Abstract
Prostaglandin E2 (PGE2) is known to have important roles in labor, but the detailed mechanism underlying the spontaneous human labor remains unknown. Here, we examined the involvement of prostaglandin biosynthetic enzymes and transporter in the accumulation of PGE2 in amniotic fluid in human labor. PGE2 and its metabolites were abundant in amniotic fluid in deliveries at term in labor (TLB), but not at term not in labor (TNL). In fetal-membrane Transwell assays, levels of PGE2 production in both maternal and fetal compartments were significantly higher in the TLB group than the TNL group. In fetal-membrane, the mRNA level of PTGES3, which encodes cytosolic prostaglandin E synthase (cPGES), was significantly higher in TLB than in TNL, but the mRNA levels of the other PGE2-synthase genes were not affected by labor. Moreover, the mRNA level of PTGS2, which encodes cyclooxygenase-2 (COX-2) in the amnion was significantly higher in TLB than in TNL. Western blot analyses revealed that the levels of COX-1 and COX-2 were comparable between the two groups, however, the level of cPGES was relatively higher in TLB than in TNL. COXs, cPGES, and prostaglandin transporter (SLCO2A1) proteins were all expressed in both chorionic trophoblasts and amniotic epithelium. These findings suggest that COXs, cPGES and SLCO2A1 contribute to PGE2 production from fetal-membrane in labor.
Introduction
Prostaglandin E2 (PGE2) is a bioactive lipid mediator and plays important roles in the induction and maintenance of labor in mammals [1,2]. PGE2 is synthesized from arachidonic acid (AA) sequentially by the actions of cyclooxygenase (COX)-1/COX-2 and three terminal PGE2 synthase (PGES) enzymes: cytosolic PGES (cPGES), microsomal PGES (mPGES)-1, and mPGES-2. In addition, PGE2 is an organic anion, and at physiological pH, the PG transporters, such as solute carrier organic anion transporter family member 2A1 (SLCO2A1), are required for its secretion. Although some studies reported the accumulation of PGE2 in amniotic fluid from pregnant women with labor [3], the detailed molecular mechanism underlying PGE2 production with labor remains largely unknown. Up-regulation of COX-2 expression has been observed in fetal-membrane, placental villous tissue, and uterine myometrium prior to the onset of spontaneous labor [4–6]. However, only a few studies have investigated the expression of PGESs in human fetal-membrane from pregnant women with labor. One study reported the increased expression of mPGES-1 in chorion from pregnant women with labor [7], but the other study reported no differences of the expression with labor [8]. The expression of mPGES-1 in fetal-membrane from pregnant women with labor is increased by infection [9]. However, the expression of cPGES with labor remains largely unclear [10].
In this study, we collected the TLB (term in labor) samples from the pregnant women with spontaneous onset of labor and the TNL (term not in labor) samples from the women before the onset of labor, and quantified PGs and their metabolites in the amnion, and examined the expression of enzymes and transporters involved in production and release of PGs. To our knowledge, this is the first study to suggest that cPGES contributes to PGE2 production from fetal-membrane in labor.
Materials and methods
Study participants
Pregnant women at term (n = 21) were classified according to the type of delivery: TLB (n = 10) or TNL (n = 11). Onset of labor was defined as the presence of regular uterine contractions with a frequency greater than one every 10 min, with cervical changes including softening and opening. All participants had been free of medication for ≥2 weeks prior to delivery, whereas those who fulfilled the diagnostic criteria for chorioamnionitis were excluded. The participants with bacterial vaginosis were also excluded, and there were no participants who had meconium in our study.
Umbilical-cord blood and fetal-membrane were collected immediately after delivery of the placenta from each participant. Umbilical-cord blood (2 ml) was collected in a heparinized tube, immediately after delivery. Fetal-membrane (amnion, chorion, and decidua) were collected with avoidance of placental-attachment sites. Fetal-membrane were collected 5 cm from the placenta and sites of membrane rupture, whereas placental tissue was collected 3 cm from the umbilical-cord attachment site. Amniotic fluid was taken by amniocentesis from the TNL group during cesarean sections, and from the TLB group during delivery. All amniotic-fluid samples were free of blood contamination.
Materials
Eicosanoid standards and deuterium-labeled eicosanoids were purchased from Cayman Chemical (Ann Arbor, MI, USA). All solvents used were HPLC or LC/MS grade, and were purchased from Wako Pure Chemical (Osaka, Japan) or Thermo Fisher Scientific (Waltham, MA, USA).
Sample preparation for LC-MS/MS analysis
Blood in heparinized tubes was kept on ice and then centrifuged at 800 × g for 5 min at 4°C to separate plasma from blood cells. Amniotic fluid was centrifuged at 1,000 × g for 10 min at 4°C. Samples of plasma and supernatants of amniotic fluid were immediately mixed with two volumes of methanol and stored at −80°C. Samples of fetal-membrane were immediately frozen in liquid nitrogen and stored at −80°C prior to homogenization with an Automill (Tokken, Chiba, Japan) and extraction of lipids with 1 ml of methanol at −20°C. After overnight incubation, the samples were centrifuged at 5,000 × g for 5 min at 4°C. The supernatants were mixed with nine volumes of water containing 0.1% formic acid, which included deuterium-labeled internal standards (Cayman Chemical). Diluted samples were loaded onto a solid-phase extraction cartridge (Waters, Milford, MA, USA) and washed serially with water containing 0.1% formic acid, 15% methanol containing 0.1% formic acid, and petroleum ether containing 0.1% formic acid. After air drying the cartridge with suction, the eicosanoids were eluted with 200 μl of methanol containing 0.1% formic acid.
LC-MS/MS analysis
Eicosanoid profiling of amniotic fluid and fetal-membranes was performed according to previously described methods, with some modifications [11–13]. Reversed-phase-LC-MS/MS used a Shimadzu liquid-chromatography system consisting of four LC-20AD pumps, a SIL-20AC autosampler, a CTO-20AC column oven, an FCV-12AH six-port switching valve, and a TSQ Quantum Ultra triple-quadrupole mass spectrometer equipped with an electrospray-ionization ion source (Thermo Fisher Scientific). A 50 μl aliquot of each sample was injected into the trap column (Opti-Guard Mini C18) at a total flow rate of 500 μl/min; 3 min after sample injection, the valve was switched to introduce the trapped sample to the analytical column (Capcell Pak C18 MGS3; Shiseido, Tokyo, Japan). Mobile phase A was water, and phase B was acetonitrile:formic acid (1000:1). The gradient conditions consisted of 37% B from 0 to 7 min, a linear gradient of 37–90% B from 7 min to 19 min, 100% B from 19 min to 21 min, and 37% B from 21 min to 22.5 min. The total flow rate was 120 μl/min, the column temperature was set at 46°C, and the column eluent was introduced directly into a TSQ Quantum Ultra mass spectrometer. All compounds were analyzed in a negative-ion-polarity mode. Eicosanoids were quantified by multiple reaction monitoring of the following transitions: PGE2, m/z 351 → 271; 15-keto-PGE2, m/z 349 → 235; 13,14-dihydro-15-keto-PGE2, m/z 351 → 175; 19-hydroxy-PGE2, m/z 367 → 189; [2H4]PGE2, m/z 355 → 275; and [2H4]13,14-dihydro-15-keto-PGE2, m/z 355 → 239. For accurate quantification, calibration curves were generated for each target eicosanoid using known reference standards and the isotope-labeled internal standard. Automated peak detection, calibration, and calculation were carried out with the Xcalibur 2.2 software package.
