Abstract
Background
BNT162b2 mRNA and ChAdOx1 nCOV-19 adenoviral vector vaccines have been rapidly rolled out in the UK from December, 2020. We aimed to determine the factors associated with vaccine coverage for both vaccines and documented the vaccine effectiveness of the BNT162b2 mRNA vaccine in a cohort of health-care workers undergoing regular asymptomatic testing.
Methods
The SIREN study is a prospective cohort study among staff (aged ≥18 years) working in publicly-funded hospitals in the UK. Participants were assigned into either the positive cohort (antibody positive or history of infection [indicated by previous positivity of antibody or PCR tests]) or the negative cohort (antibody negative with no previous positive test) at the beginning of the follow-up period. Baseline risk factors were collected at enrolment, symptom status was collected every 2 weeks, and vaccination status was collected through linkage to the National Immunisations Management System and questionnaires. Participants had fortnightly asymptomatic SARS-CoV-2 PCR testing and monthly antibody testing, and all tests (including symptomatic testing) outside SIREN were captured. Data cutoff for this analysis was Feb 5, 2021. The follow-up period was Dec 7, 2020, to Feb 5, 2021. The primary outcomes were vaccinated participants (binary ever vacinated variable; indicated by at least one vaccine dose recorded by at least one of the two vaccination data sources) for the vaccine coverage analysis and SARS-CoV-2 infection confirmed by a PCR test for the vaccine effectiveness analysis. We did a mixed-effect logistic regression analysis to identify factors associated with vaccine coverage. We used a piecewise exponential hazard mixed-effects model (shared frailty-type model) using a Poisson distribution to calculate hazard ratios to compare time-to-infection in unvaccinated and vaccinated participants and estimate the impact of the BNT162b2 vaccine on all PCR-positive infections (asymptomatic and symptomatic). This study is registered with ISRCTN, number ISRCTN11041050, and is ongoing.
Findings
23 324 participants from 104 sites (all in England) met the inclusion criteria for this analysis and were enrolled. Included participants had a median age of 46·1 years (IQR 36·0–54·1) and 19 692 (84%) were female; 8203 (35%) were assigned to the positive cohort at the start of the analysis period, and 15 121 (65%) assigned to the negative cohort. Total follow-up time was 2 calendar months and 1 106 905 person-days (396 318 vaccinated and 710 587 unvaccinated). Vaccine coverage was 89% on Feb 5, 2021, 94% of whom had BNT162b2 vaccine. Significantly lower coverage was associated with previous infection, gender, age, ethnicity, job role, and Index of Multiple Deprivation score. During follow-up, there were 977 new infections in the unvaccinated cohort, an incidence density of 14 infections per 10 000 person-days; the vaccinated cohort had 71 new infections 21 days or more after their first dose (incidence density of eight infections per 10 000 person-days) and nine infections 7 days after the second dose (incidence density four infections per 10 000 person-days). In the unvaccinated cohort, 543 (56%) participants had typical COVID-19 symptoms and 140 (14%) were asymptomatic on or 14 days before their PCR positive test date, compared with 29 (36%) with typical COVID-19 symptoms and 15 (19%) asymptomatic in the vaccinated cohort. A single dose of BNT162b2 vaccine showed vaccine effectiveness of 70% (95% CI 55–85) 21 days after first dose and 85% (74–96) 7 days after two doses in the study population.
Interpretation
Our findings show that the BNT162b2 vaccine can prevent both symptomatic and asymptomatic infection in working-age adults. This cohort was vaccinated when the dominant variant in circulation was B1.1.7 and shows effectiveness against this variant.
Funding
Public Health England, UK Department of Health and Social Care, and the National Institute for Health Research.
Research in context.
