Abstract
Background and aims
Knowledge of the proper dry weight plays a critical role in the efficiency of dialysis and the survival of hemodialysis patients. Recently, bioimpedance spectroscopy(BIS) has been widely used for set dry weight in hemodialysis patients. However, BIS is often misrepresented in clinical healthy weight. In this study, we tried to predict the clinically proper dry weight (DWCP) using machine learning for patient’s clinical information including BIS. We then analyze the factors that influence the prediction of the clinical dry weight.
Methods
As a retrospective, single center study, data of 1672 hemodialysis patients were reviewed. DWCP data were collected when the dry weight was measured using the BIS (DWBIS). The gap between the two (GapDW) was calculated and then grouped and analyzed based on gaps of 1 kg and 2 kg.
Results
Based on the gap between DWBIS and DWCP, 972, 303, and 384 patients were placed in groups with gaps of <1 kg, ≧1kg and <2 kg, and ≧2 kg, respectively. For less than 1 kg and 2 kg of GapDW, It can be seen that the average accuracies for the two groups are 83% and 72%, respectively, in usign XGBoost machine learning. As GapDW increases, it is more difficult to predict the target property. As GapDW increase, the mean values of hemoglobin, total protein, serum albumin, creatinine, phosphorus, potassium, and the fat tissue index tended to decrease. However, the height, total body water, extracellular water (ECW), and ECW to intracellular water ratio tended to increase.
Conclusions
Machine learning made it slightly easier to predict DWCP based on DWBIS under limited conditions and gave better insights into predicting DWCP. Malnutrition-related factors and ECW were important in reflecting the differences between DWBIS and DWCP.
Introduction
Accurately establishing dry weight is important in patients with end stage renal disease (ESRD) on hemodialysis. It is well known that identifying the proper dry weight is associated with the adequate efficiency of dialysis, minimizes the burden on the cardiovascular system, and improves the survival rate of dialysis patients [1–3].
In general, dry weight is(DW) defined as the lowest tolerated postdialysis weight achieved via gradual change in postdialysis weight at which there are minimal signs or symptoms of hypovolemia or hypervolemia [4].
It has been reported that DW overestimation is a major risk factor in the development of hypertension, left ventricular hypertrophy, and cardiovascular disease, therefore affecting the mortality risk of patients on hemodialysis [5–8]. Underestimation of DW can result in hypovolemia and can induce hypotension, cramps, and dizziness [9,10]. Hypovolemia can also result in a reduced blood supply to vital organs, causing ischemia and consequently contributing to the loss of residual renal function [8]. As a consequence, it is crucial to implement an efficient and precise method to determine DW in patients with ESRD on hemodialysis.
DW assessment via clinical signs, such as measurements of blood pressure, jugular venous pressure, and the presence or absence of edema, is the most commonly used method. However, this method does not reflect the patient’s underlying illnesses and decreases in muscle mass [11].
Over the years, paraclinical tools have been developed to help nephrologists assess DW. Measurements of the cardiothoracic ratio on chest X-rays has long been used but is imprecise due to its dependency on underlying cardiovascular conditions, such as cardiac hypertrophy [12]. Ultrasonic measurements of the inferior vena cava diameter and lung can also be useful tools for measuring DW but are limited because they only provide information concerning overhydration [13,14].
Recently, a method to determine the amount of body water using the bioimpedance spectroscopy (BIS) method has been developed [15]. Even though DW measurements using BIS are useful and reliable when applied to actual clinical patients, there have been reports that the measured DW was not optimal for some patients depending on the prescription of blood pressure medications and the presence of cardiovascular disease [7,16].
Recently, machine-learning-based artificial intelligence has been successfully applied in the medical field and has shown promising results in predicting complications [17–19]. Machine-learning approaches can be classified into two main types: unsupervised learning for unlabeled data and supervised learning for labeled data. The latter is more appropriate for predicting complications; decision tree-based machine-learning algorithms are especially appropriate for table-type data with labels, i.e., target factors [20–22]. However, there are few studies that use machine-learning approaches to predict DW in hemodialysis patients.
In this study, machine learning was applied to accurately determine DW. Using this approach, various clinical factors influencing patients with a large gap between the DW measured using BIS (DWBIS) and the clinically stable dry weight were identified and analyzed. Additionally, we evaluated whether a more accurate clinical dry weight could be presented using machine learning based on the various clinical factors and BIS data of the patient.
Materials and methods
Study design
The present study was approved by the institutional review board of Chungnam National University Hospital (IRB number: CNUH 2015-12-025-003). This study was entirely retrospective (using existing data and medical records available as of the date of submission of the IRB), and was permitted a consent waiver through the IRB. The study was conducted as a single center and retrospective study on dialysis patients who were treated at the Chungnam National University Hospital between January 2011 and September 2015. All data were collected from the medical records of the Chungnam National University Hospital. Information on mortality was obtained from the database of the National Health Insurance Service.
Patient selection
All patients were diagnosed with ESRD and started maintenance hemodialysis between June 2011 and December 2015. Maintenance hemodialysis is defined here as hemodialysis performed three times a week.
