Abstract
The MEN1 gene encodes MENIN, a tumor suppressor that plays a role in multiple cellular processes. Germline and somatic mutations in MEN1 have been identified in hereditary and sporadic tumors of neuroendocrine origins suggesting context-specific functions. In this review, we focus on the development of mutational Men1 in vivo models, the known cellular activities of MENIN and efforts to identify vulnerabilities in tumors with MENIN loss.
Keywords: Pancreatic neuroendocrine tumor, MENIN, MEN1, multiple endocrine neoplasia type 1, tumor suppressor gene
History of MEN1 and Discovery of MENIN
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal-dominant syndrome that classically manifests as parathyroid, pituitary and pancreatic neuroendocrine tumors. The first description of a MEN1 patient is attributed to Jakob Erdheim who described a patient with acromegaly, a pituitary adenoma, and four hypertrophic parathyroid glands on postmortem examination (Erdheim 1903; Ballard, Fame, and Hartsock 1964). In 1927, Cushing and Davidoff reported the first patient with classic findings of MEN1: adenomas were identified in the pituitary, the parathyroid glands and within a pancreatic islet (Ballard, Fame, and Hartsock 1964; Cushing 1927). Over the subsequent three decades, multiple investigators suggested the syndrome may be hereditary. In 1954, Wermer proposed that the disease was transmitted by a single autosomal dominant gene with a high degree of penetrance (Wermer 1954). This was further corroborated by Ballard and colleagues’ description of a “syndrome of multiple endocrine adenomatosis” that included six generations consisting of 42 family members. They reported endocrine neoplasia involving the pituitary (64.9%), pancreas (81.2%), parathyroids (88.2%), adrenals (37.6%) and thyroid (18.8%) (Ballard, Fame, and Hartsock 1964).
Subsequently, linkage analysis mapped MEN1 in insulinoma specimens to chromosome 11q13 (Larsson et al. 1988). By studying two brothers who inherited MEN1 syndrome from their mother, it was discovered that tumor tissue had loss of one constitutional allele at two loci on chromosome 11 when compared with matched normal tissue. The two loci spanned from band p15 to band q23 (Larsson et al. 1988). To ultimately identify the specific gene, polymorphic markers were mapped to the 11q13 region, identifying 33 candidate genes (Chandrasekharappa et al. 1997). To further narrow down the boundaries of the gene, they examined interstitial deletions and mitotic crossing-over events in patient tumor samples from hereditary MEN syndrome type I and sporadic gastrinomas that had been laser-dissected, identifying the minimal interval bounded by marker PGYM and D11S4936 as the candidate transcript for MEN1. As confirmation, the team designed primers to amplify the exons from the genomic DNA of unrelated MEN1 patients, finding mutations in 14 of the 15 patients in this candidate MEN1 transcript. Of these mutations, five were frameshift mutations, three were nonsense mutations, two were in-frame deletions and two were missense alterations (Chandrasekharappa et al. 1997). The transcript was estimated to be 2.8 kilobases and the 610-amino acid protein was named MENIN, reflecting its causality to MEN1 syndrome.
MENIN was first characterized as a nuclear protein after localization studies were performed by Guru et al. (Guru et al. 1998). With immunofluorescence and immunoblotting of subcellular fractions, they detected MENIN as a nuclear protein. Using green fluorescent protein-tagged MENIN deletion constructs transfected into HEK-293T cells, the group discovered that MENIN had at least two nuclear localization signals (NLS) and an accessory NLS (NLSa) in its C-terminal end (La et al. 2006). However, none of the known 22 missense or 3 inframe germline mutations in MEN1 localized to the NLS. Furthermore, of the 43 frameshift/nonsense mutations, 39 were truncated such that the NLS would not have been translated, which would result in a cytosolic protein.
MENIN is a well-established tumor suppressor protein, which was initially inferred from pedigree and sequencing studies (Agarwal et al. 1997; Heppner et al. 1997; Zhuang et al. 1997; Debelenko et al. 1997). By cloning and sequencing tumors from familial MEN1 syndrome, sporadic MEN1 and familial hyperparathyroidism, Agarawal et al. found forty different mutations across the MEN1 gene (Agarwal et al. 1997). The mutations predominately predicted a loss of function in the protein, thus suggesting that MEN1 is a tumor suppressor gene. Additionally, dideoxy fingerprinting (ddF) and then sequencing of amplified DNA fragments with aberrant ddF patterns on 33 sporadic parathyroid tumors identified 13 (39%) as having loss of heterozygosity (LOH) at 11q13 and seven had MEN1 mutations (Heppner et al. 1997). These data supported the tumor suppressor function of MEN1 and the biallelic loss-of-function mechanism.
MEN1 Mutations in Human Tumors
MEN1 mutations were first reported in insulinomas and gastrinomas in 1997. By using fluorescent in sito hybridization analysis on tumors from 40 patients, one copy of MEN1 was found to be deleted in 25 of 27 (93%) sporadic gastrinomas and 6 of 12 (50%) sporadic insulinomas. Of all forty tumors, only one exhibited a germline mutation (Zhuang et al. 1997). Somatic mutations of MEN1 have also been identified in 2 of 12 (17%) of sporadic insulinomas and 9 of 27 (33%) of sporadic gastrinomas (Zhuang et al. 1997). Subsequently, whole-exome sequencing of 10 non-familial pancreatic neuroendocrine tumors (pNETs), found MEN1 inactivating/missense mutations in 50% and identified mutations in 44% (30/68) of tumors in a validation set (Jiao et al. 2011). MEN1, DAXX (death-domain associated protein), ATRX (a thalassemia/mental retardation syndrome X-linked) and mTOR pathway genes (i.e. PTEN, TSC2, PIK3CA) were among the most commonly altered genes. This is in stark contrast to the genes mutated in tumors of the exocrine pancreas (e.g. TP53, KRAS, CDKN2A, TGFBR1, SMAD3, SMAD4) (Jones et al. 2008).
