Abstract
Biomechanical influences play a fundamental role in the structural, functional, and biosynthetic properties of articular cartilage. During physiologic joint loading, the contact area between two surfaces migrates due to the primary and secondary motions of the joint. It has been demonstrated that a migratory contact area plays a critical role in reducing the coefficient of friction at the cartilage surface. However, a detailed analysis of the influences that a migratory contact area plays on the structural, functional, and biosynthetic properties remain to be explored. In this study, bovine cartilage explants were placed in a biotribometer. Explants were subjected to compression and shear forces of migratory contact area, namely moving contact (MC) articulation, or stationary contact area, namely stationary contact (SC) articulation. Free swelling explants were used as control. In a separate study, bovine cartilage-bone grafts were used for frictional testing. On histologic analysis, the SC group had evidence of surface fibrillations, which was not evident in the MC group. Compared to the SC group, the MC group cartilage explants had increased chondrocyte viability, increased lubricin synthesis, and comparable proteoglycan synthesis and release. MC articulation had reduced coefficient of friction as compared to SC articulation. MC articulation led to reduced surface roughness as compared to SC articulation. In conclusion, a migratory contact area can play an important role in maintaining the structural, function, and biosynthetic properties of articular cartilage. This study provides further evidence of the importance of migratory contact area and in vitro assessment of natural joint movement, which can be further evaluated in the context of cartilage homeostasis and disease.
Keywords: Migrating contact area, moving contact articulation, chondrocyte viability, coefficient of friction, lubricin
1. Introduction
It is of critical importance to understand the structural, functional, and biologic response of cartilage to biomechanical influences. These biomechanical influences play a role in both joint homeostasis as well as degeneration. In diarthrodial joints, such as the hip and knee, articular cartilage is repeatedly subjected to high compression and shear forces (three-four times the body weight) by daily activities such as walking, running, standing up, and stair climbing [1]. The response of cartilage to specific compression and shear forces can be reproducibly analyzed using in vitro studies with cartilage explants [2–5].
Motions at the joint, such as the knee, occur upon multiple axes. These motions include the primary motion of the knee: flexion and extension. Secondary motions of the knee include: internal and external rotation, anterior and posterior translation, abduction and adduction movements, and medial and lateral translation (Figure 1A)[6]. The knee articular surface experiences a compression force due to weight bearing, and both the primary and secondary motions at the knee help dictate the shear forces experienced at the articulating surface. In addition, primary and secondary motions of the joint also create a migratory contact area. A migratory contact area alters the compression and shear forces experienced by specific regions of articular cartilage and also augments the time in which a particular area of the cartilage surface experiences these forces.
Figure 1. Physiologic joint kinematics and bioreactor set up.
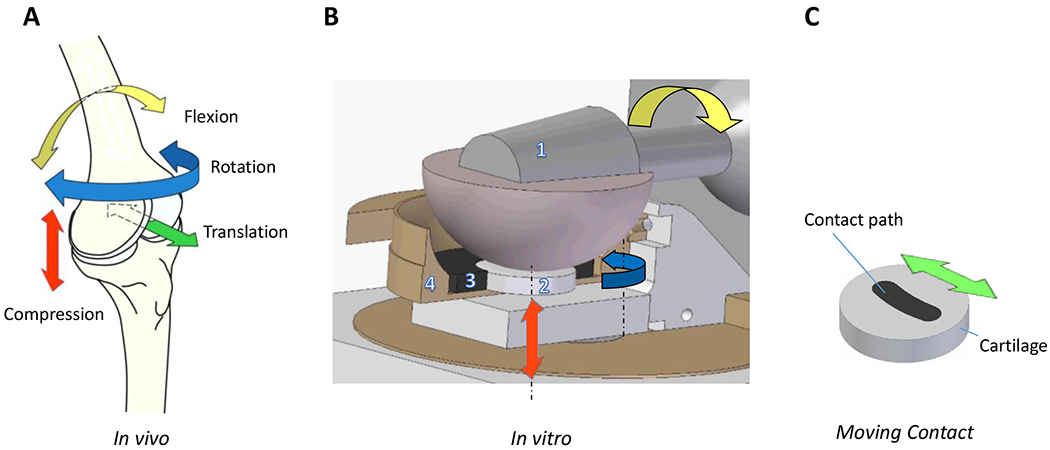
A) Representative primary and secondary motions of the knee joint (in vivo) and compression (red arrow); flexion and extension (yellow arrow); internal and external rotation (blue arrow); anterior and posterior translation (green arrow); medial/lateral translation and abduction/adduction movement not shown. B) Bioreactor set up for in vitro testing. Ceramic ball [1] provides compression (red arrow) and articulation (yellow arrow) that translate into shear forces at the explant surface (2 /grey disk). Off-center axis rotation (blue arrow) simulates secondary motions (translation and rotation). Cartilage explant is held within a porous polyethylene scaffold (3), which is held in place by a dished PEEK cup holding the testing media. Representative image shows a horizontal cross section through the ceramic ball (1) and a vertical cross section through the porous scaffold (3) and PEEK cup (4). C) Migrating contact /MC articulation: Ceramic ball moves along curvilinear contact path (black) on the cartilage explant. In SC articulation, forces and motions are confined to a circular region within the center of the cartilage explant (not shown).
Over the last decade, a description for a migratory contact area or sliding contact area has been introduced and been increasingly described and tested [7–10]. A migratory contact area not only replicates physiologic loading conditions but can also play a critical role in the frictional response of cartilage. It has been demonstrated that a migratory contact area can maintain elevated cartilage fluid interstitial pressurization and thereby reduce the frictional coefficient a cartilage; furthermore, this reduction in coefficient of friction can be substantially greater than the boundary lubrication provided by lubricants in the synovial fluid [7]. In general, with a stationary contact area, a constant fluid flow rate will equal constant pressurization; however, over time the deformation rate, flow rate, and pressure will approach zero. In migrating contact, hydrated cartilage is continuously introduced to the contact area providing the necessary fluid for pressurization. Furthermore, fluid will preferentially flow into the post-contact zone and rehydrate it for subsequent loading [7, 10]. Fluid and subsequent solute transport into cartilage can also be enhanced with sliding motion through a process coined “tribological rehydration” [11, 12]. which occurs with a migratory contact area.
The influence of both motion and combination of static/dynamic compression and shear forces has been well studied and shown to play a vital role in maintaining the homeostasis of articular cartilage [13–20]. Although this combination of compression and shear mimics joint loading forces at a given instance, its application over a single or stationary point over the cartilage surface does not replicate a migrating contact area as experienced by a joint such as the knee over time. While the role of moving or migratory contact area has been studied in regard to the mechanical properties of cartilage, limited studies have assessed the biosynthetic and functional response of articular cartilage to a moving contact (MC) vs stationary contact (SC) situation. A few studies to date have performed mechanobiological studies assessing anabolic and catabolic gene expression in response to compression and sliding forces with moving contact area [8, 9]. No studies have assessed cartilage integrity or chondrocyte viability with a comparison between a migratory and stationary contact.
