Abstract
Background
The clinical practice of platelet-rich plasma (PRP) therapy has grown significantly in recent years in multiple medical specialties. However, comparisons of PRP studies across medical fields remain challenging because of inconsistent reporting of protocols and characterization of the PRP being administered. The purpose of this systematic review was to determine the quantity of level I/II studies within each medical specialty and compare the level of study reporting across medical fields.
Methods
The Cochrane Database, PubMed, and EMBASE databases were queried for level I/II clinical studies on PRP injections across all medical specialties. From these studies, data including condition treated, PRP processing and characterization, delivery, control group, and assessed outcomes were collected.
Results
A total of 132 studies met the inclusion and exclusion criteria and involved 28 different conditions across 8 specialties (cardiothoracic surgery, cosmetic, dermatology, musculoskeletal (MSK), neurology, oral maxillofacial surgery, ophthalmology, and plastic surgery). Studies on PRP for MSK injuries made up the majority of the studies (74%), with knee osteoarthritis and tendinopathy being most commonly studied. Of the 132 studies, only 44 (33%) characterized the composition of PRP used, and only 23 (17%) reported the leukocyte component. MSK studies were more likely to use patient-reported outcome measures to assess outcomes, while studies from other specialties were more likely to use clinician- or imaging-based objective outcomes. Overall, 61% of the studies found PRP to be favorable over control treatment, with no difference in favorable reporting between MSK and other medical specialties.
Conclusions
The majority of level I/II clinical studies investigating PRP therapy across all medical specialties have been conducted for MSK injuries with knee osteoarthritis and tendinopathy being the most commonly studied conditions. Inconsistent reporting of PRP composition exists among all studies in medicine. Rigorous reporting in human clinical studies across all medical specialties is crucial for evaluating the effects of PRP and moving towards disease-specific and individualized treatment.
Introduction
The use of platelet-rich plasma (PRP) to treat a multitude of medical conditions has greatly increased over the past decade. As a strategy to deliver a higher concentration of growth factors and cytokines that initiate and regulate tissue healing, PRP therapy has been utilized for a wide range of orthopaedic injuries, including tendinopathies, osteoarthritis, and muscle injuries [1–3]. Recently, PRP has also been increasingly used for the treatment of cosmetic conditions, including hair restoration, breast augmentation, scar treatment, and dermatologic conditions [4–6]. Other reported applications of PRP therapy have included nerve regeneration, periodontal therapies, wound healing, and augmentation of surgical repairs [7–9].
Despite the widespread clinical practice of PRP in all areas of medicine, there remains uncertainty and skepticism among the medical community regarding its efficacy. Much of this skepticism can be attributed to the unawareness of the quantity and quality of evidence investigating PRP treatment, particularly across medical specialties. The practice of evidence-based medicine utilizes the strongest quality of evidence to make informed decisions on the care of individual patients. Although many randomized controlled trials investigating PRP have been conducted for musculoskeletal (MSK) conditions [1,3,10,11], the number of high-quality studies on PRP treatment from other medical specialties compared to orthopaedics, sports medicine, and other MSK fields is unknown. Furthermore, there remain deficiencies in the level of reporting in these studies, particularly regarding the processing and composition of PRP. This has led to calls within orthopaedics for minimal reporting standards in order to allow for reproducibility and comparison across studies [12–15]. Whether the level of reporting is similarly inconsistent within studies from other medical fields is unknown. Detailed reporting in clinical trials for PRP across all medical fields would be beneficial for identifying the key components of PRP and efficiently translating PRP therapy into clinically meaningful treatment.
The purpose of this systematic review was to review the current PRP literature across all medical specialties and determine 1) the quantity of level I and II studies within each medical specialty based on indication, and 2) the level of reporting in these studies with regards to PRP processing, composition, activation, delivery, and outcome assessment. Due to the majority of these studies being from the orthopaedic literature, comparisons in the level of reporting between MSK studies and those from other medical fields were performed.