Dialysis was maintained for at least 3 months after initiation. Patients who received temporarily hemodialysis due to acute renal injury were excluded. In addition, we excluded patients with unstable clinical conditions (i.e., acute infections or intensive care unit admission). Patients for whom we did not have information concerning their hydration status or who’s DW could not be confirmed using the BIS method were excluded. Patients whose antihypertensive drug prescriptions changed between the 2 weeks before and after the time their body compositions were measured were also excluded.
Body composition measurements
Body composition, including hydration status (overhydration; OH), was assessed using a portable BIS device (BCM; Fresenius Medical Care, Bad Homburg, Germany). Patient measurements were obtained on the day of dialysis prior to dialysis. All data measured using the BIS method, including extracellular water (ECW), intracellular water (ICW), total body water (TBW), lean tissue index (LTI), fat tissue index (FTI), ECW/ICW (E/I) ratio, body mass index (BMI), lean tissue mass (LTM), fat mass (FAT), adipose tissue mass (ATM), and body cell mass (BCM), were included. These fluid volumes were then used to determine the fluid overload, expressed as the OH value. OH was measured as a negative or positive value. ECW, ICW, TBW, and OH are all expressed in units of liters.
Definitions
In general, DW is defined as the lowest tolerated postdialysis weight achieved via gradual change in postdialysis weight at which there are minimal signs or symptoms of hypovolemia or hypervolemia [4]. In this study, the concept of clinically proper DW (DWCP) was used to distinguish it from DWBIS. DWCP was defined as the lowest post-dialysis weight a patient could tolerate without signs or symptoms of overhydration or dehdyration during or after dialysis. DW was defined by combining judgments on the clinical situation with reference to DW predicted by the BIS method. The clinical judgment of the appropriate dry weight used the patient’s indications and imaging studies. At the center where the study was conducted, the medical staff checks and records events from the previous dialysis to the day on the dialysis day. For example, when determining overhydration, it was included based on cases where peripheral edema, generalized edema or chest discomfort, or pleural effusion or pulmonary edema was not observed in chest PA. We checked whether musle spasm or dizziness occurred through medical records. The OH value measured using BIS is denoted OHBIS, and the DW assessed using BIS (DWBIS) was calculated from the body weight (Weight just before dialysis) minus the OHBIS value (kg). The gap between DWCP and the body weight (Weight just before dialysis) is denoted OHCP. The measurement gap between DWBIS and DWCP is the DW gap (GapDW), i.e., it is defined as the absolute value of the body weight minus DWBIS from DWCP in units of kg.
Measurements of clinical parameters
The baseline patient characteristics, its mean and standard deviation (SD) analyzed included the age on the start day of dialysis (days), sex, height (cm), initial body weight (kg), predialysis systolic/diastolic blood pressure (mmHg), and comorbidities (presence of diabetes or hypertension). Factors predicted to affect the patient’s DW were collected from the blood test results. Laboratory tests were performed within three days before and after the DWBIS measurement, and hemoglobin, blood urea nitrogen, creatinine (Cr), albumin, total calcium (TCa), phosphorus (P), sodium (Na), potassium (K), and chloride (Cl) measurements were included.
Machine-learning adjustment
The machine-learning methodology, which is currently attracting attention, was applied to predict OH (proper). Since many external sources on machine learning exist, detailed information on the introduction and methodology of machine learning is not included. Briefly, machine learning is a very effective method of learning a given data to predict a specific aim attribute (OHcp in this paper), and using it to predict the corresponding aim attribute when new patients data are inputted. A lot of research is being conducted, and it is widely used in artificial intelligence technology. In this study, we used a decision tree-based methodology suitable for application to table-type medical data, the light gradient boosting method (LightGBM). the extreme gradient boosting tree (XGBoost), and random forest methods [20–22]. These tree-based methods are more efficient for table type data structure and relatively small data set, especially, RF is better than others for given small data set. Even though deep learning is more famous in machine learning applications, it requires bigger data set for learning model and getting certain prediction accuracy. Before learning the prediction model from the collected data, outliers were first removed by pre-processing the data. To verify the prediction result, 20 random samplings were performed. In each random sample, 80% of the collected data were sampled and used for training and the remaining 20% were used for testing. The suggested values obtained from the predicted results are presented after taking the average of the 20 samplings.
Results
Baseline characteristics
The average age of the patients was 65.1 years. There were 672 (40%) men, and diabetes and hypertension were found in 1,002 (50%) and 1,328 (79%) of the patients, respectively. The average duration of dialysis was approximately 806 days.
To confirm the predicted level of machine learning according to the gap between DWBIS and DWCP, the analysis was divided into groups with differences of less than 1 kg and of more than 2 kg. In patients with a GapDW value of more than 2 kg, the proportion of women was high, the age was high, and there were many patients with diabetes (Table 1).