The aim of this study was to compare the response of articular cartilage to a MC of articulation as compared to a SC of articulation. To perform this study, we used a biotribometer as previously described [2, 21]. A rotating ceramic (alumina) ball provided compression and shear forces on the cartilage disc. This ball was then allowed to move across the cartilage explant to provide a MC situation between the ball and explant, thereby following a curvilinear path that was dictated by rotation around an off-center axis perpendicular to the explant’s surface. (Blue arrow, Figure 1 B). The length of the migratory motion was determined by the rotation amplitude (Figure 1 C). Endpoint measures between groups (SC, MC, and free-swelling control) included: histology, chondrocyte viability, friction, surface topography, proteoglycan synthesis and release, and release of lubricin into the medium. We predicted that since a MC articulation better replicates joint kinetics and has been shown to reduce the coefficient of friction at the cartilage surface, as compared to a SC articulation, it would result in better structural, functional, and biosynthetic properties of the cartilage explant.
2. Materials and Methods
Two types of articular motion were applied to the cartilage explants: one with a SC of contact to resemble the primary joint motion, and other with a MC of contact to mimic the combined effect of primary and secondary joint motions. The influence of articulation was then compared against freely swelling controls. Each condition was repeated with n = 8 cartilage explants. Responses evaluated included: i) structural, by analyzing the integrity of articular surface using histological analysis; and ii) biosynthetic, by quantifying the chondrocyte viability, lubricin synthesis, and proteoglycan synthesis and release.
Additionally, functional responses were evaluated from different sets of experiments. Topographical changes related to articulation were evaluated by measuring the surface topography of full thickness explants prior to articulation, on day 5, and post articulation, on day 12. Frictional testing was performed on a set of cartilage-bone grafts for a period of 60 minutes.
2.1. Cartilage Explants
Unless otherwise stated, all the specimens were full thickness cartilage explants, 14 mm in diameter, obtained from 6- to 8-month old bovine calf knees from the trochlear and condylar regions. The knees were acquired from a local slaughterhouse (Chiappetti Lamb & Veal Corporation, Chicago, IL) and were from animals slaughtered within 24-48 hours. During harvest, the cartilage surface was kept moist with Dulbecco’s phosphate buffered saline 1X solution. Upon retrieval, all explants were marked (notched) for anatomical orientation, washed with sterile 0.9% saline solution, and immediately placed into cell culture medium with 10% FBS at 37°C for up to 11 days. The cell culture medium was based on a 50/50 mixture of Dulbecco’s Modified Eagle’s Medium and Ham’s F12 (DMEM-F12), and contained 100 μg/mL gentamicin and 2.5 μl/mL antibiotic-antimycotic solution (100x) containing penicillin, streptomycin, and amphotericin B. An exception to the procurement technique and in the culture period was made in the case of explants used in testing the frictional response. The explants used in frictional response were procured following the above stated protocol, but the subchondral bone remained attached to the cartilage. The attached bone for these 16 explants was trimmed to a thickness of 1-2 mm and provided structural stability to the explants during frictional testing. These explants were cultured in DMEM-F12 medium with 10 % FBS at 37°C for days 1 to 5.
2.2. Articular Motion and Experimental Protocol
2.2.1. Articular Motion
An existing bio-tribometer [2] that was developed initially for tissue-engineering application [21] and based on the ball-on-disc principle, has been used to provide joint motions to the cartilage explants (Figure 1B and C). A 10 mm offset was introduced between the center of the explant and the rotation axis of the simulator station to enable a curvilinear movement for the point of contact over the explant, as shown in Figure 1C. Compressive loading was achieved by pressing a commercially available ceramic hip ball of 32 mm in diameter against the flat surface of the explant. Subsequently, the ball was rotated along an axis parallel to the explant surface, generating shear stresses on the explant. During ball rotation, the point of contact was either kept stationary or moved over the explant surface. Furthermore, control explants were allowed to swell freely during the entire period and were not subjected to any loading or articulation.
2.2.2. Experimental Protocol
All explants were cultured for the duration of the experiment in 2 mL of medium containing DMEM F12 and 10% FBS, and the conditioned medium was collected and replaced frequently. The explants were cultured from days 1 to 5 to equilibrate and stabilize their biosynthetic levels [22]. Between days 6 and 9, compressive load and articulation was applied to each explant in the articulation group, following the protocol defined ahead. The explant was mounted so that the articular motion of the ball followed the expected in vivo motion (or split line orientation). The cartilage sample was then loaded compressively to 0.8 times its original thickness by pushing against the ball, achieving 20 % compressive loading. The ball was then rotated in a sine wave for ± 30° at 1 Hz to apply articular motion. For MC articulation explants, the station hosting the explant discs was rotated in a sine wave of ± 15° at 0.1 Hz, generating a curvilinear motion of 5.2 mm for the explant discs, with respect to the ceramic ball. The path length coincides with the length of antero-posterior motion proposed in ISO 14243-3 [23]. The point of contact was kept at a standstill for the SC articulation group of explants. Articulation was applied for two one-hour cycles per day (morning/afternoon) for four consecutive days (day 6-9), achieving eight hours of loading combined with intermittent periods of free-swelling rest.
All the explants were cultured under free-swelling conditions for days 10 and 11 to examine the effect of post-articulation rest. Between days 5 and 11, the entire conditioned medium from each explant was collected every 24 hours and replaced with fresh media. The collected medium were stored in individual vials at −20 °C and analyzed at conclusion of the experiment. On day 12, all the explants were sectioned and distributed for different biochemical analysis. For the explants used to evaluate topographical changes, surface topography was measured on days 5 and 11 by following a protocol mentioned ahead in the subsection titled ‘Surface Topography’. Similarly, details for the friction testing are in the subsection titled ‘Coefficient of Friction’.
All explants used in biosynthetic analysis were cut transversally first, through the center of contact, and one half of the explant disc was evaluated for chondrocyte viability. The remaining piece was cut in two quarters, and one was used histologically to evaluate structural integrity along the longitudinal axis of the sample, following the direction of friction at the articular surface. The other quarter was used analyze proteoglycan synthesis. During distribution, proper care was taken to ensure that for articulation group explants each section contained a similarly large piece of the contact area that formed between articular cartilage and alumina ball. The controls were distributed in a manner similar to the articulation group explants.
2.3. Chondrocyte Viability Analysis
After topographical measurements, full width, 1 mm thick sections were obtained from the condylar and trochlear explants, in the anterior - posterior and in the inferior – superior directions, respectively. The sections were stained in Dulbecco’s phosphate buffered saline 1X solution containing calcein AM and ethidium homodimer-1 (LIVE/DEAD Viability/Cytotoxicity Kit; Molecular Probes, OR) and incubated at 37°C for a period of 20 minutes. The stained sections were imaged using a fluorescence-light microscope (Eclipse TE2000-S; Nikon Instruments Inc, NY), a 5× objective and a charge-coupled device (CCD) camera (SPOT RT-KE Color 3-Shot, Model 7.3x; Diagnostic Instruments, MI). ImageJ software was used to perform live and dead cell counts for the obtained images. These live and dead cell counts were used to quantify the cell viability of the superficial zone and of the total explant, which includes the superficial, middle, and deep zones. The top 15% of the cartilage thickness was defined as representative of the superficial zone of the explants [24]. The cartilage explants were 2.12 ± 0.56 mm thick and with the superficial zone defined as 15 % from the articular surface, the thickness of this zone was estimated to be 0.32 ± 0.08 mm. The chondrocyte viability was also evaluated in a depth-wise manner, by subdividing the images into 10 rectangles of equal thickness of 0.08 mm. These horizontally placed rectangles spanned the entire width of the image and were stacked along their width, with the first one aligned with the articular surface. Viability was computed as the ratio of living cells over the total number of cells (live + dead)[25]. This method did not distinguish between necrotic and apoptotic cell death.
