Abstract
Objectives
To describe and estimate the frequency of pregnancy outcomes, clinical and laboratory characteristics of vertical transmission of CHIKV in the neonate.
Study design
We performed a systematic review evaluating the clinical presentation of perinatally-acquired CHIKV infection in neonates. The search was performed using Medline (via PubMed), LILACS, Web of Science, Scielo, Google Scholar and Open grey to identify studies assessing vertical transmission of CHIKV up to November 3, 2020. There were no search restrictions regarding the study type, the publication date or language. Studies with no documented evidence of CHIKV infection in neonates (negative RT-PCR or absence of IgM) were excluded.
Results
From the 227 studies initially identified, 42 were selected as follows: 28 case reports, 7 case series, 2 cross-sectional studies and 5 cohort studies, for a total of 266 CHIKV infected neonates confirmed by serological and/or molecular tests. The vertical transmission rate was 50% in the Reunion Island outbreak, which was the subject of the majority of the studies; the premature delivery were reported in 19 (45.2%) studies; the rate of fetal distress was 19.6% of infected babies and fetal loss occurred in 2% of the cases. Approximately 68.7% of newborns were diagnosed with encephalopathy or encephalitis after perinatally acquired CHIKV. Most of the infected neonates were born healthy, developing CHIKV sepsis clinical syndrome within the first week of life.
Conclusions
We alert neonatologists to the late manifestations of neonatal CHIKV infection, relevant to the management and reduction of morbidity. A limitation of our review was that it was not possible to carry out meta-analysis due to differences in study design and the small number of participants.
1- Introduction
Chikungunya virus (CHIKV) is an arthropod-borne virus (arbovirus) of the alphavirus genus (Togaviridae family) that is believed to have originated in Africa, where it was known to circulate in sylvatic and urban cycles involving female mosquito vectors of the genus Aedes and non-human or human primates [1,2]. Since 2006, four lineages have been recognized: the West African, the East/Central/South African (ECSA), the Asian genotypes plus the Indian ocean lineage (IOL) that has diverged from the ECSA [3–5].
Vertical transmission was first observed in June 2005 in the CHIKV epidemic that affected more than a third of the population in Reunion Island from March 2005 to July 2006 [6,7]. Intrapartum transmission without placental infection, a direct consequence of maternal viremia and fetal or neonatal susceptibility to a given arbovirus, has been well documented for CHIKV in mouse models [8]. However, evidence of transplacental infection by the recovery of the genome of CHIKV in the amniotic fluid, the placenta and the fetal brain of stillborn fetuses in Reunion Island, when fetal loss occurred before 16 weeks of gestational age was reported. The mother’s chikungunya diagnosis was confirmed by RT-PCR detection of the viral genome in maternal blood two weeks before diagnosis of fetal loss; viral genome in the placenta and amniotic fluid confirmed transplacental transmission of CHIKV and its persistence after fetal death. The effect of the virus on the fetus was confirmed by further observations and intracellular detection of the virus in fetal tissues including brain and liver [9]. Humans transplacental CHIKV transmission though seems likely, but its pathophysiology remains unknown [6,10].
Reunion Island studies have shown that CHIKV could be transmitted vertically in about 50% of cases when the pregnant woman has high viral load during the early phase of labor. Fetal heart rate abnormalities and meconium-stained amniotic fluid were common during labor of pregnant women with CHIKV viremia. Neither postponed vaginal delivery nor cesarean section prevented fetal infection [6].
Neonatal infection can be severe and encephalopathy or encephalitis occurs in more than half cases who require intensive care [11]. The central nervous system involvement was associated with a massive brain swelling as evidenced by MRI findings [11].
The presentation, similar to bacterial sepsis poses a diagnostic challenge when maternal infection has not been diagnosed. Other differential diagnoses are those caused by other arboviruses and congenital infections of the TORCHZ group (toxoplasmosis, enterovirus, Listeria monocytogenes, Mycobacterium tuberculosis, parvovirus B19, Treponema pallidum, Trypanosoma cruzi, human immunodeficiency virus, varicella virus, rubella, cytomegalovirus, herpes simplex virus) [12].
As it is the case in pregnant women, there is no specific treatment for chikungunya in the neonate. The approach is symptomatic, focused on hydration, fever control and pain relief [13]. Vector control and personal protection are currently the only chikungunya prevention measures available [13].
The limited knowledge about the clinical manifestations in the neonate and pregnancy outcomes of CHIKV vertical transmission prompted us to conduct this study. We performed a systematic review of the available literature reporting the clinical and laboratory characteristics of CHIKV vertical transmission in the neonate aiming at gathering the best evidence to critically evaluate the approach following CHIKV infection in pregnancy.
2- Materials and methods
The review was conducted according to a pre-established protocol and described following PRISMA (Preferred Reporting Items for Systematics Reviews and Meta-Analyzes) recommendations [14].
2.1 Search
The literature search was performed using Medline (via PubMed), LILACS, Web of Science, Scielo, Google Scholar and Open grey to identify studies assessing vertical transmission of chikungunya virus up to November 3, 2020. Additional articles not found by the above searches were identified from the reference lists of the selected articles. No study, according to the inclusion criteria, was identified using the open gray search tool.
The search descriptors used for Medline were as follows: (((congenital chikungunya) AND (((newborn) OR (((infant OR neonatal))) AND (((mother-to-child OR congenital OR vertical transmission)))) AND chikungunya). The complete search strategies were adapted according to each data base.
2.2 Selection
Articles were selected for inclusion by two independent authors (FF and AS), with differences resolved after discussion and consensus with a third author (PB). We included studies of different design that evaluated perinatal transmission of CHIKV regarding its clinical presentation and therapeutic approach, with confirmed laboratory diagnosis in neonates (RT-PCR and/or IgM serology positive for CHIKV) and with clinical, epidemiological and/or confirmed laboratory diagnosis in pregnant women. There were no restrictions regarding publication date or language.
Initially titles and abstracts were read to exclude those that did not fall into the eligibility criteria. Subsequently, the full texts potentially eligible for inclusion were read and the same inclusion and exclusion criteria were applied. In addition, we excluded studies with no documented evidence of CHIKV infection in neonates (negative RT-PCR or absence of IgM).
2.3 Data extraction and quality assessment
The data were extracted by two authors (FF and AS), disagreements were resolved by consensus reached through a third author (PB). A standardized form developed for this review and pre-tested on three articles was used for extracting the data. The form contained the following sections: study identification (study title, author, journal, year of publication, country, language and financial institution); characteristics of the study (design, number of patients and study period); characteristics of the study population (pregnant women: diagnostic methods, clinical parameters, laboratory and treatment; neonates: age, gender, gestational age, birthweight, delivery type, place of hospitalization, diagnostic methods, clinical parameters, laboratory and treatment).
We included studies with clinical and laboratory neonatal data, as well as age in days at onset of illness and outcome in the neonate such as sequelae or death. From the selected articles, clinical, laboratory and perinatal variables of CHIKV infection were collected for pregnant women and neonates.To describe chikungunya evolution during pregnancy, the following variables were extracted: gestational age at onset of maternal disease, time of onset of disease in relation to delivery, type of delivery and outcome of pregnancy, such as death of the pregnant woman, premature delivery/preterm birth, fetal distress, miscarriage, or stillbirth.
Assessment of the methodological quality of the included studies was based on the Methodological Index for Non-randomized Studies (MINORS). The instrument consists of 12 items, the first eight being specific for non-comparative studies [15].
Because of differences in studies design and the small number of participants, a meta-analysis was deemed inappropriate and we focused on describing the studies in evidence with their results and a qualitative synthesis.