Fetal-membrane Transwell assays
Fetal-membrane Transwell assays were performed as previously described [14]. Briefly, a 3 × 3 cm square of fetal-membrane was placed in a 24 mm Transwell clear insert (Corning, Lindfield, Australia) (see Fig 3A) in 6-well culture plate containing serum-free culture medium (DMEM/Ham’s Nutrient Mixture F-12, phenol red-free, supplemented with 15 mM HEPES, pH 7.3 (Sigma-Aldrich, St Louis, MO, USA) and 0.5% fatty acid-free BSA (Sigma-Aldrich)). The maternal compartment on the decidua contained 3 ml medium and the fetal compartment on the amnion contained 2.5 ml medium. Membranes were incubated for 120 min at 37°C in an atmosphere of air containing 5% CO2. Medium (100 μl) was collected from both compartments at 0, 15, 30, and 60 min. Eicosanoids in the medium were analyzed by LC-MS/MS as described above.
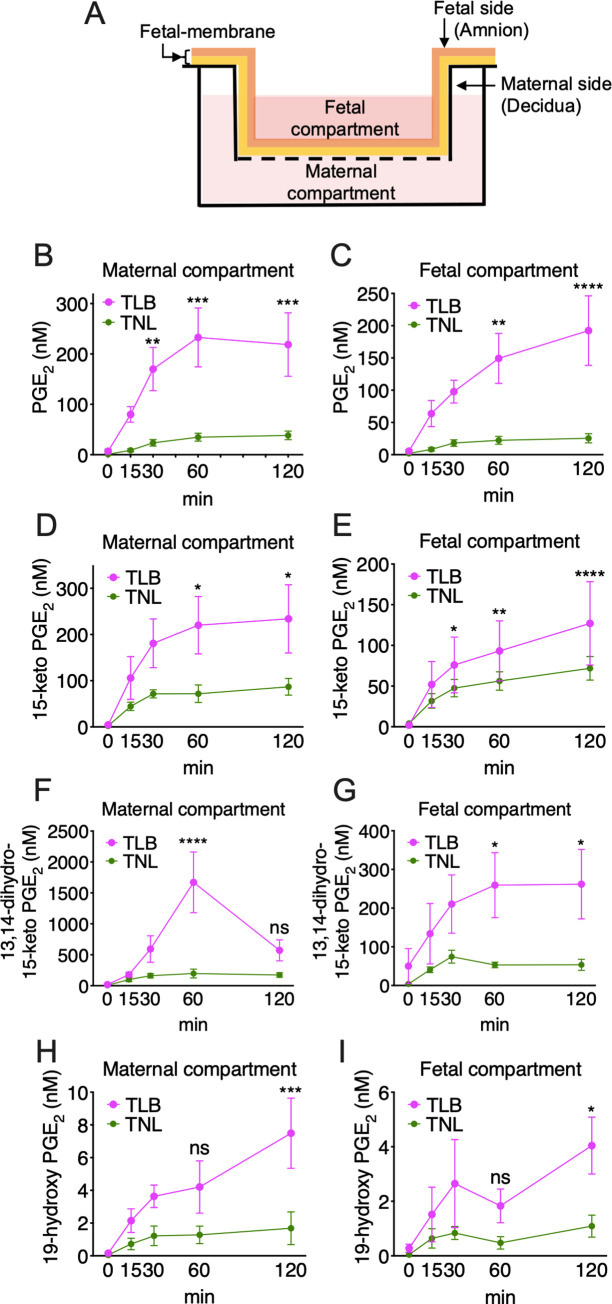
Fig 3. Production of prostaglandin E2 (PGE2) and its metabolites by fetal-membrane in vitro.
(A) Schematic presentation of fetal-membrane Transwell assay. (B-I) Accumulation of PGE2 (B and C),15-keto-PGE2 (D and E), 13,14-dihydro-15-keto-PGE2 (F and G) and 19-hydroxy-PGE2 (H and I) in maternal and fetal compartments was quantified by LC-MS/MS. Circle markers and error bars indicate the mean ± SEM. Data were analyzed by two-way ANOVA. *p <0.05, **p <0.01, ***p <0.005, ****p <0.0001. Term not in labor (TNL) samples, n = 6; term in labor (TLB) samples, n = 6.
RT-qPCR
Fetal-membranes were harvested and placed into RNAlater Solution (Ambion, Austin, TX, USA) at 4°C for 24 h, then stored at −80°C. Total RNA was extracted with RNeasy Fibrous Tissue Kit (Qiagen, Venlo, the Netherlands), and cDNA was generated with QuantiTect Reverse Transcription Kit (Qiagen). qPCR was performed with ABI TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific) on a 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). Gene expression levels were normalized to those of ACTB (β-actin) using the ΔΔCt method. The following Taqman probes were used: ACTB (Assay ID: Hs99999903_m1, Gene ID: Hs.520640), PLA2G4A (Assay ID: Hs00996912_m1, Gene ID: Hs497200), PTGS1 (Assay ID: Hs00377726_m1, Gene ID: Hs201978), PTGS2 (Assay ID: Hs00153133_m1, Gene ID: Hs196384), PTGES (Assay ID: Hs01115610_m1, Gene ID: Hs146688), PTGES2 (Assay ID: Hs00228159_m1, Gene ID: Hs495219), and PTGES3 (Assay ID: Hs00832847_gH, Gene ID: Hs50425).
Western blotting
Each fetal-membrane sample was homogenized with an Automill (Tokken, Chiba, Japan) in 2 ml of lysis buffer (50 mM Tris-HCl, pH 7.4, 1% SDS, 50 mM NaCl, 20 mM NaF, 1 mM Na3VO4, 1 mM EDTA, with protease inhibitors (Nacalai Tesque, Kyoto, Japan)). Lysates were centrifuged at 4,000 × g for 5 min at 4°C, supernatants were recovered, and their protein concentrations were determined with a BCA assay kit (Nacalai Tesque). Each sample was diluted 9:1 with SDS buffer (0.125 M Tris-HCl, pH 6.5, 5% SDS, 25% glycerol, 7.5% 2-mercaptoethanol), and proteins were denatured by incubation at 95°C for 5 min. Protein samples were separated on 12% SDS-polyacrylamide gels and transferred to PVDF membranes, which were blocked with Tris-buffered saline (TBS) containing 5% dried skimmed milk and then incubated with primary antibody against COX-1 (sc-1752, 1:1,000 dilution in TBS containing 5% dried skimmed milk; Santa Cruz Biotechnology), COX-2 (sc-1745, 1:1,000 dilution in TBS containing 5% dried skimmed milk; Santa Cruz Biotechnology), and cPGES (ab92503, 1:1,000 dilution in TBS containing 5% dried skimmed milk; Abcam), followed by HRP conjugated secondary antibody (SA00001-4, 1:2000 dilution in TBS containing 5% dried skimmed milk; PROTEINTECH GROUP, IN) for COX-1 and COX-2, and HRP-conjugated secondary antibody (NA934, 1:5,000 dilution in TBS containing 5% dried skimmed milk; GE Healthcare, Little Chalfont, UK) for cPGES. Membranes were washed with TBS-T (0.1% Tween 20 in TBS), and antibody-bound protein was visualized with a chemiluminescence reagent kit (Perkin-Elmer, Branchburg, NJ, USA) and LAS-4000 imaging system (FujiFilm, Tokyo, Japan). The membranes were re-blotted with a primary antibody against β-actin (sc-69879, 1:500 dilution in TBS containing 5% dried skimmed milk; Santa Cruz Biotechnology) and HRP-conjugated secondary antibody (NA931V, 1:5,000 dilution in TBS containing 5% dried skimmed milk; GE Healthcare). Chemiluminescence was measured with LAS 4000 film (FujiFilm).