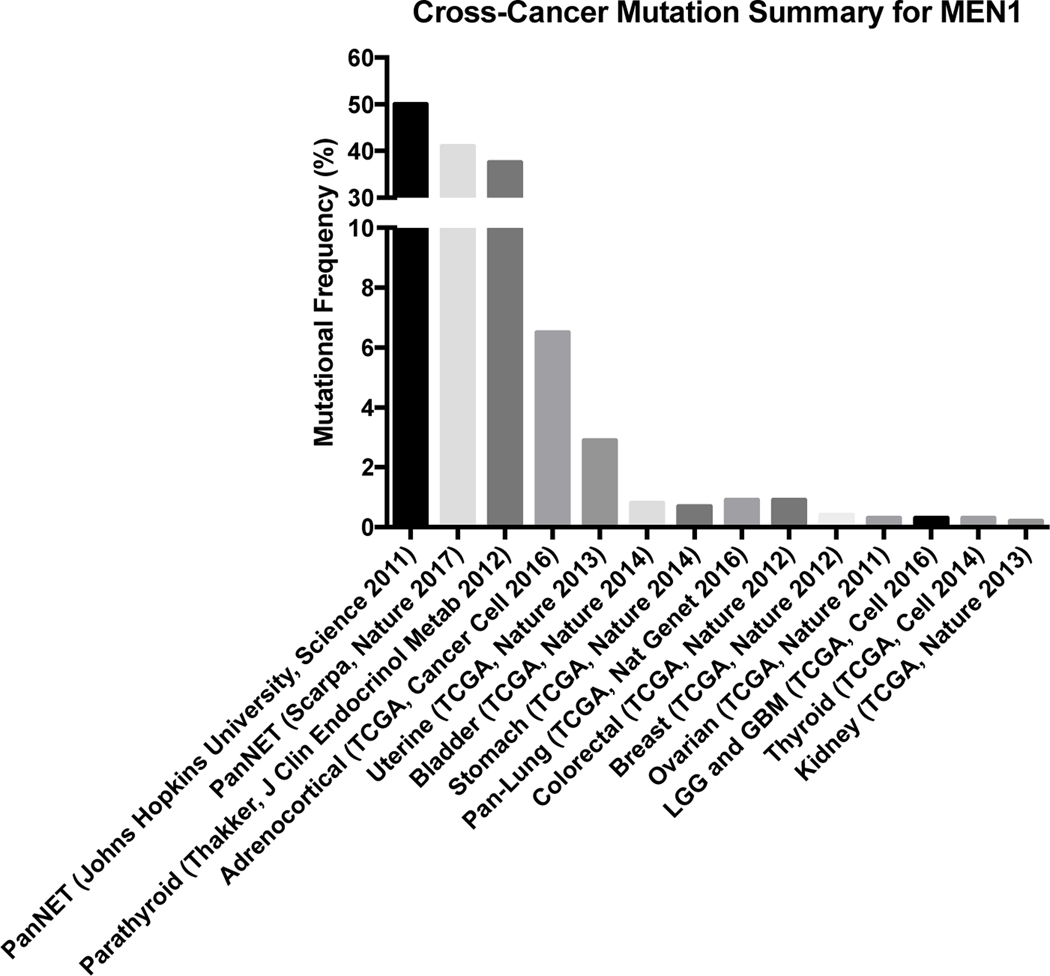
In non-neuroendocrine solid tumors, MEN1 mutations are infrequent suggesting a unique dependency of MENIN in neuroendocrine tissues. An aggregation of published analyses of the Cancer Genome Atlas (TCGA) shows that MEN1 mutations occur 0.3–6.5% of examined solid tumors (Figure 1).
Figure 1:
MEN1 mutations in cancers
Aggregation of singular studies and TCGA analyses demonstrates that MEN1 mutations are relatively infrequent in cancers with the exception of pancreatic neuroendocrine tumors and parathyroid adenomas (cbiportal.org).
Improved survival has been observed in patients with either MEN1/DAXX/ATRX mutations or MEN1 in combination with DAXX/ATRX mutation (Jiao et al. 2011). This genotype-specific survival effect is not observed in all series and may vary depending on disease stage, as loss of DAXX and ATRX has also been associated with poorer prognosis (Marinoni et al. 2014). In another study of 102 sporadic, resected primary pancreatic neuroendocrine tumors that underwent whole-genome sequencing, somatic MEN1 mutations were present in 41% of the tumors and germline mutations in 6% (Scarpa et al. 2017). The mutations were distributed across the gene without evidence of clustering. A majority of these tumors also had an inactivating copy number change in MEN1.
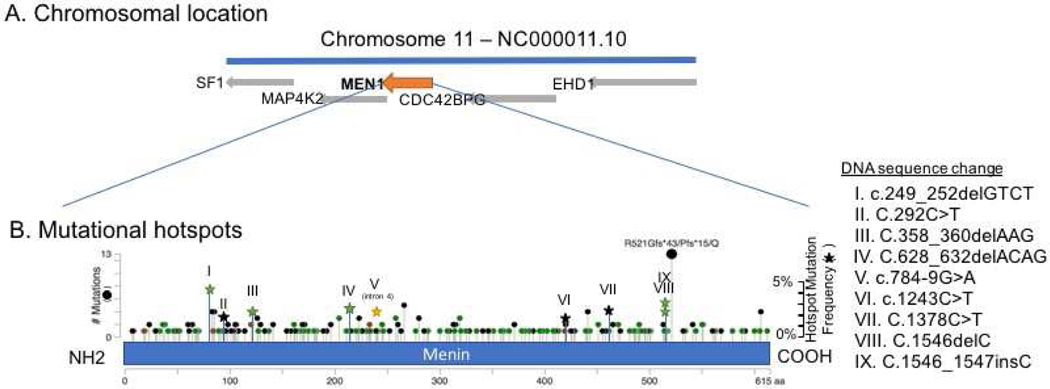
In an institutional cohort of approximately 17,000 sequenced tumors across cancer types, we observed multiple amino acid positions that have an increased frequency of mutations (Figure 2). The most frequent somatic mutation (found in multiple cancer types) occurred at codon 521 which is in proximity to the NLS although mutations at this site have not been functionally validated. MENIN has at least three NLS (NLS1, NLS2 and an accessory NLSa) and single point mutations in these regions only attenuate but do not completely block nuclear translocation of MENIN (La et al. 2006). Cytosolic localization was only achieved when all three of the NLSs were concurrently mutated. While none of these NLSs is crucial for the recruitment of MENIN to the chromatin, the key positively-charged residues at the NLSs are crucial for MENIN contacting the promoter DNA as shown by chromatin immunoprecipitation (ChIP) assay (La et al. 2006).
Figure 2.
A. MEN1 is on chromosome 11q13.1. It spans 2.8kb and is 610 amino acids long.
B. MEN1 mutations are spread diffusely across the entire gene with certain mutations occurring with slightly higher frequency than others (cbioportal.org). (Green dot: missense mutation, black dot: truncating mutation)
Mouse Models of Menin Loss
The sequence of MEN1 is highly conserved across species, from drosophila to human. In mice, the Men1 gene is approximately 6.7 kilobases, maps to chromosome 19 and is 611 amino acids long (Stewart et al. 1998). Compared to the human protein, murine Men1 has 97% amino acid sequence homology. Like human MEN1, murine Men1 has ten exons of which the first exon is not translated. Several constitutively deleted (Crabtree et al. 2001; Bertolino, Radovanovic, et al. 2003; Bertolino, Tong, Galendo, et al. 2003; Loffler et al. 2007; Harding et al. 2009; Wiedemann and Pellegata 2016) and conditionally inactivated (Bertolino, Tong, Herrera, et al. 2003) Men1 alleles have been used to study its effect in vivo (Table 1).
Table 1.