3- Results
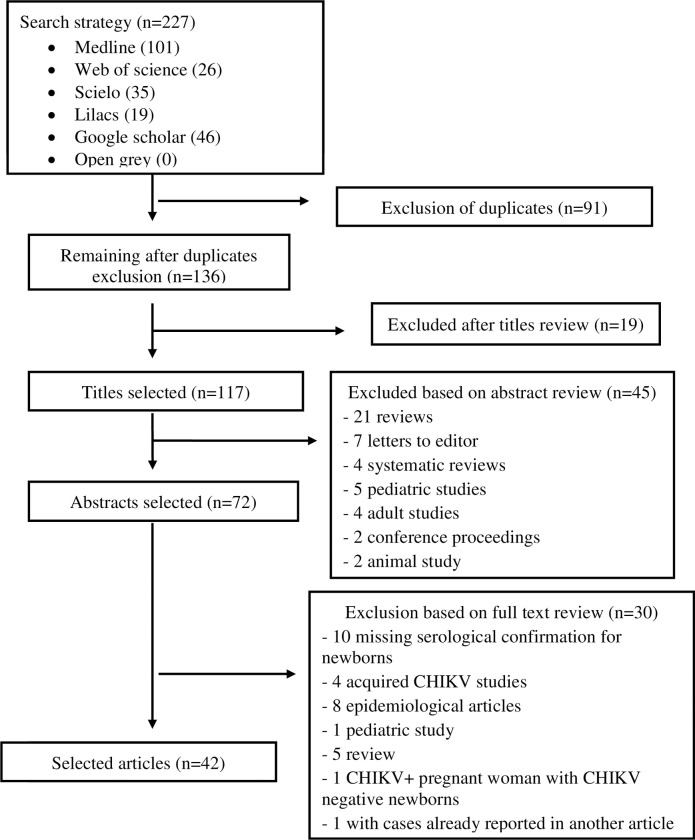
The literature search retrieved 227 publications; after exclusion of 71 duplicates, 136 titles were evaluated regarding the established inclusion and exclusion criteria. Of these, 64 were excluded based on the title (19) and abstract (45) (Fig 1). The final selection resulted in 42 articles: 28 case reports, seven case series, two cross-sectional study and five cohort studies. No new articles were added after searching the reference section of the selected articles (Fig 1).
Fig 1. Flowchart of review process.
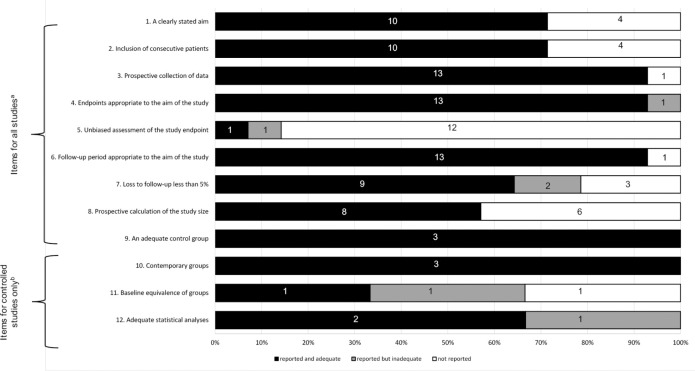
The quality of articles was assessed by MINORS instrument for all observational studies except for case reports. More than 90% of the studies presented endpoints to their aim and follow-up period appropriate and all reported an adequate control group. More than half of studies report the inclusion of consecutive patients. About 90% of the studies did not report an unbiased assessment of the study end point. Comparative studies (n = 3) achieved good quality criteria, more than 60% presented adequate statistical analyses (Fig 2).
Fig 2. Quality assessment of the studies included in the systematic review based on Methodological Index for Nonrandomized Studies (Slim et al, 2003) [15].
(A) Quality items for all observational studies. (B) Quality items for comparative studies.
In this systematic review of 42 studies that included 266 fetuses or neonates infected with CHIKV by vertical transmission, neonatal infection was severe, manifesting with neurological disease hospitalized in intensive care in 76.5% of cases. The development of life-threatening neonatal symptoms was not associated with the severity of maternal symptoms. From the maternal inoculum, it takes three to seven days for the CHIKV load to reach a significant level able to cause clinical disease. Whether the vertical transmission occurs through the syncytiotrophoblast or per mucosa during childbirth remains undiscovered. No infection was demonstrated through breastfeeding, which was postponed until the clearance of maternal symptoms.
3.1 Characteristics of selected studies
The selected studies were published between 2006 and 2020 and conducted between 2005 and 2019 (Table 1). All studies included newborn infants hospitalized in neonatal intensive care units (NICUs), with clinical chikungunya and laboratory evidence of viral infection. Cases without laboratory confirmation or postnatal chikungunya were excluded from the analysis.
Table 1. Characteristics of included studies.
| Study [reference], Author, Year | Study period, location, | Sample size (N) | Type of study | Pregnant woman CHIKV+ diagnosis | Number of vertical transmission cases, diagnosis* |
|---|---|---|---|---|---|
| [16], Correa, 2020 | ND, Brazil | 6 | Case report | Clinical-laboratory | 6, IgM+, RT-PCR |
| [17], Jebain, 2020 | ND, Brazil | 1 | Case report | Clinical-laboratory | 1, IgM+, RT-PCR |
| [18], Correa, 2020 | ND, Brazil | 3 | Case report | Clinical-laboratory | 3, IgM+, RT-PCR |
| [19], Shen, 2020 | 2019, China | 3 | Case report | Clinical-laboratory | 3, IgM+, RT-PCR |
| [20], Chandramathi, 2020 | ND, India | 1 | Case report | Clinical epidemiological | 1, IgM+ |
| [21], Di Maio Ferreira, 2019 | 2016, Brazil | 1 | Case report | Clinical-laboratory | 1, IgM+, RT-PCR |
| [22], Dorléans, 2018 | 2013–2015; Martinique and Guadaloupe | 15 | Cross-sectional | Clinical-laboratory | 15,IgM+, RT-PCR |
| [23], Kumar, 2018 | 2006, India | 2 | Case report | Clinical-laboratory | 2, IgM+ |
| [24], Kumar, 2018 | 2016; India | 6 | Case report | Clinical-laboratory | 6, IgM +, RT-PCR |
| [25], Oliveira, 2018 | 2017; Brazil | 1 | Case report | Clinical-epidemiological | 1, IgM + |
| [26], Van Enter, 2018 | 2018; Island of Curaçao | 14 | Case report | Clinical-laboratory | 3, IgM+ |
| [27], Leitão, 2018 | 2016; Brazil | 5 | Case report | clinical-laboratory | 5, RT-PCR |
| [28],Maria A, 2018 | Aug–Nov 2016; India | 10 | Case series | clinical-laboratory | 10, IgM + |
| [29], Cardona- Correa, 2017 | ND; Colombia | 1 | Case report | clinical-epidemiological | 1, IgM+ |
| [30], Evans-Gilbert, 2017 | 2014; Jamaica | 2 | Case report | clinical-laboratory | 1, RT-PCR |
| [31], Ramos, 2018 | Feb 2016; Brazil | 1 | Case report | clinical-laboratory | 1, RT-PCR |
| [32], Alvarado-Socarras, 2016 | Aug 2014-Feb 2016; Colombia | 2 | Case report | clinical-epidemiological | 1, IgM+ |
| [33], Torres, 2016 | 2014–2015; Brazil, El Salvador, Dominican Republic, Venezuela | 169 | Case Series | clinical-laboratory | 55, RT-PCR |
| [34], Karthiga, 2016 | ND; India | 2 | Case report | clinical-laboratory | 2, IgM+ |
| [35], Nigam, 2016 | ND; India | 1 | Case report | clinical-laboratory | 1, RT-PCR |
| [36],Lyra, 2016 | 2014–2016; Brazil | 2 | Case report | clinical-laboratory | 2, RT-PCR |
| [37], Bandeira, 2016 | 2015; Brazil | 1 | Case report | clinical-epidemiological | 1, RT-PCR |
| [38], Pinzon-Redondo, 2016 | Sept-Dec 2014; Colombia | 54 | Case series | ND | 2, RT-PCR |
| [39], Vasani, 2016 | ND; India | 2 | Case report | clinical-laboratory | 1, IgM+ |
| [40], Muñoz, 2014 | 2014; Colombia | 18 | Case report | ND | 2, RT-PCR |
| [41], Villamil-Gomez, 2015 | Sept 2014-Feb 2015; Colombia | 8 | Case series | clinical-laboratory | 8, IgM+ and RT-PCR |
| [42], Taksande, 2015 | ND; India | 1 | Case report | clinical-epidemiological | 1, IgM+ |
| [43], Gérardin, 2014 | 2005–2006; Reunion Island | 33 | Cohort | clinical-laboratory | 33, IgM+ and RT-PCR |
| [44], Ramful, 2014 | 2005–2006; Reunion Island | 653 | Cohort | clinical-laboratory | 1, IgM+ and RT-PCR |
| [45], Shenoy, 2012 | ND; India | 2 | Case report | clinical-laboratory | 2, RT-PCR |
| [46], Boumahni, 2012 | June 2005-March 2006; Reunion Island | 20 | Case report | clinical-laboratory | 1, IgM+ and RT-PCR |
