Abstract
Background
Tezepelumab is an anti-thymic stromal lymphopoietin monoclonal antibody in development for the treatment of severe asthma. This study assessed the functionality and performance of an accessorized pre-filled syringe (APFS) and an autoinjector (AI) for administration of tezepelumab in the clinic and at home.
Methods
This phase 3, multicenter, randomized, open-label, parallel-group study (PATH-HOME, ClinicalTrials.gov identifier: NCT03968978) was conducted in patients aged 12–80 years with asthma that was uncontrolled despite treatment with medium- to high-dose inhaled corticosteroids plus at least one additional controller medication. Patients received six subcutaneous doses of tezepelumab 210 mg via APFS or AI. The first dose was administered by a healthcare professional, and patients or caregivers administered subsequent doses. First, second, third and final doses were administered in the clinic; fourth and fifth doses were administered at home. The primary endpoint was the proportion of successful administrations of tezepelumab. Secondary endpoints included the functionality and performance of the devices, Asthma Control Questionnaire (ACQ)-6 score, pharmacokinetics and safety.
Results
Overall, 216 patients were randomized (APFS, n=111; AI, n=105). Tezepelumab was successfully administered via APFS by 91.7% of the participants (100/109) and via AI by 92.4% (97/105). Overall, 95.4–97.1% of at-home administrations were successful across device groups. Malfunction occurred in 6 of 655 dispensed APFSs and 5 of 624 dispensed AIs. Clinically meaningful improvements in ACQ-6 score were observed after 24 weeks in 81.1% and 76.2% of the patients in the APFS and AI groups, respectively. Tezepelumab pharmacokinetics were consistent between device groups and with previous studies. The most common adverse event was nasopharyngitis (9.3%). Injection-site reactions occurred in 5.7% and 0% of the patients in the AI and APFS groups, respectively.
Conclusion
This study demonstrated that the APFS and AI were functional and reliable, and performed equally well at home and in the clinic.
Keywords: accessorized pre-filled syringe, at-home administration, autoinjector, severe asthma, tezepelumab
Introduction
Tezepelumab is a human monoclonal antibody (immunoglobulin G2λ) that binds specifically to thymic stromal lymphopoietin (TSLP), preventing it from interacting with the heterodimeric TSLP receptor.1,2 TSLP is an epithelial-derived cytokine released in response to airborne irritants, including allergens, viruses and cigarette smoke, and implicated in the initiation and persistence of airway inflammation in asthma.3–6 TSLP is released by epithelial cells at the top of the inflammation cascade and is a key regulator of multiple inflammatory pathways downstream of the airway epithelium.4–6 Its expression is increased in the airways of patients with asthma and is correlated with disease severity.7,8
Tezepelumab is currently in phase 3 of clinical development for the treatment of severe asthma. In the phase 2b PATHWAY study, tezepelumab significantly reduced the annualized rate of exacerbations by up to 71% compared with placebo, and improved lung function, asthma control and patient health-related quality of life.2 Based on efficacy findings and results of exposure–response analyses in PATHWAY, tezepelumab 210 mg every 4 weeks (Q4W) was the dose regimen selected for phase 3 studies.2,9
During PATHWAY, and in ongoing phase 3 trials, tezepelumab has been administered in the clinic via subcutaneous (SC) injection, drawn from a vial and injected via a syringe. The option to administer tezepelumab for severe asthma at home will provide patients with convenient administration and a sense of control over the management of their condition. An accessorized pre-filled syringe (APFS) and an autoinjector (AI) have been developed to enable well tolerated, effective and convenient dosing of tezepelumab by caregivers and patients at home as well as by healthcare professionals (HCPs) in the clinic. An assessment of the pharmacokinetics (PK) of tezepelumab and safety of these devices was performed in a Phase 1 trial in healthy individuals (PATH-BRIDGE, ClinicalTrials.gov identifier: NCT03989544).10 PATH-BRIDGE demonstrated that tezepelumab PK after a single 210 mg SC dose were comparable when administered via vial and syringe, APFS or AI. In addition, injection-site pain was low in severity and injection-site reactions were uncommon in all device groups.10 The aim of the present study, PATH-HOME, was to assess the functionality and performance of the APFS and AI when used to administer a fixed 210 mg SC dose of tezepelumab in the clinic (by HCPs and patients or their caregivers) and at home (by patients or their caregivers).
Methods
Study Overview and Participants
This was a phase 3, multicenter, randomized, open-label, parallel-group study in adults and adolescents with severe, uncontrolled asthma (PATH-HOME, ClinicalTrials.gov identifier: NCT03968978). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice guidelines and applicable regulatory requirements. Central institutional review boards included Copernicus (Cary, NC, USA), Advarra (Toronto, ON, Canada) and Komisja Bioetyczna at Wielkopolska Izba Lekarska (Poznań, Poland). All participants (and their legal guardians where applicable) provided written informed consent before the study began.
Patients eligible to participate in the study were 12–80-year-old current non-smokers with a body weight ≥40 kg and evidence of asthma as documented by a post-bronchodilator forced expiratory volume in 1 second (FEV1) reversibility ≥12% and ≥200 mL in the previous 12 months or during screening. In addition, a pre-bronchodilator FEV1 ≥50% predicted was required at screening. For at least 6 months before screening, patients must have been receiving treatment with medium-dose or high-dose inhaled corticosteroids according to Global Initiative for Asthma 2018 guidelines11 and at least one additional controller medication used in standard clinical practice (eg, long-acting β2-agonists, leukotriene receptor antagonists or theophylline).