2.4. Determining Lubricin Released into the Medium
The lubricin released into the surrounding media during culturing were detected and quantified by following a protocol with sandwich ELISA (Enzyme Linked Immuno-Sorbent Assay) technique using a purified superficial zone protein as standard [26]. The synthesis levels obtained from day 11 medium were compared with the levels from day 5 medium, the baseline controls, to evaluate resultant influence of articulation on the biochemical activity of cartilage explants. For the ratio of day 11 over day 5 accumulation numbers, unity indicates no change in accumulation levels; a value higher than 1 indicates increased accumulation, and a value lower than 1 indicates decreased accumulation of the protein.
2.5. Proteoglycan Synthesis and Release in the Explant
2.5.1. Proteoglycan Synthesis
To evaluate the synthesis activity of the explants, a quantification assay using [35S]-sulfate as a radiolabeled precursor was used [27]. The explants were labeled by incubating with fresh media containing [35S]-sulfate for a period of 4 hours. After labeling, the radiolabeled PGs were separated from the free label by alcian blue precipitation and filtration according to the method of Masuda et al. [27] and counted in a scintillation counter. The radioactivity numbers were then normalized to per unit wet-weight of the tissue (this weight was measured prior to labeling).
2.5.2. Proteoglycan Release
Proteoglycans in the medium were quantified by using the DMMB dye binding method of Chandrasekhar et al. [28]. In this method, the collected media samples were incubated with the dye in the presence of guanidinium chloride. The addition of guanidinium chloride eliminated the interference of anionic molecules such as DNA. The dimethylmethylene blue cationic dye binds sulphated glycosaminoglycans and causes an absorbance change at 535 and 595 nm which was measured in a plate reader. The amount of glycosaminoglycans in a particular well was quantified by the absorbance value of that well. Each media sample was examined in 4 separate wells that were later averaged to obtain the glycosaminoglycans value in the given media sample. These glycosaminoglycans values from the medium were then normalized to the respective DNA of each explant, which was determined separately by using a Hoechst assay, by following a previously developed protocol [29]. For quantifying the glycosaminoglycans in the extracellular matrix, the explants were digested overnight in papain solution at 60°C. The papain solution was then processed in a manner described above to quantify the values of glycosaminoglycans, which were then normalized to the respective DNA of each explant.
2.6. Histologic Evaluation
Structural changes in the articular surface were analyzed by performing histological evaluation of the explant sections. The tissue was sectioned, fixed, dehydrated, embedded in paraffin, and stained with Safranin O and fast green (SO/FG) as contrast agent [30]. The slides were imaged using a confocal microscope (Eclipse E600; Nikon Instruments Inc.) at 25x magnification. The specimen images were qualitatively evaluated for signs of cracks and fissures at the articular surface.
2.7. Coefficient of Friction
Coefficient of friction was computed for the explants submersed in cell culture medium by measuring the normal and the tangential forces applied to the cartilage surface during articulation, using in-line miniature load cells (MTS, Neuhausen, Germany, 8414-100, 100N range, 1mV/V, serial number 1135034). The normal forces, resultant of the compressive 20% displacement applied by the ceramic ball compressing the bone-cartilage explants, were captured using a miniature load cell that was placed below the bone-cartilage explants. A miniature slide linear bearing (SKF Motion Technologies, Bethlehem, USA, LLMHS-9, having a dynamic coefficient of friction 0.002–0.003) was used to facilitate free motion of explants along the direction of the generated tangential forces. The slide was modified to allow movement only in the direction of rotation of the ceramic ball. The second miniature load cell was placed in line with the slide to capture the tangential forces, generated due to ball rotation. Both load cells were individually calibrated using dead weights; however, the losses due to the linear bearing friction were not considered and thereby omitted in calculations. For friction measurement, the ball was spun continuously in counter-clockwise direction (0 to 360°) at the rate of 12.4 deg/sec. The explants were then compressed to 0.8 times their cartilage thickness against the spinning ball. During articulation, the friction apparatus and station assembly was moved in a sinusoidal manner (±15°) with respect to the ceramic ball for the MC articulation explants, and was held still with respect to the ceramic ball for the SC articulation explants. The collected force data were filtered using Matlab (MathWorks Inc, Natick, MA USA, Matlab 6.5), and the coefficient of friction (μ) was computed as the ratio of Ftangential / Fnormal. Equivalent coefficient (μeq) was computed as an average of μ during 60th minute of articulation. Figure 2 depicts a sketch of the friction set-up.
