1910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liao Y., Smyth G.K., Shi W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 115.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 116.Akers M.D. Exploring, Analysing and Interpeting Data with Minitab 18. Compass Publishing; Seoul, Korea: 2018. [Google Scholar]
- 117.Das K., Roychoudhury A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014;2:1–13. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available in a publicly accessible repository.