Additionally, patients must have had asthma that was not well controlled, defined as an Asthma Control Questionnaire (ACQ-6) score ≥1.5 during screening or at randomization (obtained at the study site using a paper questionnaire), the occurrence of one or more exacerbations that required oral or systemic corticosteroid treatment in the previous 12 months, or an exacerbation that resulted in hospitalization for at least 24 hours in the previous 12 months. The patient or their caregiver must have been willing and able to administer study treatment, and caregivers must have been at least 21 years old. Full eligibility criteria are provided in Supplementary Table 1.
Objectives
The primary objective of this study was to assess the success of administration of tezepelumab 210 mg by SC injection with an APFS or AI in the clinic and at home. The 210 mg dose was selected to match that used in other ongoing phase 3 studies.12,13 The study had a number of secondary objectives: to assess the performance and functionality of the APFS and AI devices used to administer tezepelumab in the clinic and at home; to monitor metrics of asthma control after treatment with tezepelumab; to assess the PK (serum trough concentrations) and immunogenicity of tezepelumab administered via APFS or AI in the clinic and at home; and to assess the safety of tezepelumab administered via AFPS or AI in the clinic and at home.
Study Design
The study comprised a 2-week screening period, a 24-week treatment period and a 12-week follow-up period. During the treatment period, patients received a total of six SC doses of tezepelumab 210 mg. The first three doses were administered in the clinic (weeks 0, 4 and 8), the following two doses were administered at home (weeks 12 and 16) and the final dose was administered in the clinic (week 20) (Figure 1). An HCP administered the study drug at week 0. The patient or caregiver (the same individual in either case) was required to administer the study drug at weeks 4 (if not administered by an HCP, see below), 8, 12, 16 and 20.
Figure 1.
Study design. *Patient or caregiver to return used APFS or AI and completed questionnaire to the study site.
Abbreviations: AI, autoinjector; APFS, accessorized pre-filled syringe; EOT, end of treatment; IP, investigational product; SC, subcutaneous; V, visit.
At weeks 4 and 8, patients and caregivers were given the option of administering the study drug in the clinic under HCP supervision to ensure they understood the procedure and were capable of doing so. Patients and caregivers who were unable or unwilling to administer the study drug at week 8 discontinued participation in the study. Patients and caregivers were provided with APFS and AI instructions for use during the administration of the study drug in the clinic (under supervision) or at home, and patients attended scheduled onsite visits within 48 hours of at-home dosing. For adolescent participants, at-home administration of the study drug by either the patient or the caregiver must have been performed in the presence of an adult. At week 20, the final dose of the study drug was administered by the patient or caregiver in the clinic under HCP supervision to evaluate the administration technique.
After each dose, the individual who administered the study drug completed a questionnaire designed to indicate whether the dose was given successfully. Details of the questionnaire are provided in Supplementary Table 2. Questionnaires and used devices were returned to the study site for evaluation within 48 hours of at-home dosing. Patients attended an onsite end-of-treatment visit at week 24 and follow-up visits at weeks 30 and 36.
Study Drug and Delivery Devices
Tezepelumab was formulated at 110 mg/mL in 10 mM acetate with 3.0% (weight/volume) L-proline and 0.01% (weight/volume) polysorbate 80, at pH 5.2 for both delivery devices. An APFS is a manual drug delivery device for which the user presses a plunger to administer the full dose. The rate of injection varies according to the force applied by the user (Supplementary Figure 1A). An AI is an automated drug delivery device for which, upon activation by the user, the internal spring mechanism delivers the full dose (Supplementary Figure 1B). The APFS and AI used in this study used the same pre-filled glass syringe as the drug container. The target fill volume for the pre-filled syringe was sufficient to ensure delivery of the label claim volume (1.91 mL) and was confirmed via testing according to International Organization for Standardization 11608-1 deliverable volume requirements.14
Study Assessments and Procedures
Device Performance and Function
The primary endpoint was the proportion of HCPs and patients or caregivers who successfully administered tezepelumab with an APFS or AI in the clinic and at home. A successful administration was defined as an injection completed based on a satisfactory in vitro evaluation (visual test and functional assessment) of the returned device and a user-recorded answer of “yes” to all questions in a questionnaire on the preparation and administration of the study drug (Supplementary Table 2).
Returned devices were inspected visually for damage or disassembly, full plunger travel (indicating that a completed dose was expelled) and needle safety guard deployment. Returned devices underwent functional evaluation to challenge the needle safety guard to assess whether it deployed correctly and continued to provide protection against accidental needlestick injuries. If questionnaire responses indicated that the user could not successfully administer the study drug, the returned device underwent in vitro evaluation, taking into account information provided in the questionnaire. Device defects identified during in vitro evaluation resulted in a product complaint investigation.