| [47], Shrivastava, 2011 | ND; India | 1 | Case report | clinical-laboratory | 1, RT-PCR |
| [48], Boumahni, 2011 | ND; Reunion Island | 1 | Case report | clinical-epidemiological | 1, IgM+ and RT-PCR |
| [49], Sharanabasavesh, 2011 | 2006–2010; India | 8 | Case series | Clinical-laboratory | 8, IgM+ and RT-PCR |
| [50], Fritel, 2010 | April-Nov 2006; Reunion Island | 914 | Cohort | clinical-laboratory | 1 newborn, 2 stillborn, IgM+ and RT-PCR |
| [51], Senanayake, 2009 | April-Oct 2007; Sri Lanka | 50 | Case series | clinical-laboratory | 7, IgM+ |
| [52], Rao, 2008 | 2006; India | 2 | Case report | clinical-epidemiological | 2, RT-PCR |
| [6], Gérardin, 2008 | June 2005-Dec 2006; Reunion Island | 739 | Cohort | clinical-laboratory | 19, IgM+ and RT-PCR |
| [53], Ramful, 2007 | March 2005-April 2006; Reunion Island | 38 | Case series | clinical-laboratory | 38, IgM+ and RT-PCR |
| [9], Touret, 2006 | Dec 2005-Feb 2006; Reunion Island | 3 | Case report | clinical-laboratory | 3 fetuses <22 weeks, RT-PCR |
| [54], Paquet, 2006 | March 2005-Jan 2006; Reunion Island | 6 | Cross-sectional | clinical-laboratory | 6, IgM+ and RT-PCR |
| [10], Lenglet, 2006 | June 2005-Feb 2006; Reunion Island | 160 | Cohort | clinical-laboratory | 16, IgM+ and RT-PCR |
ND: No data.
* Diagnosis was by serology (IgM+) and/or RT-PCR.
Most studies (n = 35; 83,3%) were case reports and case series [9,16–21,23–42,45–49,51–53]. The clinical and perinatal manifestations of pregnancy and the outcome of gestation were described in ten studies [9,10,16–21,23,25,27,49–51]. Stillbirths and miscarriage associated with CHIKV vertical transmission with laboratory evidence were reported in three studies [9,50,54].
The 42 selected studies included 266 cases of vertical transmission and were conducted in countries that suffered from chikungunya epidemics, mostly Central and South America and the Caribbean, India and Reunion Island (French overseas department). Most studies in Central and South America and the Caribbean were case reports. A case series study was conducted in Latin America, which covered cases in four maternity hospitals from three different countries: Colombia, El Salvador and the Dominican Republic. Ninety-nine cases from the Dominican Republic and 35 cases from Colombia were excluded from the systematic review because they lacked laboratory confirmation [33]. Vertical transmission rates ranged from 27.7% to 48.7% in two selected studies [6,33].
3.2 Pregnancy outcome, clinical and perinatal characteristics of the pregnant women (Table 2)
Table 2. Characteristics of pregnant women, neonates and their outcomes.
| Study [reference], Author, Year | Pregnant women (N), Delivery type, Gestational age (weeks), Chikungunya start (days before delivery) | Pregnant woman manifestations (clinical and laboratory) | Fetal distress, gestation outcome | Neonates (N), Chikungunya start (days after birth), neonatal deaths | Neonatal manifestations (clinical and laboratory); sequelae |
|---|---|---|---|---|---|
| [16], Correa, 2020 | 6, C (4), V (2), 37 sem (3), 34 sem 5 d, 35 sem and 36 sem, 1 to 7 | Fever, arthralgia, rash | ND, PMT | 6, 4 to 10, N | Fever, hypoactivity, seizure, sepsis, irritability, thrombocytopenia, ND |
| [17], Jebain, 2020 | 1, ND, Term, after birth | Fever, arthralgia | ND | 1, 7, N | Bullous dermatitis, ND |
| [18], Correa, 2020 | 3, C (2) and V (1), 36 (1) and 37 (2), at birth | Fever, arthralgia, rash | ND, PMT | 3, 4,5 and 6, N | Hypoactivity, fever, rash, suctioning difficult, thrombocytopenia, longitudinal brain magnetic resonance with brain impairement; ND |
| [19], Shen, 2020 | 2, C, PMT and Term, at birth | Fever, arthralgia, rash, myalgia | ND, PMT | 3, 5 and 3, N | Fever, rash, respiratory failure, jaundice, arthralgia and hypoactivity; ND |
| [20], Chandramathi, 2020 | 1, ND, ND, 7 | Fever | ND | 1, 15, N | Apnea,fever,seizure,sepsis, jaundice, respiratory failure, thrombocytopenia, ND |
| [21], Di Maio Ferreira, 2019 | 1, C, Term, at birth | Fever, arthralgia, rash | Y, ND | 1, 6, N | Rash, fever, arthralgia, arthritis, hypoactivity, hyperpigmentation, bullous dermatitis; none |
| [22], Dorléans, 2018 | 15, ND, ND, 6 | ND | ND | 15,ND; N | ND |
| [23], Kumar, 2018 | 2,V, ND, 4 and 1 | Fever, arthralgia, rash | ND, PMT (1) | 2, 5 and 4, N | Rash, fever, arthralgia, arthritis, hypoactivity, hyperpigmentation, hemodynamic instability, respiratory distress, seizure; ND |
| [24], Kumar, 2018 | 6, ND, ND, ND | ND | ND | 6, ND, N | ND |
| [25], Oliveira, 2018 | 1, C, 34, 10 | Itching, arthralgia, arthritis, fever, headache, seizure, abdominal pain, diarrhea, cough, dyspnea and arterial hypertension, respiratory failure; thrombocytopenia and neutrophilia | ND, PMT and death | 1, at birth, 1 | Apnea,fever,seizure,sepsis, jaundice, gastrointestinal bleeding, pulmonary hemorrhage, respiratory failure; death |
| [26], Van Enter, 2018 | 14, C (2) and V(1), 35 sem 2 d (1) and ND (13), 1 (2) and 7 (11) | ND | Y, PMT (1) | 3, at birth (1) 4 (1), 5 (1); 1 | Intracranial hemorrhage, fever, rash, seizure, irritability, suctioning difficult; death |
| [27], Leitão, 2018 | 5, ND, ND, ND | Rash,arthralgia, fever,bullous dermatitis | ND | 5, 2 to 4, N | Fever,hypoactivity,encephalitis, meningoencephalitis, seizure, irritability, bullous dermatitis, hyperpigmentation intracranial hemorrhage, arthralgia thrombocytopenia; ND |
| [28],Maria A, 2018 | 10, ND, ND, ND | ND | ND | 10,ND,N | Fever, hypoactivity, respiratory distress, suctioning difficult, hemodynamic instability, encephalitis, seizure, hyperpigmentation, jaundice, thrombocytopenia, leukopenia; Neurological |
| [29], Cardona- Correa, 2017 | 1, C, 35, 3 | Rash, headache, arthralgia | N, PMT | 1, 4, N | Rash, irritability, hypoactivity, respiratory failure, intracranial hemorrhage, thrombocytopenia; neurological (optic nerve atrophy, microcephaly, epilepsy and cerebral palsy) |
