Abstract
Simple Summary
Malaria is a complex disease in part due to multiple vectors having different biological characteristics. In India, there are six primary vectors of malaria viz., Anopheles culicifacies, An. fluviatitlis, An. stephensi, An. subpictus, An. Minimus, and An. epiroticus. All these vectors have different ecological and seasonal distributions, transmission potential, and insecticide susceptibility status. In addition, except An. stephensi, all the five vectors comprise species complexes having sibling species which again differ in characteristics. Therefore, it is imperative to know the characteristics of the local vector population when it comes to planning control strategies. We carried out a study in tribal areas of Chhattisgarh state to investigate the seasonal and ecotype-wise distribution, breeding habits, sibling species composition, insecticide susceptibility, and role in the transmission of the local vector population. A high diversity of species was observed with around 16 species of Anopheles. An. culicifacies was the most dominant species and also was found to play a role in malaria transmission. The species was found to be resistant to dichlorodiphenyltrichloroethane (DDT) and Malathion, while an increasing trend of pyrethroid resistance was observed at some sites. Overall, our findings provide a picture of the characteristics of the local vector population in malaria-endemic regions.
Abstract
A study was undertaken in the villages of Korea and Bastar district (Chhattisgarh) during the years 2012–2015 to investigate the bionomics of malaria vectors and the prevalence of their sibling species complexes. Entomological surveys carried out every month included indoor resting collections, pyrethrum spray catches, light trap catches, and insecticide susceptibility status of Anopheles culicifacies using World Health Organization (WHO) methods. Anopheles culicifacies and Anopheles fluviatilis species were assayed by polymerase chain reaction (PCR) for the detection of malaria parasite, and sibling species were identified using PCR and DNA sequencing. A total of 13,186 samples of Anopheles comprising 15 species from Bastar and 16 from Korea were collected. An. Culicifacies was recorded as the most dominant species and also the only active vector at both sites. This species was found to be resistant to dichlorodiphenyltrichloroethane (DDT) and Malathion, showing signs of emerging resistance against pyrethroids. Among the sibling species of An. culicifacies, the group BCE was found in maximum numbers, while sibling species T of the An. fluviatilis was recorded to be dominant among its complex. The study provides a comprehensive view of the vector bionomics in the highly malarious regions of India that may have importance in developing vector control strategies.
Keywords: bionomics, sibling species, CDC light traps, zoophilic, vectorial capacity
1. Introduction
With malaria elimination on the agenda, there is a fresh momentum in control interventions throughout the Indian sub-continent. The renewed efforts target providing better surveillance and case management systems, improved and appropriate diagnosis, universal coverage of the population at risk with strengthened vector control interventions, and effective antimalarial drugs. However, this ambitious goal is faced with numerous challenges. Looking at the malaria incidence figures, the country accounted for approximately 0.56 million cases [1] and 77 deaths [2] in the year 2019. According to World Health Organization (WHO) [1], India, along with 29 Sub- Saharan African countries, contributes about 95% of the global malaria cases. This burden is contributed to by varied factors, including diverse topography, multiple parasite strains, and numerous species’ disease vectors. An intensified control effort designed to tune up with this diversity is urgently needed. This requires thorough information on the micro epidemiology of the disease, its vectors, and local factors influencing the overall prevalence.
In India, the majority of malaria morbidity and mortality are contributed to by eight states [3]. Chhattisgarh (CG) state stands second in the list with approximately 18% of the malaria burden and the highest number of deaths [2]. Transmission of malaria in this region is mainly due to two vectors, Anopheles culicifacies and An. fluviatilis. These species, as well as their sibling species differ in distribution, biological characteristics, vectorial capacities, and susceptibility to insecticides [4,5]. For example, An. culicifacies sibling species B is a poor vector or a non-vector, while sibling species A, C, D, and E are considered as vectors. Sibling species E is highly anthropophilic, whereas the other four are primarily zoophilic. Of the four-sibling species of the An. fluviatilis complex, species S is an efficient vector, and species T and U are poor vectors, while the role of the V form in malaria transmission remains largely unclear [6]. Although these sibling species differ widely in their physiological and behavioral characteristics, morphologically, they are indistinguishable [7]. Furthermore, reports suggest the change in vector behavior at certain places and the ecological succession of species. An. fluviatilis T was found to be playing a role in malaria transmission in Central India. Similarly, An. culicifacies, a known indoor resting species, was found parasite positive in outdoor collections. Likewise, a shift in resting preference of An. fluviatilis from outdoors to indoors was also observed [4]. These changes are of concern since a sustainable control program demands a specific and targeted approach. Hence, a comprehensive assessment of vector ecology and disease transmission is a crucial consideration in malaria control and elimination. Keeping this in view, we studied the bionomics of malaria vectors and their role in malaria transmission in south and north Chhattisgarh, regions that contribute the majority of the state’s malaria burden [8]. The results provide baseline information on vector distribution, their seasonal and ecotype-wise relative abundance, host preference, resting and breeding habits, role in malaria transmission as well as susceptibility status to insecticides in use.
2. Material and Methods
2.1. Study Sites and Population
The study was conducted in two districts, Bastar and Korea of Chhattisgarh state. Bastar is situated in the southern part of the state, while Korea lies in the northwestern part. Both the districts have dense forest cover, and the climate is tropical, with rainfall in the months of June to September. The seasons are characterized as Spring (February-March), Summer (April-June), Monsoon (July-September), Post Monsoon (October-November), and Winter (December-January) [9]. The district Bastar receives higher average annual rainfall (1323 mm) than Korea (1225 mm) [10].
The districts have undulating terrain. The river Indrawati flows through Bastar, whereas Korea is drained by the Hasdeo, Tej, Gopad, and Gopri rivers. Numerous streams originating from these rivers bifurcate the districts, and their tributaries provide permanent breeding sites for mosquitoes. District Korea comprises vast masses of forests and hills.
The population is mainly tribal. In the Korea district, the tribal population constitutes 46.2% of the total population [11], while in Bastar [12], this proportion is high as 70%. A large number of tribes still live in deep forests that either remains inaccessible or are risky to approach. Bastar and Korea are violence-prone areas and are subjected to Naxalite activities [13]. The economy is mainly agriculture and forest-based. Most of the population work as daily wage laborers in road construction or other casual work. Houses are of the kutcha type with thatched roofs, incomplete walls, poor or no ventilation, and cattle sheds near or within houses. People spend most of their time outside the house and sleep outdoors or in the verandah (walled from three sides and open from one side). Vector control activities in the study area include spraying Alphacypermethrin inside the human dwelling.