Asthma Control
Asthma control was assessed using the Asthma Control Questionnaire (ACQ)-6, which was completed at screening and during site visits at weeks 0 (baseline), 4, 8, 12, 16, 20 and 24. The ACQ-6 comprises six questions that capture asthma symptoms (night-time waking, symptoms on waking, activity limitation, shortness of breath and wheezing) and short-acting β2-agonist use (number of puffs). Questions are weighted equally and are scored from 0 (totally controlled) to 6 (severely uncontrolled).15 The mean ACQ-6 score was calculated from responses to individual questions. Mean scores ≤0.75 indicate well-controlled asthma, scores from >0.75 to <1.5 indicate partially controlled asthma and scores ≥1.5 indicate uncontrolled asthma.16 A reduction in mean ACQ-6 score ≥0.5 is considered clinically meaningful.15
PK and Immunogenicity
Blood samples for PK analysis were collected before tezepelumab administration at weeks 0, 4 and 20 of treatment, at the end of treatment (week 24) and at the final follow-up visit (week 36). Samples were analyzed using an enzyme-linked immunosorbent assay for quantification of tezepelumab in serum, with a lower limit of quantification of 10.0 ng/mL. The immunogenicity of tezepelumab was assessed by measuring anti-drug antibodies (ADAs) to tezepelumab in blood samples collected pre-dose at weeks 0 and 4, at the end of treatment (week 24) and at the final follow-up visit (week 36).
Safety Assessments
Safety assessments included monitoring of adverse events (AEs), including potential injection-site reactions, measurement of vital signs (heart rate, blood pressure), electrocardiography, physical examination and laboratory tests (clinical chemistry, hematology and urinalysis).
Statistical Considerations
Data for all analyses presented are descriptive only and no formal statistical tests were performed. Assessments relating to treatment administration, device function and performance, and asthma control were carried out for all patients who received at least one dose of tezepelumab.
For the primary endpoint (proportion of HCPs and patients or caregivers who successfully administered tezepelumab via APFS and AI in the clinic and at home), the denominator was the number of patients who received or attempted to receive study treatment at each visit. For assessment of the proportion of used or returned devices that passed functional tests and visual inspection and showed no evidence of malfunction, and for the proportion reported as malfunctioning, the denominator was the number of used and returned devices at each visit. For each of these endpoints, proportions and 95% confidence intervals (CIs) were calculated by visit for the APFS and AI separately. Data for HCPs, patients and caregivers were assessed separately for each device and by visit. For the primary endpoint, patient data were also assessed separately for adolescents.
Mean changes from baseline in ACQ-6 score were summarized descriptively and patients were categorized as being responders or non-responders to treatment, according to the device group. All patients who received tezepelumab were included in the PK, immunogenicity and safety analyses. AEs were summarized descriptively by treatment group. Geometric mean serum concentrations of tezepelumab and the corresponding coefficient of variation were calculated from log-transformed data. The incidence of ADAs and AEs were summarized descriptively for each device group.
Results
Patient Disposition, Baseline Demographics and Clinical Characteristics
A total of 216 patients were randomized to receive tezepelumab 210 mg Q4W via APFS (n=111) or AI (n=105), of whom 215 completed the study. Two patients in the APFS group discontinued treatment owing to AEs; one patient completed the study assessments and one withdrew from the study. Overall, 24 adolescent patients were included in the study. The mean (standard deviation) age of the study population was 47.2 (18.2) years and 50% were female. The mean (standard deviation) duration of asthma was 20.1 (15.5) years. The mean (standard deviation) ACQ-6 score at baseline was 2.23 (0.73) in the APFS group and 2.08 (0.62) in the AI group. Baseline demographics and clinical characteristics in both device groups were representative of the targeted clinical population (Table 1).
Table 1.
Baseline Demographics and Clinical Characteristics of Study Participants
| Demographic/Characteristic | Tezepelumab 210 mg Q4W via APFS (n=111) | Tezepelumab 210 mg Q4W via AI (n=105) | Total (N=216) |
|---|---|---|---|
| Age, years | 48.5 (18.1) | 45.8 (18.3) | 47.2 (18.2) |
| Female, n (%) | 56 (50.5) | 52 (49.5) | 108 (50.0) |
| Weight, kg | 85.3 (25.6) | 83.4 (21.5) | 84.3 (23.6) |
| BMI, kg/m2 | 30.3 (8.4) | 28.9 (6.3) | 29.6 (7.4) |
| ICS dose, n (%) | |||
| Mediuma | 59 (53.2) | 57 (54.3) | 116 (53.7) |
| Highb | 52 (46.8) | 48 (45.7) | 100 (46.3) |
| Receiving OCS, n (%) | 5 (4.5) | 5 (4.8) | 10 (4.6) |
| Pre-BD FEV1, L, | 2.2 (0.7) | 2.3 (0.8) | 2.3 (0.7) |
| FEV1 reversibility, % | 16.3 (10.7) | 20.1 (12.6) | 18.1 (11.8) |
| Duration of asthma, years | 19.8 (14.8) | 20.5 (16.2) | 20.1 (15.5) |
| Exacerbations in the previous 12 months, n (%) | |||
| 0 | 60 (54.1) | 53 (50.5) | 113 (52.3) |
| 1 | 33 (29.7) | 29 (27.6) | 62 (28.7) |
| 2 | 14 (12.6) | 14 (13.3) | 28 (13.0) |
| ≥3 | 4 (3.6) | 9 (8.6) | 13 (6.0) |
| ACQ-6 score,c mean (SD) | |||
| Screening | 2.29 (0.75) | 2.27 (0.73) | NC |
| Baseline | 2.23 (0.73) | 2.08 (0.62) | NC |
Notes: Data are mean (standard deviation) unless otherwise stated. aMedium-dose ICS: >250–500 µg/day fluticasone propionate dry powder formulation or equivalent. bHigh-dose ICS: >500 µg/day fluticasone propionate dry powder formulation or equivalent. cScores range from 0 (no impairment) to 6 (maximum impairment). Scores ≥1.5 indicate inadequately controlled asthma.