Materials and methods
Article identification and selection process
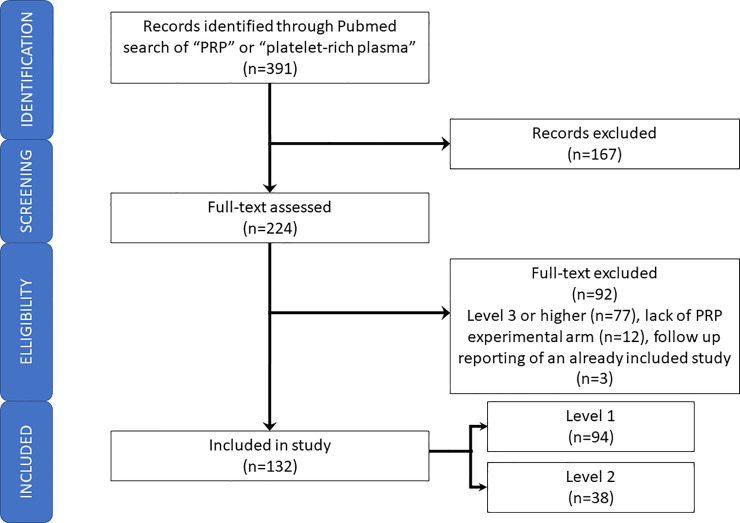
A literature search was conducted in June 2019 to identify articles pertaining to PRP therapy according to Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Fig 1) [16]. The PubMed (including MEDLINE), Cochrane, and EMBASE databases were queried using the following search terms: “platelet-rich plasma,” “platelet rich plasma,” and “PRP.” Inclusion criteria consisted of studies investigating PRP treatment in human clinical trials, comparative level I and level II studies defined by JBJS Journal’s Levels of Evidence [17] from all medical specialties, and articles in the English language. Level III-V studies, animal studies, non-comparative, meeting abstracts, book chapters, and systematic reviews and meta-analyses were excluded. After removal of duplicates, article abstracts were first reviewed to identify studies that were consistent with the inclusion and exclusion criteria. The full texts of these articles were then further assessed to determine which studies were eligible for this review. Reference lists of key articles were analyzed for studies to be included in this review. The literature search was performed by two reviewers and the selected articles were reviewed by the senior author.
Fig 1. PRISMA flowchart.
Data collection and statistical analysis
A full list of the analyzed studies can be found in S1 Appendix. From the eligible studies, the following data were collected: level of evidence according to JBJS Journal’s Levels of Evidence [17], journal name, condition treated, frequency and dosage of PRP administered, time of outcome assessment, composition of PRP (or the commercial system used), activation state of PRP and activating agent, control and other comparison groups, objective and subjective outcomes measured, and overall conclusion on efficacy or favorability of treatment. Extracted data was independently evaluated by the authors. Studies were grouped into one of the following categories based on the condition being treated: cardiothoracic surgery, cosmetic, dermatology, musculoskeletal (MSK), neurology, oral maxillofacial surgery, ophthalmology, and plastic surgery. Data on the reporting of PRP characteristics focused on the platelet and leukocyte composition. The overall conclusion on PRP efficacy or favorability was determined by the authors’ conclusion statement, typically based on statistically significant improvements seen in the outcomes of the PRP treatment group compared to control groups. Included studies were analyzed using the Cochrane Risk of Bias Tool and the Methodological Index for Non-Randomized Studies to asses for bias [18,19]. The senior author reviewed bias assessment scores. Due to the overwhelming number of MSK studies compared to studies from all other specialties, proportional data between studies from MSK and all other specialties was compared using Fisher’s exact test.
Results
Number of studies
After removal of duplicates, 391 records were examined. One hundred sixty-seven studies were removed after title screening, and 92 studies were removed after full-text examination. Among the studies excluded included those with levels of evidence III or higher (n = 77), lack of a PRP experimental arm (n = 12), and follow-up reporting of an already included study (n = 3). A total of 132 studies met the inclusion and exclusion criteria and were analyzed for this study (Fig 1). Of these studies, 94 (71%) were level I studies, and 38 (29%) were level II studies (S1 Appendix).
Among the analyzed studies, there were 28 different conditions across eight medical fields (Table 1). Studies investigating PRP treatment for MSK conditions comprised 74% of all studies. Tendinopathy (n = 29) and osteoarthritis (n = 28) were the two most commonly studied conditions. MSK studies were 76% level 1 evidence while 57% of all other studies were level 1 evidence (p<0.05). Cosmetic studies comprised 14% (n = 19) of all studies, and 53% of these were level I evidence.