2.2. Entomological Collections
Monthly longitudinal entomological surveys were carried out for a period of three years (2012–2015) in the study districts Bastar (19°15′ N, 81°40′ E) and Korea (22nd Korea (it, 81st Korea (it). Two Community Health Centers (CHCs) from each district and 4 villages in each CHC were selected in different geographical ecotypes, i.e., forest, foothill, and plains. Villages located inside deep forests, in foothills (area surrounded by hills or mountains from at least 3 sides), and plains (without forest or hills) were selected to study the difference in vector bionomics in different ecotypes. Villages in the forest ecotype have scattered houses of kutcha type with poor ventilation. Accessibility to roads is very poor, and public transport facilities are also not available. In contrast, villages located in foothill and plain ecotypes have clustered houses and have better accessibility to road and transportation.
For indoor resting collections, female Anopheles mosquito were collected from 2 fixed and 2 randomly selected human dwelling (HD) and cattle sheds (CS) located in different parts of the village using flashlight and mouth aspirators during early morning hours (0600 to 0800) for 15 min in each place as per standard WHO technique [14]. Pyrethrum spray sheet collections (PSSC) were made once a month from human dwellings (HD) randomly selected other than those selected for indoor resting collection during daytime (0600–0010) after knockdown on white sheets by space spraying of a pyrethrum solution as per WHO techniques. Center for disease control (CDC) light traps were used for indoor and outdoor collections from dawn to dusk from 1800 to 0600. Traps were hung at a fixed height of 5.5 feet indoors inside houses and outdoors near the houses and were manually emptied at hourly intervals. The collected adult female mosquitoes were assigned unique codes and stored in dried silica.
Monthly larval collections were made at all potential breeding sites, including streams, pools, tanks, irrigation fields, and ponds 500 meters around the village and inside the study villages using the standard dip technique (WHO). The larvae were reared in the lab for morphological identification. The abdomen of the blood-fed mosquito was crushed on Whatman filter paper for blood meal analysis. Insecticide susceptibility status of the field caught indoor resting adult Anopheles culicifacies mosquitoes was determined against diagnostic dosages of dichlorodiphenyltrichloroethane (DDT) 4%, Malathion 5%, Deltamethrin 0.05%, and Alphacypermethrin 0.05% following the WHO standard procedure [15]. Eight replicates, each with 15 mosquitoes, were used for each insecticide (total 120 mosquitoes for each insecticide). A tube consisting of oil-impregnated paper served as the test control. Mosquitoes were exposed to insecticides for one hour and then transferred to holding tubes for 24 h. The number of dead and alive mosquitoes was counted. The observed and corrected mortality was then calculated as
2.3. Laboratory Processing of Mosquitoes and Detection of Malaria Parasites
Collected mosquitoes were identified up to species level based on the morphological keys [16], and DNA was isolated using methods described by Coen et al. 1984 [17]. The samples were tested for the presence of malaria parasites by polymerase chain reaction (PCR) targeting the 18S rDNA region of Plasmodium. The primers and PCR conditions were as per the methodology described by Snounou et al. [18].
2.4. Identification of Sibling Species by Molecular Methods
For identification of An. culicifacies sibling species complex, D3 and ITS2 regions of ribosomal DNA were amplified and sequenced using methods described by Singh et al. 2004 [19] and Manonmani et al. 2005 [20]. Identification was also made using the methods of Goswami et al. 2006 [21] targeting the COII region of mitochondrial DNA. An. fluviatilis sibling species complex was identified using allele-specific PCR described by Singh et al. 2004 [22]. The primers and conditions of PCR were the same as described in [22].
2.5. Blood Meal Analysis for Identification of Host Preference of the Vectors
Host preference of mosquitoes was determined by gel diffusion assay against human and bovine anti-sera using the collected blood meals of Anophelines [23]. Briefly, the dried blood spots from the vector abdomen were cut and dissolved in normal saline (0.7 g/100 mL distilled water) for 4.5 h. Fifty milliliters of solution 1v(50% barbiturate buffer pH 8.6, 49.8% distilled water, and 0.2% agarose) was added to 50 mL of solution 2 (0.9% agarose in barbiturate buffer solution). Three milliliters of this mixed solution was spread on micro slides and allowed to solidify. Human and bovine test antisera and mosquito blood dissolved in normal saline were pipetted into the wells in the gel slides. A precipitation line around the wells, formed when serum came into contact with antiserum, indicated a positive result.
2.6. Data Analysis
The data were double key-entered in MS Excel 2007 (Microsoft Corporation, Redmond, Washington, DC, USA). Categorical data are presented as percentages (frequency counts) and compared using Pearson’s chi-square test. Continuous data are represented as mean (± standard deviation) and compared using ANOVA (one-way analysis of variance) with Bonferroni adjustment for multiple comparisons. Log transformed data were used in the ANOVA. Statistical analysis was done using STATA software version 8.0 (StataCorp, LP, TX, USA). Per man-hour density (PMHD) for indoor resting collections was calculated by dividing the total mosquito collected by the number of man-hours spent in the collection. The significance of differences between PMHD of two sample sets was calculated using a t-test.
3. Results:
3.1. Species Diversity, Seasonal, and Ecotype-Wise Abundance of Anophelines in the Study Sites
A total of 997 (524 h in Bastar and 473 in Korea) man-hours were spent on indoor resting mosquito collections at both the study sites. The Anopheline fauna consisted of 15 species in Bastar and 16 in Korea, comprising a total of 13,186 mosquitoes collected from both sites. The average per man-hour density of all the collected Anopheles species in indoor collections (human dwellings and cattle sheds) was 12.49. (Table 1). The diversity of Anopheles species was almost the same in both the sites, with the difference that An. stephensi and An. jamesi were collected from Korea and An. varuna from Bastar only. Species collected from both the sites (Table 1) included An. culicifacies, An. fluviatilis, An. subpictus, An. vagus, An. annularis, An. barbirostris, An. splendidus, An. Tessalatus, An. aconitus, An. nigerimus, An. pallidus, and An. theobaldi. An. culicifacies comprised the majority of the collections (38% in Bastar and 46.7% in Korea), having densities as 4.7 (95% CI 3.5–5.7) and 6.2 (95% CI 5.2–7.2) in Bastar and Korea, respectively. The average per man-hour (PMHD) density of An. culicifacies was significantly higher in Korea as compared to Bastar (p < 0.01). An. subpictus (27%) was the second most abundant species in both sites. An. fluviatilis was observed in low densities with 0.34 (95% CI 0.14–0.56) and 1.7 (95% CI 1.25–2.15) of PMHD in Bastar and Korea, respectively.