| [30], Evans-Gilbert, 2017 | 1, V, 39, ND | Fever, arthralgia | N, N | 1, 5, N | Rash, fever, thrombocytopenia, elevated GOT; none |
| [31], Ramos, 2018 | 1, C, 35,3 | Fever, disseminated intravascular coagulation, arthralgia, abdominal pain, renal failure, rhabdomyolysis | Y, PMT | 1, 3, 1 | Fever, hypoactivity, apnea, respiratory failure, cyanosis, hemodynamic instability, elevated GOT; none |
| [32], Alvarado-Socarras, 2016 | 1, C, 38,1 | Rash, fever, pelvic pain, edema | N, N | 1, 3, N | Rash, fever and irritability, thrombocytopenia, elevated GOT; none |
| [34], Karthiga, 2016 | 2, ND ND, ND | ND | ND, ND | 2, 5 and 8, N | Rash, fever and irritability; ND |
| [33], Torres, 2016 | 55, ND ND, ND | ND | ND, PMT | 55, 5 (3–9), N | El Salvador and Colombia: fever, hypoactivity, suctioning difficulty, hemodynamic instability, respiratory insufficiency, meningoencephalitis, exanthema, bullous dermatitis, hyperalgesia, limb edema, myocarditis thrombocytopenia, anemia, pleocytosis; ND |
| [35], Nigam, 2016 | 1, V 36, ND | Fever | ND, PMT | 2, 5, N | Facial hyperpigmentation, respiratory insufficiency, convulsion, intracranial hemorrhage, thrombocytopenia, anemia; neurological |
| [36],Lyra, 2016 | 1, C 36, 15 | Fever and arthralgia, thrombocytopenia | Y, PMT | 1, birth day, N | Tachypnea and pericardial effusion; none |
| [37], Bandeira, 2016 | 2, V and ND 38 and 41, 2 | One case rash, fever and arthralgia, another ND | N, N | 2, 1–4, N | One case rash, fever, respiratory failure, hemodynamic instability, hepatosplenomegaly and another fever, hypoactivity, hemodynamic inatability and grade I intracranial hemorrhage; none |
| [38], Pinzon-Redondo, 2016 | 1, V ND, 4 | Rash and fever | N, ND | 1, 4, N | rash, fever, hypoactivity, convulsion, encephalitis; ND |
| [39], Vasani, 2016 | ND, ND ND, ND | ND | ND, ND | 2, 5, N | rash, fever and diarrhea, thrombocytopenia, lymphopenia; ND |
| [40], Muñoz, 2014 | 1, V ND, 7 | Fever | ND, ND | 1, birth day, N | Respiratory insufficiency and hyperpigmentation; ND |
| [41], Villamil-Gomez, 2015 | 1, V 38, 3 | Fever, headache and myalgia | N, N | 1, 3, N | Fever and irritability; ND |
| [42], Taksande, 2015 | 1, ND ND, At birth | ND | ND, ND | 1, 6, N | ND; ND |
| [43], Gérardin, 2014 | ND, ND ND, ND | ND | ND, PMT | 33, ND, N | ND; neurological |
| [44], Ramful, 2014 | 7, V (2), C (5) 32 to 39, At birth | Rash, fever and arthralgia, edema and headache | N, PMT (3) | 8 (one twins), ND, 3 | Exanthema, fever, bullous dermatitis, respiratory failure, meningoencephalitis, gastrointestinal bleeding, hyperalgesia, edema, myocarditis, pericarditis, hemodynamic instability and enterocolitis, thrombocytopenia; none |
| [45], Shenoy, 2012 | 1, C 36, At birth | Fever | Y, PMT | 1, 4, N | Respiratory insufficiency, hemodynamic instability and septic shock; neurological |
| [46], Boumahni, 2012 | 2, C ND, ND | Fever and arthralgia | N, N | 2, 5, N | Both cases hyperpigmentation, convulsion and apnea, only case 2 respiratory insufficiency; neurological (case 2 optic nerve atrophy) |
| [47], Shrivastava, 2011 | 1, C ND, ND | Thrombocytopenia | N, N | 1, 5, N | Fever, seizure, hyperalgesia, apnea, respiratory failure, thrombocytopenia; neurological |
| [48], Boumahni, 2011 | 1, C 35, 3 | Fever and arthralgia | Y, PMT | 1, birth day, N | Fever, rash, hyperpigmentation, sucking difficulty, apnea, thrombocytopenia and elevated GOT; none |
| [49], Sharanabasavesh, 2010 | 8, ND, 1premature and 7 term, 1 | Fever | ND | 8, 5, N | Sucking difficulty, seizure, fever, encephalitis, hyperpigmentation, hepatomegaly, respiratory failure, thrombocytopenia, ND |
| [50], Fritel, 2010 | 3, ND ND, 2 (one newborn), 25 and 70 (two stillborns) | ND | ND, 2 stillborns | 1, 3, N | ND; none |
| [51], Senanayake, 2009 | 7, ND ND, 0 (4 cases) and 1st, 2nd and 3rd trimestres | Fever and arthralgia ine case, other cases ND | ND, ND | 7, 2 (one case) ND others, N | One case fever, hyperpigmentation, irritability, hypoactivity and myocarditis, 3 cases hyperpigmentation 3 cases ND; ND |
| [52], Rao, 2008 | 19, V (9), C (10) 35–40, At birth | ND | Y (14) ND (5), ND and PMT | 19, 4 (3–7), N | Intracranial hemorrhage, disseminated intravascular coagulation, hyperalgesia, hematemesis, hypovolemic shock, respiratory insufficiency, thrombocytopenia, lymphopenia, anemia, elevated GOT, hypocalcemia, altered coagulogram, fever, rash, convulsion, hypoactivity; suction difficulty; neurological and blindness |
| [6], Gérardin, 2008 | 38, V (12), C (26) 35–41, 0 (18) and 4 until 2 days post-partum, 2 asymptomatic | ND | Y (17) ND (21), ND and one PMT | 38, 4 (3–7), 1 | Fever, exanthema, hyperpigmentation, hyperalgesia, gastrointestinal and conjunctival hemorrhage, respiratory failure, necrotizing enterocolitis, diarrhea, suctioning difficulty and hemodynamic instability, thrombocytopenia, lymphopenia, hypokalemia, altered coagulogram; neurological |
| [53], Ramful, 2007 | 2, V and C ND, 1 and 4 | Fever, arthralgia and headache | ND, N | 2, 3 and 5, N | One case fever, rash, irritability, sucking difficulty, apnea, another fever, hyperpigmentation, mucositis, difficulty sucking, inadequate weight gain; none |
| [9], Touret, 2006 | 3, ND ND, ND | ND | ND, miscarriage | NA | NA |
| [54], Paquet, 2006 | 3, V 12 to 15, at 12 (1 case) and 15 (2 cases) weeks gestation | Rash, fever, arthralgia and headache | ND, miscarriage (3) | N, N, N | None; none |
| [10], Lenglet, 2006 | 16, V (9), C (7) ND, At birth | Rash, fever, arthralgia | Y (10) ND (6), N | 16, 3–7, N | Fever, ND |
ND: No data; NA: Not applicable; PMT: Premature; FD: Fetal distress; GOT: Glutamic oxaloacetic transaminase; C: Ceasarean section, V: Vaginal delivery; Y: Yes; N: None.