Immunohistochemical analysis
Fetal-membranes were collected after delivery and processed for histological analysis. Tissues were fixed in 10% formalin/PBS and embedded in paraffin, then 10 μm-thick sections were cut and fixed on New Silane III glass slides (Muto Pure Chemical, Tokyo, Japan). Immunohistochemical staining was performed with a Benchmark GX system (Ventana, Tucson, AZ, USA). The following primary antibodies were used: mouse anti-COX-1 (sc-19998, 1:30 dilution; Santa Cruz Biotechnology, Dallas, TX, USA); mouse anti-COX-2 (sc-166475, 1:150; Santa Cruz Biotechnology); mouse anti-p23 (ab92503, 1:500; Abcam, Cambridge, MA, USA); and rabbit anti-SLCO2A1 (bs-4710R, 1:250; Bioss Antibodies, Woburn, MA, USA). Secondary antibodies were anti-rabbit IgG (1:3,000; DAKO, Santa Clara, CA, USA) and anti-mouse IgG (1:3,000; DAKO). The OptiView Amplification Kit and OptiView DAB IHC Detection Kit (Ventana) were used for detection. Mouse monoclonal immunoglobulin (Ventana) was used as a negative control. Hematoxylin was used for nuclear counterstaining, and the images were taken with a light microscope (BZ9000, Keyence, Osaka, Japan).
Statistical analysis
Data in Figs 1, 2, and 4 are presented as the mean ± SEM, and were analyzed by unpaired Student’s t-tests with Welch’s correction. Data in Figs 5 and 6 are presented as the mean ± SEM, and were analyzed by unpaired Student’s t-tests. Data in Fig 3 are presented as the mean ± SEM, and were analyzed by two-way ANOVA and Bonferroni’s multiple-comparisons test. All analyses were carried out with Prism 8 software (GraphPad Software, San Diego, CA, USA).
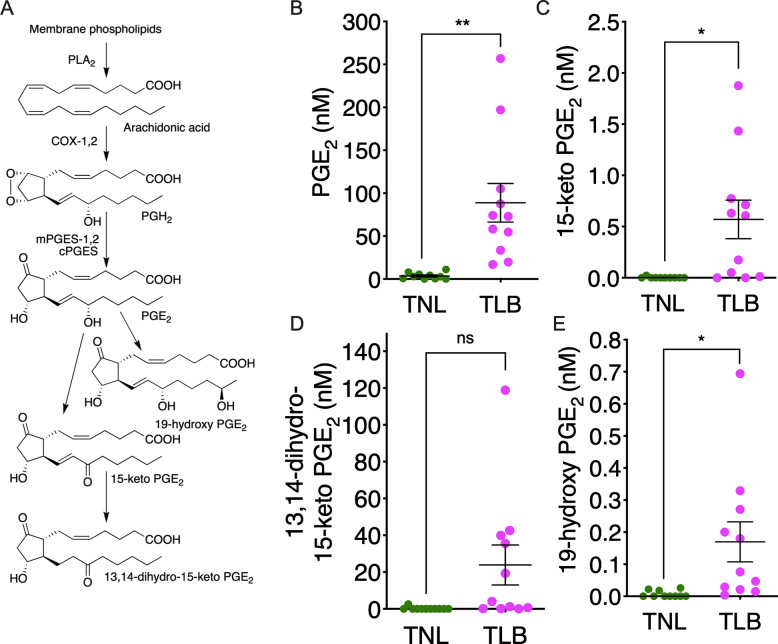
Fig 1. Levels of prostaglandin E2 (PGE2) and its metabolites in amniotic fluid.
(A) PGE2 biosynthesis and metabolism. Arachidonic acid released from membrane phospholipids by phospholipase A2 (PLA2) enzymes is acted on by either of the two cyclooxygenase (COX) isozymes, COX-1 or COX-2. The COX metabolite PGH2 is then converted to PGE2 by microsomal PGE synthase (mPGES-1), mPGES-2, or cytosolic PGE synthase (cPGES). PGE2 is then oxidized by 15-hydroxyprostaglandin dehydrogenase (15-PGDH) to form 15-keto PGE2, or by cytochrome P450 4F8 (CYP4F8) to form 19-hydroxy PGE2. (B–E) Levels of PGE2 and its metabolites in amniotic fluid from patients with term not in labor (TNL) and term in labor (TLB) deliveries were quantified by LC-MS/MS. TNL, n = 10; TLB, n = 11. Horizontal lines and error bars indicate the mean ± SEM. Data were analyzed by unpaired Student’s t-tests with Welch’s correction. *p <0.05, **p <0.01; ‘ns’, not significant.
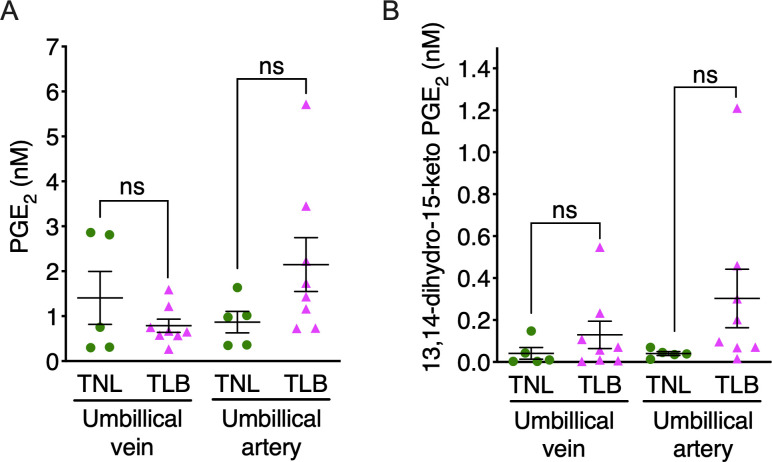
Fig 2. Levels of prostaglandin E2 (PGE2) and its metabolites in umbilical cord blood.
Levels of PGE2 and its metabolites in umbilical cord blood from patients with term not in labor (TNL) and term in labor (TLB) deliveries were quantified by LC-MS/MS. (A) PGE2 and (B) 13,14-dihydro-15-keto-PGE2 in blood from umbilical cord veins and arteries of patients with term not in labor (TNL) and term in labor (TLB) deliveries were quantified by LC-MS/MS. TNL, n = 6; TLB, n = 8. Horizontal lines and error bars indicate the mean ± SEM. Data were analyzed by unpaired Student’s t tests with Welch’s correction. ‘ns’, not significant.