Table 1.
Total number of Anopheles mosquito collected from study sites using different collection methods (Indoor resting, light trap, and pyrethrum spray sheet collections).
| Species | Bastar | Korea | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoor Resting (HD) | Indoor Resting (CS) | PMHD IRC (HD+ CS) | LT | PSSC | Indoor Resting (HD) | Indoor Resting (CS) | PMHD IRC (HD +CS) | LT | PSSC | ||
| An. culicifacies | 130 | 2342 | 4.7 | 14 | 79 | 126 | 2798 | 6.2 | 66 | 113 | 5668 |
| An. fluviatilis | 1 | 113 | 0.2 | 0 | 0 | 16 | 508 | 1.1 | 15 | 8 | 661 |
| An. subpictus | 210 | 2065 | 4.3 | 23 | 58 | 70 | 1212 | 2.7 | 101 | 62 | 3801 |
| An. jeyporiensis | 0 | 3 | 0.006 | 0 | 0 | 22 | 568 | 1.25 | 1 | 2 | 596 |
| An. varuna | 56 | 303 | 0.68 | 17 | 30 | 19 | 54 | 0.15 | 6 | 29 | 514 |
| An. annularis | 13 | 337 | 0.67 | 17 | 2 | 21 | 520 | 1.14 | 43 | 4 | 957 |
| Other Anophelines | 27 | 558 | 1.11 | 15 | 4 | 7 | 360 | 0.77 | 17 | 1 | 989 |
| Total | 437 | 5721 | 11.7 | 86 | 173 | 281 | 6020 | 13.3 | 249 | 219 | 13186 |
HD: Human dwellings; CS: Cattle sheds; LT: Light trap; PSSC: Pyrethrum Spray sheet collection, PMHD: Per man-hour density, IRC: Indoor Resting Collection.
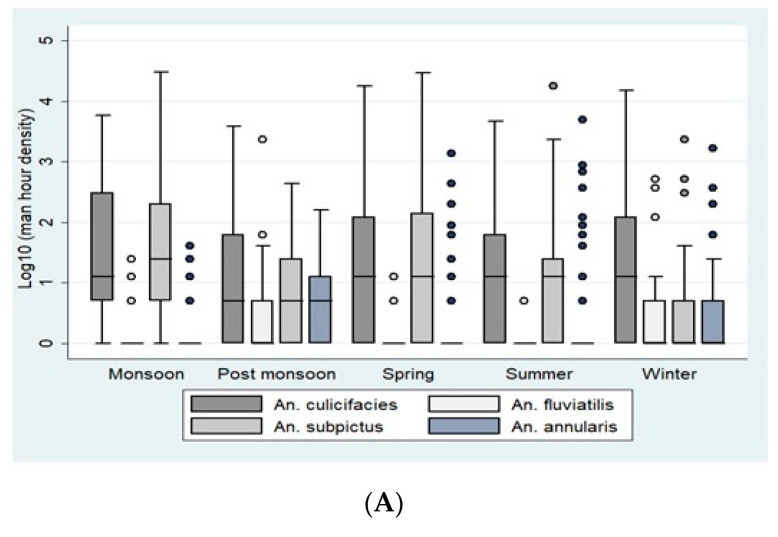
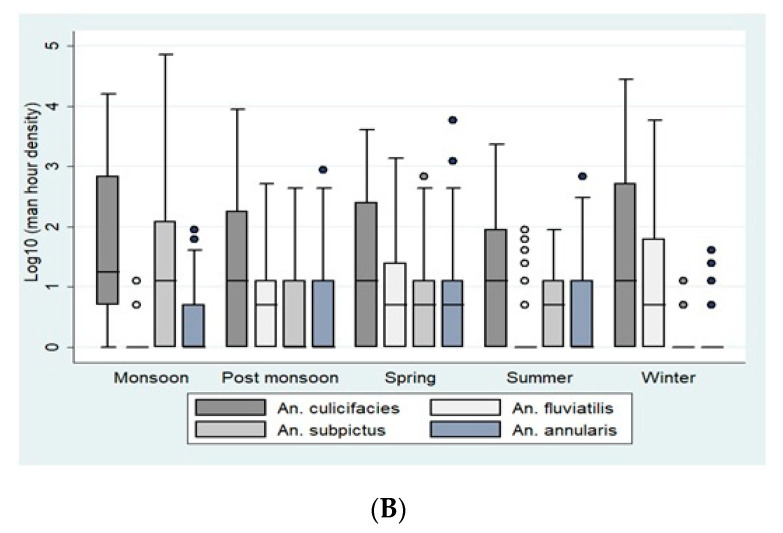
A high abundance of Anopheles mosquitoes was observed throughout the year showing seasonal peaks and dips (Figure 1A,B). The periods of monsoon and spring showed the highest densities of Anopheline at both sites, with the largest peak observed in the monsoon. The densities of An. culicifacies also peaked during monsoon with a PMHD of 8.83 (95% CI 6.7–10.9) in Bastar and 12.67 (95% CI 9.25–16.08) in Korea. However, the species was recorded in large numbers throughout the year. An. fluviatilis densities were highest during the winter season, while its abundance was also recorded during monsoon in Bastar and during spring in Korea. In Bastar, the density of An. fluviatilis during winter was 3.12 (95% CI 1.08–5.1), and in Korea, it was 6.03 (95% CI 3.1–8.8).
Figure 1.
(A). Season-wise average per man-hour density (indoor resting collections) in Bastar. (B). Season-wise average per man-hour density (indoor resting collections) in Korea district. The boxes in the plots correspond to the log-transformed values of man-hour densities of An. culicifacies, An. fluviatilis, An. subpictus, and An. annularis across different seasons. The densities are represented in the boxes as Quartile 1, median, and Quartile 3. The whiskers represent the highest and the lowest densities.