Abbreviations: AI, autoinjector; APFS, accessorized pre-filled syringe; BD, bronchodilator; BMI, body mass index; ACQ, Asthma Control Questionnaire; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroids; NC, not calculated; OCS, oral corticosteroids; Q4W, every 4 weeks.
Primary Endpoint
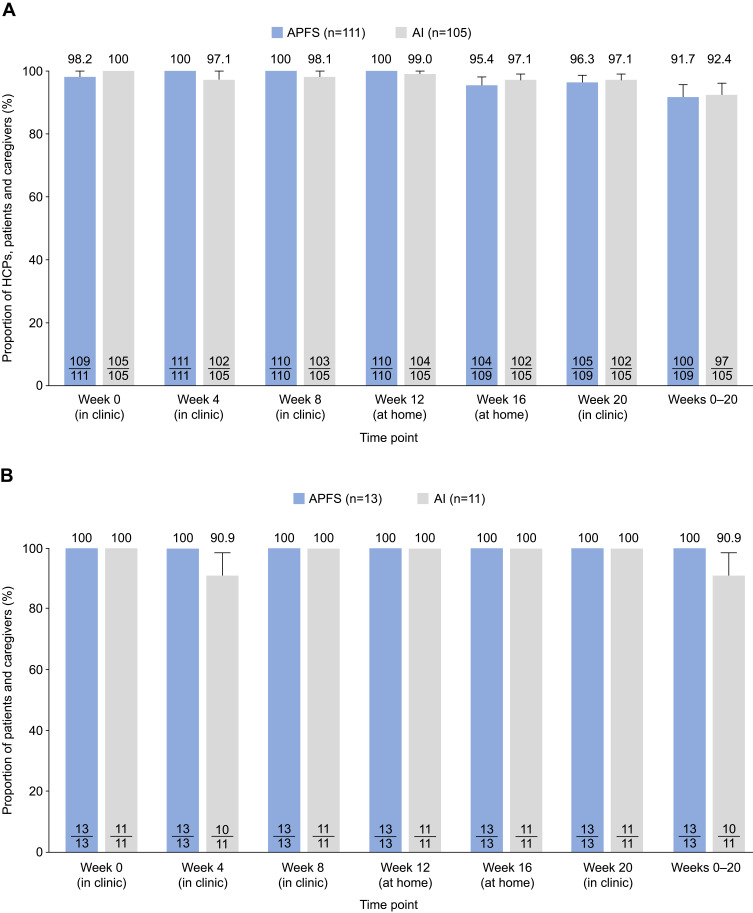
Almost all HCPs, patients and caregivers were able to administer tezepelumab successfully via APFS and AI, either in the clinic or at home. In the overall study population, from week 0 to week 20, tezepelumab was successfully administered via APFS by 91.7% of HCPs and patients or caregivers (100/109) (Figure 2A). Findings were similar in the AI group, in which tezepelumab was successfully administered by 92.4% of HCPs and patients or caregivers (97/105) (Figure 2A). At-home administration of tezepelumab at weeks 12 and 16 was successful in 95.4% of the patients or caregivers in the APFS group (104/109) and 97.1% of the patients or caregivers in the AI group (102/105).
Figure 2.
Proportion of HCPs, patients and caregivers who successfully administered tezepelumab via APFS or AI in (A) the overall population and (B) adolescents only. Values in the bars represent the number of patients who administered study drug successfully out of the total number of patients who received or attempted to receive study drug.
Abbreviations: AI, autoinjector; APFS, accessorized pre-filled syringe; HCP, healthcare professional.
When data were analyzed according to the individual who administered tezepelumab (Table 2), proportions of successful administrations via APFS were high and were similar for HCPs (98–100% in the clinic), caregivers (100% in the clinic and 93–100% at home) and patients (96–100%, both in the clinic and at home) per week at all weeks assessed. In the AI group, 100% of HCPs and 100% of caregivers successfully administered tezepelumab at all weeks assessed (Table 2). Among patients, 95–98% and 97–99% successfully administered tezepelumab via AI in the clinic and at home, respectively.
Table 2.