Select mouse models of Men1 loss
| Reference | Genetic Modification | Background Strain | Phenotype | Time to pNET | Hormonal Aberrations | LOH present? |
|---|---|---|---|---|---|---|
| Crabtree et al. 2001 | Conditional Deletion of exon 3–8 | |||||
| Men1tsm/tsm | NIH Black Swiss | Embryonic lethal (E11.5–12.5) | ||||
| Men1tsm/+ | 129Sv/ EvTacFBR | - Pancreatic islet tumors (28%) - Parathyroid adenomas (24%) - Pituitary adenomas (26%) - Adrenocortical carcinomas (20%), pheochromocytoma (74%) - Lung adenocarcinomas (22%) - Gastric neuroendocrine tumors (4%) - Prostate carcinoma (4%) - Ovarian adenoma (4%) - Thyroid follicular adenomas |
9 months | Insulin | No | |
| Men1Δ3−8/Δ3−8 (Men1tsm/tsm x EIIa-cre) | 129Sv/EvTacFBR | Embryonic lethal (E10.5-E11.5) | ||||
| Bertolino, Radovanovic, et al. 2003 | Deletion of exon 3 | |||||
| Men1T/T | 129/Ola/Sv | Embryonic lethal (E11.5–13.5) | ||||
| Men1+/T | 129/Ola/Sv | -Testis Leydig carcinoma (88%) - Pancreatic islet cell tumor adenoma 19.7%), carcinoma (60.6%) - Parathyroid adenoma (63.6%), carcinoma (3%) - Adrenal glands adenoma (27.8%), carcinoma (18%) - Extrapancreatic gastrinoma adenoma (17.6%), carcinoma (5.9%) - Pituitary gland adenoma (16.6%), - carcinoma (20%) - Mammary carcinoma (8.3%) - Thyroid tumor (6.5%) |
12–13 months | Insulin, PTH, glucagon | Yes | |
| Loffler et al. 2007 | Deletion of exon 2 | |||||
| Men1+/− | C57/129 | -Pancreatic islet cell adenoma (79.2%) - Pituitary adenomas (61.1%) -Thyroid neoplasm (15.2%) - Testis (34.4%)/ovaries (15.9%) - Parathyroid adenoma (6.9%) - Adrenal adenomas (0.8%) |
Yes | |||
| Harding et al. 2007 | Deletion of exon 1 and 2 | |||||
| Men1+/− | C57BL/6 | - Pancreatic islet cell adenoma (83.6%) - Pituitary adenomas (31.4%) - Parathyroid adenoma (41.8%) - Adrenal adenomas (11.6%) |
9–12 months | Yes | ||
| Crabtree et al. 2003 | Conditional deletion of exon 3 – 8 | |||||
| Men1ΔN/ΔN- RIP2-cre | B6;FVB;129 Sv | - Pancreatic islet cell tumor (100%) - Pituitary adenomas (12%) |
6 months | Insulin | ||
| Bertolino, Tong, Galendo, et al. 2003 | Conditional deletion of exon 3 | |||||
| Men1F/F-RipCre+ | 129/Ola/Sv | - Pancreatic islet cell tumors (100%) - Pituitary adenomas - Parathyroid adenomas |
6 months | Insulin | ||
| Biondi et al. 2004 | Conditional deletion of exon 2 | |||||
| Men1loxP/loxP-RipCre+ | C57BL/6J | - Pituitary adenomas (71.4%) - Pancreatic islet cell tumors (33.3%) |
9 months | |||
| Shen et al. 2009 | Conditional deletion of exons 3 to 8 | |||||
| Men1F/F-PDX1Cre+ | FVB;129Sv | - Pancreatic islet cell tumor | ||||
| Lines et al. 2017 | Temporal/ conditional deletion of exons 3 to 8 | |||||
| Men1L/L/RIP 2-CreER (tamoxifen-induced) | Tg(Ins2-cre/ERT)1D am/J;129S(F VB)Men1tm 1.1 Ctre/J | - Pancreatic islet cell tumor (100%) | 5 months | |||
| Shen et al. 2010 | Conditional deletion of exons 3 to 8 | |||||
| Glu-Cre;Men1 f/f (α−cell specific) | FVB;129Sv | - Pancreatic islet cell tumor | 13 months | |||
| Lu et al. 2010 | Conditional deletion of exon 3 | |||||
| Men1+/−, Me n1F/F, Men1F/F-RipCre+, Glu Cre+ (α−cell specific) | 129/Ola/Sv | - Pancreatic islet cell tumor (insulinoma)(15%) - Pancreatic a-cell tumor (glucagonoma)(30%) - Mixed insulin-glucagonoma (55%) |
8 months | Glucagon, insulin | ||
| Lin et al. 2016 | Conditional deletion of exon 3–8 of MEN1 and insertion of lacZ reporter in-frame of Kmt2a | |||||
| Kmt2af/f;Me n1f/f;RIP-Cre | 129s6, FVB/N, and C57BL/6 background | - Pancreatic islet cell tumor | 6 months | Insulin | ||
| Gillam et al. 2015 | Conditional eletion of exon 3–8 of MEN1 and deletion of exon 1–2 of CDK4 and three point mutations | |||||
| Men1+/−; Cdk4−/− | FVB/N, 129S6 and C57BL/6; 129sv | - No neuroendocrine tumors | 15 months | |||
| Men1+/−; Cdk2−/− | FVB/N, 129S6 and C57BL/6; 129sv | - Pituitary tumor (52%) - Pancreatic islet cell tumor (65%) |
15 months | |||
Mouse Models of Men1 Conventional Knockout
The earliest murine model of Men1 knockouts was developed at the National Human Genome Research Institute by Crabtree et al (Crabtree et al. 2001). The group created a targeted Men1 gene by inserting a floxed PGK-neomycin cassette into intron 2 and a third loxP site in intron 8 (Men1Δ3−8/+). The homozygous phenotype was found to be embryonic lethal, with developmental delay and exhibited cranio-facial defects by E11.5–12.5. Heterozygous mice developed islet cell hyperplasia and pancreatic islet tumors at 9 months. By 16 months, larger and multi-focal islet tumors were observed. 20/71 mice (28%) developed a pancreatic islet tumor by the age of 22 months and disease progression from hyperplastic islet cells tumor formation correlated with worsening of insulin elevation (2.4–15 ng/ml versus 15–39 ng/ml) and loss of heterozygosity at the Men1 locus. Comparable to human MEN1 syndrome, these animals also had tumors in the parathyroid, pituitary and adrenal glands (carcinoma and pheochromocytoma) in 7–26% of the animals.
In 2003, Bertolino et al. created a mouse model of constitutive Men1 deletion spanning exon 3 (Men11/Δ) (Bertolino, Radovanovic, et al. 2003). Once again, the Men1Δ/Δ genotype were embryonic lethal with defects found in organogenesis of the neural tube, heart and liver. The heterozygous animals exhibited multiple endocrine neoplasms around one year of age mimicking the human condition, with pancreatic islet cell tumors, gastrinomas, parathyroid adenomas, pituitary adenomas, and thyroid hyperplasia and tumors (Bertolino, Tong, Galendo, et al. 2003). However, islet cell tumors were found to progress to carcinoma and then metastasis in aged mice.
Loffler et al. developed the third conventional mouse model of Men1 knockout (Loffler et al. 2007). Exon 2, including the translation start site, was deleted from Men1. By 270 days, 33% of the heterozygous animals had pancreatic islet hyperplasia and 58% had an adenoma. Pancreatic islet adenomas were observed in 72% by 360 days, 83% by 450 days, and >90% by 540 days. Other organs that developed a phenotype include pituitary gland, parathyroid, thyroid, testes, ovaries and adrenal glands.
Harding et al. followed in 2009 with a conventional mouse model knocking out exons 1 and 2 of Men1 (Harding et al. 2009). Heterozygous animals had a worse survival compared to wildtype animals and developed a broad spectrum of endocrine tumors. Men1+/− animals developed pancreatic tumors (9 months), pituitary tumors (12 months) and parathyroid adenomas and adrenal cortical tumors (15 months).
Mouse Models of Men1 Conditional Inactivation
As homozygous knockout of the Men1 gene led to embryonic lethality, it was desirable to develop conditional targeting of the Men1 gene to investigate the function of menin in various organs in temporally controllable manner. Using their Men1 floxed exon 3 to 8 transgene, Crabtree et al. crossed mice with floxed Men1 loci with mice expressing a Cre-recombinase transgene under the rat insulin promoter (RIP) to specifically delete Men1 in β-cells (Crabtree et al. 2003). These animals developed pancreatic neuroendocrine tumors at 6 months of age.
Bertolino et al. also created a model of Men1 deletion (Men1+/Δ) that was specific to β-cells (RIP-Cre) (Bertolino, Tong, Herrera, et al. 2003). They observed islet hyperplasia around two months with progression to dysplasia and insulinoma by six months of age. By ten months, 100% penetrance in tumor formation was observed. Like the previous murine models, insulin levels were correlated with the degree of malignant transformation in the β-cells. Similarly, Biondi et al. induced conditional deletion of exon 2 of Men1 with RIP-Cre (Men1loxP/loxP-RipCre+) (Biondi et al. 2004) By. four months, β-cells hyperplasia was observed and by 9 months, the animals developed insulinomas (33.3%) and prolactinomas (71.4%).