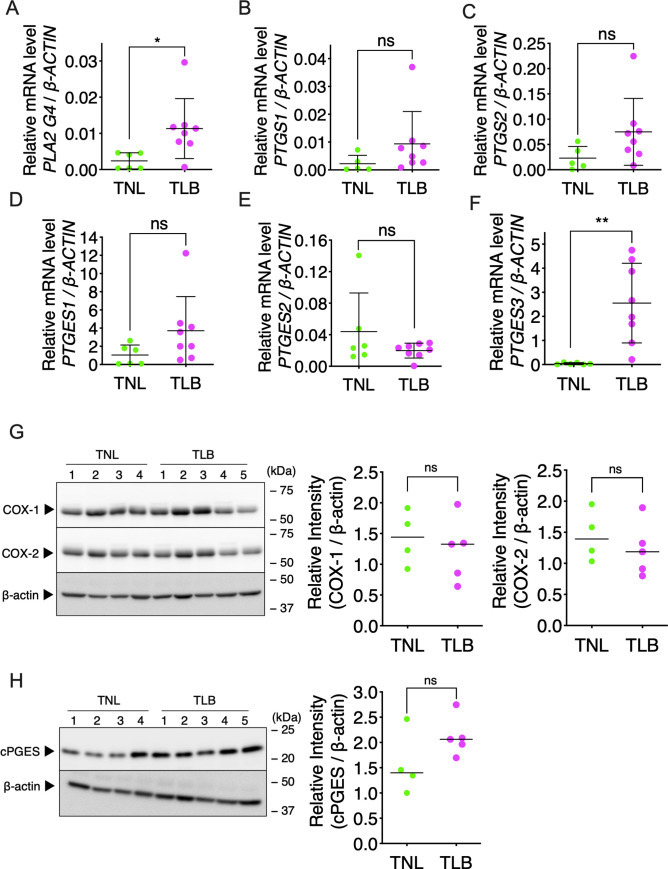
Fig 4. Expression of genes of the prostaglandin E2 (PGE2) biosynthetic enzymes in fetal-membrane.
Relative levels of mRNAs encoding genes involved in PGE2 biosynthesis in fetal-membrane of patients with term not in labor (TNL) or term in labor (TLB) deliveries were measured by RT-qPCR. (A) PLA2G4A, (B) PTGS1, (C) PTGS2, (D) PTGES1, (E) PTGES2, (F) PTGES3. Horizontal lines and error bars indicate the mean ± SEM. TNL, n = 6; TLB, n = 8. Western blot analysis of COX-1 and COX-2 (G), and cPGES (H) in fetal-membrane. TNL, n = 4; TLB, n = 5. Data were analyzed by unpaired Student’s t-tests with Welch’s correction. *p <0.05, **p <0.01; ‘ns’, not significant.
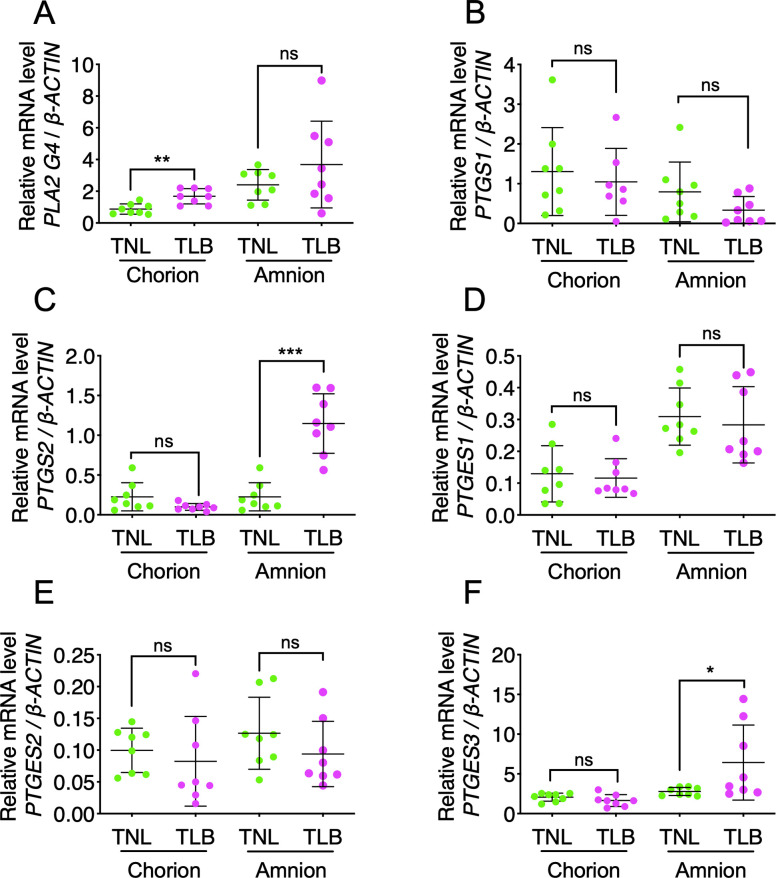
Fig 5. Expression of genes of the prostaglandin E2 (PGE2) biosynthetic enzymes in the amnion and the chorion.
Relative levels of mRNAs encoding genes involved in PGE2 biosynthesis in the amnion and the chorion of patients with term not in labor (TNL) or term in labor (TLB) deliveries were measured by RT-qPCR. (A) PLA2G4A, (B) PTGS1, (C) PTGS2, (D) PTGES1, (E) PTGES2, (F) PTGES3. Horizontal lines and error bars indicate the mean ± SEM. TNL, n = 8; TLB, n = 8. Data were analyzed by unpaired Student’s t-tests. *p <0.05, **p <0.01, ***p <0.005; ‘ns’, not significant.
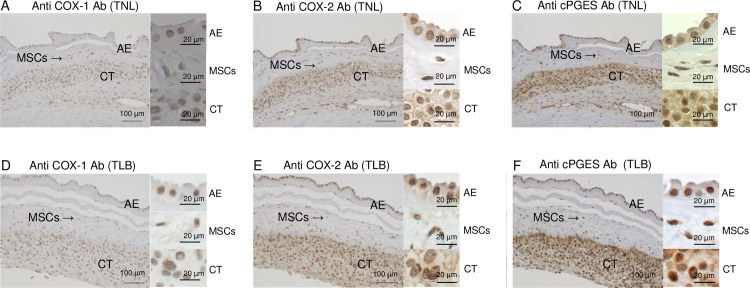
Fig 6. Immunohistochemical analyses of prostaglandin E2 (PGE2) biosynthetic enzymes proteins in fetal-membrane.
Immunohistochemical staining of fetal-membrane with antibodies (Ab) specific for cyclooxygenase-1 (COX-1) (A, D), cyclooxygenase-2 (COX-2) (B, E), and cytosolic PGE synthase (cPGES) (C, F). Hematoxylin was used for nuclear counterstaining. Membrane sections were from patients with term not in labor (TNL; A–C) and term in labor (TLB; D–F) deliveries. Scale bars = 100 μm (Left; low-power field), Scale bars = 20 μm (Right: high-power field). AE, amniotic epithelium; MSCs, mesenchymal cells; CT, chorionic trophoblast.