Evidence before this study
We searched PubMed and medRxiv for articles in English published between Oct 1, 2020, and Feb 20, 2021, using the keywords (vaccin* OR immunis* OR immuniz* OR chadox1 OR chadox1 ncov-19 OR azd1222 OR bnt162b2) AND (coronavirus OR sars-cov-2 OR sarscov2 OR Severe Acute Respiratory Syndrome Coronavirus 2 OR COVID OR COVID-19) AND (effectiveness OR vaccine effectiveness OR VE), limited to “human” studies. We selected articles that included asymptomatic and symptomatic SARS-CoV-2 results after vaccination. We found one article for ChAdOx1, which stated that it reduced all (symptomatic or asymptomatic) infection by 51·9% (95% CI 42·0–60·1). Three studies from Israel showed that those who attended symptomatic testing had reduced infections 2 weeks post-vaccination with the BNT162b2 vaccine); a single cohort study of health-care workers in Israel reported vaccine effectiveness of 75% (95% CI 72–84) against all SARS-CoV-2 infections and 85% (71–92) against symptomatic infection from days 15–28 after the first dose of the BNT162b2 vaccine. A large matched control study based on data from the mass vaccination campaign in Israel estimated vaccine effectiveness of 46% (95% CI 40–51) against infection and 57% (50–63) against symptomatic infection after one dose. No data were available on asymptomatic infection through routinely collected swabs asymptomatic testing for the BNT162b2 vaccine.
Added value of this study
The SIREN study is a large, established cohort study in health-care workers that enables accurate measurement of asymptomatic and symptomatic infection rates in the vaccinated and unvaccinated population. It measured the impact of a single dose of vaccine over the first 8-week period in a cohort of health-care workers with high coverage of the BNT62b2 vaccine. We have estimated the vaccine effectiveness against all (symptomatic and asymptomatic) infection for the BNT162b2 vaccine to be 70% 21 days after the first dose, which increased to 85% 7 days after the second dose.
Implications of all the available evidence
Our findings provide strong evidence that vaccinating working-age adults will substantially reduce SARS-CoV-2 infection. Our findings after 2 months of vaccine introduction show that the BNT62b2 vaccine protects against both symptomatic and asymptomatic infection, and, therefore, might help to reduce transmission of infection in the population. However, conclusions on this prevention of transmission require more follow-up time and stratified analysis. Vaccination does not eliminate infection risk completely and, therefore, personal protective equipment, non-pharmaceutical interventions, and regular asymptomatic testing will need to be continued until prevalence of SARS-CoV-2 is extremely low to reduce the risk of transmission in health-care settings.
Introduction
Since WHO declared the emergence of coronavirus disease 2019 (COVID-19) a pandemic on March 11, 2020, over 2·4 million people have died worldwide,1 including over 120 000 people in the UK.2 There has been an unprecedented international effort by private and public institutions to develop a vaccine against its causative agent, the Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).3 In less than a year, three COVID-19 vaccine candidates have been granted Emergency Use Authorisation by the UK Medicines and Healthcare products Regulatory Agency,4 with several more in the development pipeline. The BNT162b2 mRNA (Pfizer-BioNTech) and ChAdOx1 nCoV-19 adenoviral (AZD1222; Oxford-AstraZeneca) COVID-19 vaccines were approved on Dec 2, 2020, and Dec 30, 2020, respectively, based on interim analyses from phase 3 randomised controlled trials,5, 6 and were deployed for use in the UK within 7 days of authorisation.
Following advice from the Joint Committee on Vaccination and Immunisation (JCVI), the UK Government selected a vaccination strategy with the aim of rapidly reducing hospitalisations, severe outcomes, and preventable deaths from COVID-19.7 The initial phase targeted individuals at high-risk of severe COVID-19, such as care home residents and their carers, people aged 80 years and over, and front-line health-care workers, recognising this group's particular high exposure and potential role in transmission. On December 30, the JCVI published their recommendation to delay the second dose of the deployed coronavirus vaccines by up to 12 weeks with the aim of optimising the public health impact of the vaccination campaign in the population by doubling the number of people who would receive the first dose.8 By Feb 19, 2021, more than 17·2 million people (25% of the population) in the UK had been vaccinated with their first dose.9 However, population-level vaccine effectiveness studies are needed to assess the real-world impact of coronavirus vaccination and inform developments of the public health policy.
The SARS-CoV-2 Immunity and Reinfection Evaluation (SIREN) Study is a large, multicentre prospective cohort study of health-care workers and support staff in publicly-funded National Health Service (NHS) hospitals in the UK.10 SIREN initially investigated the effect of previous infection on protection against reinfection and was amended to investigate COVID-19 vaccine effectiveness in January, 2021.
