Abstract
Spontaneous pneumomediastinum is defined as having an etiology that is not related to surgery, trauma, or mechanical ventilation. Precipitating causes of spontaneous pneumomediastinum include coughing, exercise, vomiting, infection, underlying lung diseases such as asthma, and illicit drugs. Symptoms include chest pain, shortness of breath, and dysphagia. A 54-year-old man presented with 2 weeks of shortness of breath, cough, and fever. He was admitted for severe SARS-CoV-2 pneumonia and acute hypoxic respiratory failure requiring non-rebreather mask. Chest imaging on admission showed bilateral peripheral consolidations and pneumomediastinum with subcutaneous emphysema. No precipitating event was identified. He did not require initiation of positive pressure ventilation throughout his admission. On hospital day 7, chest imaging showed resolution of pneumomediastinum and subcutaneous emphysema, and he was successfully discharged on oxygen therapy. Spontaneous pneumomediastinum is a rare complication of severe acute respiratory syndrome coronavirus 2 infection. Spontaneous pneumomediastinum is typically benign and self-limiting, requiring only supportive treatment.
Keywords: COVID-19, severe acute respiratory syndrome coronavirus 2, spontaneous pneumomediastinum
Introduction
Novel Coronavirus 2019 (COVID-19), also known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been in the worldwide spotlight since its first cases were reported in Wuhan, China, in December 2019. Symptoms associated with the virus include fever, cough, shortness of breath, gastrointestinal symptoms, loss of smell and taste, and complications such as severe pneumonia and acute respiratory distress syndrome (ARDS).1,2 Imaging features often include peripheral ground-glass opacities bilaterally and/or peripheral consolidation that can be multilobar.3 Spontaneous pneumomediastinum (SPM), which excludes exposure to mechanical ventilation as a precipitating cause, is an uncommon presentation and complication of the novel COVID-19 infection. However, it reportedly has presented in infected patients regardless of age or comorbidities.4–7 We present a rare case of SPM diagnosed at presentation in a patient with SARS-CoV-2 pneumonia who did not have exposure to positive pressure ventilation throughout his entire course.
Case presentation
A 54-year-old Hispanic man with hypertension presented to our emergency department with a 2-week history of fever, chills, cough, and progressively worsening dyspnea. The patient reported SARS-CoV-2 positive contacts at home. He denied abdominal pain, nausea, vomiting, diarrhea, and any tobacco, alcohol, or illicit drug use. The patient had previously seen his primary care physician for these symptoms within the past week and reported that he had tested positive for SARS-CoV-2 via polymerase chain reaction (PCR). His SARS-CoV-2 PCR done in the emergency department on the day of admission was negative. This was presumed a false negative test due to the significant SARS-CoV-2 contacts at home, positive test obtained by his primary care physician, and clinical presentation.
Upon presentation to the emergency department, the patient was febrile to 38°C, tachycardic, tachypneic, and hypoxic to 77%, and using his accessory muscles of respiration. On examination, crepitus was palpated along the supraclavicular region and neck bilaterally but not along the sternum. The patient endorsed chest pain with palpation. His respiratory exam was notable for bilateral crackles extending to the mid lung fields bilaterally. His oxygen saturation improved to 95% on 8 L of oxygen via a non-rebreather mask at 100% FiO2.
Blood test results showed elevated leukocytes at 17,200 (4500–10,000 cells/µL) with neutrophils 15,700 (1800–8000 cells/µL) and lymphocytes 900 (1200–3300 cells/µL), procalcitonin level of 0.27 (⩽0.09 ng/mL), and C-reactive protein concentration of 23.6 (0–0.74 mg/dL). Arterial blood gas at admission revealed metabolic alkalosis with arterial pH of 7.47, partial pressure of oxygen of 93 mm Hg, arterial bicarbonate of 29.8 (22–26 mmol/L), and normal partial pressure of carbon dioxide. Chest X-ray done at admission showed confluent bilateral ground-glass opacities and pneumomediastinum with subcutaneous emphysema at the base of the neck (Figure 1). No pleural effusion, pleural thickening, or pneumothorax was noted. Computed tomography (CT) thorax without contrast confirmed bilateral peripheral consolidation and revealed diffuse pneumomediastinum (Figures 2 and 3).
Figure 1.