Study approval
The patients were pregnant women who delivered at Juntendo University between March and May 2015. The total number of the patients with singleton pregnancy and full-term delivery was 218, and the patients with planned cesarean section was 25. Ten patients with breech presentation were recruited to the TNL group and agreed to participate in our study. The total number of the patients with spontaneous delivery was 112, and the 28 patients were excluded due to underlying disease, medication, preterm rupture of membrane, or bacterial vaginosis during pregnancy. Eleven patients were recruited the TLB group and agreed to participate in our study. The patients were all provided written informed consent prior to sample collection under a protocol approved by the Juntendo University Research Ethics Committee (13–131). All methods were performed in accordance with the Declaration of Helsinki.
Results
Study participants
The study included 21 pregnant women at term, 10 with TLB deliveries and 11 with TNL deliveries. Maternal characteristics are summarized in Table 1. There are no significant differences in maternal age, gestational age, or gravidity between the women in the TNL and TLB groups.
Table 1. Maternal characteristics of the study participants.
| TNL (n = 10) | TLB (n = 11) | p-value | |
|---|---|---|---|
| Maternal age (years) | 36.3 (27–42) | 34.9 (30–40) | 0.39 |
| Gestational age at delivery (weeks) | 383/7 (38–39) | 385/7 (38–39) | 0.13 |
| Gravidity | 1.0 (0–1) | 0.5 (0–2) | 0.85 |
| Parity | 0.0 (0–1) | 0.0 (0–1) | 0.64 |
Values are expressed as the median (full range). p-values of maternal age and gestational age were determined by unpaired Student’s t-tests, and p-values of gravidity and parity were determined by Mann-Whitney U test. TNL, term not in labor; TLB, term in labor.
PGE2 and its metabolites in amniotic fluid
The biosynthetic and metabolic pathways of PGE2 are shown in Fig 1A. Levels of PGE2 and its metabolites in amniotic fluid from women delivering at TNL or TLB were measured by LC-MS/MS. The mean PGE2 concentration in amniotic fluid was significantly higher in TLB (89.0 nM) than in TNL (3.6 nM, p <0.01) (Fig 1B). Concentrations of the following PGE2 metabolites in amniotic fluid were also significantly higher in TLB than in TNL: 15-keto-PGE2 (0.6 nM versus 0.0 nM, p <0.05) (Fig 1C), 13,14-dihydro-15-keto-PGE2 (23.9 nM versus 0.2 nM, p = 0.05) (Fig 1D), and 19-hydroxy-PGE2 (0.2 nM versus 0.0 nM, p <0.05) (Fig 1E).
PGE2 and its metabolites in umbilical-cord blood
To examine whether PGE2 and its metabolites were produced by placental villous tissue, their concentrations were measured in blood plasma of the umbilical artery (UmA) and vein (UmV) by LC-MS/MS (Fig 2). In UmV plasma, the mean concentrations of PGE2 (1.4 nM versus 0.8 nM in the TNL and TLB groups, respectively, p = 0.23) (Fig 2A) and 13,14-dihydro-15-keto-PGE2 (0.0 nM versus 0.1 nM, in the TNL and TLB groups, respectively, p = 0.33) (Fig 2B) were low when compared with amniotic fluid, and did not differ significantly. Similarly, the median concentrations in UmA plasma of PGE2 (0.9 nM versus 2.1 nM, p = 0.13) (Fig 2A) and 13,14-dihydro-15-keto-PGE2 (Fig 2B) (0.0 nM versus 0.3 nM, p = 0.17) were comparable between TNL and TLB groups. As umbilical-cord blood consists of the blood flow from the placenta, these data suggest that placental villous tissue does not produce large amounts of PGE2 or its metabolites.
PGE2 and its metabolites are produced by fetal-membrane
We next investigated PG production by fetal-membrane, which cover a large surface area of the uterus and placenta, with one side (the amniotic epithelium) in direct contact with the amniotic fluid. Release of PGE2 from the fetal-membrane to the maternal and fetal compartments was measured in Transwell assays (Fig 3A). PGE2 concentrations in both maternal (decidual) and fetal (amniotic) compartments in the TLB group were rapidly increased compared to the TNL group (Fig 3B and 3C). The concentrations of PGE2 metabolites 15-keto-PGE2 (maternal compartment, p <0.05; fetal compartment, p = 0.65) (Fig 3D and 3E), 13,14-dihydro-15-keto-PGE2 (maternal compartment, p = 0.48; fetal compartment, p <0.05) (Fig 3F and 3G), and 19-hydroxy-PGE2 (maternal compartment, p <0.001; fetal compartment, p <0.05) at 120 min (Fig 3H and 3I) were also higher in TLB than in TNL. These findings suggested that PGE2 and its metabolites are biosynthesized in fetal-membranes and released rapidly into the amniotic fluid.
Expression of PGE2-related genes and enzymes in fetal-membrane
To clarify the enzymes related to PGE2 production and its release into amniotic fluid, we performed quantitative reverse-transcription PCR (RT-qPCR) analysis of the fetal-membrane for expression of genes encoding various PGE2 biosynthetic enzymes. The expression levels of PLA2G4A mRNA, which encodes cytosolic phospholipase A2α (cPLA2α), were significantly higher in TLB than in TNL (p <0.05) (Fig 4A). No significant differences were observed in the expression of PTGS1 mRNA, which encodes COX-1 (p = 0.13), or of PTGS2 mRNA, which encodes COX-2 (p = 0.07), between the TNL and TLB samples (Fig 4B and 4C). Notably, among the genes encoding the three PGES isozymes, only PTGES3, which encodes cPGES, showed a significant difference, with higher expression in TLB than in TNL fetal-membrane (p <0.005) (Fig 4F); by contrast, the levels of PTGES1 (p = 0.09) and PTGES2 (p = 0.28) mRNAs were similar in the two groups (Fig 4D and 4E). Western blotting of fetal-membrane extracts showed that COX-1 and COX-2 protein levels were similar between TNL and TLB (Fig 4G), however, cPGES protein levels were relatively higher in TLB than in TNL (Fig 4H). Although the patient of TNL1, 2, and 3 had no operation before pregnancy, the patient of TNL4 had the uterine operation before pregnancy. The higher expression of cPGES in TNL4 might be due to the uterine operation.
To precisely determine the site of PGE2 production, the fetal-membranes of 8 patients in TNL and 7 patients in TLB were separated into the amnion and the chorion, and the expression of PGE2-related genes was compared between TNL and TLB. There were no significant differences between the women in the TNL and TLB groups with respect to the following variables: maternal age (34.0 years versus 34.5 years, respectively, p = 0.74), gestational age (386/7 weeks versus 391/7 weeks, p = 0.37), gravidity (0.0 versus 0.0, p > 0.99), or parity (0.0 versus 0.0, p = 0.44). (p-values of maternal age and gestational age were determined by unpaired Student’s t-tests, and p-values of gravidity and parity were determined by Mann-Whitney U test.) The mRNA expression of chorionic PLA2G4A, amniotic PTGS2, and amniotic PTGES3 was significantly higher in the TLB than in the TNL (p <0.01, p <0.005, and p <0.05, respectively) (Fig 5A, 5C, and 5F) The levels of PTGS1, PTGES1, and PTGES2 mRNAs were similar on both the chorion and amnion of the TNL and TLB samples (Fig 5B, 5D, and 5E). These data suggest that PGE2 is preferably produced from the amnion of fetal-membrane.