Table 1. Baseline characteristics.
| Group | Total N = 1,672 | GAP (<1 kg) N = 972 | GAP (≧1kg, <2 kg) N = 303 | GAP (>2 kg) N = 384 | ||||
|---|---|---|---|---|---|---|---|---|
| Feature | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Age | 65.01 | 12.23 | 64.91 | 12.44 | 65.56 | 11.58 | 64.84 | 12.30 |
| Gender | 672 (40%) | 0.49 | 408 (42%) | 0.49 | 119 (39%) | 0.49 | 142 (37%) | 0.48 |
| Height (cm) | 161.46 | 8.51 | 160.98 | 8.25 | 161.14 | 8.62 | 162.69 | 8.92 |
| Weight (kg) | 60.50 | 11.30 | 60.24 | 11.24 | 60.34 | 10.39 | 61.15 | 12.07 |
| Vintage of HD (day) | 806.79 | 826.20 | 786.98 | 809.59 | 837.01 | 808.27 | 827.89 | 873.59 |
| DM | 1,002 (50%) | 0.49 | 561 (58%) | 0.49 | 185 (61%) | 0.49 | 247 (64%) | 0.48 |
| HTN | 1,328 (79%) | 0.40 | 797 (82%) | 0.38 | 240 (79%) | 0.41 | 279 (73%) | 0.45 |
| Hb (g/dL) | 10.13 | 1.55 | 10.25 | 1.50 | 10.15 | 1.59 | 9.83 | 1.61 |
| Total protein (g/dL) | 6.34 | 0.70 | 6.37 | 0.66 | 6.43 | 0.64 | 6.19 | 0.81 |
| Albumin (g/dL) | 3.38 | 0.57 | 3.46 | 0.55 | 3.39 | 0.49 | 3.17 | 0.65 |
| BUN (mg/dL) | 56.05 | 23.65 | 57.72 | 23.21 | 53.94 | 23.29 | 52.95 | 24.07 |
| Cr (mg/dL) | 7.68 | 3.37 | 7.98 | 3.26 | 7.73 | 3.35 | 6.89 | 3.55 |
| Tca (mg/dL) | 8.36 | 0.79 | 8.36 | 0.77 | 8.33 | 0.73 | 8.37 | 0.88 |
| P (mg/dL) | 4.35 | 1.57 | 4.43 | 1.55 | 4.41 | 1.66 | 4.10 | 1.48 |
| Na (mEq/L) | 137.06 | 3.97 | 137.18 | 3.95 | 136.70 | 3.71 | 137.03 | 4.15 |
| K (mEq/L) | 4.74 | 0.92 | 4.77 | 0.90 | 4.81 | 0.96 | 4.60 | 0.96 |
| Cl (mEq/L) | 101.34 | 5.16 | 101.59 | 5.16 | 100.69 | 5.06 | 101.21 | 5.13 |
| TBW (L) | 32.00 | 7.11 | 31.62 | 6.90 | 32.08 | 6.81 | 32.85 | 7.79 |
| ECW (L) | 15.54 | 3.51 | 15.19 | 3.32 | 15.59 | 3.23 | 16.37 | 3.99 |
| ICW (L) | 16.45 | 4.20 | 16.41 | 4.03 | 16.48 | 4.08 | 16.51 | 4.71 |
| E/I | 0.97 | 0.19 | 0.94 | 0.16 | 0.97 | 0.18 | 1.03 | 0.24 |
| BMI (kg/m2) | 23.16 | 3.80 | 23.18 | 3.78 | 23.20 | 3.52 | 23.09 | 4.06 |
| LTI (kg/m2) | 13.05 | 3.42 | 13.07 | 3.24 | 13.18 | 3.32 | 12.92 | 3.92 |
| FTI (kg/m2) | 9.11 | 4.61 | 9.23 | 4.56 | 8.96 | 4.52 | 8.88 | 4.83 |
| LTM (kg/m2) | 34.39 | 10.74 | 34.31 | 10.34 | 34.59 | 10.60 | 34.41 | 11.85 |
| FAT (kg) | 18.70 | 9.67 | 18.74 | 9.48 | 18.29 | 9.04 | 18.71 | 10.52 |
| ATM (kg) | 21.85 | 10.79 | 22.08 | 10.56 | 21.34 | 10.50 | 21.57 | 11.64 |
| BCM (kg) | 18.93 | 7.28 | 18.92 | 6.99 | 19.13 | 7.18 | 18.82 | 8.08 |
| DWBIS (kg) | 58.41 | 11.19 | 58.44 | 10.88 | 58.12 | 10.32 | 58.35 | 12.54 |
| OHBIS (kg) | 2.11 | 2.40 | 1.77 | 1.91 | 2.17 | 2.07 | 2.94 | 3.32 |
| DWCP (kg) | 58.63 | 10.94 | 58.44 | 10.86 | 58.44 | 10.16 | 59.29 | 11.50 |
| GapDW (kg) | 0.22 | 3.07 | 0.00 | 0.48 | 0.32 | 1.50 | 0.94 | 4.79 |
| OHCP (kg) | 1.87 | 3.17 | 1.80 | 2.15 | 1.91 | 2.07 | 1.87 | 4.00 |
Abbreviations: SD, standard deviation; HD, Hemodialysis; DM, diabetes mellitus; HTN, hypertension; Hb, hemoglobin; BUN, blood urea nitrogen; Cr, serum creatinine; TCa, serum total calcium; P, serum phosphorus; Na, sodium; K, serum potassium; Cl, serum chloride; TBW, total body water; ECW, extracellular water; ICW, intracellular water; E/I, extracellular water to intracellular water ratio; BMI, body mass index; LTI, lean tissue index; FTI, fat tissue index; lean tissue mass, LTM; FAT, fat mass; ATM, adipose tissue mass; BCM, body cell mass; DW, dry weight; OH, overhydration.