Figure 2. Sketch of the set-up for the friction measurements.
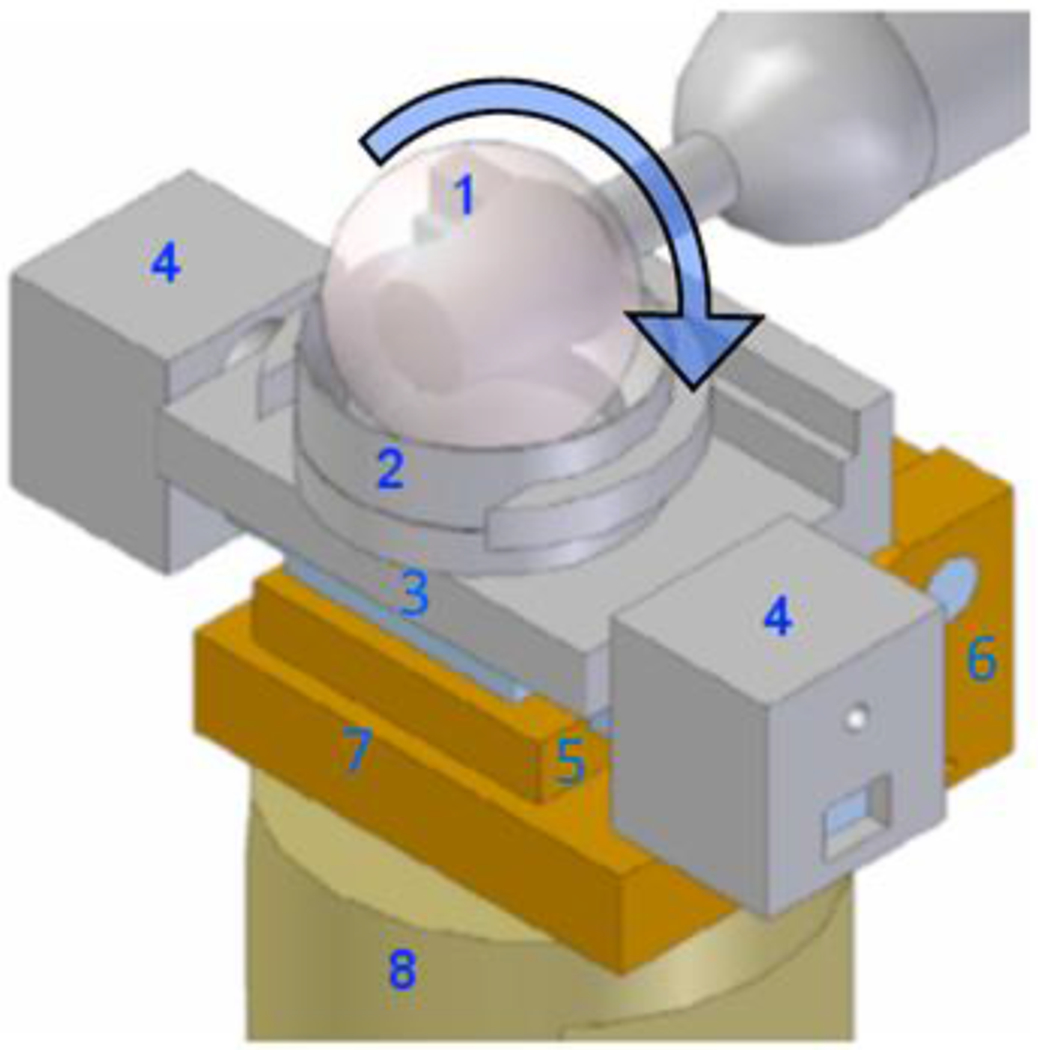
A 32 mm diameter CoCrMo ball (1) rotates equatorially against a bovine articular cartilage plug held in the explant holder (2) mounted onto a sliding stage (3) that is free to slide laterally on a linear bearing. Movements of the sliding stage are prevented by lateral constraints (4) that house the load cells measuring tangential force. The jack plate (5), moving around hinge joint (6), rests on a load cell (embedded in bottom plate (7)) that measures normal force. The whole friction device is mounted onto a single stage (8) of the four-station bioreactor (Figure originally published in [42])
2.8. Change in Surface Topography
The articular surface of the explants was prepared for measurement following a protocol described previously [31], and 15 (3x5 grid with a 5% overlap) topography measurements were made per explant, on days 5 and 11 using a 20× Mirau interferometric objective and a scanning white light interferometry microscope (NewView 6300; Zygo Corp., Middlefield, CT). The areal surface roughness, expressed as the arithmetic average Ra, was computed after correcting for geometry, typically a cylinder or a plane. The topography data was otherwise unfiltered. The change in surface roughness, Ra change was computed by subtracting the day 5 values from the day 11 values.
2.9. Statistics
The correlation between different groups was evaluated using GraphPad Prism 5.00 for Mac (GraphPad Software, San Diego California USA). Cell viability differences between groups, biosynthetic, and functional analyses were performed using ANOVA with Newman-Keuls post test. Differences in the coefficient of friction for the moving point and stationary point articulation groups were evaluated using unpaired, two-tailed t-tests. All the data were first evaluated for Gaussian distribution and analyzed respectively. The multifactorial optimization experiments were designed and analyzed by ANOVA using Design Expert, version 6.0 (Stat-Ease, Inc., Minneapolis, MN). The significance level was set to p < 0.05. Unless otherwise stated, the ± values following means are standard error of means.
3. Results
3.1. MC articulation had reduced cartilage degeneration as compared to SC articulation
SO/FG stained sections were analyzed from the MC group, SC group, and control group. The MC group had minimal to no surface irregularities or fissures. Only in the SC group were fibrillations readily apparent at the articular surface. The MC group was histologically similar to the control group. None of the groups had significant cartilage degeneration in lower depths of the cartilage explant. Representative images are shown in Figure 3.
Figure 3. Representative histological SO/FG stained sections of the cartilage explant surface.

The explant subjected to SC articulation showed discontinuity and fissures on the surface, while the explant subjected to MC articulation retained normal surface morphology, similar to the control group explant. Black arrows represent surface fibrillation.
3.2. MC articulation had increased chondrocyte viability compared to SC articulation
In the superficial zone of the cartilage explant, MC articulation resulted in greater cell viability (chondrocyte viability 55.5 ± 3.17 % live) as compared to SC articulation (37.16 ± 4.47) (p<0.001). There was no difference in cell viability between the control group (61.07 ± 3.25) and the MC group in the superficial zone. There was a decrease in chondrocyte viability between the SC group and the control group (p<0.001) Figure 4A.
Figure 4. Chondrocyte cell viability.
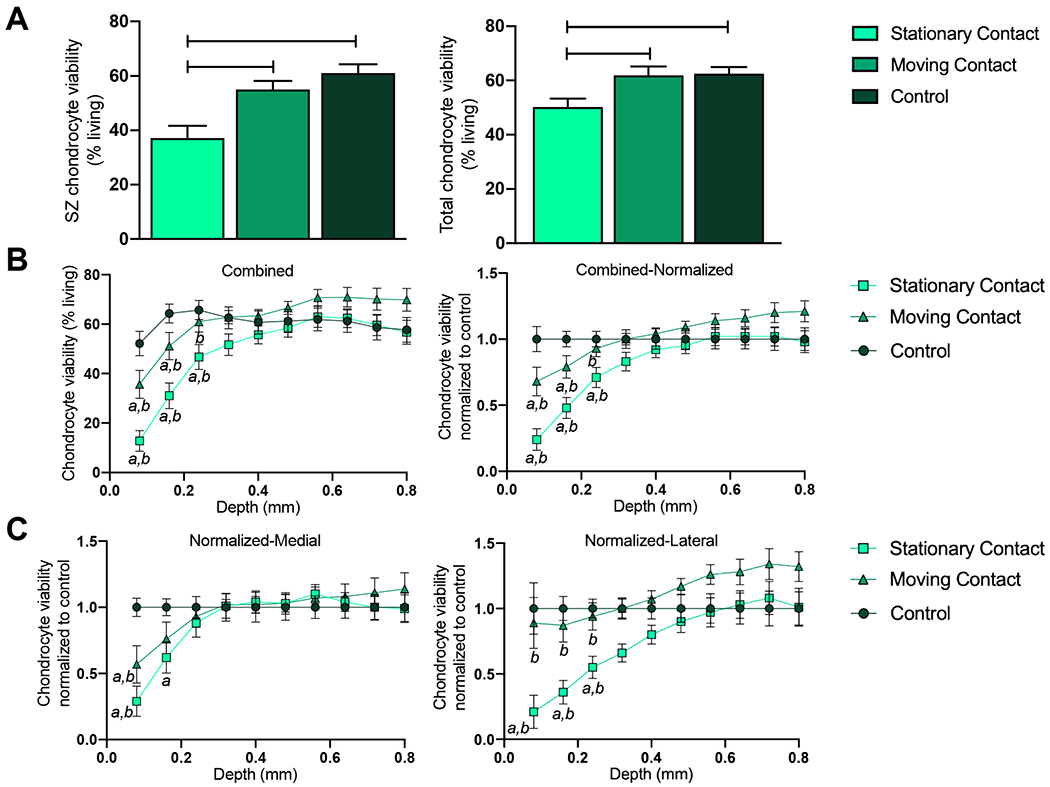
A) Superficial zone chondrocyte viability was reduced in the SC articulation group as compared to the MC or control group; furthermore total chondrocyte viability was reduced in the SC group as compared to the MC or control group. B) Combined analysis (medial and lateral sides) shows that MC articulation helps retain chondrocyte viability, while explants subjected to SC articulation suffer a greater loss in chondrocyte viability up to a greater depth when compared to control group. C) In both the medial and lateral explants, SC articulation reduced chondrocyte viability. Data represented as Mean ± SEM; *p<0.05, **p<0.01, ***p<0.001; a = p <0.05 between control; b = p<0.05 between SC and MC.