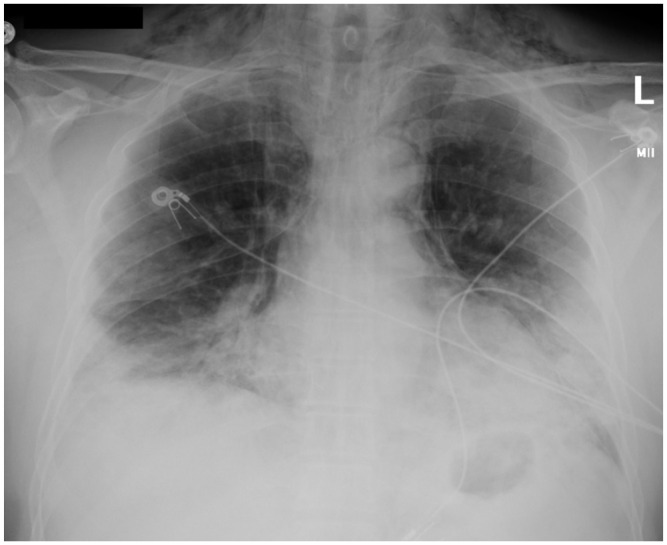
Initial chest X-ray. This is a chest radiograph showing evidence of pneumomediastinum, subcutaneous emphysema tracking into the neck base soft tissues, and diffuse ground-glass and consolidative opacities bilaterally and peripherally.
Figure 2.

Chest computerized tomography (coronal). This is a coronal chest CT showing bilateral peripheral consolidations with diffuse pneumomediastinum and subcutaneous emphysema.
Figure 3.

Chest computerized tomography (axial). This is an axial chest CT showing bilateral peripheral consolidations with diffuse pneumomediastinum and subcutaneous emphysema.
The patient was hospitalized for acute hypoxic respiratory failure and SPM secondary to SARS-CoV-2 pneumonia and treated per our hospital protocol with Ceftriaxone and Azithromycin for 5 days, Remdesivir for 5 days, and intravenous Dexamethasone for 10 days. He was initially admitted to the medical intensive care unit (ICU) and downgraded on day 3 of admission after demonstrating stable oxygen saturations on a non-rebreather mask at 100% FiO2. He was eventually weaned down to nasal cannula on day 6 of admission. Chest X-ray performed on hospital day 7 showed radiographic resolution of pneumomediastinum and subcutaneous emphysema with persistent bibasilar infiltrates (Figure 4). He was discharged home on day 8 of admission with 2 L/min of nasal cannula with oxygen saturations above 94%. He remained afebrile and hemodynamically stable throughout his hospital course.
Figure 4.

Chest X-ray on discharge. This is a chest radiograph performed on hospital day 7 that showed resolution of pneumomediastinum and subcutaneous emphysema.
Discussion
Pneumomediastinum can be categorized as spontaneous or traumatic. SPM is defined as having a cause that is non-surgical, atraumatic, and not related to mechanical ventilation.8 Symptoms of SPM include chest pain, shortness of breath, cough, and dysphagia.4 A wide variety of causes of SPM have been described including intense exercise, coughing, vomiting, infection, primary lung disease such as asthma, interstitial lung disease, and inhalation irritants (e.g. tobacco and illicit drugs).9,10 Viral lung infections are rare causes of SPM.4 SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), however, have been shown to cause desquamation of the pneumocytes that make up the alveolar wall.11 Rupture of the alveoli and the subsequent air leak is the likely initial event leading to pneumomediastinum.
We reviewed seven cases (including this case) of SPM in patients with SARS-CoV-2 who were not exposed to positive pressure ventilation (Table 1). There appears to be a propensity of SPM that occurs in males with SARS-CoV-2 infection. There does not appear to be a correlation of incidence of SPM with severity of hypoxia nor age. The time from symptom onset to diagnosis of SPM is widely variable with a mean of 15 days. None of the cases had a diagnosis of primary lung disease. SPM does not appear to confer an increased mortality based on our review, although larger observational studies would need to be performed.
Table 1.