Prediction of clinical DW using machine learning
The accuracy of the results when applying machine learning to all the collected data is shown in Table 2. As can be seen from the results, the prediction accuracy of machine learning for OHCP was very low, less than 40%, and the maximum error for OHCP was very high at 1.5 kg. It was difficult to obtain a valid machine-learning-based predictive model, which is required for OHCP prediction.
Table 2. Prediction using machine learning.
| LightGBM Test Accuracy (%) (OH-CP(MAE)) | XGBoost Test Accuracy (%) (OH-CP(MAE)) | Random Forest Test Accuracy (%) (OH-CP(MAE)) | ||
|---|---|---|---|---|
| GapDW < 1 kg | Mean | 81.25% (547.0 g) | 82.89% (515.5 g) | 79.52% (570.3 g) |
| Max | 84.57% (499.1 g) | 86.33% (459.9 g) | 83.11% (530.1 g) | |
| Min | 73.85% (622.1 g) | 75.21% (596.1 g) | 70.44% (656.2 g) | |
| GapDW < 2 kg | Mean | 69.71% (770.6 g) | 72.02% (734.6 g) | 71.00% (747.3 g) |
| Max | 78.33% (693.9 g) | 80.06% (674.3 g) | 79.03% (682.2 g) | |
| Min | 53.76% (876.9 g) | 55.84% (831.0 g) | 54.18% (857.0 g) | |
| Total | Mean | 23.58% (1,351.9 g) | 28.54% (1,287.5 g) | 30.82% (1,250.5 g) |
| Max | 35.85% (1,224.6 g) | 38.83% (1,178.8 g) | 39.36% (1,139.4 g) | |
| Min | 11.19% (1,448.7 g) | 21.01% (1,404.2 g) | 21.43% (1,377.9 g) |
All collected data (1,672 patients) were included. CP(MAE) indicates the clinically proper mean absolute error.
We classified and extracted two groups based on GapDW (|DWCP−DWBIS|) from the collected data. One group contained data where GapDW is less than 1 kg, and the other contained data where GapDW is less than 2 kg. The results of applying machine learning to these two groups are presented in Table 2. It can be seen that the average accuracies for the two groups are 83% and 72%, respectively, in the case of XGBoost; in particular, the accuracy for the GapDW < 1 kg patients was dramatically improved compared to the prediction result for the entire dataset. It is obvious from this result that, when data with large GapDW are included and as GapDW increases, it is increasingly difficult to predict the target property (OHCP) as a BIS measurement property.
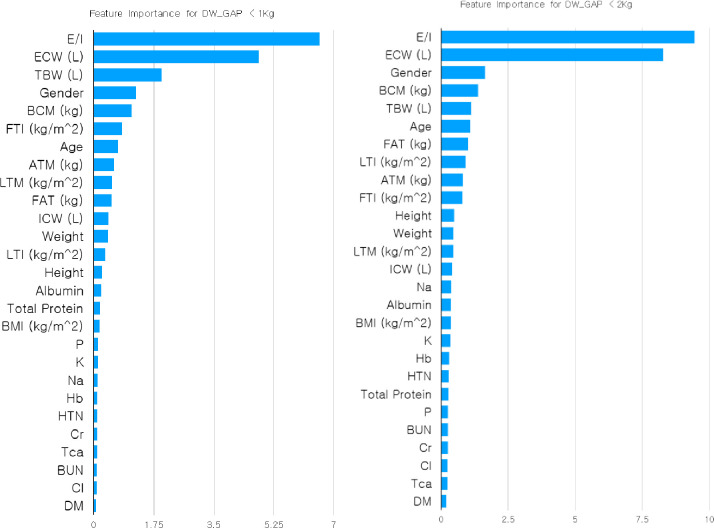
In terms of machine learning, the difference between the two groups can also be confirmed via the feature importance of the machine-learning methodology. Comparing the feature importance of the two groups (Group 1: GapDW < 1 kg and Group 2: GapDW < 2 kg) reveals very different patterns, as shown in Fig 1. In the feature importance of Group 1, E/I and ECW (L) play important roles in learning the machine-learning predictive model and predicting the target feature; the other features, especially TBW (L), appear to play a secondary role. Conversely, Group 2, which includes patients with GapDW between 1 kg and 2 kg, shows a further enhancement in the importance of E/I and ECW (L). In addition, the roles of features of low importance in Group 1 are slightly increased in Group 2 and distributed downward. Therefore, the data (1 kg ≦ GapDW < 2 kg) newly included in Group 2 changes the properties of the dataset and appears to play a role in lowering the accuracy of the prediction.