Another conditionally inactivated Men1 murine model was created by Shen et al. and relies on a Cre recombinase specifically driven by a pancreatic progenitor promoter, PDX1 (pancreatic and duodenal homeobox 1) (Shen et al. 2009). The Men1 allele is floxed from exon 3 to 8 (Men1f/f). Although the Pdx1 promoter expresses Cre recombinase in both endocrine and exocrine pancreatic cells, only endocrine tumors manifested, indicating the specific impact of the Men1 gene deletion on certain endocrine tissues. These mice have elevated serum insulin levels by 5 months and then develop an insulinoma by 10–12 months.
Recently, Lines et al. described a temporally controlled model of MEN1 pancreatic neuroendocrine tumor using tamoxifen-inducible Cre recombinase under the control of rat insulin promoter (Lines et al. 2017). Exons 3 and 8 of Men1 were floxed by loxP sites. The mice were aged to three months and then fed a tamoxifen diet for 5 days. They were then subsequently aged until at least 5 months and necropsies were performed. 100% of animals fed tamoxifen had pancreatic NETs and significantly larger non-malignant islet cells.
Interest in knocking out Men1 in the endocrine islets of Langerhans has been not limited to β-cells. Ablation of exons of Men1 alleles in α-cells has been achieved by two groups with the use of a Cre recombinase under control of a rat glucagon promoter (Shen et al. 2010) or a proglucagon promoter (Lu et al. 2010). Although the absence of Men1 was proven to be limited to α-cells, Shen et al. observed only β-cell insulinomas where as the animals developed by Lu et al. exhibited α-cell glucagonomas, β-cell insulinomas and tumors that consisted of both α-cells and β-cells. To elucidate why insulinomas develop in light of ablating menin in α-cells only, Lu et al. performed cell-lineage analysis by staining for a reporter fluorescent protein marker and concluded that α-cells trans-differentiate into β-cells. On the contrary, Shen et al. performed fluorescence-activated cell sorting to isolate α-cells or β-cells and detected Men1 deletion only α-cells.
Double Knockout Mouse Models of Men1 Conditional Inactivation
With the discovery of additional interacting partners of menin, several mouse models knocking out both menin and the interacting partner have been developed. As discussed in the following sections, Kmt2a (MLL1) is a histone H3 lysine 4 specific methyltransferase that associates with menin and promotes leukemogenesis. However, the role of Kmt2a is largely not understood in the pathogenesis of pancreatic neuroendocrine tumor. As such, Lin et al. developed a Kmt2af/f;Men1f/f;RIP-Cre animal (Lin et al. 2016). Kmt2af/f;RIP-Cre animals exhibited islet hyperplasia at 10 months but even with later time points, no frank tumors developed. With the addition of Men1f/f, Kmt2af/f;Men1f/f;RIP-Cre animals had a significantly worsened survival compared to Men1f/f;RIP-Cre animals (334 days vs. 274 days). Hyperinsulinemia was used to approximate time of tumor formation and higher levels of insulin were achieved in the animals deficient of Kmt2a at 6 months than Men1f/f;RIP-Cre animals. Islet cell tumors were also more numerous, larger and higher grade in Kmt2af/f;Men1f/f;RIP-Cre animals as compared to Men1f/f;RIP-Cre animals.
When Gillam et al. noted hypoplasia in pituitary and pancreatic islet cells with the ablation of Cdk4, they became interested in studying this in the context of neuroendocrine tumorigenesis. As such, they made animals that were heterozygous in Men1 with the addition of homozygous knockout of either Cdk4 or Cdk2, a CDK which did not exhibit any neuroendocrine effects. Compared to the Men1+/− animals which developed pituitary (45%) and islet cell tumors (62%), Men1+/−;Cdk4−/− animals did not develop neuroendocrine tumors at 15 months. In addition, Men1+/−;Cdk2−/− animals developed both types of tumors, suggesting that Cdk4 is required for neuroendocrine tumorigenesis secondary to Men1 loss (Gillam et al. 2015).
MENIN Function
Across species, MENIN is highly conserved and does not share homology with any other family of proteins. The delineated activities of MENIN are diverse and often context specific. MENIN is thought to play a role in GTPase activity, transcriptional regulation and cell cycle regulation, among others.
GTPase Activity and Transcriptional Regulation
Within its N-terminus, MENIN contains five consensus sequence motifs characteristic of GTPases. In the presence of nm23, a tumor metastasis suppressor, MENIN acts as a GTPase (Yaguchi et al. 2002). Individually, MENIN and nm23 both have very low affinity to GTP and GDP and no GTPase activity. As a corollary, MENIN does not influence the functions of nm23 (i.e. NDP kinase, protein kinase, or Rad-GAP activity). Moreover, the crystal structure of MENIN and its interacting proteins have been solved using crystallography but no GTPase-like folding or domain has been identified (Huang et al. 2012; Murai et al. 2011). Thus, the significance of the GTPase activity for MENIN remains undefined.
The discovery of the interaction of MENIN and activator protein 1 factor (AP1) JunD were the first hints suggesting that MENIN played a role in cell cycle regulation. Unique among the AP1 proteins, JunD is an inhibitor of cell growth (Shaulian and Karin 2001). Transcription factor JunD was identified as an interacting partner with the N-terminus of MENIN via a yeast two-hybrid screen (Gobl et al. 1999; Agarwal et al. 1999). MENIN does not interact with any other member of the AP1 family. In the presence of mutant MENIN, JunD-mediated transcription was not repressed and with the addition of a histone deacetylase inhibitor, MENIN lost its ability to repress JunD-mediated transcription. This paradoxical repression of JunD, a suppressor of growth, by MENIN, remained without biologic significance until it was discovered that JunD had growth promoting activity in the absence of MENIN (Agarwal et al. 2003). In vitro cell growth assays in JunD-null, Men1 WT immortalized fibroblasts showed a rescue of the growth inhibition phenotype with overexpression of wildtype JunD whereas overexpression of mutant JunD, which could not bind to Men1, exhibited growth promotion. In immortalized fibroblasts that were JunD WT, Men1 null, overexpression of wildtype JunD exhibited a more transformed-like morphology but no change in growth index. Furthermore, it was further corroborated that the mechanism through which MENIN represses JUND is through recruitment of histone deacetylase (Kim et al. 2003).
Coimmunoprecipitation experiments have shown that MENIN also interacts with the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) family of transcriptional factors, which induce a cellular stress response (e.g. to ionizing radiation, mitogens, growth factors and cytokines) (Heppner et al. 2001). MENIN was shown to repress NF-kB transactivation in a dose-dependent manner.
Menin is implicated in histone modification through its interactions with mixed lineage leukemia protein 1 (MLL1, also called KMT2A) (Huang et al. 2012), mixed lineage leukemia protein 2 (MLL2, also called MLL4, KMT2B, or KMT2D) and trithorax protein absent, small homeotic 2 (Ash2L) (Hughes et al. 2004). Menin was shown to physically associate with Set-domain containing histone methyltransferase (HMTase) complexes MLL2 and Ash2L by nondenaturing immunoprecipitation and mass spectrometry (Hughes et al. 2004). The menin-associated HMTase specifically methylates histone H3 on lysine 4 (H3K4). In cells retrovirally infected with mutant Men1, HMTase activity significantly decreases. Since menin also interacts with Rpb2, the menin-HMTase complex likely associates with transcriptional activation complexes. Using microarray hybridization, gene expression of Men1 wildtype and null embryos revealed null embryos had a significant decrease in expression of Men1 and developmental genes Hoxc6, Cyt19 and Hoxc8 with menin binding specifically to the Hoxc8 locus. Huang et al. discovered that menin binds to MLL1, a H3K4 methyltransferase, and JunD in the same pocket (Huang et al. 2012). However, contrary to the transcription repressing activity of the menin-JunD complex, menin-MLL1 complexes associate with the HOXA9 promoter which consequently upregulates transcription and promotes leukemic transformation (Yokoyama et al. 2005).