Proportion of HCPs, Patients and Caregivers Who Successfully Administered Tezepelumab via APFS or AI
| Time Point (Location) | Tezepelumab 210 mg Q4W via APFS (N=111) | Tezepelumab 210 mg Q4W via AI (N=105) | ||||
|---|---|---|---|---|---|---|
| N | n (%) | 95% CI | N | n (%) | 95% CI | |
| HCPs | ||||||
| Week 0 (in clinic) | 111 | 109 (98.2) | 93.7–99.5 | 105 | 105 (100) | 96.5–100 |
| Week 4 (in clinic) | 50 | 50 (100) | 92.9–100 | 46 | 46 (100) | 92.3–100 |
| Patients | ||||||
| Week 4 (in clinic) | 54 | 54 (100) | 93.4–100 | 56 | 53 (94.6) | 85.4–98.2 |
| Week 8 (in clinic) | 96 | 96 (100) | 96.2–100 | 94 | 92 (97.9) | 92.6–99.4 |
| Week 12 (at home) | 96 | 96 (100) | 96.2–100 | 94 | 93 (98.9) | 94.2–99.8 |
| Week 16 (at home) | 95 | 91 (95.8) | 89.7–98.4 | 94 | 91 (96.8) | 91.0–98.9 |
| Week 20 (in clinic) | 95 | 91 (95.8) | 89.7–98.4 | 94 | 91 (96.8) | 91.0–98.9 |
| Weeks 8–20 combined | 95a | 89 (93.7) | 86.9–97.1 | 94a | 89 (94.7) | 88.2–97.7 |
| Caregivers | ||||||
| Week 4 (in clinic) | 7 | 7 (100) | 64.6–100 | 3 | 3 (100) | 43.9–100 |
| Week 8 (in clinic) | 14 | 14 (100) | 78.5–100 | 11 | 11 (100) | 74.1–100 |
| Week 12 (at home) | 14 | 14 (100) | 78.5–100 | 11 | 11 (100) | 74.1–100 |
| Week 16 (at home) | 14 | 13 (92.9) | 68.5–98.7 | 11 | 11 (100) | 74.1–100 |
| Week 20 (in clinic) | 14 | 14 (100) | 78.5–100 | 10 | 10 (100) | 72.3–100 |
| Weeks 8–20 combined | 14a | 13 (92.9) | 68.5–98.7 | 10a | 10 (100) | 72.3–100 |
Note: aThe number of patients who received or attempted to receive study drug at all specified visits.
Abbreviations: AI, autoinjector; APFS, accessorized pre-filled syringe; CI, confidence interval; HCP, healthcare professional; Q4W, every 4 weeks.
When data were analyzed in the adolescent population only, 100% of the adolescents (n=13) successfully administered tezepelumab via APFS at all weeks assessed (Figure 2B). In the AI group, one adolescent patient was unable to administer tezepelumab successfully at week 4. At all other weeks assessed, 100% of the adolescent patients (n=11) successfully administered tezepelumab via AI.
Device Performance and Function
Overall, 99.1% of the dispensed and returned APFSs (649/655) and 99.2% of the dispensed and returned AIs (619/624) passed functional tests and visual inspection and showed no evidence of malfunction. Of the six APFSs reported as malfunctioning (Table 3), three were used in the clinic (two by HCPs and one by a patient) and three were used at home (two by patients and one by a caregiver). In the AI group, all five devices reported as malfunctioning were used in the clinic by patients (no devices were reported as malfunctioning during at-home use). No mechanical or design-related issues were found during in vitro evaluation of the devices that were reported as malfunctioning.
Table 3.
Proportion of Devices Reported as Malfunctioning
| APFS (N=655) | AI (N=624) | |
|---|---|---|
| Reported as malfunctioning, n/N (%)a,b | 6/655 (0.9) | 5/624 (0.8) |
| Administered in the clinic, n/N (%) | ||
| All | 3/438 (0.7) | 5/418 (1.2) |
| By HCP | 2/161 (1.2) | 0/152 (0) |
| By patient | 1/242 (0.4) | 5/242 (2.1) |
| By caregiver | 0/35 (0) | 0/24 (0) |
| Administered at home, n/N (%) | ||
| All | 3/217 (1.4) | 0/206 (0) |
| By patient | 2/189 (1.1) | 0/184 (0) |
| By caregiver | 1/28 (3.6) | 0/22 (0) |
Notes: aTwelve kits were lost and were not evaluated after administration of study drug. These kits are not included in this analysis. bN refers to the cumulative number of used and returned devices over relevant visits.
Abbreviations: AI, autoinjector; APFS, accessorized pre-filled syringe; HCP, healthcare professional.
Asthma Control
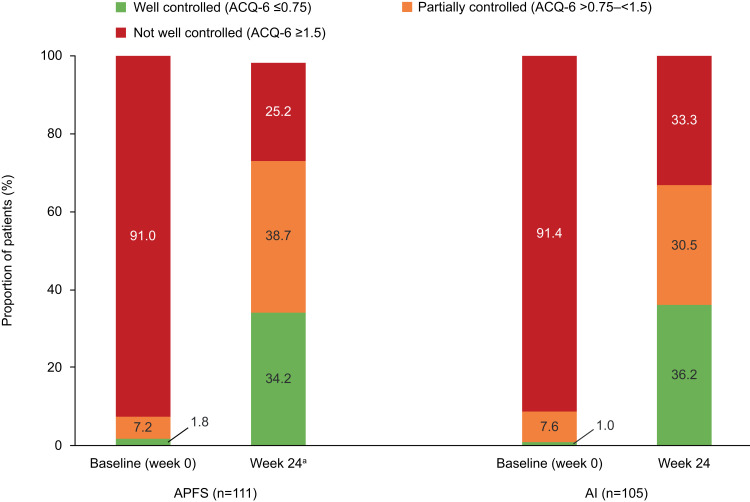
At week 24, in the APFS group, the proportions of patients with well-controlled (mean ACQ-6 score ≤0.75) and partially controlled (mean ACQ-6 score >0.75–<1.5) asthma increased compared with week 0/baseline (34.2% vs 1.8% and 38.7% vs 7.2%, respectively). Consequently, the proportion of patients with asthma that was not well controlled at week 24 decreased compared with baseline (25.2% vs 91.0%) (Figure 3). Findings were similar in the AI group: at week 24, 36.2% and 30.5% of patients had well controlled or partially controlled asthma, respectively, compared with 1.0% and 7.6% at baseline, and 33.3% of patients had asthma that was not well controlled compared with 91.4% at baseline (Figure 3).