In the total cartilage explant, MC articulation resulted in greater cell viability (62.58 ± 2.37) as compared to SC articulation (50.30 ± 3.08) (p<0.05), There was no difference in cell viability between the control group (61.95±15.02) and the MC group. There was a decrease in chondrocyte viability in the SC group as compared to the control group (p<0.01) Figure 4A.
The chondrocyte viability at various depths of the cartilage explant were measured. MC articulation result in greater cell viability up to a depth of 0.24 mm as compared to SC viability (p<0.05), There was a decrease in cell viability between the MC group and control group up to a depth of 0.16 mm (p<0.05). There was a decrease in chondrocyte viability between the SC group and the control group up to a depth of 0.24 mm (p<0.01) Figure 4B.
Assessment of explants harvested from different compartments of the knee (medial or lateral) were analyzed separately. In medial explants, MC articulation resulted in greater cell viability up to a depth of 0.08 mm as compared to stationary point viability (p<0.05). There was a decrease in cell viability between the MC medial group as compared to the control medial group up to a depth of 0.08 mm. There was a decrease in chondrocyte viability in the SC group compared to the control group up to a depth of 0.16 mm (p<0.05). In lateral explants, MC articulation resulted in greater cell viability up to a depth of 0.24 mm as compared to SC viability (p<0.05). There was no change in cell viability between the MC lateral group and control lateral group. There was a decrease in chondrocyte viability in the SC group as compared to the control group up to a depth of 0.24 mm (p<0.05) Figure 4C.
3.3. MC articulation had increased lubricin synthesis and comparable proteoglycan synthesis and release compared to SC articulation
For relative amounts of lubricin released into the conditioned medium, explants subjected to MC articulation (Ratio day11/day5 = 2.89 ± 0.38) had significantly higher lubricin synthesis (p<0.05) as compared to both the stationary point articulation (0.96 ± 0.27) and the control (1.58 ± 0.41) explants. No difference was observed between the SC articulation and the control explants for lubricin in the conditioned medium Figure 5A. Evaluation of the levels of proteoglycan synthesized and released into the conditioned medium showed that there was no difference (p>0.05) in any of the three groups. Proteoglycan synthesis for explants from the three groups was similar: MC articulation (proteoglycan synthesis 1252 ± 192 CPM/WW mg); stationary point articulation (1267 ± 197); controls (1373 ± 138). Also, proteoglycan released into the medium by explants from the three groups was similar: MC articulation (proteoglycan release 828 ± 74 μg/ml); SC articulation (861 ± 48); controls (758 ± 47) (Figure 5B).
Figure 5. Chondrocyte biosynthetic response.
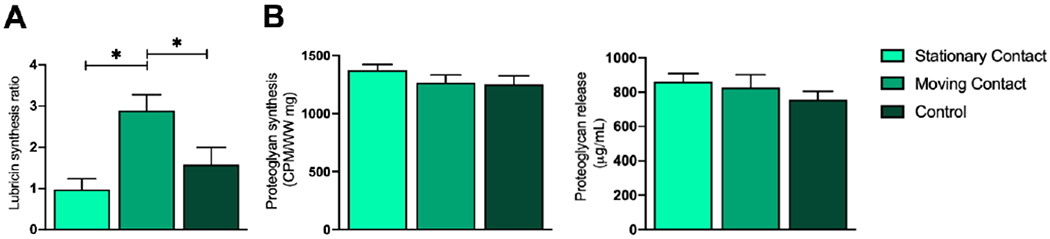
A) Lubricin release was increased in the MC group as compared to the SC or control group. B) Proteoglycan synthesis and release between the SC, MC, and control group was similar. Data represented as Mean ± SEM; *p<0.05.
3.4. MC articulation had reduced coefficient of friction and reduced surface roughness
Bone-cartilage explants subjected to MC articulation and 60 minutes of friction testing had lower values for the coefficient of friction over time, as compared to the explants with SC articulation Figure 6A. The equivalent peak coefficient of friction for MC articulation (0.09 ± 0.004) is significantly lower than the equivalent coefficient of friction for SC articulation (0.30 ± 0.009) (p<0.05), as seen in Figure 6B.
Figure 6. Influence of articulation type on coefficient of friction and surface roughness of articular cartilage.
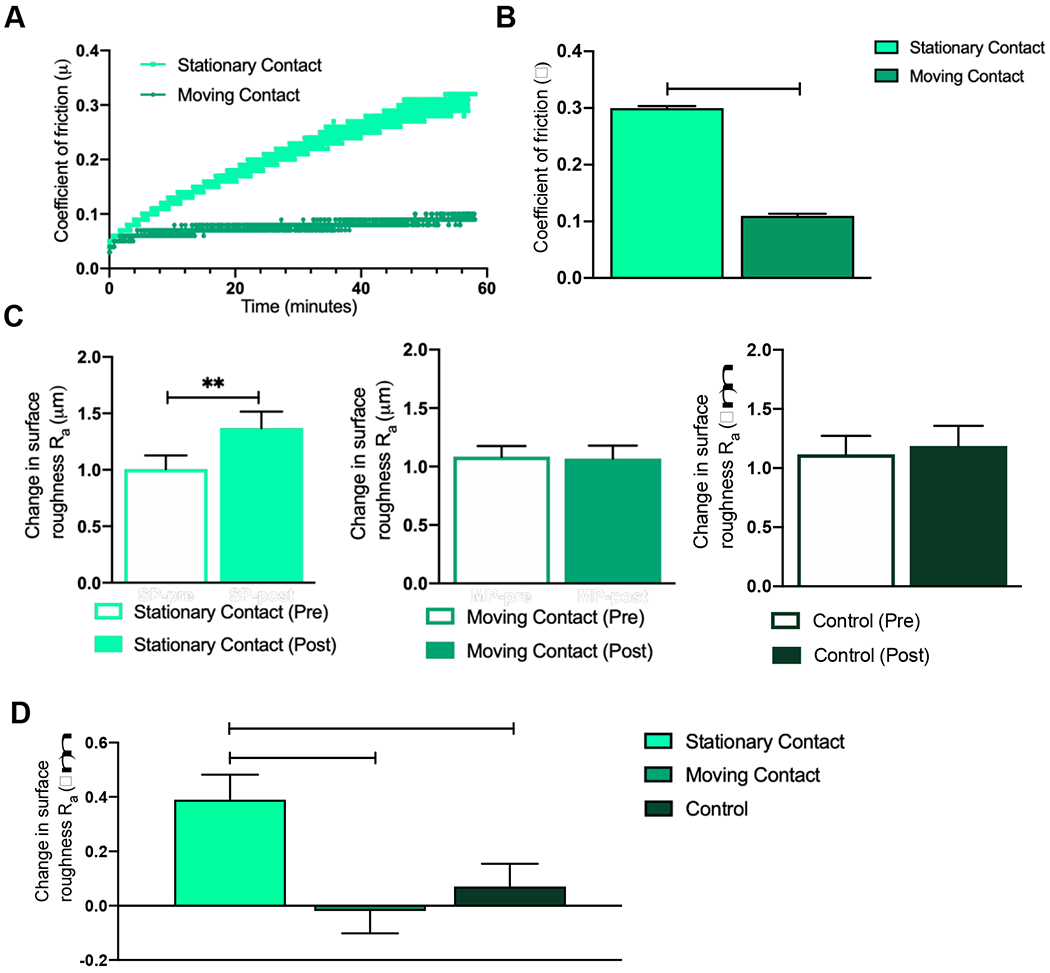
A) Change in coefficient of friction over time is higher in the SC group as compared to the MC group. B) SC group had the highest coefficient of friction over 60 minutes as compared to the MC group. C) Change in surface roughness was increased in the SC group but not increased in the MC or control group. D) Change in surface roughness was increased in the SC group as compared to the MC or control group. Data represented as Mean ± SEM; *p<0.05, **p<0.01.