Patients with COVID-19 who developed spontaneous pneumomediastinum without preceding positive pressure ventilation or concomitant pneumothorax.
| Article | Age (years) | Gender | Comorbidities | Oxygen saturation at presentation | Days from symptom onset to pneumomediastinum | Patient outcome |
|---|---|---|---|---|---|---|
| Kolani et al.4 | 23 | Female | None | 98% on room air | Unknownb | Survived |
| Wegner et al.5 | 44 | Male | None | 91% on room air | Not reported | Survived |
| Goldman et al.6 | 64 | Male | Diabetes obstructive sleep apneaa | 94% on room air | 26 days | Survived |
| Zhou et al.7 | 38 | Male | None | Not reported | 12 days | Survived |
| Lacroix et al.12 | 57 | Male | None | Not reported | 14 days | Not reported |
| Mohan and Tauseen13 | 49 | Male | Diabetes hypertension | 85% on room air | 8 days | Survived |
This is a table summarizing pertinent information in six other cases of spontaneous pneumomediastinum caused by SARS-CoV-2 infection. These six cases were not exposed to positive pressure ventilation. Pertinent data include age, gender, comorbidities, oxygen saturation at presentation, days of symptom onset to pneumomediastinum, and patient outcome.
Patient was non-compliant with CPAP therapy as an outpatient.
Patient was asymptomatic at presentation.
Treatment of SPM involves supportive measures such as analgesics, rest, and oxygen.9 Treating the underlying cause or precipitating factor if indicated, such as antibiotics for pneumonia or corticosteroids for asthma exacerbation, is crucial. SPM itself is often benign and self-limiting without need for invasive interventions.5
Our patient highlights a rare SARS-CoV-2 complication of SPM. Aside from SARS-CoV-2, the patient did not have any predisposing factors for the development of pneumomediastinum, and he did not have exposure to positive pressure ventilation. He was critically ill with acute hypoxic respiratory failure requiring non-rebreather mask, but showed marked improvement while on SARS-CoV-2 treatment. On hospital day 7 (day 21 of symptoms), his chest X-ray (CXR) showed resolution of his SPM and subcutaneous emphysema. He survived his hospital course and was discharged successfully on 2 L of oxygen.
Conclusion
SPM is a rare complication of SARS-CoV-2 infection. It is diagnosed by radiographic imaging with treatment that is mainly supportive. Larger observational studies are needed to make conclusions about SPM and SARS-CoV-2, such as impact on prognosis, a potential marker of severity, time to development and resolution of SPM, prevalence, and effect of SARS-CoV-2-directed treatment.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Duong T Hua  https://orcid.org/0000-0001-7636-751X
https://orcid.org/0000-0001-7636-751X
References
- 1. Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 2020; 215(1): 87–93. [DOI] [PubMed] [Google Scholar]
- 2. Zhai P, Ding Y, Wu X, et al. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents 2020; 55(5): 105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kong W, Agarwal PP. Chest imaging appearance of COVID-19 infection. Radiol Cardiothorac Imaging 2020; 2(1): e200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kolani S, Houari N, Haloua M, et al. Spontaneous pneumomediastinum occurring in the SARS-COV-2 infection. IDCases 2020; 21: e00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wegner U, Jeffery G, Abrajan O, et al. Spontaneous pneumomediastinum associated with SARS-CoV-2: infrequent complication of the novel disease. Cureus 2020; 12: e9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldman N, Ketheeswaran B, Wilson H. COVID-19-associated pneumomediastinum. Clin Med 2020; 20: 91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou C, Gao C, Xie Y, et al. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis 2020; 20: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med 2008; 102(9): 1329–1334. [DOI] [PubMed] [Google Scholar]
- 9. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007; 31(6): 1110–1114. [DOI] [PubMed] [Google Scholar]
- 10. Dionísio P, Martins L, Moreira S, et al. Spontaneous pneumomediastinum: experience in 18 patients during the last 12 years. J Bras Pneumol 2017; 43(2): 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol 2015; 235(2): 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lacroix M, Graiess F, Monnier-Cholley L, et al. SARS-CoV-2 pulmonary infection revealed by subcutaneous emphysema and pneumomediastinum. Intensive Care Med 2020; 46(8): 1620–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohan V, Tauseen RA. Spontaneous pneumomediastinum in COVID-19. BMJ Case Rep 2020; 13: e236519. [DOI] [PMC free article] [PubMed] [Google Scholar]