Figure 3.
Proportion of patients with well-controlled, partially controlled and not well-controlled asthma at baseline and at week 24, by device group. aData were missing for two patients in the APFS group and the mean ACQ-6 score was not calculated for these patients.
Abbreviations: ACQ-6, Asthma Control Questionnaire; AI, autoinjector; APFS, accessorized pre-filled syringe.
A high proportion of responders (patients with a decrease in mean ACQ-6 score ≥0.5 from baseline to week 24) was observed in both the APFS (81.1%, 90/111) and AI (76.2%, 80/105) groups.
Tezepelumab PK
Serum concentrations of tezepelumab were consistent between device groups at each measurement time point (Supplementary Table 3).
Tezepelumab Immunogenicity
ADAs were present in two patients (1.8%) in the APFS group and in 11 patients (10.5%) in the AI group (Supplementary Table 4) at baseline and/or post-baseline. Treatment-emergent ADAs were present in two patients (1.8%) in the APFS group and eight patients (7.6%) in the AI group. The ADA response was low in magnitude, with a median within-participant maximum titer below the minimum reportable value of the assay (67.2) for both devices. In ADA-positive participants, no apparent impact of ADAs on PK was observed.
Tezepelumab Safety
At least one AE was reported in 107 of 216 patients (49.5%), with similar frequencies found across the two device groups (APFS, 46.8%; AI, 52.4%; Table 4). Overall, the most frequently reported AE was nasopharyngitis (9.3%). None of the AEs were related to device function. Treatment-related AEs were reported in 2.8% of patients overall (APFS, 0%; AI, 5.7%). Injection-site reactions were reported in six patients (2.8%) overall, all of whom were in the AI group.
Table 4.
Proportions of Patients Who Reported AEs During the Treatment Period
| AE Category | APFS (n=111) | AI (n=105) | Total (N=216) |
|---|---|---|---|
| All AEs | 52 (46.8) | 55 (52.4) | 107 (49.5) |
| AEs related to device malfunction | 0 (0) | 0 (0) | 0 (0) |
| AEs related to study druga | 0 (0) | 6 (5.7) | 6 (2.8) |
| Injection-site reactions | 0 (0) | 6 (5.7) | 6 (2.8) |
| AEs by severity | |||
| Mild | 32 (28.8) | 27 (25.7) | 59 (27.3) |
| Moderate | 18 (16.2) | 26 (24.8) | 44 (20.4) |
| Severe | 2 (1.8) | 2 (1.9) | 4 (1.9) |
| Serious AEs | 3 (2.7) | 3 (2.9) | 6 (2.8) |
| AE leading to death | 0 (0) | 0 (0) | 0 (0) |
| AEs leading to treatment discontinuation | 2 (1.8) | 0 (0) | 2 (0.9) |
| AEs by preferred termb | |||
| Nasopharyngitis | 8 (7.2) | 12 (11.4) | 20 (9.3) |
| Upper respiratory tract infection | 7 (6.3) | 5 (4.8) | 12 (5.6) |
| Asthma | 5 (4.5) | 5 (4.8) | 10 (4.6) |
Notes: Data are number of patients (%). aAs judged by the investigator. bAEs by Medical Dictionary for Regulatory Activities (MedDRA) preferred term experienced by >3% of the patients overall. Patients with multiple AEs categorized under the same preferred term were only counted once for that preferred term.
Abbreviations: AE, adverse event; AI, autoinjector; APFS, accessorized pre-filled syringe.
The majority of AEs were mild (27.3%) or moderate (20.4%) in severity. Serious AEs occurred in three patients in each device group and comprised a total of six events per group. In the APFS group, there were two incidences of infection, one of pneumonia, one of pancreatitis, one of hemoptysis and one of renal colic. In the AI group, there was one incidence of infection, three of asthma, one of pneumothorax and one of psychogenic seizure. None of these events were considered by the investigator to be related to study treatment.
There were no clinically meaningful trends in changes in clinical chemistry values or vital signs, or notable differences between the two device groups. Hematology assessments demonstrated a mean (standard deviation) reduction from baseline to week 24 in blood eosinophil count of 78 (144) cells/µL in the APFS group and 81 (219) cells/µL in the AI group.
Discussion
An APFS and AI have been developed to facilitate convenient administration of tezepelumab by HCPs in the clinic and by patients and caregivers at home. In this study, tezepelumab was successfully administered using the APFS and AI both in the clinic and at home by HCPs, patients and their caregivers.
Over 91% of HCPs, patients and caregivers were able to administer tezepelumab successfully both in the clinic and at home, and the proportions of successful administrations were comparable between devices and settings. In addition, this study demonstrated for the first time that adolescents can successfully administer tezepelumab using the APFS and AI: all 24 adolescent study participants (except for one patient in the AI group at week 4) were able to administer tezepelumab successfully both at home and in the clinic using the two devices. Some previous studies of at-home use of biologics have included adults (≥18 years old) only.17,18 Adolescents participated in the phase 3 NAVIGATOR study of the efficacy and safety of tezepelumab,12 so this age group was also included in the current study.