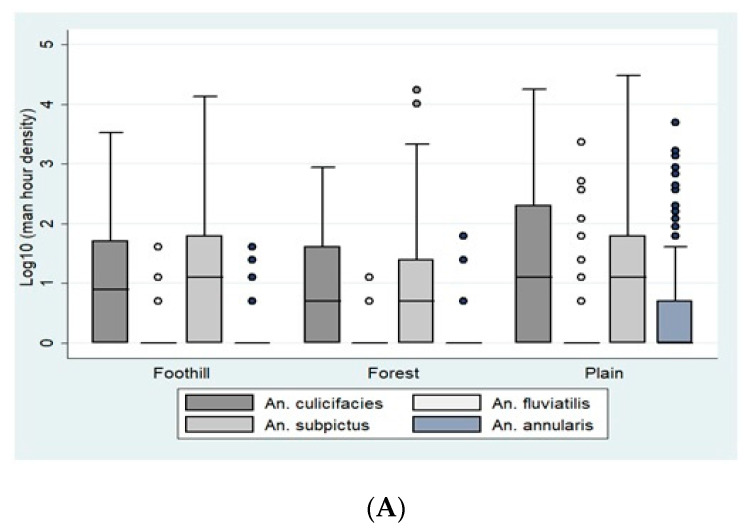
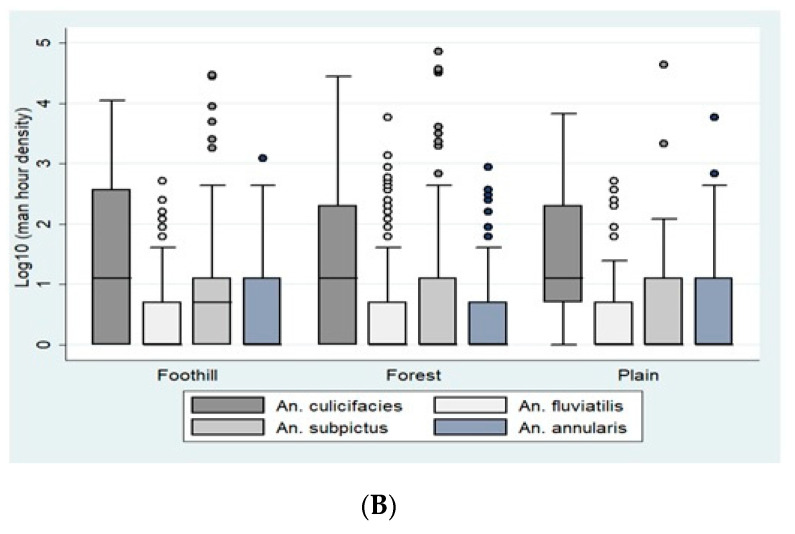
Ecotype-wise analysis of Anopheline distribution in Bastar showed that An. culicifacies was most abundant in the plains (85%) with a mean density of 9.01 (95% CI 7.5–10.4), while its densities and the density of other Anopheline was low in forest and foothill ecotypes. Maximum numbers of An. fluviatilis in Bastar were recorded from the plains (Mean 3.42; 95% CI 1.2–5.5). In the Korea district, the forest ecotype had the maximum density of mosquitoes with An. culicifacies being the most abundant. The average PMHD of An. culicifacies in the forest ecotype of Korea was 10.2 (95% CI 8.01–12.39), while in the plain, it was 7.81 (95% CI 5.9–9.2) and 9.65 (95% CI 7.6–11.6) in the foothill ecotype. The density of An. fluviatilis in this site was higher in forest ecotypes (Mean 5.05; 95% CI 3.3–6.7), comprising 54% of the collections (Figure 2A,B).
Figure 2.
(A). Ecotype-wise per man-hour density (Indoor Resting Collection) in district Bastar. (B). Ecotype-wise per man-hour density (Indoor Resting Collection) in district Korea. The boxes in the plots correspond to the log-transformed values of man-hour densities of An. Culicifacies, An. Fluviatilis, An. Subpictus, and An. annularis across different ecotypes, including foothill, forest, and plain. The densities are represented in the boxes as Quartile 1, median, and Quartile 3. The whiskers represent the highest and the lowest densities
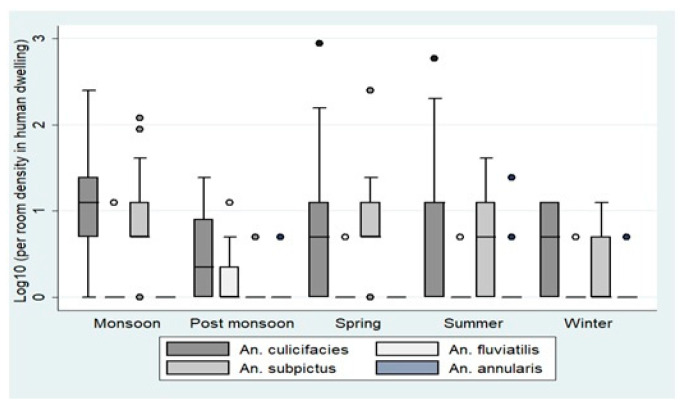
Pyrethrum spray sheet collections conducted in houses at each site recorded 10 Anopheles species from Bastar and 8 from Korea. An. culicifacies was the most abundant species at both sites. An. subpictus, An. Vagus, and An. annularis were also collected in large proportions. Other species observed, though in few numbers, included An. barbirostris and An. splendidus, while An. tessalatus and An. aconitus were recorded from Bastar and An. jeyporiensis from Korea only. The month-wise distribution of An. culicifacies showed peaks during March-April and July-August. An. fluviatilis was mainly found from November to March (Figure 3).
Figure 3.
Seasonal pyrethrum spray catches in human dwellings in districts Bastar and Korea. The boxes in the plots correspond to the log-transformed values of per room density in human dwellings across different seasons. The densities are represented as Quartile 1, median, and Quartile 3. The whiskers represent the highest and the lowest densities.
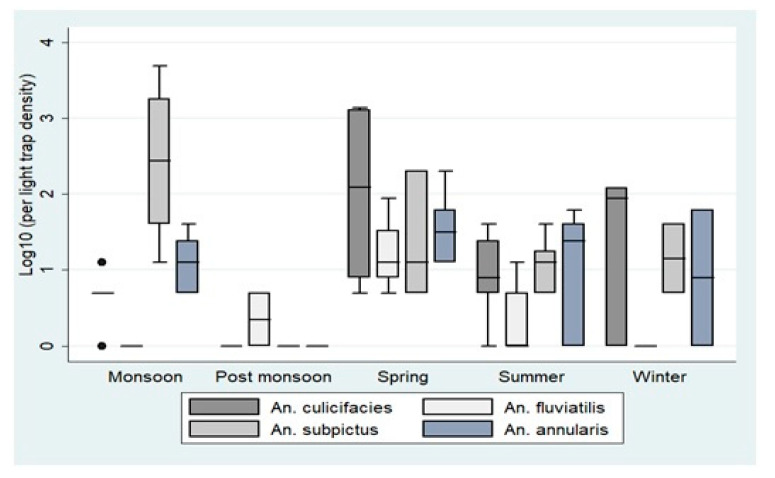
In light trap catches, 12 nights in Bastar and 17 nights in Korea were spent for collections; 71 CDC light traps in the former and 203 in the later site (Figure 4). In Bastar An. culicifacies, An. subpictus, An. Vagus, and An. annularis were recorded, while in Korea, in addition, An. fluviatilis and An. jeyporiensis were also caught. An. subpictus was the dominant species in night catches at both sites. An. culicifacies comprised 19.7% of collections in Bastar and 31.5% in the Korea district. The proportion of An. fluviatilis was only 7%. Season-wise distribution studies showed the maximum abundance of Anopheline in the monsoon, while the highest night catches of An. culicifacies and An. fluviatilis were recorded in spring.