Table 1. Number of Level I and Level II studies investigating platelet-rich plasma treatment organized by medical specialty.
| Specialty | Condition | Level I | Level II |
|---|---|---|---|
| Cardiothoracic Surgery (1) | Blood Loss | 1 | 0 |
| Cosmetic (19) | Alopecia | 5 | 1 |
| Fat graft | 0 | 1 | |
| Hair Regrowth | 2 | 0 | |
| Hyperpigmentation | 0 | 1 | |
| Scars/Stretch marks | 3 | 6 | |
| Dermatology (3) | Psoriasis | 0 | 1 |
| Vitiligo | 1 | 1 | |
| Musculoskeletal (97) | ACL Reconstruction | 1 | 0 |
| Arthroplasty/Arthroscopy | 0 | 2 | |
| Back Pain | 1 | 0 | |
| Carpal Tunnel | 1 | 0 | |
| Disk Degeneration | 1 | 0 | |
| Fracture | 4 | 0 | |
| Lumbar Facet Syndrome | 1 | 0 | |
| Meniscus Repair | 2 | 0 | |
| Muscle Injury | 4 | 1 | |
| Osteoarthritis | 21 | 7 | |
| Plantar Fasciitis | 7 | 5 | |
| Rotator Cuff Repair | 6 | 2 | |
| Sprain | 1 | 1 | |
| Tendinopathy | 24 | 5 | |
| Neurology (3) | Carpal Tunnel | 1 | 1 |
| Neuropathy | 1 | 0 | |
| Ophthalmology (1) | Retinitis Pigmentosa | 0 | 1 |
| Oral Maxillofacial Surgery (6) | Temporomandibular Joint Disorder | 6 | 0 |
| Plastic Surgery (2) | Breast Reconstruction | 0 | 1 |
| Wound Closure | 0 | 1 |
Reporting of PRP composition and administration
Among all studies, 44 studies (33%) provided details on PRP processing or characteristics of PRP being studied. Within specialty, 37% of MSK studies reported composition details, and 23% of studies from other specialties reported composition details (p = 0.15). Only 23 of the 44 studies also reported details on the leukocyte concentration (Table 2). No studies from the fields of cardiothoracic surgery, ophthalmology, oral maxillofacial surgery, and plastic surgery provided details on platelet or leukocyte counts. A total of 57 studies (43%) indicated that the PRP was activated prior to administration. Calcium was used as the activator in 80% of these studies (Table 3). Among MSK studies, 36% used an activator, whereas 63% of studies from other specialties used an activator (p<0.01). Regarding PRP administration, 84% of studies reported details on quantity, volume, and dosing interval of PRP injections. The quantity of PRP treatments administered ranged from 1 to 9, and the total volume of PRP administered in a single setting ranged from 0.1 to 22 mL. The treatment window ranged from a single injection to serial doses over the span of one year.
Table 2. Percentage of studies reporting details on composition of platelet-rich plasma used.
| Specialty (Total Number of Studies) | Composition | Platelet Concentration | Leukocyte Concentration |
|---|---|---|---|
| Cosmetic (19) | 30% (5) | 100% (5/5) | 20% (1/5) |
| Dermatology (3) | 33% (1) | 100% (1/1) | 0% (0/1) |
| Musculoskeletal (97) | 37% (36) | 100% (36/36) | 56% (20/36) |
| Neurology (3) | 67% (2) | 100% (2/2) | 100% (2/2) |
| Other (10) | 0% (0) | 0% (0/0) | 0% (0/0) |
| TOTAL (132) | 34% (44) | 100% (44/44) | 52% (23/44) |
Table 3. Studies using an activator for platelet-rich plasma.