Fig 1. Feature importance for the two groups (Group 1: GapDW < 1 kg and Group 2: GapDW < 2 kg).
The factors affecting GapDW
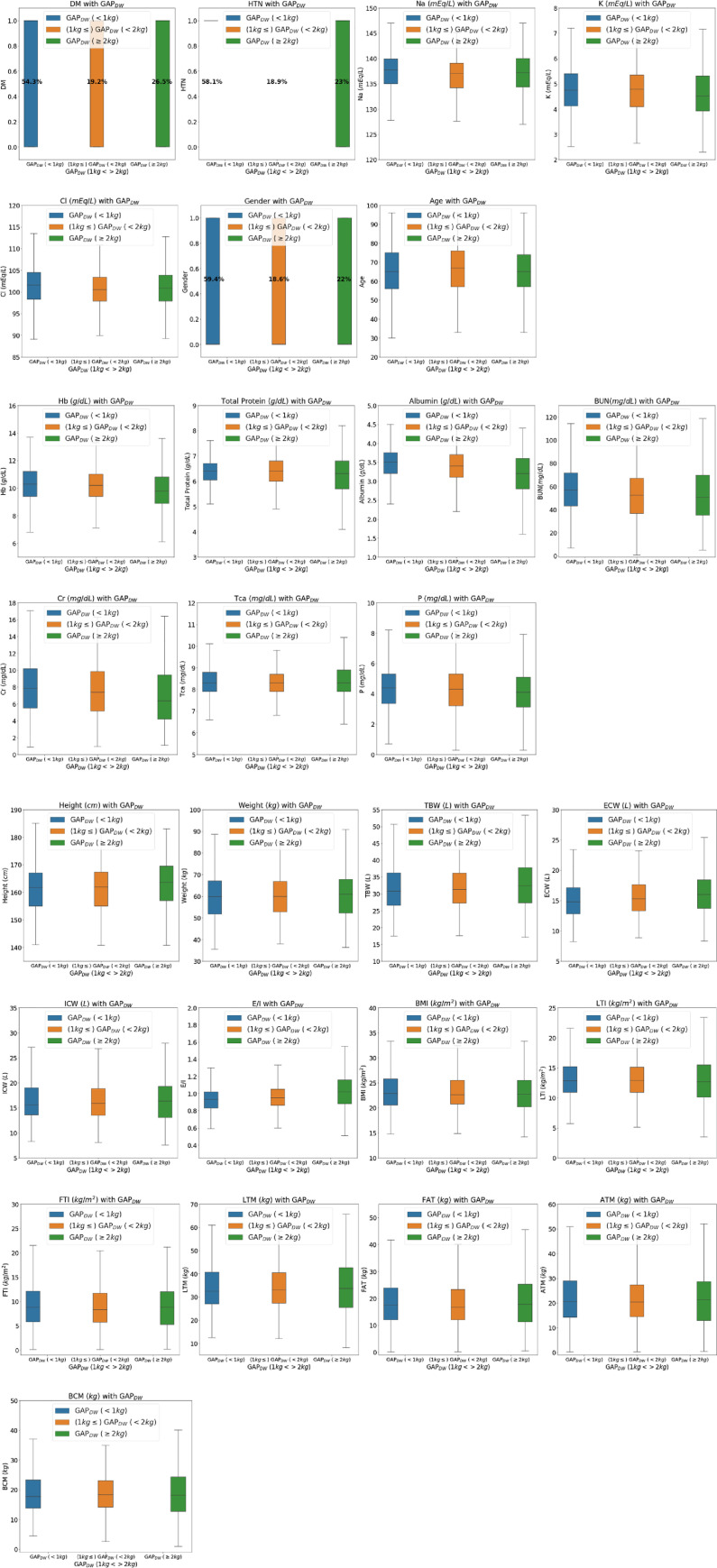
To compare the clinical differences from the point of view of the data distribution, box plots were used to compare the clinical factors in groups with GapDW less than 1 kg, between 1 kg and 2 kg, and greater than 2 kg (Fig 2). In box plot, the mean values of hemoglobin, total protein, serum albumin, serum creatinine, phosphorus, serum sodium, serum potassium, and FTI tended to decrease, as the GapDW increased. The height, TBW, ECW, and E/I ratio tended to increase as the GapDW increased. LTI and BMI showed no change in their trends for the three groups.
Fig 2.
Distribution of the data according to GapDW: (A) clinical data; (B) blood chemistry; and (C) BIS parameters.
Discussion
In this study, we derived a more clinically accurate DW using machine learning based on volume status information measured using BIS as well as clinical information concerning the patients.
In addition, it was confirmed that patient’s blood chemistry, including hemoglobin, total protein, albumin, creatinine, phosphorus, and potassium, was a factor that caused a gap between DWBIS and DWCP.
It is important to maintain adequate hydration during the treatment of patients with ESRD on hemodialysis. Determining the amount and rate of water removal by targeting the appropriate patient DW during dialysis treatment is a major part of the dialysis regimen. In general, the DW evaluation is determined by a clinical decision. DW is defined as the lowest weight that is tolerable to a patient without symptoms or the occurrence of hypotension [23].