In the primary in vitro articulation study, it was observed that the cartilage explants with MC articulation did not differ in surface roughness between pre articulation (day 5) (Ra = 1.08 ± 0.09 μm) and post articulation (day 11) (Ra = 1.07 ± 0.11 μm). Similarly, the controls did not differ in surface roughness between pre articulation (Ra = 1.09 ± 0.15 μm) and post articulation (Ra = 1.26 ± 0.17 μm). However, the explants with SC articulation showed an increase (p<0.01) in surface roughness between pre articulation (Ra = 1.01 ± 0.12 μm) and post articulation (Ra = 1.37 ± 0.15 μm) Figure 6C.
Upon one-way ANOVA for change in surface roughness (Ra change = Ra day 11 – Ra day 5 μm) for the three groups, it was observed that the explants with MC articulation (Ra change = −0.02 ± 0.27 μm) were not different (p>0.05) from the controls (Ra change = 0.07 ± 0.28 μm). However, the explants with SC articulation (Ra change = 0.39 ± 0.32 μm) had an increase in surface roughness as compared to both the explants with MC articulation (p<0.01) and the controls (p<0.05), as seen in Figure 6D.
4. Discussion
The results of this study demonstrated that a migrating or moving contact area plays an important and beneficial role in cartilage homeostasis by influencing structural, functional, and biosynthetic properties of the articular cartilage. Detailed evaluation of the combined analysis of the biosynthetic, the structural, and the functional data of MC explants, emphasizes the importance of a migrating contact area and the roles it plays in maintaining cartilage homeostasis. Continuously moving the point of contact has a three-fold effect of enlarging the load-bearing region thereby distributing the applied stresses over a larger area, shortening the loading time for individual regions inside this large area, and increasing external hydrodynamic (EHD) pressure thereby facilitating fluid and solute transport into cartilage.
The first effect of the moving point of contact is an expanded load-bearing region where more cartilage is being stimulated actively by the compressive stresses. It has been shown for articular cartilage that load bearing regions have higher biosynthetic activity compared to the non load-bearing regions within the joint [32]. Broader load-bearing region would thus increase the biosynthetic response of articular cartilage. In the present study, explants in the MC group had higher lubricin release in the medium with similar superficial zone viability numbers as the controls. On the contrary, explants in the stationary group did not differ from controls in the lubricin release in the medium, despite having lower superficial zone viability numbers than the controls. Together, these results indicate an increased biosynthetic response of the chondrocytes from articulating explants.
The second effect of the MC during articulation is the shortening of loading time, ensuring cyclic periodic loading of cartilage for short durations. Articular cartilage has been shown to use interstitial fluid pressure, extra-cellular matrix components, and chondrocytes to support the applied joint forces [1, 33, 34]. Among these, interstitial fluid pressure supports majority (up to 95%) of the applied load, and has a high threshold for compressive forces. Extra-cellular matrix components and chondrocytes, together, support the remaining 5% [33]. The interstitial fluid pressure has a key limitation that its support of the applied load is for a short duration, lasting up to a few minutes. When applied for a longer duration, compressive forces lead to exudation of interstitial fluid from the extra-cellular matrix, resulting in loss of interstitial fluid pressure [35]. Also worth noting is the depth dependent, inhomogeneous exudation of interstitial fluid. The fluid mostly exudes from the superficial zone, a primary fluid reservoir and over time is then redistributed within the tissue to sustain the applied load [1]
The effect of loading time was seen on the articulation explants. Contact areas with MC articulation were loaded for shorter durations and interstitial fluid pressure supported the forces and maintained low friction at the articular surface. Support of applied stresses by interstitial fluid pressure can also be attributed for the continuity observed at the articular surface and the sustained surface topography after articulation. On the other hand, explants with SC articulation were loaded for longer durations, exuding the interstitial fluid and thereby the interstitial fluid pressure. For these SC articulation explants, applied loads were eventually supported by the extra-cellular matrix components and the chondrocytes. Loading these components leads to higher friction forces [35], which is likely responsible for the high percentage of dead chondrocytes near the articular surface, and also for the fibrillations at the articular surface. Change in the load carrying mechanism of cartilage may also alter the lubrication modes at the articular surface. Although the exact impact on lubrication was not investigated, the current study shows that explants with SC articulation develop higher values for the coefficient of friction with time. Our results support previous investigation into migratory contact area, which suggested that migrating contact areas maintain elevated cartilage interstitial fluid pressurization, which has a dramatic effect of lowering the coefficient of friction [7]. In the SC group, the high friction at the articular surface can be the reason for surface fissures and cracks and for the increase in surface roughness post articulation.
A third effect of MC during articulation is the influence of increased EHD pressure, which is a result of sliding of the ceramic ball on the cartilage. It has been suggested that sliding can produce a phenomenon termed “tribological rehydration” [11,12]. During the sliding motion, fluid flows into a convergent wedge around the point of contact resulting in increased EHD pressure. This pressure drives fluid into the cartilage surface and increases the available interstitial fluid. The increased fluid pressurization results in decreased friction [11]. Furthermore, during this process of tribological rehydration, increased solute transport is facilitated. Therefore, sliding could also be an important mediator of nutrient transport into cartilage and play a role in cartilage and chondrocyte homeostasis [36]. However, it should be noted that the rehydration effect is mainly driven by sliding speed and only secondarily by reciprocation [37]. Hence, for higher sliding speeds, it is conceivable that tribological rehydration occurs also for SC.
This study applied 20% compressive strain rather than a controlled load to the cartilage explants. Hence, in SC mode, the applied load is reduced over time. This load reduction, which will not occur to the same extent in MC mode, may have embellished the SC results. Another potential drawback of the study could be articulation of the cartilage explant against a non-physiological material. Articulation by a hard, non-compliant material, the ceramic ball, could be a reason for the low cell viability numbers in the superficial zone (0.32mm from cartilage surface) for explants with SC articulation. However, for the explants with MC articulation, this loss in viability is only seen for the top layer (0.08mm from cartilage surface).