All included studies (n = 42) reported CHIKV vertical transmission confirmed by laboratory and clinical-epidemiological evidence in pregnant women (Table 1). The maternal infection diagnosis was confirmed by molecular and serological tests in 31 (73.8%) studies [6,9,10,16–28,30,31,33–35,39,41,43–45,47–51,53,54] and in 243 (96.8%) women.
The CHIMERE cohort study on Reunion Island reported five miscarriages and five stillbirths; of the later tested by RT-PCR performed on placenta and amniotic fluid (AF) samples, two were CHIKV positive, one was negative and the remaining two were not tested [50]. One miscarriage (<22 weeks) was tested negative. For the two fetuses tested positive in the amniotic fluid, maternal symptoms of CHIKV infection began about 25 to 70 days before fetal loss. A previous report from Reunion Island identified three fetal losses from pregnant women who presented CHIKV clinical manifestations at about two, three and four weeks respectively before the detection of fetal death on routine ultrasound examination. CHIKV infection was confirmed by molecular examination (RT-PCR) of the placenta, AF, and fetal brain [9]. A case series study during an epidemic in Sri Lanka reported 50 pregnant women with serological proof of CHIKV infection, and among these one single case of first trimester miscarriage and one stillbirth at 33 weeks [51]. The fetal losses were not virologically analyzed in this study.
3.2.1 Pregnancy outcomes (Table 2)
In two studies, a case of maternal death occurred. In one them, the death occured four days postpartum, with rhabdomyolysis, disseminated intravascular coagulation (DIC) and acute renal failure [30]. In the other one, a 28-year-old pregnant woman with hypertension presented with symptoms compatible with an arboviral disease at 34 weeks’ gestation. She developed preeclampsia with severe respiratory failure which resulted in the emergency cesarean section, and the patient died 12 days after the onset of symptoms [25].
Two cohort studies [6,50] reported six miscarriages (<22 weeks of gestational age) and two stillbirths (> 22 weeks of gestational age) [50]. Premature deliveries/preterm births were reported in 19 (45.2%) studies. One of these showed a median gestational age at childbirth of 38 weeks (35–41 weeks) [6]. Latin American studies reported a mean gestational age of 38 and 39 weeks [33,41]. Among these studies, there were related 18 premature deliveries/preterm births and 58 full-term deliveries/full-term neonates. No congenital malformation was described.
3.2.2 Perinatal characteristics (Table 2)
In one single case report, the onset of chikungunya illness for the pregnant woman began 15 days before delivery [35]. In twelve studies (28.5%), the timing of onset of symptoms before childbirth was not reported. The other studies showed that symptoms started during the perinatal period (from 10 day before delivery until 2 day postpartum), therefore maternal chikungunya onset ranged from 15 days before delivery to two days after delivery with 158 mothers of infants infected with CHIKV presenting chikungunya signs during the perinatal period. The gestational age of chikungunya infection in the pregnant woman was not described in 20 (47.6%) of the studies. Among others, the majority of CHIKV infection was reported in the second and third trimester, mainly in the intrapartum period [50,51,54]. In 19 studies (45,2%) childbirth by cesarean delivery was postponed, whereas in 16 studies (38%) it was vaginal delivery. Fetal distress was observed in 9 (21.4%) studies, of which 66,4% were deliveries by cesarean section. The rate of fetal distress among infected pregnant women was 49 (19.6%). In total, 75 cases of cesarean delivery were reported, 48 of which were due to fetal distress. The number of vaginal deliveries was 96 cases and in 158 cases the birth route was not reported.
3.3 Clinical and perinatal characteristics of the neonates (Table 2)
All studies described newborn infants with CHIKV clinical manifestations and laboratory, serological and molecular tests confirming the vertical transmission. The clinical and laboratory evidence, and in some cases the outcome of vertical transmission and the therapeutic approach for these neonates were reported. A total of 266 confirmed infected neonates were reported by serological and/or molecular examination, in addition to two stillbirths and four miscarriages.
3.3.1 Clinical characteristics (Tables 2 and 3)
Table 3. Characteristics of 42 mother-to-child congenital chikungunya studies.
| Clinical findings | Manifestation | N of studies (%) |
|---|---|---|
| Fever | 24 (57.1%) | |
| Neurological | Hipoactivity Irritability Poor feeding Encephalopaty/encephalithis Meningoencephalitis Seizures Intracranial hemorrhage |
24 (57.1%) |
| Dermatological | Maculopapular eruption Hyperpigmentation Bullous dermatoses Skin scaling |
24 (57.1%) |
| Respiratory | Apnea Respiratory failure |
24 (57.1%) |
| Hematological | Thrombocytopenia Disseminated coagulation intravascular |
17 (40.4%) |
| Cardiovascular | Hemodynamic instability Myocarditis Pericardial effusion Pericarditis |
11 (26.1%) |
| Gastrintestinal | Enterocolitis Diarrhea Mucositis Gastrointestinal bleeding |
9 (21.4%) |
| Sequels | Neurological | 9 (21.4%) |
| Skeletal muscle | Hyperalgesia Diffuser lower limb edema |
7 (16.6%) |
| Death | 5 (11.9%) | |
| Miscarriages | 2 (4.75%) | |
| Stillbirth | 2 (4.75%) |
Neonatal sepsis was the most common CHIKV clinical presentation in the neonate; it was reported in 36 (85.7%) studies and 250 cases.
In all studies the neonates were admitted to the NICU. The first signs and symptoms appeared between the first and the fifteenth day of life. The signs and symptoms reported in the 42 studies were in decreasing order: 24 (57.1%) presenting fever and rash, (187 cases with fever and 147 cases with rash); in 24 (57.1%) studies there were 183 cases with neurological signs characterized by irritability, seizures, meningoencephalitis, hypotonia and encephalitis, and 111 of respiratory failure characterized by apnea and the need for oxygen therapy; 11(26.1%) studies reported 137 cases of hemodynamic instability; nine (21.4%) studies reported 65 cases with gastrointestinal findings; seven (16.6%) studies reported 123 cases of hyperalgesia. Other signs and symptoms such as septic shock, bullous dermatosis, hyperpigmentation, intracranial hemorrhage, pericarditis, myocarditis, pericardial effusion, coronary dilatation, diarrhea and necrotizing enterocolitis were also reported (Table 2).
Nosocomial sepsis complicating maternal-neonatal chikungunya was reported twice with detection of Staphylococcus sp. and Klebsiella pneumonia in blood culture, and CHIKV viral genome by RT-PCR as well [36,53].
3.4 Laboratory characteristics (Table 2)
There were few laboratory findings reported. Seventeen studies (40.4%) reported 74 cases of thrombocytopenia (5,000–150,000 mm3) and five (11.9%) studies reported 41 cases of increased aspartate aminotransferase. Other relevant laboratory findings were anemia and prothrombin time enlargement.
3.5 Therapeutic approach
Therapeutic management of the neonates consisted of symptomatic measures in all studies, all cases being admitted to the NICU for supportive cares due to the severity of chikungunya presentation. The median length of hospital stay was 18 days (3–69 days). The use of antibiotic therapy was reported in 23 (54.7%) studies, corresponding to 28 cases. Respiratory failure and apnea, leading to invasive mechanical ventilation was reported in 15 (35.7%) studies or 48 cases. Transfusion of blood products (fresh frozen plasma and/or platelet concentrates and/or packed red blood cells) was performed in seven (16.6%) studies or 45 neonates. The use of vasoactive amines and/or volumetric expansion in patients with hemodynamic instability was reported in six (14.2%) studies or 24 cases. Pain assessment was done through a pain scale for the neonates in one study [9]. Neonates received analgesics for pain relief in three studies [5,9,40].