| Specialty (Total Number of Studies) | % of Studies Using Activator | Calcium (# of Studies) | Thrombin (# of Studies) | Other (# of Studies) |
|---|---|---|---|---|
| Cardiothoracic Surgery (1) | 100% (1) | 0 | 1 | 0 |
| Cosmetic (19) | 74% (14) | 13 | 0 | 1 |
| Dermatology (3) | 67% (2) | 2 | 0 | 0 |
| Musculoskeletal (97) | 36% (35) | 26 | 5 | 4 |
| Neurology (3) | 0% (0) | |||
| Ophthalmology (1) | 0% (0) | |||
| Oral Maxillofacial Surgery (6) | 50% (3) | 2 | 0 | 1 |
| Plastic Surgery (2) | 100% (2) | 2 | 0 | 0 |
Reporting of outcomes
The majority of subjective outcomes reported consisted of patient-reported outcome measures (PROMs), including visual analogue scale (VAS) pain scores and joint- and disease-specific functional outcome measures. A variety of validated and non-validated scores were used depending on the condition being studied. The vast majority (93%) of MSK studies used at least one PROM in their study (Table 4). Of these, 66% of them reported scores from PROMs favoring PRP over other treatment groups. In contrast, only 49% of studies from all other specialties used a PROM to assess outcomes (p<0.01).
Table 4. Percentage of studies reporting subjective and objective outcomes.
| Specialty (Total Number of Studies) | Studies Reporting Subjective Outcomes | Studies Reporting Favorable Subjective Outcomes for PRP | Studies Reporting Objective Outcomes | Studies Reporting Favorable Objective Outcomes for PRP |
|---|---|---|---|---|
| Musculoskeletal (97) | 93% (90) | 66% (60/90) | 57% (56) | 48% (28/56) |
| Cosmetic (19) | 63% (12) | 58% (7/12) | 74% (14) | 64% (14/19) |
| Other (16) | 31% (5) | 60% (3/5) | 56% (9) | 78% (7/9) |
Objective outcomes assessed included imaging studies, examination-based quantification, and time to return to play. Among MSK studies, 57% reported at least one objective outcome measure, and 48% of these studies demonstrated favorability of PRP on an objective outcome measure over other treatments (Table 4). Among studies from all other specialties, 66% used an objective outcome measure (p = 0.43).
Overall favorability of PRP compared to control treatment
Overall, 61% of the studies found PRP to be favorable over control treatment (Table 5). In 33 studies, PRP was administered as an adjunctive treatment and compared to primary treatment without PRP. These primary treatments ranged from other nonoperative therapies to surgery. Among studies utilizing PRP as adjunctive therapy, 66% found PRP to be favorable compared to controls. Among these studies comparing PRP to saline, 50% found PRP to be favorable compared to saline. With regards to specialty, 58% of MSK studies found PRP to be favorable over control, while 67% of studies from all other specialties found PRP to be favorable over control (p = 0.42). Among the few studies from dermatology, neurology, ophthalmology, and plastic surgery, 100% studies found PRP to be favorable over control.
Table 5. Percentage of studies reporting favorability of platelet-rich plasma over control treatment.
| Specialty (Total Number of Studies) | Primary Treatment (PRP used as adjunct therapy) | Saline | Nonprocedural Treatment (e.g., physical therapy) | Hyaluronic Acid | Procedure | Other | TOTAL |
|---|---|---|---|---|---|---|---|
| Musculoskeletal (97) | 64% (14/22) | 44% (8/18) | 70% (14/20) | 53% (9/17) | 67% (4/6) | 57% (8/14) | 58% (57/97) |
| Cosmetic (19) | 60% (3/5) | 71% (5/7) | 33% (1/3) | 50% (2/4) | 58% (11/19) | ||
| Oral Maxillofacial Surgery (6) | 100% (1/1) | 0% (0/1) | 100% (2/2) | 50% (1/2) | 67% (4/6) | ||
| Dermatology (3) | 100% (3/3) | 100% (3/3) | |||||
| Neurology (3) | 100% (2/2) | 100% (1/1) | 100% (3/3) | ||||
| Plastic Surgery (2) | 100% (1/1) | 100% (1/1) | 100% (2/2) | ||||
| Ophthalmology (1) | 100% (1/1) | 100% (1/1) | |||||
| Cardiothoracic Surgery (1) | 0% (0/1) | 0% (0/1) | |||||
| TOTAL (132) | 66% (22/33) | 50% (13/26) | 73% (16/22) | 60% (12/20) | 55% (6/11) | 60% (12/20) | 61% (81/132) |
Risk of bias
Majority of studies were assessed using the Cochranes Risk of Bias Tool, 80% (n = 106). Among these studies, 30% (n = 32) were assessed to be “Low” risk of bias, 25% (n = 26) were found to have “Some Concerns”, and 45% (n = 48) were assessed to be “High” risk of bias (S2 Appendix). The remaining 20% (n = 26) were assessed using MINORS with an average score of 17.4, SD = 2.96 (S3 Appendix).