Menin’s transcription and growth-suppressing role in pancreatic beta cells has been shown to rely on its interaction with protein arginine methyltransferase 5 (PRMT5) to downregulate the Hedgehog signaling pathway (Gurung et al. 2013). PRMT5, which suppresses transcription through its histone arginine methyltransferase activity, was shown to coelute with menin by chromatography, followed by mass spectroscopic analysis. PRMT5 is recruited by menin to the promoter of Gas1, a crucial factor for Sonic hedgehog (Shh) ligand to bind to its receptor patched homolog 1 (PTCH1). By increasing the histone H4 arginine 3 dimethylation at the Gas1 promoter, PRMT5 suppresses Gas1 expression and subsequently, Hedgehog-signaling mediated growth. Menin-mediated recruitment of PRMT5 has also been described to the GLI1 promoter (Gurung, Feng, and Hua 2013), which is downstream of GAS1 and smoothened (SMO). These findings raise the interesting possibility that pNETs with mutated MEN1 and high Hedgehog signaling could be treated with a Hedgehog inhibitor.
In neuroendocrine cell lines, Menin interacts with death-domain-associated protein (DAXX) to enhance histone H3 lysine9 trimethylation (H3K9me3) resulting in suppression of the membrane metallo-endopeptidase Mme promoter (Feng et al. 2017). This interaction has clinical implications as DAXX is mutated in 40% of human neuroendocrine tumors (Jiao et al. 2011). By cDNA microarray analysis, Mme, DCN and Gria3 were identified to be upregulated in Men1- or Daxx-null mouse embryonic fibroblasts. Increased Mme expression has been associated with larger tumor size, higher ki-67 index and metastasis (Deschamps et al. 2006). Menin binds to Daxx in its C-terminal region with one mutation associated with MEN1 tumor syndrome resulting in the failure of menin to bind Daxx. Menin and Daxx enhance histone 3 lysine 9 trimethylation. Without this suppression of Mme, cell proliferation of neuroendocrine tumors goes unchecked. Either with shRNAs or drug (Thiorphan), decreasing levels of Mme significantly decreases tumors size.
Cell Cycle Regulation
Inspired by the differential expression of other tumor suppressors in accordance with the cell cycle (i.e. BRCA1 (Gudas et al. 1996) and BRCA1 (Bertwistle et al. 1997)), Kaji et al. investigated the levels and localization of menin in conjunction with JunD at various points of the cell cycle in rat pituitary GH4C1 cells (Kaji et al. 1999). Menin was mostly localized to the nucleus. Higher levels of menin were present during quiescence but levels drop after entry of the cell cycle. As the cell enters S phase, levels of menin increase again. JunD levels corresponded with levels of menin.
Later in 2005, after menin was found to interact with MLL2 through regulation of Hox expression, Milne et al. hypothesized that menin may additionally regulate cell growth by interfacing with cyclin dependent kinase inhibitors, specifically p18Ink4c and p27Kip1 (Milne et al. 2005). Chromatin immunoprecipitation revealed that menin binds directly to the coding sequences of p18Ink4c and p27Kip1. In fibroblasts deficient in menin or MLL2, expression of p18Ink4c and p27Kip1 decreased and cell growth increased. Although MLL2 is dependent on menin to bind to p18Ink4c and p27Kip1 loci, menin seems to recruit MLL2 to particular loci in order to regulate transcription (Milne et al. 2005). In a separate study by Schnepp et al., cells deficient of menin were found to decrease the levels of protein and RNA of p18Ink4c and p27Kip1, increase CDK2 activity and accelerate G0/G1 to S phase entry (Schnepp et al. 2006). With the restoration of menin by retrovirus, expression of p18Ink4c and p27Kip1 and cell cycle progression both normalized.
As a tumor repressor, MENIN was hypothesized to play a role in the TGF-ß pathway, which is crucial in cell growth inhibition (Kaji et al. 2001). Using antisense RNA to inactivate menin, TGF-ß was found to less efficiently inhibit rat anterior pituitary cell growth through reduced transcriptional activity. Smad2, Smad3 and Smad4 are the canonical downstream effectors of the TGF-ß pathway, translocating into the nucleus upon phosphorylation to act as transcriptional activators. By coimmunoprecipitation, menin was found to specifically interact with Smad3. Inactivation of menin interrupts Smad3 binding with DNA, thus preventing transcriptional activation. The loss of Men1 in neuroendocrine tumors may prevent TGF-ß from halting the cell cycle when exposed to stress and thus contribute to tumor formation (Kaji et al. 2001). Of note, interaction with JunD and NF-kB suggests that menin acts as a transcriptional repressor but its interaction with Smad3 indicates that it can also act as a transcriptional activator.
MENIN in Leukemia
As opposed to its tumor repressing functions in endocrine organs, MENIN is critical for the leukemogenic activity of mixed lineage leukemia (MLL) gene in acute human leukemia. MLL chromosomal translocations form a diverse cohort of over 40 MLL-fusion proteins that constitutively activate MLL’s transcriptional effector properties (Mitterbauer-Hohendanner and Mannhalter 2004). MLL is a histone methyltransferase that maintains embryonic Hox gene expression (Yu et al. 1998). However, MLL fusions lose histone methyltransferase activity of MLL but gain other histone modification functions, resulting in oncogenicity. Interestingly, MENIN is the only interacting partner of MLL to maintain its interaction to the MLL-fusion protein (Yokoyama et al. 2004). Moreover, MENIN and wildtype MLL are also crucial for maintenance of MLL fusion protein-induced acute myeloid leukemia (Thiel et al. 2010; Thiel et al. 2013).
MENIN was found to be an interacting partner with MLL by immunopurification (Hughes et al. 2004). It associates with MLL oncoproteins on HOX gene promoters via a conserved, high-affinity MENIN binding motif (hMBM) (Yokoyama et al. 2005). Without a functioning hMBM, cells were incapable of clonogenic activity and oncogenic transformation (Yokoyama et al. 2005; Caslini et al. 2007). As such, the interaction between MENIN and MLL served to be a druggable target.
High-throughput screening identified a small-molecule MI-1 (with MI-2 and MI-3, as analogs), which could reversibly inhibit the MENIN-MLL binding motif (Grembecka et al. 2012). Treatment with MI-2 and MI-3 showed significant growth arrest, inhibition of transformation and induced hematopoietic differentiation in vitro. Further modifications of this MI small molecule yielded MI-463 and MI-503 which showed growth inhibition of patient-derived leukemia xenografts (Borkin et al. 2015).
Targeting MENIN Loss
Because MENIN is multifunctional and is context specific in its actions, there are considerable challenges to developing a robust therapeutic strategy. In order to target tumors with MENIN loss, focus has turned to orthogonal pathways that contain druggable targets.