3.6 Neonate outcomes (Table 2)
Neurological sequelae (cognitive and/or motor and/or ophthalmologic dysfunction) of CHIKV vertical transmission were reported among infants in nine (21.4%) studies for a total of 106 cases; no information was reported in the remaining studies (n = 33; 78.5%). Few studies reported the infant follow-up time by a multidisciplinary team. Five studies (11.9%) described a total of 9 neonatal deaths; three of these newborns developed necrotizing enterocolitis, and the others had septic shock as the cause of death.
Only in twelve studies (28.5%), magnetic resonance imagery (MRI) was done, demonstrating the evolution of lesions from edema to cavitation or subcortical atrophy [5,16,18,29,55].
4- Discussion/Conclusions
This review confirms the severity of neonatal cases due to the vertical transmission of CHIKV. Maternal-neonatal chikungunya presents as a neurological disease or a neonatal sepsis that requires intensive support and upstream, the surveillance of CHIKV positive pregnant women all throughout pregnancy, and especially, during childbirth.
Vertical transmission of CHIKV was first reported during the 2005–2006 epidemic on Reunion Island, the location of most of the studies included here. It was demonstrated that the effects of CHIKV infection occurred almost invariably during maternal viremia in the peripartum period with a vertical transmission rate of about 50% and with a high viral load in the placenta [7,56]. As newborns infected in the peripartum period are born with very low or even undetectable viremia, placental microtransfusion seems unlikely as expected neonatal viremia would be close to that of the mother. In all cases, vertical transmission occurred regardless of the delivery route (75 cesareans and 96 natural deliveries). Premature delivery was related in 45.2% of studies. Most of the infected neonates were born healthy, developing CHIK clinical signs and symptoms within the first week of life. Fetal distress was reported in 25% of infected babies and was the cause of a cesarean delivery indication, reported in 69.2% of cases in one study [6]. Fetal loss (miscarriage and stillbirth) was reported in 4.75% of the studies. These rates were reported to be much lower in a recent meta-analysis that evaluated the risk for mother-to-child transmission (15.5%), antepartum fetal deaths (0.3%), and neonatal deaths (0.6%) [57]. In our review, the vertical transmission rate could not be estimated because neonatal confirmed CHIKV infection was an inclusion criteria of the study. The quantitative analysis of the study is impaired since the denominator of the number of cases on Reunion Island is unknown due to the absence of birth records from other places on the Island, with only data from the Saint Pierre region in the south of the Island. Saint Pierre is responsible for 40% of the total births on the island, but the epidemic also affected the eastern region and we do not have these data. In view of the above, the quantitative analysis data do not correspond to the reality of the epidemic in Reunion Island, impairing a meta-analysis.
The approach of this systematic review differs from the recent meta-analysis, published in 2018, as the latter is a study of an epidemiological nature while this review is aimed at the medical public and provides additional information for obstetricians and neonatologists complementing the meta-analysis information.
A limitation of this review was that the designs of the papers that we selected differed, with the majority being case reports, which hampered quantitative analysis. A strength is the clinical characterization of signs and symptoms associated with the vertical transmission of CHIKV, which is critical for improving the diagnosis of suspected cases and the appropriate therapeutic management of infected neonates. A novel contribution of this study is that it provides insights about arboviruses in endemic areas where the lack of adequate diagnosis and clinical management are associated with increased morbidity. Our review confirms that CHIKV can cause significant morbidity and also death in infected fetuses and neonates after maternal infections during gestation, which supports the previous meta-analysis [57].
To the best of our knowledge, there are no cohort studies in the literature that has reported vertical transmission with other CHIKV genotype than the IOL (mutation of the ECSA genome virus). Only three cohort studies [6,50,51] described vertical transmission. The main limitations of this review were the restriction of data to areas currently suffering or threatened by CHIKV epidemics; and the heterogeneity of mostly selected case reports and series, that prevented meta-analysis or statistical comparisons. The handling of these vertical cases is based on the opinion and experience of experts rather than higher rated evidence.
Despite laboratory confirmation of CHIKV vertical transmission in all neonates, some pregnant women only had clinical-epidemiological diagnosis, demonstrating the difficulty of simultaneous viral detection or serological conversion in the mother-child pair [33].
No correlation of the presence or severity of maternal symptoms with neonatal outcomes was reported.
The main clinical manifestations in neonates were those of neonatal sepsis: hypoactivity, irritability, sucking difficulty, apnea, hemodynamic instability and respiratory failure. In addition, most of the neonates presented fever, rash, hyperpigmentation, hyperalgesia, neurological manifestations and hemorrhagic phenomena. Convulsion, the main neurological manifestation in these neonates, could be due to encephalitis and intracranial hemorrhage. Cerebral edema may increase permeability of the blood-brain barrier without virus-induced damage of the central nervous system, but a direct virus-induced encephalitis cannot be ruled out. Magnetic resonance imaging showed severe white matter injury that has been well characterized in a 3 stage: cytotoxic brain edema (ischemia), vasogenic edema (reperfusion) and mass reduction (demyelination) [6,16,43].
The intensity of thrombocytopenia was associated with severe neonatal disease as central nervous system hemorrhages, namely scattered cerebrum parenchyma petechiae associated with a clinical context of disseminated intravascular coagulation syndrome.
Cutaneous hyperpigmentation in the convalescence phase was related to maternal CHIKV infection in the first trimester of gestation and usually disappeared around 6–7 months of life [51]. It demonstrates the ability of the virus to cross the barrier of placenta and points out an important sign of infection during early pregnancy.
The main chikungunya laboratory finding reported in the literature, lymphopenia was related to viremia and was defined when the lymphocyte count was lower than 1,000 cells/mm3. However, in this review it was not the most frequent laboratory abnormality; instead, increased levels of alanine aminotransferase and aspartate aminotransferase and hypocalcemia in the neonatal blood were the most often reported. Erythrocyte sedimentation rate was generally increased and correlated with active disease [58]. Thrombocytopenia (platelets <150000 mm3) was a frequent laboratory finding in the cases reported here, associated with the clinical picture of cerebral, gastrointestinal and conjunctival hemorrhages [53,58].
For differential diagnosis of CHIKV vertical transmission with other arboviral diseases sharing similar outcomes during gestation (miscarriage, preterm birth, fetal distress), sepsis should alert neonatologists and evoke chikungunya. Hyperalgesia, difficult to evaluate, and irritability, although common in neonatal CHIKV, were not specific and could be due to neonatal sepsis of other etiology. Although clinical and epidemiological criteria are important for the clinical and therapeutic approach of these neonates, especially in epidemic-affected areas, there are no specific clinical criteria that define neonatal CHIKV infection. Laboratory diagnosis is essential for the confirmation of infection and to differentiate chikungunya from other causes of fever in the neonate.
In only one study a pain scale was used for monitoring the infected neonates [59]. Arthralgia was little reported in the articles evaluated because pain was not assessed, therefore it is important to routinely establish neonatal pain monitoring in neonatal ICUs [58,60,61]. In fact, arthralgia should be assessed in neonates, mainly in those presenting limb edema with engulfing ankles and wrists. It was even proposed that suckling difficulties were due to mandibular painful involvement, mimicking trismus in some cases [6].
In conclusion, this systematic study reports the impact of CHIKV maternal infections during gestation on the fetuses and the newborn infants. The cases included in this review indicate that CHIKV congenital infection can be severe and therefore differential diagnosis of sepsis should be considered in affected areas, regardless of the presence or not of mother’s clinical manifestation. Neonatologists should be aware of late manifestations and babies of symptomatic mothers should be kept in the maternity ward for a week for clinical and laboratory surveillance through serial blood count with platelet count monitoring. Whether the neonates become symptomatic or present with lymphopenia or thrombocytopenia, they should be transferred urgently to a NICU.