Discussion
The present systematic review found that the vast majority of published level I and II clinical studies investigating PRP therapy across all medical specialties have been conducted for MSK conditions. Among all studies analyzed, only 33% reported details on the composition of PRP used, and only 17% reported the leukocyte component of PRP used. MSK studies were more likely to use PROMs as their primary outcome measure, while studies from other specialties were more likely to use objective outcomes. Overall, 61% of the studies found PRP to be favorable over control treatment, with no difference in favorable reporting between MSK and other medical specialties.
In recent years, there has been an explosion in the clinical practice of PRP treatment despite little evidence to support its use for most indications. Platelets, which contain various growth factors and cytokines that initiate and regulate healing, play a role in the normal mechanisms for tissue repair. As such, PRP, with a platelet concentration above the baseline of autologous blood, is thought to deliver more proteins, cytokines, and other bioactive factors to augment or promote the healing process. However, uncertainty remains as to the critical platelet concentration that correlates with appreciable augmentation of healing, which may vary depending on the mode of delivery, tissues involved, and pathology being treated. Moreover, positive clinical responses to PRP may be attributed to anti-inflammatory effects rather than a predominantly anabolic response as PRP therapy was originally intended to induce [1,20,21]. Improved understanding of the underlying structural and compositional deficiencies of the injured tissue, along with characterization of the specific components in PRP that are beneficial in the pathophysiology being treated, will help to define how or if PRP can be symptom-modifying and/or structure-modifying [1].
As a minimally manipulated tissue and autologous blood product, PRP has avoided the regulatory hurdles of extensive preclinical and clinical trial testing, and as a result, its clinical practice may always outpace the supporting scientific data. Rigorous, controlled, human clinical studies on PRP therapy are therefore crucial in the evaluation process, not only to evaluate its efficacy, but also to aid in defining the critical components within the PRP that are responsible for clinical improvement. Within orthopaedics, there has been numerous calls for minimal reporting standards in clinical studies regarding PRP preparation and composition in order to allow for comparison among studies and reproducibility [12–15]. However, among other medical specialties, similar calls for minimal reporting standards appear to be lacking. Even with the orthopaedic call for minimal reporting standards, this study found that only a third of studies among all specialties, including MSK specialties, provided details on the PRP processing and characteristics, with only half of those studies performing leukocyte analysis. The clinical ramifications and cellular effects of leukocyte-rich versus leukocyte-poor PRP continue to be debated. Although some believe that leukocyte (neutrophil)-rich PRP is associated with pro-inflammatory effects due to an elevated level of catabolic cytokines which antagonize the anabolic factors contained within the platelets [22,23], others have reported the opposite [21]. The differences in the concentrations of bioactive molecules, including interleukin 1 receptor antagonist, platelet-derived growth factor, and matrix metalloproteinases, within leukocyte-rich PRP versus leukocyte-poor PRP make leukocyte reporting one of key elements in the clinical evaluation of PRP [21]. Furthermore, because the majority of commercially available PRP systems do not allow for user-titration of the leukocyte component, and because leukocyte concentration appears to important depending on the disease being treated [1,24], the lack of leukocyte reporting among current PRP studies only adds to this debate and makes it difficult to delineate the optimal PRP formulation to use. We propose that future PRP studies include the following PRP characterization details; concentration of platelets, activation status/activator used, leukocyte concentration, volume of therapeutic dose, and number of doses administered. The wide variability in the reporting of PRP processing, composition, activation, and delivery in the highest level of evidence clinical studies all contribute to the uncertainty and skepticism of PRP therapy within the medical community, and systematic standardization of reporting and classification systems, not just in orthopaedics but among all medical specialties, is a necessary step in translating PRP therapy into clinically meaningful treatment.