We aimed to describe the factors associated with both BNT162b2 and ChAdOx1 nCoV-19 vaccine coverage and early vaccine effectiveness of the BNT162b2 vaccine against all (asymptomatic and symptomatic) infection in this large-scale cohort of health-care workers in the UK. This Article presents an interim analysis of the primary study objective, with data collected from English hospitals up to Feb 5, 2021. Testing data from Scotland and Northern Ireland were not available at data cutoff. Wales began recruitment after Dec 7, 2020.
Methods
Study design and participants
The SIREN study was a prospective cohort study among staff working in publicly-funded NHS hospitals across the UK.
Health-care workers, support staff, and administrative staff (aged ≥18 years) working at hospital sites participating in SIREN who could provide written informed consent and anticipated remaining engaged in follow-up for 12 months were eligible to join SIREN. Participants were excluded from this analysis if they had no PCR tests after Dec 7, 2020, or had insufficient PCR and antibody data to complete cohort assignment on Dec 7, 2020, which included any participants who enrolled after this date.
Participants were assigned into either the positive cohort (antibody-positive or history of infection [indicated by previous positivity of antibody or PCR tests]) or the negative cohort (antibody negative with no previous positive test) at the beginning of the follow-up period (Dec 7, 2020).
The study protocol was approved by the Berkshire Research Ethics Committee on May 22, 2020; the vaccine amendment was approved on Dec 23, 2020. The SIREN protocol is described elsewhere.11
Data sources and measurement
Vaccination data were obtained directly from participants completing the enrolment and follow-up questionnaires and from linkage on personal identifiable information (NHS number, surname, date of birth, and postcode) to the National Immunisation Management System, the registry of COVID-19 vaccination in England.
SIREN participants had asymptomatic PCR testing (anterior nasal swabs or combined nose and oropharyngeal swabs) every 14 days and monthly antibody testing at their site of enrolment, with a variety of assays used across sites. As per government guidelines, hospitals introduced twice weekly asymptomatic testing using a lateral flow device, Innova SARS-CoV-2 Antigen Rapid Qualitative Test (Innova),12 to all front-line health-care workers for twice weekly asymptomatic testing in November, 2020. All positive lateral flow tests were confirmed by PCR. Participant consent was given for the release of all SARS-CoV-2 PCR and antibody test results before or after enrolment to the study team through the Public Health England (PHE) national laboratory testing surveillance system. The SIREN Structured Query Language database runs automated data linkage with the laboratory surveillance system daily to extract new positive and negative test results.
Participants were requested to complete online questionnaires at enrolment and every 14 days, capturing data on demographics, symptoms, testing, and exposures (household, community, and occupational). Index of Multiple Deprivation (IMD), which is a measure of neighbourhood relative deprivation calculated by the Office of National Statistics, was obtained through linkage with participant postcodes.
Typical COVID-19 symptoms were fever, cough, or change or loss of taste or smell. Participants were recorded as having other symptoms if they reported any of the following: shortness of breath, sore throat, runny nose, headache, muscle aches, extreme fatigue, diarrhoea, nausea or vomiting, or small itchy red patches on fingers or toes, on the follow-up questionnaire with a symptom onset date within 14 days before or after the PCR positive sample date.
We extracted data from all sources on Feb 8, 2021.
Bias reduction
We collected data on potential confounders, including site and participant demographics, to enable adjusted analysis. We restricted our analysis to one manufacturer only, where sufficient follow-up time had accrued. To avoid misclassification of exposure, if a participant had an unreliable date of a second dose (eg, a second dose administered before a first dose or administered less than 19 days after the first dose) their follow-up time was censored at the date of the suspect second dose. We used the sample date of a PCR positive result as the event date, which might have introduced some misclassification of vaccination status relative to infection or onset in the period shortly after vaccination. This possibility informed our decision to calculate cumulative vaccine effectiveness after suitable intervals (21 days post-first dose and 7 days post-second dose) to focus on infections acquired since vaccination after a sufficient interval for biological protection.
Person-time at risk
Follow-up for all participants started on Dec 7, 2020, the day before vaccine roll-out began, with all participants having at least 1 day of follow-up unvaccinated. Participants moved from unvaccinated to vaccinated within their assigned cohort on the date of their first vaccination dose. Participants contributed person-time to follow-up until either an event of interest (ie, a new positive PCR result in the negative cohort or a reinfection in the positive cohort), the date of the suspect second dose for those with an unreliable date of second dose, the date of their first dose for those vaccinated with the ChAdOx1 vaccine, or the censored date. We defined the end of follow-up in those who were not positive cases as the date of a negative test or Feb 5, 2021, if the test was after this date, to avoid immortal time bias. Because symptomatic testing was done at any time during the presentation of symptoms, the most recent days could be biased towards symptomatic testing; therefore, the end of follow-up was defined at a date 2 days before the last date samples were available.