Localization of COX-1, COX-2, and cPGES in fetal-membrane
To identify PGE2-producing cells, fetal-membrane were immunohistochemically stained with antibodies to COX-1, COX-2, and cPGES. Anti-cPGES antibody was also used for western blotting (Fig 4G). These enzymes were mainly expressed in chorionic trophoblasts, mesenchymal cells, and amniotic epithelium (Fig 6). These results suggest that COX-1 and/or COX-2, and cPGES in these cells are involved in the production of PGE2.
Expression and localization of PG transporter in fetal-membrane
PGE2 is transported to the extracellular space and exerts its effects by binding to target receptors [15]. The PG transporter SLCO2A1 transports several substrates including PGE2 [16]. To investigate the expression of SLCO2A1 in chorionic trophoblasts, mesenchymal cells, and amniotic epithelium, we performed RT-qPCR analysis and immunohistochemistry (Fig 7). SLCO2A1 mRNA was detected in fetal-membrane, but its levels of expression did not differ between TNL and TLB (Fig 7A). Similarly, immunohistochemical analysis of SLCO2A1 in fetal-membrane revealed similar staining in chorionic trophoblasts, mesenchymal cells, and amniotic epithelium in TNL and TLB samples (Fig 7B and 7C). These results suggest that cPGES, and SLCO2A1 in chorionic trophoblasts, mesenchymal cells, and amniotic epithelium are involved in the production and release of PGE2.
Fig 7. Expression of the prostaglandin transporter in fetal-membrane.
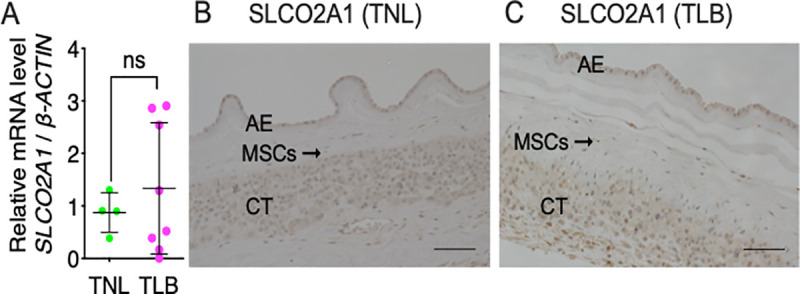
(A) Relative levels of mRNA encoding SLCO2A1 in fetal-membrane of patients with term not in labor (TNL) or term in labor (TLB) deliveries were measured by RT-qPCR. Horizontal lines and error bars indicate the mean ± SEM. Data were analyzed by unpaired Student’s t-tests. ‘ns’, not significant. TNL, n = 4; TLB, n = 8. (B, C) Immunohistochemical staining of fetal-membrane from patients with (B) TNL and (C) TLB deliveries with an anti-SLCO2A1 antibody. Hematoxylin was used for nuclear counterstaining. Scale bars = 100 μm. AE, amnion epithelium; MSCs, mesenchymal cells; CT, chorion trophoblast.
Discussion
PGE2 is known to play an important role in human labor. Preventing the PGE2 biosynthesis by administration of a nonselective cyclooxygenase (COX) inhibitor effectively delays parturition in mice [17]. Celecoxib, a selective inhibitor of COX-2, can also delay human preterm labor [18]. Vaginal administration of PGE2 tablets at term increases the likelihood of a transvaginal delivery within 24 h [19], and vaginal and cervical application of PGE2 is more effective than intravenous injection of oxytocin for induction of vaginal delivery within 24 h [20]. The administration of PGE2 for labor induction is permitted by the Japan Society of Obstetrics and Gynecology guidelines and is commonly used in the clinical situation in Japan [21].
Recently, PGs and their metabolites were shown to accumulate in amniotic fluid in TLB deliveries [3], but the detailed molecular mechanism underlying PGs production during human spontaneous labor remains unclear. To determine the enzymes and transporter(s) responsible for the production and accumulation of PGE2 in the amniotic fluid, we conducted the current study. Firstly, we confirmed that high levels of PGE2 and its metabolites accumulate in the amniotic fluid of TLB, but not in that of TNL. The PGE2 concentration was much higher in amniotic fluid than in umbilical cord blood, suggesting the importance of amniotic PGE2 in inducing labor. It was previously reported that the major site of PG synthesis in human pregnancy is fetal-membrane [2]. The fetal-membrane, which consists of the chorion and amnion, is a major source of amniotic fluid [22]. The amnion consists of a single layer of epithelial cells and subepithelial mesenchymal layer, and directly contacts with amniotic fluid. The chorion consists of trophoblast cells and decidua, and directly contacts with the maternal myometrium. In agreement with previous reports [23,24], we confirmed that the level of PGE2 produced from fetal-membrane was significantly higher in TLB than TNL. RT-PCR analysis (Fig 5) and immunohistochemical staining (Figs 6 and 7) suggest that both amnion and the chorion are able to produce and release PGE2. Because the amnion directly contacts amniotic fluid, it may contribute to PGE2 accumulation amniotic fluid during labor. There was no correlation between the amniotic PGE2 concentration and the length of labor or the number of examinations. We also showed that SLCO2A1 is expressed in term fetal-membrane, which is consistent with the previous report [15]. 15-hydroxyprostaglandin dehydrogenase (PGDH) is an important enzyme in PG inactivation, and it converts PGE2 into its inactive form, 15-keto PGE2 [25]. Because PGDH is not expressed in the amniotic epithelium at term [26], intact PGE2 escapes from inactivation and accumulates in amniotic fluid.
PGE2 induces uterine contractions by binding to G-protein-coupled PGE2 receptors (EPs) expressed in the uterine myometrium [27]. Four EPs bind PGE2, with Kd values ranging from 1 nM to 40 nM [28]; EP1 and EP3 are highly expressed in human myometrium at term [29]. We identified a median concentration of PGE2 in amniotic fluid in the TLB group of ~70 nM, which is sufficient to activate these EP receptors. The fetal-membrane Transwell assay showed both fetal and maternal side of the TLB fetal-membrane produced a high concentration of PGE2. As the chorion exposes to the uterine myometrium, EPs in uterine myometrium may be activated by the accumulated PGE2 in amniotic fluid.