Activating mutations in Kras are frequently observed in pancreatic ductal adenocarcinoma and not pNET, suggesting Kras and its effectors are suppressed in these tissues (Chamberlain et al. 2014). Mice with islet-specific expression of the gain of function mutant, KrasG12D, exhibited slower proliferation of ß-cells as compared to control animals. However, in animals with overexpressed KrasG12D but decreased Men1, there was an increase in proliferation of ß-cells suggesting that the Kras-induced growth suppression in endocrine ß-cells is dependent on Men1. Since Kras both activates MAPK pathway-mediated cell proliferation and RASSF1A-mediated cell growth suppression, MEN1-mediated suppression of the MAPK pathway is crucial to maintain physiologic balance in ß-cells. Currently, the exact mechanism of MEN1-mediated suppression of the MAPK pathway is unknown. However, pharmacologic targeting to re-instate MAPK pathway suppression in the context of a Men1 mutation has potential clinical relevance. Recently, it has also been reported that menin suppresses glucagon-like peptide 1 (GLP-1)/PKA-induced phosphorylation of FOXO1 and CREB, two transcription factors, to suppress ß-cells proliferation (Muhammad et al. 2017). It is not yet known whether this pathway is relevant to ß-cell proliferation induced by menin deletion or only to ß-cell regulation in diabetic stress conditions.
The Wnt/ß-catenin pathway has been thought to play a role in pancreatic development and cell proliferation (Rulifson et al. 2007; Murtaugh et al. 2005). Moreover, pNETs with mutations in MEN1 exhibit increased active ß-catenin and decreased phosphorylated ß-catenin (Jiang et al. 2014). This finding was reproduced in animals that were Men1-deficient. In addition, in Men1-deficient animals, concurrent knockout of ß-catenin resulted in smaller insulinoma size and reduced rates of tumorigenesis. Treatment with a small-molecule antagonist of the TCF/ß-catenin complex, PKF115–584, in Men1-deficient animals resulted in reduced hyperinsulinemia and Ki67 staining. Men1-deficient ß-cells treated with PKF115–584 showed significantly suppressed replication in vitro.
As previously discussed, the discovery of MENIN and DAXX interaction resulting in suppressed Mme (Feng et al. 2017) yields a potentially targetable interaction for patients with neuroendocrine tumors. Similarly, the physiologic interaction between MENIN and PRMT5 represses GLI1 and GAS1 (mediators of the Hedgehog pathway), which may serve as additional pharmacologic targets. A Hedgehog signaling inhibitor, GDC-0449, was used in MEN1f/f - Ripcre mice which develop insulinomas (Gurung et al. 2013) and found to decrease serum insulin levels. Treatment with GANT-61, a small molecule inhibitor of GLI1, resulted in a decrease in cell proliferation in MEN1 null cells as well as a reduction in C-MYC protein levels (Gurung, Feng, and Hua 2013).
Conclusion
MENIN is a multi-functional protein with pivotal roles in physiologic endocrine organ maintenance and neuroendocrine tumorigenesis. With diverse functions including transcription regulation, histone modification, and cell cycle regulation, MENIN holds immense promise in increasing understanding of pathogenesis and its interacting partners may provide new therapeutic targets for patients with somatic or germline MEN1 mutations.
Highlights for review.
MEN1 encodes MENIN, a tumor suppressor that is altered in select cancers.
Pancreatic neuroendocrine and parathyroid tumors are enriched with MEN1 mutations.
Conventional and conditional mouse models of Men1 loss produce PanNETs.
MENIN has GTPase activity and regulates transcription and the cell cycle.
Acknowledgements:
This research was funded in part through the Rubenstein Center for Pancreatic Cancer Research, Cycle for Survival and the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Agarwal SK, Guru SC, Heppner C, Erdos MR, Collins RM, Park SY, Saggar S, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ, and Burns AL. 1999. ‘Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription’, Cell, 96: 143–52. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Kester MB, Debelenko LV, Heppner C, Emmert-Buck MR, Skarulis MC, Doppman JL, Kim YS, Lubensky IA, Zhuang Z, Green JS, Guru SC, Manickam P, Olufemi SE, Liotta LA, Chandrasekharappa SC, Collins FS, Spiegel AM, Burns AL, and Marx SJ. 1997. ‘Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states’, Hum Mol Genet, 6: 1169–75. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Novotny EA, Crabtree JS, Weitzman JB, Yaniv M, Burns AL, Chandrasekharappa SC, Collins FS, Spiegel AM, and Marx SJ. 2003. ‘Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter’, Proc Natl Acad Sci U S A, 100: 10770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard HS, Fame B, and Hartsock RJ. 1964. ‘Familial Multiple Endocrine Adenoma-Peptic Ulcer Complex’, Medicine (Baltimore), 43: 481–516. [PubMed] [Google Scholar]
- Bertolino P, Radovanovic I, Casse H, Aguzzi A, Wang ZQ, and Zhang CX. 2003. ‘Genetic ablation of the tumor suppressor menin causes lethality at mid-gestation with defects in multiple organs’, Mech Dev, 120: 549–60. [DOI] [PubMed] [Google Scholar]
- Bertolino P, Tong WM, Galendo D, Wang ZQ, and Zhang CX. 2003. ‘Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1’, Mol Endocrinol, 17: 1880–92. [DOI] [PubMed] [Google Scholar]
- Bertolino P, Tong WM, Herrera PL, Casse H, Zhang CX, and Wang ZQ. 2003. ‘Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice’, Cancer Res, 63: 4836–41. [PubMed] [Google Scholar]
- Bertwistle D, Swift S, Marston NJ, Jackson LE, Crossland S, Crompton MR, Marshall CJ, and Ashworth A. 1997. ‘Nuclear location and cell cycle regulation of the BRCA2 protein’, Cancer Res, 57: 5485–8. [PubMed] [Google Scholar]
- Biondi CA, Gartside MG, Waring P, Loffler KA, Stark MS, Magnuson MA, Kay GF, and Hayward NK. 2004. ‘Conditional inactivation of the MEN1 gene leads to pancreatic and pituitary tumorigenesis but does not affect normal development of these tissues’, Mol Cell Biol, 24: 3125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkin D, He S, Miao H, Kempinska K, Pollock J, Chase J, Purohit T, Malik B, Zhao T, Wang J, Wen B, Zong H, Jones M, Danet-Desnoyers G, Guzman ML, Talpaz M, Bixby DL, Sun D, Hess JL, Muntean AG, Maillard I, Cierpicki T, and Grembecka J. 2015. ‘Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo’, Cancer Cell, 27: 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caslini C, Yang Z, El-Osta M, Milne TA, Slany RK, and Hess JL. 2007. ‘Interaction of MLL amino terminal sequences with menin is required for transformation’, Cancer Res, 67: 7275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain CE, Scheel DW, McGlynn K, Kim H, Miyatsuka T, Wang J, Nguyen V, Zhao S, Mavropoulos A, Abraham AG, O’Neill E, Ku GM, Cobb MH, Martin GR, and German MS. 2014. ‘Menin determines K-RAS proliferative outputs in endocrine cells’, J Clin Invest, 124: 4093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, and Marx SJ. 1997. ‘Positional cloning of the gene for multiple endocrine neoplasia-type 1’, Science, 276: 404–7. [DOI] [PubMed] [Google Scholar]