Several methods have been proposed to measure the volume status, and blood tests including atrial or brain natriuretic peptide levels, inferior vena cava diameter measurements using ultrasound, and blood pressure measurements have been used. However, DW results using these tools are not accurate [24,25]. BIS or multifrequency bioimpedance analysis was introduced to establish DW in dialysis patients [15]. Using a method to distinguish between ECW and ICW over multiple frequencies, it is possible to more accurately predict DW by measuring the volume status of a dialysis patient [26]. However, sometimes, dialysis targeting DW using the BIS method can result in a hypotensive status or peripheral and pulmonary edema in dialysis patients [16].
In this study, we tried to correct the gap between DWBIS and DWCP using various clinical data gathered from patients. The application of machine learning to the BIS-based data and clinical parameters resulted in accuracy compatible with that of predicting OHCP using DWBIS alone; however, the mean absolute error (MAE) for OHCP was improved.
In particular, when only data with a GapDW value of less than 1 kg were analyzed as a dataset using machine learning, a mean accuracy of 83.26% and a maximum accuracy of 86.65% were found using the XGBoost method and the predicted difference from OHCP was up to 458 ml. For OHCP, the average measurement range was 731 ml and it was more predictable for DWCP than for BIS-based data alone. When using all of the data, the method was very inaccurate when predicting OHCP; however, because BIS-based data play an important role in the prediction, when there was too much of a difference with respect to the clinical DW, or when data that accurately reflect the volume status were used, the machine-learning prediction was thought to be inaccurate overall.
In view of the GapDW groups, the box plots indicated that the GapDW difference and older ages did not show a tendency but that the importance of age increased in the group with a GapDW difference of more than 2 kg. In a previous study, when comparing the hydration status of elderly dialysis patients and young dialysis patients, it was reported that ICW is low, ECW/TBW tends to be low, and a larger hydration status is shown. In the distribution of the actual data, an age trend was not observed but it was confirmed that the importance of the features analyzed by machine learning is useful for applications to actual clinical data.
Box plots were used to reveal the distributions of the BIS-based data and the clinical data according to the gap differences based on GapDW. In the box plots, the mean value of ECW showed a tendency to increase as GapDW increased. Conversely, the overall distribution of hemoglobin, serum total protein, serum albumin, serum creatinine, phosphorus, and serum potassium had lower values as GapDW increased.
The ECW value reflects overhydration. As GapDW increases, ECW tends to increase. As the degree of overhydration increases, the prediction accuracy of DWBIS is expected to decrease.
Previous studies have shown that hypoalbuminemia (low serum albumin concentration) is associated with overhydration in patients undergoing maintenance dialysis. ECW increased in patients with low serum albumin concentrations, and similar results were shown regardless of the dialysis method. In addition, when hypoalbuminemia is present, overall nutritional weight, BMI, BUN, serum creatinine and potassium levels, and dietary protein intake are normal [27–29]. Hypophosphatemia is significantly associated with overhydration. In addition, it has been reported to be related to higher age and lower serum albumin, creatinine, hemoglobin, and serum calcium levels [30].
In previous studies, a relationship between overhydration and low hemoglobin concentration was reported and anemia tended to worsen when overhydration was severe. In addition to chronic kidney disease, more than half of patients with advanced heart failure who could show volume overload showed anemia and it was reported that this anemia was caused by a dilution effect due to fluid overload [31,32]. There are limitations to directly using anemia as a measure of overhydration; however, it can be considered as a factor affecting the increase in GapDW.
In several previous studies, serum creatinine was used as a biomarker for muscle metabolism when assessing muscle mass in patients with ESRD on dialysis. Early studies estimating muscle mass based on creatinine kinetics demonstrated good correlation with other estimates of muscle mass [33,34]. In addition, serum creatinine has been significantly correlated with LTM [35]. Based on these findings, lower predialysis serum creatinine levels are predicted to affect the GapDW increase.
Hypokalemia is used as an indicator of malnutrition, and its association with malnutrition via albumin and total protein can be estimated [36]. Hypokalemia showed a tendency to drop significantly in the group with GapDW of more than 2 kg. Based on these results, caution is needed when interpreting and applying DWBIS to patients with hypokalemia and/or malnutrition.
These blood chemistry findings may not be related to malnutrition. Even though there was no tendency in LTI and BMI according to the GapDW increase in the box plots, these blood chemistry findings are associated with a decrease in the effective circulating volume and an increase in interstitial edema, consequently making the prediction of DWCP difficult.
There are some limitations to this study. First, the DW measurements using the BIS device were performed by a single person; therefore, there is a possibility that an error in the measurement result may be included. Second, because the amount of data for the group with GapDW of more than 2 kg is small, it is not suitable for training a machine-learning predictive model for OHCP. However, it is important to obtain a predictive model and proper factors other than the BIS factors to improve the treatment of patients in this group. Third, in this study, an analysis by gender and age was not performed. Such an analysis in the future will enable a more detailed approach for these patient groups. Fourth, it was not possible to evaluate whether the external cohort population showed the same pattern as a single-center study. Fifth, the patient’s mortality and morbidity were not analyzed in relation to water control through more accurate measurement of dry weight. In future studies, prospective studies and multicenter studies will be conducted, and the long-term prognosis of patients will need to be analyzed.