This study characterizes the clinical presentation in the neonate of perinatally-acquired CHIKV infection during childbirth, which is critical for a better clinical awareness aimed at diagnosis improvement, early and appropriate therapeutic management. Therapeutic interventions aimed at clearing maternal or early neonatal viremia, such as anti-CHIKV hyperimmune immunoglobulins, should be mandatory to prevent neurological damage and lifelong disability in infected neonates.
Since now we have all the articles, we can contact the authors and prepare a data extraction form with all the variables of interest for a meta-analysis plan of individual data.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The production of this manuscript was supported by Centro Nacional de Pesquisa (CNPQ award number 307282/2017-1) and Fundação Carlos Chagas de Amparo à pesquisa do Estado do Rio de Janeiro (FAPERJ- E-26/202.862/2018). JR is an independent consultant, but has no other affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Robinson M.C., An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I. Clinical features. Trans R Soc Trop Med Hyg, 1955. 49(1): p. 28–32. 10.1016/0035-9203(55)90080-8 [DOI] [PubMed] [Google Scholar]
- 2.Ross R.W., The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond), 1956. 54(2): p. 177–91. 10.1017/s0022172400044442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumsden WHR, An epidemic of virus disease in southern province, Tanganyika territory, in 1952–53. II: General description and epidemiology. Trans R Soc Trop Med Hyg, 1955. 49(1): p. 33–55. 10.1016/0035-9203(55)90081-x [DOI] [PubMed] [Google Scholar]
- 4.Pialoux G., et al., Chikungunya, an epidemic arbovirosis. Lancet Infect Dis, 2007. 7(5): p. 319–27. 10.1016/S1473-3099(07)70107-X [DOI] [PubMed] [Google Scholar]
- 5.Burt F.J., et al., Chikungunya: a re-emerging virus. Lancet, 2012. 379(9816): p. 662–71. 10.1016/S0140-6736(11)60281-X [DOI] [PubMed] [Google Scholar]
- 6.Gérardin P., et al., Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Reunion. PLoS Med, 2008. 5(3): p. e60. 10.1371/journal.pmed.0050060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gérardin P., et al., Estimating Chikungunya prevalence in La Réunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis, 2008,8:99. 10.1186/1471-2334-8-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couderc T., et al., A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog, 2008. 4(2): p. e29. 10.1371/journal.ppat.0040029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touret Y., et al., [Early maternal-fetal transmission of the Chikungunya virus]. Presse Med, 2006. 35(11 Pt 1): p. 1656–8. 10.1016/S0755-4982(06)74874-6 [DOI] [PubMed] [Google Scholar]
- 10.Lenglet Y., et al., [Chikungunya infection in pregnancy: Evidence for intrauterine infection in pregnant women and vertical transmission in the parturient. Survey of the Reunion Island outbreak]. J Gynecol Obstet Biol Reprod (Paris), 2006. 35(6): p. 578–83. 10.1016/s0368-2315(06)76447-x [DOI] [PubMed] [Google Scholar]
- 11.Charlier C., et al., Arboviruses and pregnancy: maternal, fetal, and neonatal effects. The Lancet Child & Adolescent Health, 2017. 1(2): p. 134–146. [DOI] [PubMed] [Google Scholar]
- 12.Coyne C.B. and Lazear H.M., Zika virus—reigniting the TORCH. Nat Rev Microbiol, 2016. 14(11): p. 707–715. 10.1038/nrmicro.2016.125 [DOI] [PubMed] [Google Scholar]
- 13.Gérardin P., La fièvre Chikungunya chez l’enfant: de l’épidémiologie au traitement. mt pédiatrie, 2012. 15(2): p. 49–56. [Google Scholar]
- 14.Liberati A., et al., The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med, 2009. 6(7): p. e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slim K, Nini E, Forestier D, et al. Methodological Index for non-randomized studies (MINORS); development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 16.Corrêa DG, Freddi T A L, Werner H, Lopes FPPL, Moreira MEL, Di Maio Ferreira FCPA, Lopes JMA, Rueda-Lopes FC, Cruz LCH. Brain MR imaging of patients with perinatal chikungunya vírus infection. AJNR 2019. 10.3174/ajnr.A6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jebain J, Jr AS, Lupi O, Tyring SK. Perinatal chikungunya induced scalded skin syndrome. ID Cases 22(2020. e 00969). 10.1016/j.idcr.2020.e.00969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correa DG, Di Maio FCPA, Junior LCHC, Brasil P et al. Longitudinal brain magnetic resonance imaging of children with perinatal chikungunya encephalitis. The neuroradiology journal, 2020, 0(0) 1–6. 10.1177/1971400920959070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen JY, Li M, Xie L, Mao JR et al. Perinatal vertical transmission of chikungunya virusin Ruili, a town on th border between China and Burm. Virologia Sinica–Springer 10.1007/s12250-020-00245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandramathi J, Prabhu A, Kumar A. The “Chik sign” in neonatal chikungunya. Journal of brasilian society of tropical medicine Vol.:53:e20200157:2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Maio Ferreira FCPA, Silva ASV, Bispo de Filippis AM and Brasil P. Late Identification of CHIKV in the CNS. JPIDS 2019:8 374–377. 10.1093/jpids/piy135 [DOI] [PubMed] [Google Scholar]
- 22.Dorléans F, Hoen B, Najioullah F, Hermann–Storck C et al. Outbreak of chikungunya in the French Caribbean Island of Martinique and Guadaloupe: Finding froma hospital-based surveillance system (2013–2015). Am J Trop Hyg, 98 (6), 2018, pp. 1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Poonia AK, Agrawal K. Neonatal chikungunya encephalopathy. Pediatric Oncall Journal April–June 2019. 16 (2):53–54. [Google Scholar]
- 24.Kumar S, Agraval G, Wazir S, Kumar A et al. Experience of perinatal and neonatal chikungunya virus infection in a terciary care neonatal center during outbreak in North India in 2016. A case series. Journal of Tropical Pediatrics 2019, 65, 169–175. 10.1093/tropej/fmy032 [DOI] [PubMed] [Google Scholar]
- 25.Oliveira RMAB Barreto FKA, Maia AMPC Gomes IP et al. Maternal and infant death after probable vertical transmission of chikungunya virus in Brazil–case report. BMC Infectious Diseases (2018) 18:333. 10.1186/s12879-018-3243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Enter BJD, Huibers MHW, Van Rooij L, Steingrover R et al. Perinatal outcomes in vertically infected neonates during a chikungunya outbreak on the Island of Curaçao. Am J Trop Med Hyg, 99(6) 2018, pp.1415–1418. 10.4269/ajtmh.17-0957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leitão MCA, Arrais NMR, Filgueira FA, Bezerra MTAL et al. Cases of Chikungunya by vertical transmission in a University hospital in thre half of 2016. Respediatr-2019. v9n2-05. [Google Scholar]
- 28.Maria A, Vallamkonda N, Shukla A, Bhatt A and Sachdev N. Encephalitic presentation of Neonatal Chikungunya: A Case Series. Indian Pediatrics August 15, 2018; volume 55. [PubMed] [Google Scholar]