Figure 4.
Seasonal light trap catches in districts Bastar and Korea. The boxes in the plots correspond to the log-transformed values per light trap catch density across different seasons. The densities are represented in the boxes as Quartile 1, median, and Quartile 3. The whiskers represent the highest and the lowest densities.
3.2. Vector Incrimination
A total of 5668 samples of An. culicifacies (3103 from Korea and 2565 from Bastar) and 661 An. fluviatilis (547 from Korea and 114 from Bastar) were tested for the presence of malaria parasites. A total of five An. culicifacies mosquitoes were found infected, three with Plasmodium falciparum (Pf) and two with Plasmodium vivax (Pv). Both the Pv positive mosquito and two of the Pf positive were collected from Korea. Of these, one Pf positive mosquito was collected in a pyrethrum spray sheet, while the rest of the three from cattle sheds. One notable observation was that all the parasite-positive mosquitoes from Korea were collected in May in two successive years. Only one Pf positive mosquito was from Bastar collected from a cattle shed in the month of March. None of the An. fluviatilis was positive for malaria infection (Table 2). All the parasite-infected An. culicifacies belonged to the “BCE type” sibling species group.
Table 2.
Ecotype and season-wise Anopheles mosquito diagnosed for malaria parasites (Plasmodium falciparum and P. vivax) by polymerase chain reaction (PCR) method.
| Site | Ecotype | Season | An. culicifacies (no. of Mosquito Positive/no. of Mosquito Tested) | An. fluviatilis no. of Mosquito Positive/no. of Mosquito Tested) | Total no. of Mosquito Positive/no. of Mosquito Tested) |
|---|---|---|---|---|---|
| Bastar | Forest | Summer | 0/39 | 0 | 0/39 |
| Monsoon | 0/50 | 0 | 0/50 | ||
| Post Monsoon | 0/18 | 0/3 | 0/21 | ||
| Winter | 0/16 | 0/3 | 0/19 | ||
| Spring | 0/58 | 0 | 0/58 | ||
| Foothill | Summer | 0/27 | 0 | 0/27 | |
| Monsoon | 0/102 | 0/3 | 0/105 | ||
| Post Monsoon | 0/30 | 0/5 | 0/35 | ||
| Winter | 0/14 | 0/2 | 0/16 | ||
| Spring | 0/39 | 0/2 | 0/41 | ||
| Plain | Summer | 0/511 | 0/2 | 0/513 | |
| Monsoon | 0/528 | 0/3 | 0/531 | ||
| Post Monsoon | 0/228 | 0/41 | 0/269 | ||
| Winter | 0/458 | 0/45 | 0/503 | ||
| Spring | 1/447 | 0/5 | 1/452 | ||
| Total (Bastar) | 1/2565 | 0/114 | 1/2679 | ||
| Korea | Forest | Summer | 2/227 | 0/21 | 2/248 |
| Monsoon | 0/485 | 0/4 | 0/489 | ||
| Post Monsoon | 0/185 | 0/50 | 0/235 | ||
| Winter | 0/238 | 0/115 | 0/353 | ||
| Spring | 0/362 | 0/108 | 0/470 | ||
| Foothill | Summer | 2/223 | 0/14 | 2/237 | |
| Monsoon | 1/298 | 0/2 | 0/300 | ||
| Post Monsoon | 0/141 | 0/35 | 0/176 | ||
| Winter | 0/117 | 0/43 | 0/160 | ||
| Spring | 0/186 | 0/44 | 0/230 | ||
| Plain | Summer | 0/126 | 0/4 | 0/130 | |
| Monsoon | 0/142 | 0 | 0/142 | ||
| Post Monsoon | 0/119 | 0/15 | 0/134 | ||
| Winter | 0/129 | 0/23 | 0/152 | ||
| Spring | 0/125 | 0/69 | 0/194 | ||
| Total (Korea) | 4/3103 | 0/547 | 4/3650 | ||
| Total (Korea and Bastar) | 5/5668 | 0/661 | 5/6329 | ||
3.3. Sibling Species Distribution in the Study Sites
Sibling species identification was made by PCR and DNA sequencing targeting D3, Internal transcribed spacer (ITS2), and Cytochrome c oxidase subunit 2 (COII) regions. However, we could not identify all five-sibling species of An. culicifacies due to the lack of an accurate molecular identification technique. The species was identified into two groups only: A/D and B/C/E. Of 600 samples tested, group B/C/E was the most abundant, comprising 87%. Analysis of An. fluviatilis sibling species complex by 28S rDNA allele-specific PCR revealed that sibling species T was dominant (70%) at both sites. Sibling species S comprised only 4% of the total An. fluviatilis tested from both Korea and Bastar.
3.4. Host Preference of An. culicifacies and An. fluviatilis
Out of 223 An. culicifacies and 75 An. Fluviatilis blood-fed mosquito tested for host blood meal preference, seven were found having human blood, while remaining 291 were positive with the bovine serum. In Bastar district, five An. culicifacies mosquitoes and none of the An. fluviatilis were human blood positive. While in the Korea district, one sample of both the vectors was found positive for human blood.
3.5. Anopheline Fauna Composition of Breeding Sites
A total of 777 Anopheles mosquito larvae were collected, representing nine species in Bastar and 12 species in the Korea district from potential breeding sites, such as ditches, ponds, river pool, running water, seepage water, stream, pool, sandy pit, rice field, rocky pit, and rock stream. An. culicifacies was the most abundant species representing 36% and 45.1% of total collections from Korea and Bastar, respectively. This species was found breeding mainly in streams, seepage water, and rock pits and showed peaks of relative abundance in March (34.8%) and September (13.8%); large numbers of larvae were also obtained in June. In addition, An. culicifacies, An. subpictus was found in abundance (26% in Bastar and 17.5% in Korea). An. fluviatilis occurred in low densities, accounting for 3.1% in Korea and 1.67% in Bastar, and breeding mainly in seepage water, rock pits, and streams from October to April.