Multiple enzymic reactions are required for PGE2 biosynthesis from AA. cPLA2s are a group of enzymes that hydrolyze the phospholipids to release fatty acids including AA [30]. Cyclooxygenases are responsible for the generation of the PG precursor PGH2 and indispensable for PGE2 production [31]. In agreement with the previous report [32], immunohistochemistry showed the expression of COX-1, COX-2, and cPGES in the amniotic epithelium, mesenchymal cells in the amnion, and chorionic trophoblasts in the chorion. Some studies suggested that the PGE2 biosynthesis in the fetal-membrane is mediated by an increase of PTGS2 mRNA expression in amnion cells [33]. Among the three PGE2 synthases, cPGES is less well understood. cPGES was originally purified from the cytosolic fraction of lipopolysaccharide-treated rat brain and has been shown to be ubiquitously expressed [34]. Although its roles in vivo remain unclear, cPGES-deficient mice die before birth (possibly as a result of lung aplasia), with abnormal skin and lung morphology with reduced PGE2 content in the lungs compared with wild-type mice [35]. By contrast, mPGES-1 deficient mice [36] and mPGES-2 deficient mice [37] develop and deliver normally. Taken together, these results suggest that cPGES contributes to PGE2 production in fetal-membrane in human labor.
In clinical practice, uterine contractions in patients with weak labor are frequently augmented by artificial rupture of the amniotic membrane (ROM) [38]. Previous studies have reported the relationship between the release of PGs at amniotomy and the subsequent onset of labor [39,40]. The relationship between ROM/amniotomy and augmentation of labor could be explained by the high level of PGE2 in amniotic fluid. In addition, extra-amniotic PGE2 administration can also achieve a 25% reduction in the length of induced labor [41]. This extra-amniotic PGE2 might also enhance myometrial contractions following SLCO2A1-mediated transport across the membranes of myometrium.
The major limitation in our study is that the fetal-membrane was taken after labor, and we could not exclude the possibility that PGE2 was produced after labor. Another limitation is that the amniocentesis was performed transvaginally from the TLB group, but that was performed transabdominally from TNL. Because the transabdominal amniocentesis may induce serious complications, we could not collect the amniotic fluid during pregnancy. Thus, we could not exclude the effects of cervicovaginal secretions and bacteria in TLB subjects. To minimize these effects, we washed the vagina with the saline and wiped it with sterilized gauze. In addition, we excluded the patients who were positive for the cultured examination of the vaginal swab. In summary, our results suggest that cPGES, and SLCO2A1 contribute to PGE2 production from fetal-membrane in labor.
Supporting information
(TIF)
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by MEXT/JSPS KAKENHI Grant Numbers 15KK0320, 16K08596, 19K07357 (to T. O.), 15H05904, 15H04708, 18H02627, 19KK0199 (to T.Y.), 18K06923 (to K.S.) and by grants from AMED CREST (20gm1210006), the Mishima Kaiun Memorial Foundation (to TO), the Ono Medical Research Foundation, the Uehara Memorial Foundation (to TY). The study was supported in part by a Grant-in-Aid (S1311011) from the Foundation of Strategic Research Projects in Private Universities from the MEXT, and a grant from the Institute for Environmental and Gender-Specific Medicine (to TY).
References
- 1.Sugimoto Y, Inazumi T, Tsuchiya S. Roles of prostaglandin receptors in female reproduction. J Biochem. 2015;157(2):73–80. 10.1093/jb/mvu081 . [DOI] [PubMed] [Google Scholar]
- 2.Gibb W. The role of prostaglandins in human parturition. Ann Med. 1998;30(3):235–41. 10.3109/07853899809005850 . [DOI] [PubMed] [Google Scholar]
- 3.Maddipati KR, Romero R, Chaiworapongsa T, Zhou SL, Xu Z, Tarca AL, et al. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J. 2014;28(11):4835–46. 10.1096/fj.14-254383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mijovic JE, Zakar T, Nairn TK, Olson DM. Prostaglandin endoperoxide H synthase (PGHS) activity and PGHS-1 and -2 messenger ribonucleic acid abundance in human chorion throughout gestation and with preterm labor. J Clin Endocrinol Metab. 1998;83(4):1358–67. 10.1210/jcem.83.4.4692 . [DOI] [PubMed] [Google Scholar]
- 5.Slater DM, Berger LC, Newton R, Moore GE, Bennett PR. Expression of cyclooxygenase types 1 and 2 in human fetal-membrane at term. Am J Obstet Gynecol. 1995;172(1 Pt 1):77–82. 10.1016/0002-9378(95)90087-x . [DOI] [PubMed] [Google Scholar]
- 6.Zuo J, Lei ZM, Rao CV, Pietrantoni M, Cook VD. Differential cyclooxygenase-1 and -2 gene expression in human myometria from preterm and term deliveries. J Clin Endocrinol Metab. 1994;79(3):894–9. 10.1210/jcem.79.3.8077379 . [DOI] [PubMed] [Google Scholar]
- 7.Alfaidy N, Sun M, Challis JR, Gibb W. Expression of membrane prostaglandin E synthase in human placenta and fetal-membrane and effect of labor. Endocrine. 2003;20(3):219–25. Epub 2003/05/02. 10.1385/ENDO:20:3:219 . [DOI] [PubMed] [Google Scholar]
- 8.Meadows JW, Eis AL, Brockman DE, Myatt L. Expression and localization of prostaglandin E synthase isoforms in human fetal-membrane in term and preterm labor. J Clin Endocrinol Metab. 2003;88(1):433–9. 10.1210/jc.2002-021061 . [DOI] [PubMed] [Google Scholar]
- 9.Premyslova M, Li W, Alfaidy N, Bocking AD, Campbell K, Gibb W, et al. Differential expression and regulation of microsomal prostaglandin E(2) synthase in human fetal-membrane and placenta with infection and in cultured trophoblast cells. J Clin Endocrinol Metab. 2003;88(12):6040–7. Epub 2003/12/13. 10.1210/jc.2003-030618 . [DOI] [PubMed] [Google Scholar]
- 10.Astle S, Newton R, Thornton S, Vatish M, Slater DM. Expression and regulation of prostaglandin E synthase isoforms in human myometrium with labour. Mol Hum Reprod. 2007;13(1):69–75. 10.1093/molehr/gal093 . [DOI] [PubMed] [Google Scholar]
- 11.Matsunobu T, Okuno T, Yokoyama C, Yokomizo T. Thromboxane A synthase-independent production of 12-hydroxyheptadecatrienoic acid, a BLT2 ligand. J Lipid Res. 2013;54(11):2979–87. 10.1194/jlr.M037754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kita Y, Takahashi T, Uozumi N, Shimizu T. A multiplex quantitation method for eicosanoids and platelet-activating factor using column-switching reversed-phase liquid chromatography-tandem mass spectrometry. Anal Biochem. 2005;342(1):134–43. 10.1016/j.ab.2005.03.048 . [DOI] [PubMed] [Google Scholar]