A very small proportion of devices that were dispensed and returned were reported as malfunctioning (0.9% of APFSs and 0.8% of AIs). The very small proportion of device malfunctions supports that the instructions for use provided to HCPs, patients and caregivers provided adequate guidance to these individuals on how to administer subcutaneous tezepelumab successfully both in the clinic and at home. At-home use of these devices to administer tezepelumab is strongly supported by the fact that no AI malfunctions and only three APFS malfunctions were reported during dosing in this setting.
Improvements from baseline to week 24 in asthma control were observed in both device groups, with the majority of patients having asthma that was well controlled or partially controlled at the end of the treatment period. In addition, the proportions of ACQ-6 responders in both device groups were high (APFS, 81%; AI, 76%). These findings suggest that administration of tezepelumab by patients or caregivers was sufficient to achieve improvements in ACQ-6 comparable to those observed in the phase 2b PATHWAY study, during which tezepelumab was administered by an HCP in the clinic.19
Tezepelumab PK were consistent between devices and were comparable with the findings from previous clinical studies, including PATHWAY,2 PATH-BRIDGE10 and an additional phase 1 study in healthy individuals.20 Together with the comparable improvements in asthma control observed in the present study and in the PATHWAY study with tezepelumab treatment, these data indicate that exposure to tezepelumab after at-home self-administration using an APFS or AI is sufficient to obtain the clinical benefit observed in the PATHWAY study.
The overall proportions of participants who tested positive for ADAs (APFS, 1.8%; AI, 10.5%) and treatment-emergent ADAs (APFS, 1.8%; AI, 7.6%) in this study were low. In ADA-positive patients, the majority of ADA maximum titers were below the limit of detection. In addition, no AEs were associated with ADAs. The proportion of treatment-emergent ADAs in the APFS group was comparable in the present study and the phase 1 PATH-BRIDGE study (1.8% vs 1.0%, respectively)10 but was slightly higher in the AI group in the present study (7.6% vs 0%). Low immunogenicity to tezepelumab has been observed in previous studies. In two previous studies in healthy volunteers, no participants tested positive for treatment-emergent ADAs,20,21 and in the PATHWAY phase 2b study in patients with severe asthma, 0.8–3.7% of the patients treated with tezepelumab tested positive for treatment-emergent ADAs.2
The safety profile of tezepelumab administered by both devices was favorable and the incidence of AEs was low and comparable between the two groups (APFS, 46.8%; AI, 52.4%). In both device groups, the majority of AEs were mild or moderate in severity, and in the APFS group, none of the AEs reported were considered by the investigator to be related to treatment. The incidence of AEs observed in the present study was lower than that observed in the phase 2b PATHWAY study (66.0%), in which tezepelumab was administered by HCPs in a clinical setting only.2
In the present study, no patients in the APFS group reported injection-site reactions. In the AI group, a small proportion of patients (6 out of 105, 5.7%) experienced injection-site reactions, which was slightly higher than the proportion in the PATH-BRIDGE study in healthy individuals (2.8%).10 The majority of these injection-site reactions were mild to moderate in intensity and were transient. The occurrence of injection-site reactions after administration of tezepelumab in this study, and in previous studies (PATHWAY, PATH-BRIDGE), irrespective of the device used, appeared to be relatively low compared with that found in studies of some other biologics in severe asthma, in which the reported occurrence ranged from 2–45%.22–24
Consistent with observations in the PATHWAY study,25 hematology assessments in the present study demonstrated a decrease from baseline to the end of treatment in blood eosinophil counts in both device groups. These data indicate that reductions in blood eosinophil counts after treatment with tezepelumab were consistently observed across studies in which this outcome was investigated. Furthermore, reductions in blood eosinophil counts have been demonstrated to be associated with improvements in clinical outcomes in patients with severe asthma.26
The findings of the PATH-HOME study are strengthened by the inclusion of both adult and adolescent patients and by the high retention of participants (only one patient did not complete the study). In addition, detailed assessment of the functionality and performance of each device after use supports their ability to be used in the clinic and at home. A limitation of the study was that some kits were lost and therefore could not be evaluated (by visual test or functional evaluation) after use. In addition, patient preference for either device was not assessed. The impact of the COVID-19 pandemic on the PATH-HOME study is considered to be low. All study sites were able to dose the study drug in the clinic when required, and only 15 follow-up visits were virtual.
Conclusion
This study demonstrated that SC dosing of tezepelumab via APFS or AI to adults and adolescents with severe, uncontrolled asthma, at home or in the clinic, is a well-tolerated and reliable method of administration. The results of this study support the use of an APFS or AI both at home and in the clinic, providing patients and HCPs with greater choice and the convenient option of at-home treatment administration.
Acknowledgments
Medical writing support was provided by Laura Knapp, PhD, of PharmaGenesis London, London, UK, with funding from AstraZeneca and Amgen Inc.
Funding Statement
This study was funded by AstraZeneca and Amgen Inc.