In a similar argument, increase in lubricin in the conditioned media may be influenced by erosion or rubbing off of the protein by the ceramic ball. Assuming that lubricin coats the articular surface with an even thickness, it can be argued that a moving contact point would cover larger area and can thus have higher rubbing off of lubricin, compared to the stationary contact point with a comparatively smaller area. Such an argument has to be taken with care since it has been shown that lubricin does not uniformly coat the articular surface, and load bearing regions have a higher concentration of lubricin than the non-load bearing regions [32, 38]; what may actually refute this argument is the observation that higher amounts of lubricin were released into the media by MC group explants on days 10 and 11 during rest after articulation (data not shown). Increase of lubricin on a day of rest indicates synthetic activity of the chondrocytes, and this data is consistent with a previous study [39]where a trend was observed during post-test culture period for an increase in lubricin synthesis. There was no change in the release of lubricin for explants with a stationary contact point.
Although hyaluronic acid (HA) is a major component of synovial fluid contributing to its viscosity and lubricating properties, we did not add it to the culture medium out of concern that it may interfere with biological outcome measures. It has been our own experience as well as those of others that in the presence of HA cell viability in cartilage explants suffers, and PG/GAG analysis using the DMMB assay becomes less reliable [40]. These methodological difficulties are in stark contrast to the known role of HA in cartilage boundary lubrication, where HA-phospholipid complexes facilitate hydration lubrication [41]. We therefore started to work on a medium that contains HA-phospholipid vesicles in order to better simulate the lubricating properties of synovial fluid and at the same time maintain healthy culture conditions. We published preliminary results on the rheology and friction diminishing effects of this new cell culture mixture [42], which now much better simulates synovial fluid, and we also found that cell viability can be maintained. We therefore believe, in the future, cell culture media containing HA-phospholipid vesicles may become the lubricant of choice for cartilage explant studies.
Summarizing the study results, we can conclude that moving the contact point during articulation has been shown to be beneficial to the cartilage explants, in terms of maintaining the viability, stimulating the biosynthetic response of chondrocytes, and sustaining the functional properties of the cartilage explants. The advantage of moving the contact point over the cartilage surface can be manifold. The shorter period of loading may lead to retaining the interstitial fluid for longer periods and thereby maintaining interstitial fluid pressure which would then bear majority of applied load and effectively reduce the coefficient of friction [7, 34]. Moving the contact point may induce fluid flow [11, 36, 43], thereby also supplying essential nutrients to the tissue during articular motion. A MC articulation also provides greater contact area on the cartilage to potentially stimulate a greater biosynthetic response. Tribological research studies, which model cartilage articulation with a stationary contact – as for example by rubbing an osteochondral plug against a flat counterface – , need to consider that the set-up may differentially affect the structural, functional, and biosynthetic response of the tissue in vitro, and may be problematic for long term wear testing. Further research into this area could provide important insights for in vitro testing of cartilage with implications for both joint homeostasis and disease.
Acknowledgements
This study was funded in part by a research training grant from the National Institutes of Health NIH/NIAMS T32AR073157 (Trainee JLH). The authors thank Dr. Chubinskaya, Rush University Medical Center for support and discussion related to histology.
Funding
NIH/NIAMS T32AR073157 (PI Rick Sumner)
Abbreviations:
- MC
moving contact
- SC
stationary contact
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest. Partial funding was obtained through a National Institutes of Health Training grant with Dr. John Hamilton as the grantee (NIH/NIAMS T32AR073157 (PI Rick Sumner; co-director Markus Wimmer). The sponsor had no influence on study design, data analysis, and writing of the manuscript.
References
- [1].Mow VC, Wang CC, Some bioengineering considerations for tissue engineering of articular cartilage, Clin Orthop Relat Res (367 Suppl) (1999) S204–23. [DOI] [PubMed] [Google Scholar]
- [2].Wimmer MA, Grad S, Kaup T, Hanni M, Schneider E, Gogolewski S, Alini M, Tribology approach to the engineering and study of articular cartilage, Tissue Eng 10(9-10) (2004) 1436–45. 10.1089/ten.2004.10.1436 [DOI] [PubMed] [Google Scholar]
- [3].Trevino RL, Pacione CA, Malfait AM, Chubinskaya S, Wimmer MA, Development of a Cartilage Shear-Damage Model to Investigate the Impact of Surface Injury on Chondrocytes and Extracellular Matrix Wear, Cartilage 8(4) (2017) 444–455. 10.1177/1947603516681133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Trevino RL, Stoia J, Laurent MP, Pacione CA, Chubinskaya S, Wimmer MA, Establishing a Live Cartilage-on-Cartilage Interface for Tribological Testing, Biotribology (Oxf) 9 (2017) 1–11. 10.1016/j.biotri.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shekhawat VK, Schmid TM, Pennekamp PH, Pacione CA, Chubinskaya S, Wimmer MA, Implications of trauma and subsequent articulation on the release of Proteoglycan-4 and tissue response in adult human ankle cartilage, Journal of orthopaedic research : official publication of the Orthopaedic Research Society 35(3) (2017) 667–676. 10.1002/jor.23397 [DOI] [PubMed] [Google Scholar]
- [6].Andriacchi TP, Stanwyck TS, Galante JO, Knee biomechanics and total knee replacement, J Arthroplasty 1(3) (1986) 211–9. 10.1016/s0883-5403(86)80033-x [DOI] [PubMed] [Google Scholar]
- [7].Caligaris M, Ateshian GA, Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction, Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 16(10) (2008) 1220–7. 10.1016/j.joca.2008.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schatti OR, Gallo LM, Torzilli PA, A Model to Study Articular Cartilage Mechanical and Biological Responses to Sliding Loads, Ann Biomed Eng 44(8) (2016) 2577–88. 10.1007/s10439-015-1543-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schatti OR, Markova M, Torzilli PA, Gallo LM, Mechanical Loading of Cartilage Explants with Compression and Sliding Motion Modulates Gene Expression of Lubricin and Catabolic Enzymes, Cartilage 6(3) (2015) 185–93. 10.1177/1947603515581680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bonnevie ED, Baro V, Wang L, Burris DL, In-situ studies of cartilage microtribology: roles of speed and contact area, Tribol Lett 41(1) (2011) 83–95. 10.1007/s11249-010-9687-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moore AC, Burris DL, Tribological rehydration of cartilage and its potential role in preserving joint health, Osteoarthritis Cartilage 25(1) (2017) 99–107. 10.1016/j.joca.2016.09.018 [DOI] [PubMed] [Google Scholar]