3.6. Status of Susceptibility to Insecticides in An. culicifacies
Insecticide susceptibility status of An. culicifacies was determined in districts Bastar and Korea for two and three successive years, respectively (Table 3). The species was resistant against DDT and Malathion in both the districts with corrected mortality of 6.6 ± 2.8 and 65.8 ± 3.4, respectively. Resistance was recorded against deltamethrin (73.7 ± 6.35) and alpha-cypermethrin (78 ± 5.05) in district Bastar. However, in Korea, the species remained susceptible to alpha-cypermethrin, having a mortality rate of 98.6 ± 0.7, with possible resistance to deltamethrin (91.5 ± 1).
Table 3.
Insecticide susceptibility status of An. culicifacies against dichlorodiphenyltrichloroethane (DDT), Malathion, Alphacypermethrin, and Deltamethrin in districts Bastar and Korea of Chhattisgarh state during 2014–2016.
| District | Year | % Mortality (R/S) | |||
| DDT (4%) | Malathion (5%) | Alphacypermethrin (0.05%) | Deltamethrin (0.05%) | ||
| Bastar | 2014 | 6.6 (R) | 76.2(R) | 88.1 (PR) | 86.4 (PR) |
| 2015 | Nd | Nd | 78(R) | 73.7(R) | |
| Korea | 2014 | 12.9 (R) | 73.4 (R) | 100 (S) | 92.7 (PR) |
| 2015 | Nd | Nd | 98.6 (S) | 91.5 (PR) | |
| 2016 | 15 (R) | 65.8(R) | 100 (S) | 97.4 (S) | |
R and S refer to resistant and susceptible status of An. culicifacies; nd: not done; PR: possible resistance.
4. Discussion
The present study provides information on the bionomics of malaria vectors in different ecotypes through all seasons in two distant districts situated at opposite ends of the malaria-endemic state Chhattisgarh. Studies on vectors in this region have been limited [8]. Our work demonstrated high Anopheline species abundance and diversity in both the study sites. An. culicifacies was incriminated as the only vector and was the most abundant species collected throughout the year. An. fluviatilis was caught in low densities, contrary to the previous findings where this species was dominant, whereas the former was obtained in few numbers. However, in other parts of Central India, An. culicifacies has been documented to be the most prevalent species [24]. Mishra et al. when carried out a study similar to the present one in the forested areas of Central India as a part of the Malaria Elimination demonstration project, An. culicifacies was collected in maximum numbers, highest densities recorded in the month of July.
In the present study, the prevalence of An. culicifacies showed two peaks, first in February–March and second in August–September, coinciding with the paddy cultivation regimes in the study villages. An. fluviatilis densities were recorded in the winter season (November–February). Seasonal distribution of parasite-infected mosquito showed that most of the infected An. culicifacies was found in the month of May, i.e., the summer season. Actually, the spray campaigns are organized pre- and post-monsoon (June–July and September–October). As the effectiveness of sprayed insecticides declines, the mosquito density increases. This, along with other contributing factors, may be the reason for high mosquito density and disease transmission during summer. Ecotype-wise analysis of species distribution showed that the density of An. culicifacies and other Anopheles species were highest in plains in the district Bastar, while in Korea, the forest ecotype had the maximum densities of all the Anopheline species. Previous studies undertaken established distinct differences in intensity of malaria transmission in plain and forest ecotypes. Observations similar to ours have been made in Madhya Pradesh [25].
Despite observations made in previous studies [8], no An. fluviatilis mosquitoes were found to be infected with the malaria parasite. This requires further investigations as the present findings might be due to the low abundance of the species or due to collection/trapping bias. An. culicifacies was the only species recorded to transmit malaria in both sites. However, the infection rates were low despite the fact that the study sites selected are highly malarious. Cases of mixed infections and also a rare case of quadruple infection have been recorded in the periods when infection rates in our collections remained zero. This indicates the existence of high levels of malaria incidences even in the presence of low transmission rates. Similar findings have been made previously [26,27,28]. Our results highlight that probably many malaria cases were due to a single infective bite. This is also suggestive of the outdoor resting habit of vectors due to indoor spraying operations, thus the need for outdoor control measures. A total of 6329 mosquito specimens were tested, and out of this, five were found positive by PCR. This showed that active transmission is going on in this area. Although there are a few glitches reported in the literature, such as the inhibition of PCR results when whole mosquitoes are used for DNA isolation, the removal of the head and thorax seemed to negate the inhibition, suggesting the presence of inhibitors in these body parts. [29].
The study revealed that both An. culicifacies and An. fluviatilis are strongly zoophilic in the study area. They prefer to rest in cattle sheds that are in the vicinity of human dwellings and are generally dark, damp, and humid. An. culicifacies is an established zoophilic vector [8], whereas zoophily in An. fluviatilis could be due to the predominance of sibling species T in the area. This kind of behavior in vector species provides them additional benefit as they avoid the insecticide-treated wall surfaces of human dwellings. This also increases the survival probability of infectious mosquitoes as they escape control interventions which somehow pose a threat to vector control activities.
The Vector control program in Chhattisgarh state employs DDT and pyrethroid-based indoor residual spray (IRS) and long-lasting insecticidal nets (LLINs). We evaluated the susceptibility of An. culicifacies to insecticides in current use. The species was found to be resistant to DDT and Malathion in both the study sites. There seemed to be an increasing trend of pyrethroid resistance in Bastar, while the vector remained susceptible or possibly resistant in Korea. Studies conducted in other parts of Chhattisgarh state also report resistance against one or more classes of insecticides [30,31]. In the adjoining state Madhya Pradesh, An. culicifacies was found to be resistant against DDT, Malathion, Alphacypermethrin with possible resistance against Deltamethrin [24]. Resistance against pyrethroids has been widespread in the states of Odisha, Chhattisgarh, and Madhya Pradesh [5]. With only a few insecticides available presently, the development of resistance against all the categories of insecticides in vectors is of concern. However, the epidemiological impact of insecticide resistance remains largely disputed. Studies report that although vectors have developed resistance to DDT and pyrethroid, the excito-repellant effect of these insecticides provides sufficient protection against mosquito bites. But there are examples of malaria case resurgence due to the development of insecticide resistance in the vector [32,33]. Therefore, there is a compelling need to monitor the effectiveness of control strategies continuously. In addition, detection of genetic markers responsible for resistance in vectors is needed to monitor the spread of resistance.