- 29.Cardona-Correa S.E., Castano-Jaramillo L.M., and Quevedo-Velez A., [Vertical transmission of chikungunya virus infection. Case Report]. Rev Chil Pediatr, 2017. 88(2): p. 285–288. 10.4067/S0370-41062017000200015 [DOI] [PubMed] [Google Scholar]
- 30.Evans-Gilbert T., Chikungunya and Neonatal Immunity: Fatal Vertically Transmitted Chikungunya Infection. Am J Trop Med Hyg, 2017. 96(4): p. 913–915. 10.4269/ajtmh.16-0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos R., et al., Perinatal Chikungunya Virus-associated Encephalitis Leading to Postnatal-Onset Microcephaly and Optic Atrophy. Pediatr Infect Dis J, 2018. 37(1): p. 94–95. 10.1097/INF.0000000000001690 [DOI] [PubMed] [Google Scholar]
- 32.Alvarado-Socarras J.L., et al., Congenital and Neonatal Chikungunya in Colombia. Journal of the Pediatric Infectious Diseases Society, 2016. 5(3): p. e17–e20. 10.1093/jpids/piw021 [DOI] [PubMed] [Google Scholar]
- 33.Torres J.R., et al., Congenital and perinatal complications of chikungunya fever: a Latin American experience. Int J Infect Dis, 2016. 51: p. 85–88. 10.1016/j.ijid.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 34.Karthiga V., Kommu P.P., and Krishnan L., Perinatal chikungunya in twins. J Pediatr Neurosci, 2016. 11(3): p. 223–224. 10.4103/1817-1745.193369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nigam A., et al., Vertical Transmission of Chikungunya Manifesting as Foetal Pericardial Effusion. J Assoc Physicians India, 2016. 64(12): p. 76–79. [PubMed] [Google Scholar]
- 36.Lyra P.P., et al., Congenital Chikungunya Virus Infection after an Outbreak in Salvador, Bahia, Brazil. AJP Rep, 2016. 6(3): p. e299–300. 10.1055/s-0036-1587323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandeira A.C., et al., Neonatal encephalitis due to Chikungunya vertical transmission: First report in Brazil. IDCases, 2016. 5: p. 57–59. 10.1016/j.idcr.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinzon-Redondo H., et al., Risk Factors for Severity of Chikungunya in Children: A Prospective Assessment. Pediatr Infect Dis J, 2016. 35(6): p. 702–4. 10.1097/INF.0000000000001135 [DOI] [PubMed] [Google Scholar]
- 39.Vasani R., et al., Congenital Chikungunya—A Cause of Neonatal Hyperpigmentation. Pediatr Dermatol, 2016. 33(2): p. 209–12. 10.1111/pde.12650 [DOI] [PubMed] [Google Scholar]
- 40.Muñoz C.M., et al., Manifestaciones mucocutáneas atípicas por fiebre por el virus del chikungunya en neonatos y lactantes de Cúcuta, Los Patios y Villa del Rosario, Norte de Santander, Colombia, 2014. Biomédica, 2016. 36: p. 368–77. 10.7705/biomedica.v36i3.2760 [DOI] [PubMed] [Google Scholar]
- 41.Villamil-Gomez W., et al., Congenital Chikungunya Virus Infection in Sincelejo, Colombia: A Case Series. J Trop Pediatr, 2015. 61(5): p. 386–92. 10.1093/tropej/fmv051 [DOI] [PubMed] [Google Scholar]
- 42.Taksande A. and Vilhekar K.Y., Neonatal Chikungunya Infection. J Prevent Infect Control 2015. 1(1): p. 8. [Google Scholar]
- 43.Gérardin P., et al., Neurocognitive Outcome of Children Exposed to Perinatal Mother-to-Child Chikungunya Virus Infection: The CHIMERE Cohort Study on Reunion Island. PLoS Neglected Tropical Diseases, 2014. 8(7): p. e2996. 10.1371/journal.pntd.0002996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramful D., et al., Antibody Kinetics in infants exposed ti Chikungunya virus infection during pregnancy revels absence of congenital infection. J Infect Dis, 2014. 209(11): p.1726–3. 10.1093/infdis/jit814 [DOI] [PubMed] [Google Scholar]
- 45.Shenoy S. and Pradeep G.C., Neurodevelopmental outcome of neonates with vertically transmitted Chikungunya fever with encephalopathy. Indian Pediatr, 2012. 49(3): p. 238–40. [PubMed] [Google Scholar]
- 46.Boumahni B. and Bintner M., Devenir à 5 ans des infections materno-foetales à vírus Chikungunya. Med Trop, 2012. 72: p. 94–96. [PubMed] [Google Scholar]
- 47.Shrivastava A., et al., Management of a vertically transmitted neonatal Chikungunya thrombocytopenia. Indian J Pediatr, 2011. 78(8): p. 1008–9. 10.1007/s12098-011-0371-7 [DOI] [PubMed] [Google Scholar]
- 48.Boumahni B., et al., [Maternal-fetal chikungunya infection associated with Bernard-Soulier syndrome]. Arch Pediatr, 2011. 18(3): p. 272–5. 10.1016/j.arcped.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 49.Mangalgi SM, Shenoy S, Maralusiddappa PG, Aprameya IV. Neonatal Chikungunya. Journal Chikungunya. Journal of Pediatrics Sciences 2011;3(2):e7. [Google Scholar]
- 50.Fritel X., et al., Chikungunya virus infection during pregnancy, Reunion, France, 2006. Emerg Infect Dis, 2010. 16(3): p. 418–25. 10.3201/eid1603.091403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senanayake M.P., et al., Vertical transmission in chikungunya infection. Ceylon Med J, 2009. 54(2): p. 47–50. 10.4038/cmj.v54i2.865 [DOI] [PubMed] [Google Scholar]
- 52.Rao G., Khan Y.Z., and Chitnis D.S., Chikungunya infection in neonates. Indian Pediatr, 2008. 45(3): p. 240–2. [PubMed] [Google Scholar]
- 53.Ramful D; et al. ; Mother-to-child transmission of Chikungunya virus infection. Pediatr Infect Dis J, 2007.26(9): p.811–5. 10.1097/INF.0b013e3180616d4f [DOI] [PubMed] [Google Scholar]
- 54.Paquet C., et al., Chikungunya outbreak in Reunion: epidemiology and surveillance, 2005 to early January 2006. Euro Surveill, 2006. 11(2): p. E060202.3. 10.2807/esw.11.05.02891-en [DOI] [PubMed] [Google Scholar]
- 55.Guinsburg R, Balda RC, Berenguel RC, et al. Behavioral pain scales assessment in neonates. J Pediatr (RIO J) 1997;73:411e418. 10.2223/jped.571 [DOI] [PubMed] [Google Scholar]
- 56.Robillard P.Y., et al., [Vertical maternal fetal transmission of the chikungunya virus. Ten cases among 84 pregnant women]. Presse Med, 2006. 35(5 Pt 1): p. 785–8. 10.1016/s0755-4982(06)74690-5 [DOI] [PubMed] [Google Scholar]
- 57.Contopoulos-Ioannidis D, Newman-Linsay S, Chow C, LaBeaud AD (2018) Mother-to-child transmission of Chikungunya virus; a Systematic review and meta-analysis. PloS Negl Trop Dis 12(6):e0006510. 10.1371/journal.pntd.0006510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weaver S.C. and Lecuit M., Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med, 2015. 372(13): p. 1231–9. 10.1056/NEJMra1406035 [DOI] [PubMed] [Google Scholar]
- 59.Motta GCP, Scardosim JM, Cunha MLC. Neonatal Infant pain Scale:Cross-Cultural Adaptation and Validation in Brazil. JPainsymman.2015.03.019. 10.1016/j.jpainsymman.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 60.Rollé A., et al., Severe Sepsis and Septic Shock Associated with Chikungunya Virus Infection, Guadeloupe, 2014. Emerging Infectious Diseases, 2016. 22(5): p. 891–894. 10.3201/eid2205.151449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hudson-Barr D, Capper-Michel B, Lambert S, Palermo TM, Morbeto K, Lombardo S. Validation of he Pain Assessment in Neonates (PAIN) scale with Neonatal Infant Pain Scale (NIPS). Neonatal Netw 200;21:15e21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.