Other than the disparity in the number of published studies per specialty, this study found that MSK studies (93%) were more likely measure outcomes using PROMs compared to studies from other specialties (49%). Although some diseases may not be as amenable to subjective outcome measurement, in most scenarios, PRP is often being administered as a self-paying treatment option for cosmesis and quality of life. Therefore, PROMs to gauge satisfaction and other measures of quality of care from a patient’s perspective should be a necessary, if not the primary, outcome in all PRP studies. Although PROMs exist in dermatology and aesthetic surgery [25–27], they may not be as widely used and validated compared to the myriad MSK PROMs [28]. In non-MSK studies, objective measures were more often the primary outcome measure assessed (e.g., maintenance of breast volume on imaging and clinical evaluation after PRP-supplemented breast reconstruction, quantitative assessment of hair growth and density after PRP injection for alopecia). However, these metrics don’t capture the patient’s perception of their health status, which clinicians should consider as the gold-standard outcome for evaluation.
There are several limitations to this review. Data on the reporting of other suggested criteria for PRP treatment evaluation, such as whole blood storage, whole blood characteristics, and patient usage of anti-inflammatory or anti-platelet medications, were not specifically elicited in this study. Given the wide variability or lack of reporting of PRP processing and composition, it is hypothesized that the reporting on whole blood details would be similarly scarce among all studies. Finally, this review of the literature would be subject to publication bias as 45% of the studies assessed using Cochrane were found to have “High” risk of bias. This adds to the call for standardization of not only composition of PRP but also the design and reporting of PRP studies.
In summary, the vast majority of level I and II clinical studies investigating PRP have been conducted for MSK injuries, with only a handful of studies conducted for conditions in other medical specialties. Studies that reported details on PRP processing and composition were in the minority, and PROMs were not often used as an outcome measure in non-MSK studies. Because the clinical practice of PRP may always outpace the supporting scientific data, rigorous reporting in human clinical studies across all medical specialties is crucial for evaluating the effects of PRP and moving towards disease-specific and individualized treatment.
Supporting information
(PDF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
Investigation performed at the University of California, Irvine, Irvine, CA.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Wang D, Rodeo SA. Platelet-Rich Plasma in Orthopaedic Surgery: A Critical Analysis Review. JBJS Rev. 2017;5(9):e7. 10.2106/JBJS.RVW.17.00024 [DOI] [PubMed] [Google Scholar]
- 2.Mishra A, Woodall J, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113–25. 10.1016/j.csm.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick J, Bulsara MK, O’Donnell J, McCrory PR, Zheng MH. The Effectiveness of Platelet-Rich Plasma Injections in Gluteal Tendinopathy: A Randomized, Double-Blind Controlled Trial Comparing a Single Platelet-Rich Plasma Injection With a Single Corticosteroid Injection. Am J Sports Med. 2018;46(4):933–9. 10.1177/0363546517745525 [DOI] [PubMed] [Google Scholar]
- 4.Hausauer AK, Humphrey S. The Physician’s Guide to Platelet-Rich Plasma in Dermatologic Surgery Part II: Clinical Evidence. Dermatol Surg. 2020;46(4):447–56. 10.1097/DSS.0000000000002148 [DOI] [PubMed] [Google Scholar]
- 5.Gupta AK, Versteeg SG, Rapaport J, Hausauer AK, Shear NH, Piguet V. The Efficacy of Platelet-Rich Plasma in the Field of Hair Restoration and Facial Aesthetics-A Systematic Review and Meta-analysis. J Cutan Med Surg. 2019;23(2):185–203. 10.1177/1203475418818073 [DOI] [PubMed] [Google Scholar]
- 6.Samadi P, Sheykhhasan M, Khoshinani HM. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast Surg. 2019;43(3):803–14. 10.1007/s00266-018-1293-9 [DOI] [PubMed] [Google Scholar]