- Crabtree JS, Scacheri PC, Ward JM, Garrett-Beal L, Emmert-Buck MR, Edgemon KA, Lorang D, Libutti SK, Chandrasekharappa SC, Marx SJ, Spiegel AM, and Collins FS. 2001. ‘A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors’, Proc Natl Acad Sci U S A, 98: 1118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JS, Scacheri PC, Ward JM, McNally SR, Swain GP, Montagna C, Hager JH, Hanahan D, Edlund H, Magnuson MA, Garrett-Beal L, Burns AL, Ried T, Chandrasekharappa SC, Marx SJ, Spiegel AM, and Collins FS. 2003. ‘Of mice and MEN1: Insulinomas in a conditional mouse knockout’, Mol Cell Biol, 23: 6075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing H, and Davidoff LM 1927. The pathological findings in four autopsied cases of acromegaly with a discussion of their significance. (The Rockefeller Institute for Medical Research: New York: ). [Google Scholar]
- Debelenko LV, Brambilla E, Agarwal SK, Swalwell JI, Kester MB, Lubensky IA, Zhuang Z, Guru SC, Manickam P, Olufemi SE, Chandrasekharappa SC, Crabtree JS, Kim YS, Heppner C, Burns AL, Spiegel AM, Marx SJ, Liotta LA, Collins FS, Travis WD, and Emmert-Buck MR. 1997. ‘Identification of MEN1 gene mutations in sporadic carcinoid tumors of the lung’, Hum Mol Genet, 6: 2285–90. [DOI] [PubMed] [Google Scholar]
- Deschamps L, Handra-Luca A, O’Toole D, Sauvanet A, Ruszniewski P, Belghiti J, Bedossa P, and Couvelard A. 2006. ‘CD10 expression in pancreatic endocrine tumors: correlation with prognostic factors and survival’, Hum Pathol, 37: 802–8. [DOI] [PubMed] [Google Scholar]
- Erdheim J. 1903. ‘Zur normalen und pathologischen histologie der glandula thyreoides, parathyreoidea und hypophysis’, Beitr. Path. Anat, 33. [Google Scholar]
- Feng Z, Wang L, Sun Y, Jiang Z, Domsic J, An C, Xing B, Tian J, Liu X, Metz DC, Yang X, Marmorstein R, Ma X, and Hua X. 2017. ‘Menin and Daxx Interact to Suppress Neuroendocrine Tumors through Epigenetic Control of the Membrane MetalloEndopeptidase’, Cancer Res, 77: 401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam MP, Nimbalkar D, Sun L, Christov K, Ray D, Kaldis P, Liu X, and Kiyokawa H. 2015. ‘MEN1 tumorigenesis in the pituitary and pancreatic islet requires Cdk4 but not Cdk2’, Oncogene, 34: 932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobl AE, Berg M, Lopez-Egido JR, Oberg K, Skogseid B, and Westin G. 1999. ‘Menin represses JunD-activated transcription by a histone deacetylase-dependent mechanism’, Biochim Biophys Acta, 1447: 51–6. [DOI] [PubMed] [Google Scholar]
- Grembecka J, He S, Shi A, Purohit T, Muntean AG, Sorenson RJ, Showalter HD, Murai MJ, Belcher AM, Hartley T, Hess JL, and Cierpicki T. 2012. ‘Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia’, Nat Chem Biol, 8: 277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas JM, Li T, Nguyen H, Jensen D, Rauscher FJ 3rd, and Cowan KH. 1996. ‘Cell cycle regulation of BRCA1 messenger RNA in human breast epithelial cells’, Cell Growth Differ, 7: 717–23. [PubMed] [Google Scholar]
- Guru SC, Goldsmith PK, Burns AL, Marx SJ, Spiegel AM, Collins FS, and Chandrasekharappa SC. 1998. ‘Menin, the product of the MEN1 gene, is a nuclear protein’, Proc Natl Acad Sci U S A, 95: 1630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung B, Feng Z, and Hua X. 2013. ‘Menin directly represses Gli1 expression independent of canonical Hedgehog signaling’, Mol Cancer Res, 11: 1215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung B, Feng Z, Iwamoto DV, Thiel A, Jin G, Fan CM, Ng JM, Curran T, and Hua X. 2013. ‘Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome’, Cancer Res, 73: 2650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding B, Lemos MC, Reed AA, Walls GV, Jeyabalan J, Bowl MR, Tateossian H, Sullivan N, Hough T, Fraser WD, Ansorge O, Cheeseman MT, and Thakker RV. 2009. ‘Multiple endocrine neoplasia type 1 knockout mice develop parathyroid, pancreatic, pituitary and adrenal tumours with hypercalcaemia, hypophosphataemia and hypercorticosteronaemia’, Endocr Relat Cancer, 16: 1313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner C, Bilimoria KY, Agarwal SK, Kester M, Whitty LJ, Guru SC, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ, and Burns AL. 2001. ‘The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation’, Oncogene, 20: 4917–25. [DOI] [PubMed] [Google Scholar]
- Heppner C, Kester MB, Agarwal SK, Debelenko LV, Emmert-Buck MR, Guru SC, Manickam P, Olufemi SE, Skarulis MC, Doppman JL, Alexander RH, Kim YS, Saggar SK, Lubensky IA, Zhuang Z, Liotta LA, Chandrasekharappa SC, Collins FS, Spiegel AM, Burns AL, and Marx SJ. 1997. ‘Somatic mutation of the MEN1 gene in parathyroid tumours’, Nat Genet, 16: 375–8. [DOI] [PubMed] [Google Scholar]
- Huang J, Gurung B, Wan B, Matkar S, Veniaminova NA, Wan K, Merchant JL, Hua X, and Lei M. 2012. ‘The same pocket in menin binds both MLL and JUND but has opposite effects on transcription’, Nature, 482: 542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, Hayes DN, Shanmugam KS, Bhattacharjee A, Biondi CA, Kay GF, Hayward NK, Hess JL, and Meyerson M. 2004. ‘Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus’, Mol Cell, 13: 587–97. [DOI] [PubMed] [Google Scholar]
- Jiang X, Cao Y, Li F, Su Y, Li Y, Peng Y, Cheng Y, Zhang C, Wang W, and Ning G. 2014. ‘Targeting beta-catenin signaling for therapeutic intervention in MEN1-deficient pancreatic neuroendocrine tumours’, Nat Commun, 5: 5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA Jr., Vogelstein B, Kinzler KW, Hruban RH, and Papadopoulos N. 2011. ‘DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors’, Science, 331: 1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, and Kinzler KW. 2008. ‘Core signaling pathways in human pancreatic cancers revealed by global genomic analyses’, Science, 321: 1801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji H, Canaff L, Goltzman D, and Hendy GN. 1999. ‘Cell cycle regulation of menin expression’, Cancer Res, 59: 5097–101. [PubMed] [Google Scholar]
- Kaji H, Canaff L, Lebrun JJ, Goltzman D, and Hendy GN. 2001. ‘Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling’, Proc Natl Acad Sci U S A, 98: 3837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee JE, Cho EJ, Liu JO, and Youn HD. 2003. ‘Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex’, Cancer Res, 63: 6135–9. [PubMed] [Google Scholar]
- La P, Desmond A, Hou Z, Silva AC, Schnepp RW, and Hua X. 2006. ‘Tumor suppressor menin: the essential role of nuclear localization signal domains in coordinating gene expression’, Oncogene, 25: 3537–46. [DOI] [PubMed] [Google Scholar]
- Larsson C, Skogseid B, Oberg K, Nakamura Y, and Nordenskjold M. 1988. ‘Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma’, Nature, 332: 85–7. [DOI] [PubMed] [Google Scholar]