In conclusion, we found the feature importance of groups according to differences in GapDW. It was confirmed that ECW and malnutrition-related blood chemistry findings, including hemoglobin, serum total protein, serum albumin, serum creatinine, serum phosphorus, and serum potassium, were important features. Machine learning made better predictions of DWCP based on DWBIS in patients with GapDW of less than 2 kg. It is difficult to predict DWCP using machine learning for patients with GapDW of more than 2 kg; therefore, it is necessary to analyze additional appropriate features for future extreme cases.
In the future, if more patients are included to increase the prediction accuracy using machine learning, this technique will be helpful in establishing the appropriate DW for patients. Machine-learning predictive models can be helpful to establish aOHCP.
Data Availability
Data cannot be shared publicly because data contains potentially identifying or sensitive patient information. Data are available from the Institutional Data Access / Ethics Committee of Chungnam National University Hospital (contact via irb@cnuh.co.kr) for researchers who meet the criteria for access to confidential data.
Funding Statement
This research was supported by National Institute for Mathematical Sciences (NIMS) grant funded by the Korea government, 2021 (No. NIMS-B21910000) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1AB03035061). However, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chang S-T, Chen C-C, Chen C-L, Cheng H-W, Chung C-M, Yang T-Y. Changes of the cardiac architectures and functions for chronic hemodialysis patients with dry weight determined by echocardiography. Blood Purif. 2004;22(4):351–359. 10.1159/000080031 [DOI] [PubMed] [Google Scholar]
- 2.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24(5):1574–1579. 10.1093/ndt/gfn707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charra B. Does empirical long slow dialysis result in better survival? If so, how and why? ASAIO J. 1993. [PubMed] [Google Scholar]
- 4.Sinha AD, Agarwal R. Opinion: can chronic volume overload be recognized and prevented in hemodialysis patients? The pitfalls of the clinical examination in assessing volume status. Semin Dial. 2009;22(5):480–482. 10.1111/j.1525-139X.2009.00641.x [DOI] [PubMed] [Google Scholar]
- 5.Onofriescu M, Siriopol D, Voroneanu L, Hogas S, Nistor I, Apetrii M, et al. Overhydration, cardiac function and survival in hemodialysis patients. PLoS One. 2015;10(8):e0135691. 10.1371/journal.pone.0135691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vertes V, Cangiano JL, Berman LB, Gould A. Hypertension in end-stage renal disease. NEJM. 1969;280(18):978–981. 10.1056/NEJM196905012801802 [DOI] [PubMed] [Google Scholar]
- 7.Fishbane S, Natke E, Maesaka JK. Role of volume overload in dialysis-refractory hypertension. Am J Kidney Dis. 1996;28(2):257–261. 10.1016/s0272-6386(96)90309-1 [DOI] [PubMed] [Google Scholar]
- 8.Amato R, Hlebovy D, King B, Salai P. Fluid removal. Obtaining estimated dry weight during hemodialysis. ANNA Core Curriculum for Nephrology Nursing. 2008:692–703. [Google Scholar]
- 9.Sands JJ, Usvyat LA, Sullivan T, Segal JH, Zabetakis P, Kotanko P, et al. Intradialytic hypotension: Frequency, sources of variation and correlation with clinical outcome. Hemodial Int. 2014;18(2):415–422. 10.1111/hdi.12138 [DOI] [PubMed] [Google Scholar]
- 10.Daugirdas JT, Blake PG, Ing TS. Handbook of dialysis. 4th ed: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 11.San Miguel S. Haemodialysis dry weight assessment: A literature review. Ren Soc Aust J. 2010;6(1):19–24. [Google Scholar]
- 12.Poggi A, Maggiore Q. Cardiothoracic ratio as a guide to ultrafiltration therapy in dialyzed patients. Int J Artif Organs. 1980;3(6):332–337. [PubMed] [Google Scholar]
- 13.Vitturi N, Dugo M, Soattin M, Simoni F, Maresca L, Zagatti R, et al. Lung ultrasound during hemodialysis: the role in the assessment of volume status. Int Urol Nephrol. 2014;46(1):169–174. 10.1007/s11255-013-0500-5 [DOI] [PubMed] [Google Scholar]
- 14.Agarwal R, Bouldin JM, Light RP, Garg A. Inferior vena cava diameter and left atrial diameter measure volume but not dry weight. Clin J Am Soc Nephrol. 2011;6(5):1066–1072. 10.2215/CJN.09321010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passauer J, Petrov H, Schleser A, Leicht J, Pucalka K. Evaluation of clinical dry weight assessment in haemodialysis patients using bioimpedance spectroscopy: a cross-sectional study. Nephrol Dial Transplant. 2010;25(2):545–551. 10.1093/ndt/gfp517 [DOI] [PubMed] [Google Scholar]
- 16.Chamney PW, Krämer M, Rode C, Kleinekofort W, Wizemann V. A new technique for establishing dry weight in hemodialysis patients via whole body bioimpedance. Kidney Int. 2002;61(6):2250–2258. 10.1046/j.1523-1755.2002.00377.x [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Liu T, Shepherd S, Paiva W. Correction: Using Statistical and Machine Learning Methods to Evaluate the Prognostic Accuracy of SIRS and qSOFA. Healthc Inform Res. 2018;24(3):250. 10.4258/hir.2018.24.3.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desautels T, Hoffman J, Barton C, Mao Q, Jay M, Calvert J, et al. Pediatric severe sepsis prediction using machine learning. bioRxiv. 2017:223289. [Google Scholar]
- 19.Arefan D, Mohamed AA, Berg WA, Zuley ML, Sumkin JH, Wu S. Deep learning modeling using normal mammograms for predicting breast cancer risk. Med Phys. 2020;47(1):110–118. 10.1002/mp.13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke G, Meng Q, Finley T, Wang T, Chen W, Ma W, et al., editors. Lightgbm: A highly efficient gradient boosting decision tree. Adv Neural Inf Process Syst; 2017. [Google Scholar]
- 21.Chen T, Guestrin C, editors. Xgboost: A scalable tree boosting system. Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining; 2016 August; San Francisco California USA: Association for Computing Machinery, New York, NY, United States.