- 7.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;10(1):23. 10.1186/1742-4933-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Wang Y, Sun J. The effect of platelet-rich plasma on arthroscopic double-row rotator cuff repair: a clinical study with 12-month follow-up. Acta Orthop Traumatol Turc. 2016;50(2):191–7. 10.3944/AOTT.2015.15.0113 [DOI] [PubMed] [Google Scholar]
- 9.Anjayani S, Wirohadidjojo YW, Adam AM, Suwandi D, Seweng A, Amiruddin MD. Sensory improvement of leprosy peripheral neuropathy in patients treated with perineural injection of platelet-rich plasma. Int J Dermatol. 2014;53(1):109–13. 10.1111/ijd.12162 [DOI] [PubMed] [Google Scholar]
- 10.Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am J Sports Med. 2020:363546520909397. 10.1177/0363546520909397 [DOI] [PubMed] [Google Scholar]
- 11.Johal H, Khan M, Yung SP, Dhillon MS, Fu FH, Bedi A, et al. Impact of Platelet-Rich Plasma Use on Pain in Orthopaedic Surgery: A Systematic Review and Meta-analysis. Sports Health. 2019;11(4):355–66. 10.1177/1941738119834972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chahla J, Cinque ME, Piuzzi NS, Mannava S, Geeslin AG, Murray IR, et al. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J Bone Joint Surg Am. 2017;99(20):1769–79. 10.2106/JBJS.16.01374 [DOI] [PubMed] [Google Scholar]
- 13.Murray IR, Geeslin AG, Goudie EB, Petrigliano FA, LaPrade RF. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): Platelet-Rich Plasma and Mesenchymal Stem Cells. J Bone Joint Surg Am. 2017;99(10):809–19. 10.2106/JBJS.16.00793 [DOI] [PubMed] [Google Scholar]
- 14.Rodeo S. The Need for Minimum Reporting Standards for Studies of "Biologics" in Sports Medicine. Am J Sports Med. 2019;47(11):2531–2. 10.1177/0363546519872219 [DOI] [PubMed] [Google Scholar]
- 15.Chu CR, Rodeo S, Bhutani N, Goodrich LR, Huard J, Irrgang J, et al. Optimizing Clinical Use of Biologics in Orthopaedic Surgery: Consensus Recommendations From the 2018 AAOS/NIH U-13 Conference. J Am Acad Orthop Surg. 2019;27(2):e50–e63. 10.5435/JAAOS-D-18-00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Joint Surg Am. 2015;97(1):1–2. 10.2106/JBJS.N.01112 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. Published 2011 Oct 18. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 20.Abdul Ameer LA, Raheem ZJ, Abdulrazaq SS, Ali BG, Nasser MM, Khairi AWA. The anti-inflammatory effect of the platelet-rich plasma in the periodontal pocket. Eur J Dent. 2018;12(4):528–31. 10.4103/ejd.ejd_49_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler CG, Van Sloun R, Gonzalez S, Whitney KE, DePhillipo NN, Kennedy MI, et al. Characterization of Growth Factors, Cytokines, and Chemokines in Bone Marrow Concentrate and Platelet-Rich Plasma: A Prospective Analysis. Am J Sports Med. 2019;47(9):2174–87. 10.1177/0363546519832003 [DOI] [PubMed] [Google Scholar]
- 22.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135–40. 10.1177/0363546511417792 [DOI] [PubMed] [Google Scholar]
- 23.Dragoo JL, Braun HJ, Durham JL, Ridley BA, Odegaard JI, Luong R, et al. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med. 2012;40(6):1274–81. 10.1177/0363546512442334 [DOI] [PubMed] [Google Scholar]
- 24.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr Rev Musculoskelet Med. 2018;11(4):624–34. 10.1007/s12178-018-9527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori S, Lee EH. Beyond the physician’s perspective: A review of patient-reported outcomes in dermatologic surgery and cosmetic dermatology. Int J Womens Dermatol. 2019;5(1):21–6. 10.1016/j.ijwd.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danilla S, Cuevas P, Aedo S, Dominguez C, Jara R, Calderon ME, et al. Introducing the Body-QoL(R): A New Patient-Reported Outcome Instrument for Measuring Body Satisfaction-Related Quality of Life in Aesthetic and Post-bariatric Body Contouring Patients. Aesthetic Plast Surg. 2016;40(1):19–29. 10.1007/s00266-015-0586-5 [DOI] [PubMed] [Google Scholar]
- 27.Vaidya TS, Mori S, Khoshab N, Dusza SW, Bander T, Matros E, et al. Patient-reported Aesthetic Satisfaction following Facial Skin Cancer Surgery Using the FACE-Q Skin Cancer Module. Plast Reconstr Surg Glob Open. 2019;7(9):e2423. 10.1097/GOX.0000000000002423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pusic AL, Lemaine V, Klassen AF, Scott AM, Cano SJ. Patient-reported outcome measures in plastic surgery: use and interpretation in evidence-based medicine. Plast Reconstr Surg. 2011;127(3):1361–7. 10.1097/PRS.0b013e3182063276 [DOI] [PubMed] [Google Scholar]