- [12].Graham BT, Moore AC, Burris DL, Price C, Detrimental effects of long sedentary bouts on the biomechanical response of cartilage to sliding, Connect Tissue Res 61(3–4) (2020) 375–388. 10.1080/03008207.2019.1673382 [DOI] [PubMed] [Google Scholar]
- [13].Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD, Biosynthetic response of cartilage explants to dynamic compression, J Orthop Res 7(5) (1989) 619–36. 10.1002/jor.1100070502 [DOI] [PubMed] [Google Scholar]
- [14].Park S, Hung CT, Ateshian GA, Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels, Osteoarthritis Cartilage 12(1) (2004) 65–73. 10.1016/j.joca.2003.08.005 [DOI] [PubMed] [Google Scholar]
- [15].Nugent GE, Schmidt TA, Schumacher BL, Voegtline MS, Bae WC, Jadin KD, Sah RL, Static and dynamic compression regulate cartilage metabolism of PRoteoGlycan 4 (PRG4), Biorheology 43(3–4) (2006) 191–200. [PubMed] [Google Scholar]
- [16].Nugent GE, Aneloski NM, Schmidt TA, Schumacher BL, Voegtline MS, Sah RL, Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4, Arthritis Rheum 54(6) (2006) 1888–96. 10.1002/art.21831 [DOI] [PubMed] [Google Scholar]
- [17].Mow V, “Functional tissue engineering”, Conf Proc IEEE Eng Med Biol Soc 1 (2006) nil 16–7. [Google Scholar]
- [18].Krishnan R, Mariner EN, Ateshian GA, Effect of dynamic loading on the frictional response of bovine articular cartilage, J Biomech 38(8) (2005) 1665–73. 10.1016/j.jbiomech.2004.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dowson D, Lubrication and wear of joints, Physiotherapy 59(4) (1973) 104–6. [PubMed] [Google Scholar]
- [20].Basalo IM, Mauck RL, Kelly TA, Nicoll SB, Chen FH, Hung CT, Ateshian GA, Cartilage interstitial fluid load support in unconfined compression following enzymatic digestion, J Biomech Eng 126(6) (2004) 779–86. 10.1115/1.1824123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grad S, Lee CR, Goma K, Gogolewski S, Wimmer MA, Alini M, Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds, Tissue Eng 11(1-2) (2005)249–56. 10.1089/ten.2005.11.249 [DOI] [PubMed] [Google Scholar]
- [22].Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Banes AJ, Guilak F, The effects of static and intermittent compression on nitric oxide production in articular cartilage explants, J Orthop Res 19(4) (2001) 729–37. 10.1016/S0736-0266(00)00049-8 [DOI] [PubMed] [Google Scholar]
- [23].ISO 14243-3:2014 Implants for surgery – Wear of total knee-joint prostheses – Part3: Loading and displacement parameters for wear-testing machines with displacement control and corresponding environmental conditions for test. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- [24].Buckwalter JA, Articular cartilage, Instr Course Lect 32 (1983) 349–70. [PubMed] [Google Scholar]
- [25].Shekhawat VK, Laurent MP, Muehleman C, Wimmer MA, Surface topography of viable articular cartilage measured with scanning white light interferometry, Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 17(9) (2009) 1197–203. 10.1016/j.joca.2009.03.013 [DOI] [PubMed] [Google Scholar]
- [26].Su JL, Schumacher BL, Lindley KM, Soloveychik V, Burkhart W, Triantafillou JA, Kuettner K, Schmid T, Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein, Hybridoma 20(3) (2001) 149–57. 10.1089/027245701750293475 [DOI] [PubMed] [Google Scholar]
- [27].Masuda K, Shirota H, Thonar EJ, Quantification of 35S-labeled proteoglycans complexed to alcian blue by rapid filtration in multiwell plates, Anal Biochem 217(2) (1994) 167–75. 10.1006/abio.1994.1105 [DOI] [PubMed] [Google Scholar]
- [28].Chandrasekhar S, Esterman MA, Hoffman HA, Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride, Anal Biochem 161(1) (1987) 103–8. 10.1016/0003-2697(87)90658-0 [DOI] [PubMed] [Google Scholar]
- [29].McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL, Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258, Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 10(7) (2002) 580–7. 10.1053/joca.2002.0794 [DOI] [PubMed] [Google Scholar]
- [30].Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB, Osteoarthritis cartilage histopathology: grading and staging, Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 14(1) (2006) 13–29. 10.1016/j.joca.2005.07.014 [DOI] [PubMed] [Google Scholar]
- [31].Shekhawat VK, Laurent M, Muehleman C, Wimmer MA, Characterizing the Surface Topography of Viable Cartilage Explants - A Novel Application of the Scanning White Light Interferometer, Proceedings of the 2008 Summer Bioengineering Conference, Marco Islands, FL, 2008. [Google Scholar]
- [32].Nugent-Derfus GE, Takara T, O'Neill JK, Cahill SB, Gortz S, Pong T, Inoue H, Aneloski NM, Wang WW, Vega KI, Klein TJ, Hsieh-Bonassera ND, Bae WC, Burke JD, Bugbee WD, Sah RL, Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4, Osteoarthritis and Cartilage 15(5) (2007) 566–574. 10.1016/j.joca.2006.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Park S, Krishnan R, Nicoll SB, Ateshian GA, Cartilage interstitial fluid load support in unconfined compression, J Biomech 36(12) (2003) 1785–96. 10.1016/s0021-9290(03)00231-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Soltz MA, Ateshian GA, Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage, Ann Biomed Eng 28(2) (2000) 150–9. 10.1114/1.239 [DOI] [PubMed] [Google Scholar]
- [35].Ateshian GA, The role of interstitial fluid pressurization in articular cartilage lubrication, J Biomech 42(9) (2009) 1163–76. 10.1016/j.jbiomech.2009.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Graham BT, Moore AC, Burris DL, Price C, Sliding enhances fluid and solute transport into buried articular cartilage contacts, Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 25(12) (2017) 2100–2107. 10.1016/j.joca.2017.08.014 [DOI] [PubMed] [Google Scholar]
- [37].Burris DL, Ramsey L, Graham BT, Price C, Moore AC, How Sliding and Hydrodynamics Contribute to Articular Cartilage Fluid and Lubrication Recovery, Tribology Letters 67(2) (2019) 46. 10.1007/s11249-019-1158-7 [DOI] [Google Scholar]
- [38].Li Z, Yao S, Alini M, Grad S, Different response of articular chondrocyte subpopulations to surface motion, Osteoarthritis Cartilage (2007). 10.1016/j.joca.2007.03.001 [DOI] [PubMed] [Google Scholar]
- [39].Shekhawat VW; Alini M, Madsen L; Schmid T, Effect of articular motion on cartilage-bone explants, Trans Orthop Res Soc. 31:1506 (2006). [Google Scholar]
- [40].Warren S, A critical analysis of the 1,9-dimethylene blue assay for sulfated glycosaminoglycans in synovial fluid University of Guelph (2000). [Google Scholar]
- [41].Lin W, Liu Z, Kampf N, Klein J, The Role of Hyaluronic Acid in Cartilage Boundary Lubrication, Cells 9(7) (2020). 10.3390/cells9071606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Veselack T, Aldebert G, Trunfio-Sfarghiu AM, Schmid TM, Laurent MP, Wimmer MA, Phospholipid Vesicles in Media for Tribological Studies against Live Cartilage, Lubricants 6(1) (2018). 10.3390/lubricants6010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Morita Y, Tomita N, Aoki H, Wakitani S, Tamada Y, Suguro T, Ikeuchi K, Evaluation of dynamic visco-elastic properties during cartilage regenerating process in vitro, Biomed Mater Eng 13(4) (2003) 345–53. [PubMed] [Google Scholar]