Abbreviations
ACQ-6, Asthma Control Questionnaire; ADA, antidrug antibody; AE, adverse event; AI, autoinjector; APFS, accessorized pre-filled syringe; CI, confidence interval; FEV1, forced expiratory volume in 1 second; HCP, healthcare professional; PK, pharmacokinetics; Q4W, every 4 weeks; SC, subcutaneous; TSLP, thymic stromal lymphopoietin.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics and Consent Statement
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice guidelines and applicable regulatory requirements. Central institutional review boards included Copernicus (Cary, NC, USA), Advarra (Toronto, ON, Canada) and Komisja Bioetyczna at Wielkopolska Izba Lekarska (Poznań, Poland). All participants (and their legal guardians where applicable) provided written informed consent before the study began.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Sady Alpizar is an employee of Clinical Research Trials of Florida and received personal fees from AstraZeneca during the conduct of the PATH-HOME study. Ayman Megally, Claudia Chen, Abhi Raj and Gene Colice are employees of AstraZeneca. John Downie is an employee of Amgen Inc. The authors report no other conflicts of interest in this work.
References
- 1.Gauvreau GM, O’Byrne PM, Boulet L-P, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102–2110. doi: 10.1056/NEJMoa1402895 [DOI] [PubMed] [Google Scholar]
- 2.Corren J, Parnes J, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–946. doi: 10.1056/NEJMoa1704064 [DOI] [PubMed] [Google Scholar]
- 3.Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792. doi: 10.1080/14728222.2020.1783242 [DOI] [PubMed] [Google Scholar]
- 4.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680. doi: 10.1038/ni805 [DOI] [PubMed] [Google Scholar]
- 5.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204(2):253–258. doi: 10.1084/jem.20062211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitajima M, Lee H-C, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41(7):1862–1871. doi: 10.1002/eji.201041195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790–2798. doi: 10.4049/jimmunol.181.4.2790 [DOI] [PubMed] [Google Scholar]
- 8.Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174(12):8183–8190. doi: 10.4049/jimmunol.174.12.8183 [DOI] [PubMed] [Google Scholar]
- 9.Ly N, Zheng Y, Griffiths JM, van der Merwe R, Agoram B, Roskos L. Exposure-response analysis of tezepelumab in patients with severe asthma to guide phase 3 dose selection. Eur Respir J. 2018;52:PA1688. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Abuqayyas L, Megally M, et al. Tezepelumab pharmacokinetics, safety and tolerability after administration via vial-and-syringe, accessorized pre-filled syringe, or autoinjector: a randomized trial in healthy volunteers. Clin Ther. 2020. [DOI] [PubMed] [Google Scholar]
- 11.Global Initative for Asthma. Global strategy for asthma management and prevention; 2018. Available from: https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf. Accessed October12, 2020.
- 12.Menzies-Gow A, Colice G, Griffiths JM, et al. NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020;21(1):266. doi: 10.1186/s12931-020-01526-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wechsler ME, Colice G, Griffiths JM, et al. SOURCE: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir Res. 2020;21(1):264. doi: 10.1186/s12931-020-01503-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Organization for Standardization. ISO 11608-1:2014. Needle-based injection systems for medical use – requirements and test methods – part 1: needle-based injection systems; 2014. Available from: https://www.iso.org/standard/65021.html. Accessed November17, 2020.
- 15.American Thoracic Society. Asthma control questionnaire. Available from: http://www.thoracic.org/members/assemblies/assemblies/srn/questionaires/acq.php. Accessed October13, 2020.
- 16.Juniper EF, Bousquet J, Abetz L, Bateman ED; GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the asthma control questionnaire. Respir Med. 2006;100(4):616–621. doi: 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 17.Ferguson GT, Cole J, Aurivillius M, et al. Single-use autoinjector functionality and reliability for at-home administration of benralizumab for patients with severe asthma: GRECO trial results. J Asthma Allergy. 2019;12:363–373. doi: 10.2147/JAA.S224266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson GT, Mansur AH, Jacobs JS, et al. Assessment of an accessorized pre-filled syringe for home-administered benralizumab in severe asthma. J Asthma Allergy. 2018;11:63–72. doi: 10.2147/JAA.S157762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corren J, Garcia Gil E, Griffiths JM, et al. Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol. 2020:S1081-1206(1020)31108-X. [DOI] [PubMed] [Google Scholar]
- 20.Parnes JR, Sullivan JT, Chen L, Dias C. Pharmacokinetics, safety, and tolerability of tezepelumab (AMG 157) in healthy and atopic dermatitis adult subjects. Clin Pharmacol Ther. 2019;106(2):441–449. doi: 10.1002/cpt.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto K, Matsuki S, Irie S, et al. A phase 1, randomized, placebo-controlled study to evaluate the safety, tolerability, pharmacokinetics, and immunogenicity of subcutaneous tezepelumab in healthy Japanese men. Clin Pharmacol Drug Dev. 2020;9(7):833–840. doi: 10.1002/cpdd.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AstraZeneca. Fasenra® (benralizumab) prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf. Accessed November17, 2020.
- 23.Genentech Inc. Xolair®(omalizumab) prescribing information; 2003. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/omalgen062003LB.pdf. Accessed November17, 2020.
- 24.GlaxoSmithKline. Nucala®(mepolizumab) prescribing information; 2015. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125526Orig1s000Lbl.pdf. Accessed November17, 2020.
- 25.Pham TH, Ren P, Parnes JR, Griffiths JM. Tezepelumab reduces multiple key inflammatory biomarkers in patients with severe, uncontrolled asthma in the Phase 2b PATHWAY Study. Am J Respir Crit Care Med. 2019;199:A2677. [Google Scholar]
- 26.Harrison TW, Chanez P, Menzella F, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med. 2020. doi: 10.1016/S2213-2600(1020)30414-30418 [DOI] [PubMed] [Google Scholar]