- 22.Ho TK, editor Random decision forests. Proceedings of 3rd international conference on document analysis and recognition; 1995 Aug 14–16; Montreal, Quebec, Canada, Canada: IEEE; 2002.
- 23.Jaeger JQ, Mehta RL. Assessment of dry weight in hemodialysis: an overview. J Am Soc Nephrol. 1999;10(2):392–403. [DOI] [PubMed] [Google Scholar]
- 24.Lee SW, Song JH, Kim GA, Lim HJ, Kim M-J. Plasma brain natriuretic peptide concentration on assessment of hydration status in hemodialysis patient. Am J Kidney Dis. 2003;41(6):1257–1266. 10.1016/s0272-6386(03)00358-5 [DOI] [PubMed] [Google Scholar]
- 25.Ando Y, Yanagiba S, Asano Y. The inferior vena cava diameter as a marker of dry weight in chronic hemodialyzed patients. Artificial Organs. 1995;19(12):1237–1242. 10.1111/j.1525-1594.1995.tb02292.x [DOI] [PubMed] [Google Scholar]
- 26.Cox-Reijven PL, Kooman JP, Soeters PB, van der Sande FM, Leunissen KM. Role of bioimpedance spectroscopy in assessment of body water compartments in hemodialysis patients. Am J Kidney Dis. 2001;38(4):832–838. 10.1053/ajkd.2001.27703 [DOI] [PubMed] [Google Scholar]
- 27.Dumler F. Hypoalbuminemia is a marker of overhydration in chronic maintenance patients on dialysis. ASAIO J. 2003;49(3):282–286. 10.1097/01.mat.0000065465.52748.bb [DOI] [PubMed] [Google Scholar]
- 28.Zaki DS, Mohamed RR, Mohammed NA, Abdel-Zaher RB. Assessment of malnutrition status in hemodialysis patients. Clinical Medicine and Diagnostics. 2019;1:0.20. [Google Scholar]
- 29.Jones CH, Akbani H, Croft DC, Worth DP. The relationship between serum albumin and hydration status in hemodialysis patients. J Ren Nutr. 2002;12(4):209–212. 10.1053/jren.2002.35295 [DOI] [PubMed] [Google Scholar]
- 30.Garagarza C, Valente A, Caetano C, Oliveira T, Ponce P, Silva AP. Hypophosphatemia: nutritional status, body composition, and mortality in hemodialysis patients. International urology nephrology. 2017;49(7):1243–1250. 10.1007/s11255-017-1558-2 [DOI] [PubMed] [Google Scholar]
- 31.Hung SC, Kuo KL, Peng CH, Wu CH, Wang YC, Tarng DC. Association of fluid retention with anemia and clinical outcomes among patients with chronic kidney disease. J Am Heart Assoc. 2015;4(1):e001480. 10.1161/JAHA.114.001480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Androne A-S, Katz SD, Lund L, LaManca J, Hudaihed A, Hryniewicz K, et al. Hemodilution is common in patients with advanced heart failure. Circ. 2003;107(2):226–229. 10.1161/01.cir.0000052623.16194.80 [DOI] [PubMed] [Google Scholar]
- 33.Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle. 2013;4(1):19–29. 10.1007/s13539-012-0079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, et al. Associations of body mass index and weight loss with mortality in transplant‐waitlisted maintenance hemodialysis patients. Am J Transplant. 2011;11(4):725–736. 10.1111/j.1600-6143.2011.03468.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinge E, Lindergård B, Nilsson–Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. 1999;59(8):587–592. 10.1080/00365519950185076 [DOI] [PubMed] [Google Scholar]
- 36.Nakhoul GN, Huang H, Arrigain S, Jolly SE, Schold JD, Nally JV Jr, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. J Am Soc Nephrol. 2015;41(6):456–463. 10.1159/000437151 [DOI] [PMC free article] [PubMed] [Google Scholar]