The findings of the present study provide insight into nearly all the aspects of vector bionomics in the study area. However, there are certain limitations to the study. The habitat parameters of breeding sites could not be determined so as the results generated are informative only of the vector bionomics of the sites. The number of parasite-positive mosquitoes were few. Therefore, the entomological inoculation rate (EIR) could not be calculated. Second, due to the unavailability of the insectary strain of An. culicifacies, we had to use field-collected adult mosquitoes of mixed physiological age in the insecticide susceptibility assay. The study site is almost 700 km far from the institute. The emergence of enough larvae from the field and their maintenance was almost impossible in this field setting. Therefore, we used the field-caught mosquitoes for the susceptibility assay irrespective of their age, etc.
The unreliability of the available molecular techniques for identification of sibling species of An. culicifacies proved to be a hurdle in the identification of the species up to the subspecies level. However, the species was accurately grouped into “BCE” and “AD” types. BCE was the dominant type. Nearly 97% of An. culicifacies tested were found positive for bovine blood. Sibling species B and C are known to be zoophilic [34], suggesting the predominance of these two-sibling species in the area. All the malaria parasite-positive mosquito belonged to the “BCE type”, indicating the probable role of sibling species C in malaria transmission as B is a poor- or non-vector. Sibling species C is an established vector in other parts of central India [4].
Based on observations made in this study, the following suggestions are put forward for further research and implementation of vector control:
Continuous monitoring of insecticide resistance and elucidation and characterization of the underlying mechanisms in vectors is required to evaluate its impact on control interventions and malaria transmission.
There is a need to strengthen vector control in the study areas. The high densities of vector species despite control interventions point towards the need for studies to evaluate the effectiveness of such measures. The efficacy, household acceptability, and feasibility of LLINs should be assessed. The target dose of the insecticide spray formulation and the residual efficacy of the applied insecticides should be monitored. An appropriate combination of IRS, LLINs, and larval control activities is needed to bring down the vector densities effectively.
Insecticides spray campaigns should be targeted to cover the months of February- April and October–November as malaria transmission occurs during these periods also. The IRS should be directed towards cattle shelters also as the vectors in our study were found to be predominantly zoophilic.
The bionomics of vector species differs globally. The responses of these vectors to control interventions differ likewise. Similarly, various biological, cultural, and environmental factors regulate the dynamics of disease transmission in an area. Therefore, to have the greatest impacts, the efforts to control should be channeled as per the needs specific to a country, village, or region.
5. Conclusions
The present study discusses important aspects of vector bionomics, including the seasonal abundance, host and habitat preferences, insecticide susceptibility, sibling species complexes as well as malaria transmission potentials. The complete Anopheline fauna of the region was studied, and sibling species identified. However, the biological characteristics of individual sibling species, as well as their role in malaria transmission, need to be further assessed to help the policymakers design suitable strategies.
Acknowledgments
We would like to thank to the villagers for their support during mosquito collection. Financial support for this work was provided by the Indian Council of Medical Research (ICMR), New Delhi, India. T.I.K. thanks the University Grants Commission for the Research fellowship.
Author Contributions
Conceptualization: T.I.K., G.C., A.T., and P.K.B.; Performed the experiments: T.I.K., J.K.N., A.K.V., and A.K.M. Data analysis: T.I.K., J.K.N., A.K.V., A.K.M., S.K.C. Wrote the manuscript: P.K.B., T.I.K., J.K.N., and Critical review: P.K.B.; G.C., A.K.M., S.K.C., A.T., and P.K.B. All authors have read and agreed to the published version of the manuscript.
Funding
Indian Council of Medical Research, New Delhi, India.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data that support the finding are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organisation World Malaria Report. [(accessed on 27 January 2021)];2020 Available online: https://www.who.int/publications/i/item/9789240015791.
- 2.National Vector Borne Disease Control Programme (NVBDCP) Malaria Situation in India 2019. [(accessed on 28 January 2021)]; Available online: https://nvbdcp.gov.in/WriteReadData/l892s/63783729891610104793.pdf.
- 3.Bharti P.K., Chandel H.S., Ahmad A., Krishna S., Udhayakumar V., Singh N. Prevalence of pfhrp2 and/or pfhrp3 Gene Deletion in Plasmodium falciparum Population in Eight Highly Endemic States in India. PLoS ONE. 2016;11:e0157949. doi: 10.1371/journal.pone.0157949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh N., Mishra A.K., Chand S.K., Bharti P.K., Singh M.P., Nanda N., Singh O.P., Sodagiri K., Udhyakumar V. Relative Abundance and Plasmodium Infection Rates of Malaria Vectors in and around Jabalpur, a malaria Endemic Region in Madhya Pradesh State, Central India. PLoS ONE. 2015;10:e0126932. doi: 10.1371/journal.pone.0126932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbarao S.K., Nanda N., Rahi M., Raghavendra K. Biology and bionomics of malaria vectors in India: Existing information and what more needs to be known for strategizing elimination of malaria. Malar. J. 2019;18:396. doi: 10.1186/s12936-019-3011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dev V., Sharma V.P. The dominant mosquito vectors of human malaria in India. Anopheles mosquitoes- New insights into new malaria vectors. IntechOpen. 2013:239–271. doi: 10.5772/55215. [DOI] [Google Scholar]
- 7.Das M., Das B., Patra A.P., Tripathy H.K., Mohapatra N., Kar S.K., Hazra R.K. Anopheles culicifacies sibling species in Odisha, eastern India: First appearance of Anopheles culicifacies E and its vectorial role in malaria transmission. Trop. Med. Int. Health. 2013;18:810–821. doi: 10.1111/tmi.12112. [DOI] [PubMed] [Google Scholar]
- 8.Nanda N., Bhatt R.M., Sharma S.N., Rana P.K., Kar N.P., Sharma A., Adak T. Prevalence and incrimination of Anopheles fluviatilis species S (Diptera: Culicidae) in a malaria endemic forest area of Chhattisgarh state, central India. Parasites Vectors. 2012;5:215. doi: 10.1186/1756-3305-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N., Chand S.K., Bharti P.K., Singh M.P., Chand G., Mishra A.K., Shukla M.M., Mahulia M.M., Sharma R.K. Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS ONE. 2013;8:e73730. doi: 10.1371/journal.pone.0073730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhuarya S.K., Chaudhary J.L., Manikandan N., Khalkho D. Spatial Analysis of Rainfall and Rainy Days in Chhattisgarh State, India. J. Agric. Phys. 2015;15:140–149. [Google Scholar]
- 11.District Highlights and Important Statistics Census District Korea 2011. [(accessed on 24 June 2019)]; Available online: https://cdn.s3waas.gov.in/s37750ca3559e5b8e1f44210283368fc16/uploads/2016/09/2018041066.pdf.