- 13.Okuno T, Gijon MA, Zarini S, Martin SA, Barkley RM, Johnson CA, et al. Altered eicosanoid production and phospholipid remodeling during cell culture. J Lipid Res. 2018;59(3):542–9. Epub 2018/01/22. 10.1194/jlr.M083030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinson LF, Ireland DJ, Kemp MW, Payne MS, Stock SJ, Newnham JP, et al. Effects of cytokine-suppressive anti-inflammatory drugs on inflammatory activation in ex vivo human and ovine fetal-membrane. Reproduction. 2014;147(3):313–20. 10.1530/REP-13-0576 . [DOI] [PubMed] [Google Scholar]
- 15.Banu SK, Arosh JA, Chapdelaine P, Fortier MA. Expression of prostaglandin transporter in the bovine uterus and fetal-membrane during pregnancy. Biol Reprod. 2005;73(2):230–6. 10.1095/biolreprod.105.039925 . [DOI] [PubMed] [Google Scholar]
- 16.Lu R, Kanai N, Bao Y, Schuster VL. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA(hPGT). J Clin Invest. 1996;98(5):1142–9. 10.1172/JCI118897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuboi K, Iwane A, Nakazawa S, Sugimoto Y, Ichikawa A. Role of prostaglandin H2 synthase 2 in murine parturition: study on ovariectomy-induced parturition in prostaglandin F receptor-deficient mice. Biol Reprod. 2003;69(1):195–201. 10.1095/biolreprod.102.013870 . [DOI] [PubMed] [Google Scholar]
- 18.Stika CS, Gross GA, Leguizamon G, Gerber S, Levy R, Mathur A, et al. A prospective randomized safety trial of celecoxib for treatment of preterm labor. Am J Obstet Gynecol. 2002;187(3):653–60. 10.1067/mob.2002.125281 . [DOI] [PubMed] [Google Scholar]
- 19.Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2014;(6):CD003101. 10.1002/14651858.CD003101.pub3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozurkewich EL, Chilimigras JL, Berman DR, Perni UC, Romero VC, King VJ, et al. Methods of induction of labour: a systematic review. BMC Pregnancy Childbirth. 2011;11:84. 10.1186/1471-2393-11-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A I, M S, S A, K O, Y O, M O, et al. Guidlines for Obstetrical Practice in Japan 2020 edition [In Japanese]. Japan Society of Obstetrics and Gynecology, Japan Association of Obstetricians and Gynecologists; 2020. [Google Scholar]
- 22.Myatt L, Sun K. Role of fetal-membrane in signaling of fetal maturation and parturition. Int J Dev Biol. 2010;54(2–3):545–53. Epub 2009/11/20. 10.1387/ijdb.082771lm . [DOI] [PubMed] [Google Scholar]
- 23.Gibb W, Sun M. Localization of prostaglandin H synthase type 2 protein and mRNA in term human fetal-membrane and decidua. J Endocrinol. 1996;150(3):497–503. Epub 1996/09/01. 10.1677/joe.0.1500497 . [DOI] [PubMed] [Google Scholar]
- 24.Mijovic JE, Zakar T, Nairn TK, Olson DM. Prostaglandin-endoperoxide H synthase-2 expression and activity increases with term labor in human chorion. Am J Physiol. 1997;272(5 Pt 1):E832–40. Epub 1997/05/01. 10.1152/ajpendo.1997.272.5.E832 . [DOI] [PubMed] [Google Scholar]
- 25.Kishore AH, Owens D, Word RA. Prostaglandin E2 regulates its own inactivating enzyme, 15-PGDH, by EP2 receptor-mediated cervical cell-specific mechanisms. J Clin Endocrinol Metab. 2014;99(3):1006–18. Epub 2014/01/30. 10.1210/jc.2013-3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung PY, Walton JC, Tai HH, Riley SC, Challis JR. Localization of 15-hydroxy prostaglandin dehydrogenase in human fetal-membrane, decidua, and placenta during pregnancy. Gynecol Obstet Invest. 1992;33(3):142–6. 10.1159/000294868 . [DOI] [PubMed] [Google Scholar]
- 27.Senior J, Marshall K, Sangha R, Clayton JK. In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. Br J Pharmacol. 1993;108(2):501–6. 10.1111/j.1476-5381.1993.tb12832.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–7. 10.1074/jbc.R600038200 . [DOI] [PubMed] [Google Scholar]
- 29.Arulkumaran S, Kandola MK, Hoffman B, Hanyaloglu AC, Johnson MR, Bennett PR. The roles of prostaglandin EP 1 and 3 receptors in the control of human myometrial contractility. J Clin Endocrinol Metab. 2012;97(2):489–98. 10.1210/jc.2011-1991 . [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, Taketomi Y. Secreted phospholipase A2 and mast cells. Allergol Int. 2015;64(1):4–10. Epub 2015/01/13. 10.1016/j.alit.2014.07.005 . [DOI] [PubMed] [Google Scholar]
- 31.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. Epub 2000/08/31. 10.1146/annurev.biochem.69.1.145 . [DOI] [PubMed] [Google Scholar]
- 32.Phillips RJ, Fortier MA, Lopez Bernal A. Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregnancy Childbirth. 2014;14:241. 10.1186/1471-2393-14-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vrachnis N, Karavolos S, Iliodromiti Z, Sifakis S, Siristatidis C, Mastorakos G, et al. Review: Impact of mediators present in amniotic fluid on preterm labour. In Vivo. 2012;26(5):799–812. Epub 2012/09/06. . [PubMed] [Google Scholar]
- 34.Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem. 2000;275(42):32775–82. 10.1074/jbc.M003504200 . [DOI] [PubMed] [Google Scholar]
- 35.Nakabayashi M, Asami M, Nakatani A. Efficacy of antithrombin replacement therapy in severe early-onset preeclampsia. Semin Thromb Hemost. 1999;25(5):463–6. Epub 2000/01/07. 10.1055/s-2007-994951 . [DOI] [PubMed] [Google Scholar]
- 36.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100(15):9044–9. Epub 2003/07/02. 10.1073/pnas.1332766100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jania LA, Chandrasekharan S, Backlund MG, Foley NA, Snouwaert J, Wang IM, et al. Microsomal prostaglandin E synthase-2 is not essential for in vivo prostaglandin E2 biosynthesis. Prostaglandins Other Lipid Mediat. 2009;88(3–4):73–81. Epub 2008/11/18. 10.1016/j.prostaglandins.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiersch L, Rosen H, Salzer L, Aviram A, Ben-Haroush A, Yogev Y. Does artificial rupturing of membranes in the active phase of labor enhance myometrial electrical activity? J Matern Fetal Neonatal Med. 2015;28(5):515–8. 10.3109/14767058.2014.927431 . [DOI] [PubMed] [Google Scholar]
- 39.Sellers SM, Mitchell MD, Anderson AB, Turnbull AC. The relation between the release of prostaglandins at amniotomy and the subsequent onset of labour. Br J Obstet Gynaecol. 1981;88(12):1211–6. Epub 1981/12/01. 10.1111/j.1471-0528.1981.tb01199.x . [DOI] [PubMed] [Google Scholar]
- 40.Mitchell MD, Flint AP, Bibby J, Brunt J, Arnold JM, Anderson AB, et al. Rapid increases in plasma prostaglandin concentrations after vaginal examination and amniotomy. Br Med J. 1977;2(6096):1183–5. Epub 1977/11/05. 10.1136/bmj.2.6096.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calder AA, Embrey MP, Tait T. Ripening of the cervix with extra-amniotic prostaglandin E2 in viscous gel before induction of labour. Br J Obstet Gynaecol. 1977;84(4):264–8. Epub 1977/04/01. 10.1111/j.1471-0528.1977.tb12574.x . [DOI] [PubMed] [Google Scholar]