- Lin W, Francis JM, Li H, Gao X, Pedamallu CS, Ernst P, and Meyerson M. 2016. ‘Kmt2a cooperates with menin to suppress tumorigenesis in mouse pancreatic islets’, Cancer Biol Ther, 17: 1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines KE, Vas Nunes RP, Frost M, Yates CJ, Stevenson M, and Thakker RV. 2017. ‘A MEN1 pancreatic neuroendocrine tumour mouse model under temporal control’, Endocr Connect, 6: 232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler KA, Biondi CA, Gartside M, Waring P, Stark M, Serewko-Auret MM, Muller HK, Hayward NK, and Kay GF. 2007. ‘Broad tumor spectrum in a mouse model of multiple endocrine neoplasia type 1’, Int J Cancer, 120: 259–67. [DOI] [PubMed] [Google Scholar]
- Lu J, Herrera PL, Carreira C, Bonnavion R, Seigne C, Calender A, Bertolino P, and Zhang CX. 2010. ‘Alpha cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development’, Gastroenterology, 138: 1954–65. [DOI] [PubMed] [Google Scholar]
- Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, Hunger F, Pasquinelli S, Speel EJ, and Perren A. 2014. ‘Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors’, Gastroenterology, 146: 453–60 e5. [DOI] [PubMed] [Google Scholar]
- Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, Schnepp RW, Krankel C, Livolsi VA, Gibbs D, Hua X, Roeder RG, Meyerson M, and Hess JL. 2005. ‘Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors’, Proc Natl Acad Sci U S A, 102: 749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterbauer-Hohendanner G, and Mannhalter C. 2004. ‘The biological and clinical significance of MLL abnormalities in haematological malignancies’, Eur J Clin Invest, 34 Suppl 2: 12–24. [DOI] [PubMed] [Google Scholar]
- Muhammad AB, Xing B, Liu C, Naji A, Ma X, Simmons RA, and Hua X. 2017. ‘Menin and PRMT5 suppress GLP1 receptor transcript and PKA-mediated phosphorylation of FOXO1 and CREB’, Am J Physiol Endocrinol Metab: ajpendo 00241 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai MJ, Chruszcz M, Reddy G, Grembecka J, and Cierpicki T. 2011. ‘Crystal structure of menin reveals binding site for mixed lineage leukemia (MLL) protein’, J Biol Chem, 286: 31742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Law AC, Dor Y, and Melton DA. 2005. ‘Beta-catenin is essential for pancreatic acinar but not islet development’, Development, 132: 4663–74. [DOI] [PubMed] [Google Scholar]
- Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, and Kim SK. 2007. ‘Wnt signaling regulates pancreatic beta cell proliferation’, Proc Natl Acad Sci U S A, 104: 6247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, Lawlor RT, Johns AL, Miller DK, Mafficini A, Rusev B, Scardoni M, Antonello D, Barbi S, Sikora KO, Cingarlini S, Vicentini C, McKay S, Quinn MC, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, McLean S, Nourse C, Nourbakhsh E, Wilson PJ, Anderson MJ, Fink JL, Newell F, Waddell N, Holmes O, Kazakoff SH, Leonard C, Wood S, Xu Q, Nagaraj SH, Amato E, Dalai I, Bersani S, Cataldo I, Dei Tos AP, Capelli P, Davi MV, Landoni L, Malpaga A, Miotto M, Whitehall VL, Leggett BA, Harris JL, Harris J, Jones MD, Humphris J, Chantrill LA, Chin V, Nagrial AM, Pajic CJ Scarlett, Pinho A, Rooman I, Toon C, Wu J, Pinese M, Cowley M, Barbour A, Mawson A, Humphrey ES, Colvin EK, Chou A, Lovell JA, Jamieson NB, Duthie F, Gingras MC, Fisher WE, Dagg RA, Lau LM, Lee M, Pickett HA, Reddel RR, Samra JS, Kench JG, Merrett ND, Epari K, Nguyen NQ, Zeps, Falconi M, Simbolo M, Butturini G, Van Buren G, Partelli S, Fassan M, Initiative Australian Pancreatic Cancer Genome, Khanna KK, Gill AJ, Wheeler DA, Gibbs RA, Musgrove EA, Bassi C, Tortora G, Pederzoli P, Pearson JV, Waddell N, Biankin AV, and Grimmond SM. 2017. ‘Whole-genome landscape of pancreatic neuroendocrine tumours’, Nature, 543: 65–71. [DOI] [PubMed] [Google Scholar]
- Schnepp RW, Chen YX, Wang H, Cash T, Silva A, Diehl JA, Brown E, and Hua X. 2006. ‘Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells’, Cancer Res, 66: 5707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, and Karin M. 2001. ‘AP-1 in cell proliferation and survival’, Oncogene, 20: 2390–400. [DOI] [PubMed] [Google Scholar]
- Shen HC, He M, Powell A, Adem A, Lorang D, Heller C, Grover AC, Ylaya K, Hewitt SM, Marx SJ, Spiegel AM, and Libutti SK. 2009. ‘Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1’, Cancer Res, 69: 1858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, Ylaya K, Pechhold K, Wilson A, Adem A, Hewitt SM, and Libutti SK. 2010. ‘Multiple endocrine neoplasia type 1 deletion in pancreatic alpha-cells leads to development of insulinomas in mice’, Endocrinology, 151: 4024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C, Parente F, Piehl F, Farnebo F, Quincey D, Silins G, Bergman L, Carle GF, Lemmens I, Grimmond S, Xian CZ, Khodei S, Teh BT, Lagercrantz J, Siggers P, Calender A, Van de Vem V, Kas K, Weber G, Hayward N, Gaudray P, and Larsson C. 1998. ‘Characterization of the mouse Men1 gene and its expression during development’, Oncogene, 17: 2485–93. [DOI] [PubMed] [Google Scholar]
- Thiel AT, Blessington P, Zou T, Feather D, Wu X, Yan J, Zhang H, Liu Z, Ernst P, Koretzky GA, and Hua X. 2010. ‘MLL-AF9-induced leukemogenesis requires coexpression of the wild-type Mll allele’, Cancer Cell, 17: 148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel AT, Feng Z, Pant DK, Chodosh LA, and Hua X. 2013. ‘The trithorax protein partner menin acts in tandem with EZH2 to suppress C/EBPalpha and differentiation in MLL-AF9 leukemia’, Haematologica, 98: 918–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermer P. 1954. ‘Genetic aspects of adenomatosis of endocrine glands’, Am J Med, 16: 363–71. [DOI] [PubMed] [Google Scholar]
- Wiedemann T, and Pellegata NS. 2016. ‘Animal models of multiple endocrine neoplasia’, Mol Cell Endocrinol, 421: 49–59. [DOI] [PubMed] [Google Scholar]
- Yaguchi H, Ohkura N, Tsukada T, and Yamaguchi K. 2002. ‘Menin, the multiple endocrine neoplasia type 1 gene product, exhibits GTP-hydrolyzing activity in the presence of the tumor metastasis suppressor nm23’, J Biol Chem, 277: 38197–204. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, and Cleary ML. 2005. ‘The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis’, Cell, 123: 207–18. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, Herr W, and Cleary ML. 2004. ‘Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression’, Mol Cell Biol, 24: 5639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BD, Hanson RD, Hess JL, Horning SE, and Korsmeyer SJ. 1998. ‘MLL, a mammalian trithorax-group gene, functions as a transcriptional maintenance factor in morphogenesis’, Proc Natl Acad Sci U S A, 95: 10632–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Vortmeyer AO, Pack S, Huang S, Pham TA, Wang C, Park WS, Agarwal SK, Debelenko LV, Kester M, Guru SC, Manickam P, Olufemi SE, Yu F, Heppner C, Crabtree JS, Skarulis MC, Venzon DJ, Emmert-Buck MR, Spiegel AM, Chandrasekharappa SC, Collins FS, Burns AL, Marx SJ, Lubensky IA, and et al. 1997. ‘Somatic mutations of the MEN1 tumor suppressor gene in sporadic gastrinomas and insulinomas’, Cancer Res, 57: 4682–6. [PubMed] [Google Scholar]