- 12.About District Bastar Government. [(accessed on 24 June 2019)]; Available online: https://bastar.gov.in/en/
- 13.Krishna S., Bhandari S., Bharti P.K., Basak S., Singh N. A rare case of quadruple malaria infection from the highly malaria-endemic area of Bastar, Chhattisgarh, India. PLoSNegl Trop. Dis. 2017;11:e0005558. doi: 10.1371/journal.pntd.0005558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . Manual on Practical Entomology in Malaria. World Health Organization offset publication; Geneva, Switzerland: 1975. p. 13. Part II. [Google Scholar]
- 15.WHO . Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. 2nd ed. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 16.Christophers S.R., Sinton J.A., Covell G. Synoptic Table for the Identification of the Anopheline Mosquitoes of India. 2nd ed. Government of India Press; Calcutta, India: 1931. p. 31. Health Bulletin No. 10. Malaria Burease No. 2. [Google Scholar]
- 17.Coen E.S., Thoday J.M., Dover G. Rate of turnover of structural variants in the rDNA gene family of Drosophila melanogaster. Nature. 1982;295:564–568. doi: 10.1038/295564a0. [DOI] [PubMed] [Google Scholar]
- 18.Snounou G., Viriyakosol S., Zhu X.P., Jarra W., Pinheiro L., do Rosario V.E., Thaithong S., Brown K.N. High sensitivity of detection of human malaria parasites by use of nested polymerase chain reaction amplification. Mol. Biochem. Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 19.Singh O.P., Goswami G., Nanda N., Raghavendra K., Chandra D., Subbarao S.K. An allele-specific polymerase chain reaction assay for the differentiation of members of the Anopheles culicifacies complex. J. Biosci. 2004;29:275–280. doi: 10.1007/BF02702609. [DOI] [PubMed] [Google Scholar]
- 20.Manonmani A.M., Sadanandane C., Sahu S.S., Mathivanan A., Jambulingam P. rDNA-ITS2-PCR assay for grouping the cryptic species of Anopheles culicifacies complex (Diptera: Culicidae) Acta Trop. 2007;104:72–77. doi: 10.1016/j.actatropica.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Goswami G., Singh O.P., Nanda N., Raghavendra K., Gakhar S.K., Subbarao S.K. Identification of all members of the Anopheles culicifacies complex using allele-specific polymerase chain reaction assays. Am. J. Trop. Med. Hyg. 2006;75:454–460. doi: 10.4269/ajtmh.2006.75.454. [DOI] [PubMed] [Google Scholar]
- 22.Singh O.P., Chandra D., Nanda N., Raghavendra K., Sunil S., Sharma S.K., Dua V.K., Subbarao S.K. Differentiation of members of the Anopheles fluviatilisspecies complex by an allele-specific polymerase chain reaction based on 28S ribosomal DNA sequences. Am. J. Trop. Med. Hyg. 2004;70:27–32. doi: 10.4269/ajtmh.2004.70.27. [DOI] [PubMed] [Google Scholar]
- 23.Collins R.T., Dash B.K., Agarwala R.S., Dhal K.B. An adaptation of the gel diffusion technique for identifying the source of mosquito blood meals. Indian J. Malariol. 1986;23:81–89. [PubMed] [Google Scholar]
- 24.Mishra A.K., Bharti P.K., Vishwakarma A., Nisar S., Rajvanshi H., Sharma R.K., Saha K.B., Shukla M.M., Jayswar H., Das A., et al. A study of malaria vector surveillance as part of the Malaria Elimination Demonstration Project in Mandla, Madhya Pradesh. Malar. J. 2020;19:447. doi: 10.1186/s12936-020-03517-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chand G., Chaudhary N.K., Soan V., Kaushal L.S., Sharma R.K., Singh N. Transmission dynamics & epidemiology of malaria in two tribal districts in Madhya Pradesh, India. Indian J. Med. Res. 2015;141:556–566. doi: 10.4103/0971-5916.159513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beier J.C., Killeen G.F., Githure J.I. Entomological inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- 27.Hay S.I., Guerra C.A., Tatem A.J., Atkinson P.M., Snow R.W. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert V., Le Goff G., Andrianaivolambo L., Randimby F.M., Domarle O., Randrianarivelojosia M., Raharimanga V., Raveloson A., Ravaonjanahary C., Ariey F. Moderate transmission but high prevalence of malaria in Madagascar. Int. J. Parasitol. 2006;36:1273–1281. doi: 10.1016/j.ijpara.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Arez A.P., Lopes D., Pinto J., Franco A.S., Snounou G., do Rosário V.E. Plasmodium sp.: Optimal protocols for PCR detection of low parasite numbers from mosquito (Anopheles sp.) samples. Exp. Parasitol. 2000;94:269–272. doi: 10.1006/expr.2000.4496. [DOI] [PubMed] [Google Scholar]
- 30.Kona M.P., Kamaraju R., Donnelly M.J., Bhatt R.M., Nanda N., Chourasia M.K., Swain D.K., Suman S., Uragayala S., Kleinschmidt I., et al. Characterization and monitoring of deltamethrin-resistance in Anopheles culicifacies in the presence of a long-lasting insecticide-treated net intervention. Malar. J. 2018;17:414. doi: 10.1186/s12936-018-2557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatt R.M., Sharma S.N., Barik T.K., Raghavendra K. Status of insecticide resistance in malaria vector, Anopheles culicifacies in Chhattisgarh state, India. J. Vector Borne Dis. 2012;49:36–38. [PubMed] [Google Scholar]
- 32.Trape J.F., Tall A., Diagne N., Ndiath O., Ly A.B., Faye J., Dieye-Ba F., Roucher C., Bouganali C., Badiane A., et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: A longitudinal study. Lancet Infect. Dis. 2011;11:925–932. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 33.Maharaj R., Mthembu D.J., Sharp B.L. Impact of DDT re-introduction on malaria transmission in KwaZulu-Natal. S. Afr. Med. J. 2005;95:871–874. [PubMed] [Google Scholar]
- 34.Waite J.L., Swain S., Lynch P.A., Sharma S.K., Haque M.A., Montgomery J., Thomas M.B. Increasing the potential for malaria elimination by targeting zoophilic vectors. Sci. Rep. 2017;7:40551. doi: 10.1038/srep40551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the finding are contained within the article.