Outcomes
The primary outcome for the vaccine coverage analysis was the binary ever vaccinated variable. Participants were categorised as ever vaccinated if they had at least one vaccine dose recorded between Dec 8, 2020, and Feb 5, 2021, from at least one of the two vaccination data sources available. Data on vaccination date, manufacturer, and batch number was available for each dose.
The primary outcome for the vaccine effectiveness analysis was SARS-CoV-2 infection confirmed by a PCR test, defined as a new PCR positive result during follow-up for the negative cohort and a reinfection during the follow-up in the positive cohort, irrespective of symptom status (appendix 1 p 2).10
Infections were described by their symptom status 14 days before and 14 days either side of their PCR-positive test date.
Statistical analysis
Before vaccine introduction, we calculated the precision of effectiveness estimates on an estimated cohort size of 40 000 participants, with 65% seronegativity at baseline, coverage averaging 75% in the follow-up period, and incidence in the follow-up period ranging from 0·5% to 5%. Precision estimates around effectiveness of 60% and 90% gave 95% CIs ranging from the widest for a vaccine effectiveness of 60% (95% CI 39–74) to the narrowest for a vaccine effectiveness of 90% (88–92).
For vaccine coverage, we used mixed-effect multivariable logistic regression models (with hospital site as a random effect) to assess confounding between demographic and occupational risk factors on the primary outcome of ever vaccinated. We used a backwards stepwise approach, removing variables from the model sequentially, with those with the least effect at univariable analysis removed first. We tested goodness of fit (likelihood ratio tests) after each change. Only the variables with strong evidence of an association with vaccine coverage were retained in the final model.
We used a piecewise exponential hazard mixed-effects model (shared frailty-type model) and Poisson distribution to calculate hazard ratios to compare time-to-infection in unvaccinated and vaccinated participants to estimate the impact of the BNT162b2 vaccine on infection (including asymptomatic and symptomatic as the primary outcome). A specific characteristic of the impact of the BNT162b2 vaccine on infection in this study is that the main covariate of interest (vaccination) changes as time elapses and the effect of vaccine changes over follow-up time. The grouping on or smoothing over vaccination day are two most applied approaches to handle the time-dependent covariates. We grouped on the time to vaccination and divided follow-up time into unvaccinated and 11 post-vaccination time intervals. For a piecewise exponential hazard approach, we divided the follow-up time into as many intervals as there were unique failure times with each starting at (just after) a failure time and ending at (just after) the next failure time.13 Holford showed that the likelihood of the piecewise exponential survival model is proportional to that of Poisson model when the data are expanded appropriately.14 We fitted the models by Poisson regression with a log link, using COVID-19 infection as response, log of exposure times as an offset, and dummies for the time intervals as explanatory variables to allow for different piecewise constant hazards.14 The proportional hazards in this model is an assumption. However, most time intervals based on unique calendar times of events are of 1 day in length, which should provide sufficient flexibility to model how the hazard varies over time. Similar results were obtained by approximating the baseline hazard with an orthogonal polynomial of degree 6 of the follow-up time. This alternative approach is useful if there is a very large number of risk sets.
We then extended the piecewise exponential hazard model by introducing the NHS hospital site as a random effect into the linear predictor to account for the extra variation and associated correlation that was not explained by risk and covariates variables. Some individuals are more susceptible to the COVID-19 infection than can be explained by their observed covariates (referred to as frailty here). Therefore, the piecewise exponential hazard mixed-effects model was further extended by including individual within the site as an addition random effect. The results (data not shown) did not support heterogeneity among individuals after controlling for site effect; therefore, our final model does not include individuals. The hospital trust random effect was assumed to be normal, and this implies that frailty (exp(hospital trusts)) distribution was log-normal. The model fitting approach also provided estimates of the baseline hazard rates (appendix 1 p 15). The fixed covariates in the model were age, ethnicity, comorbidities, region, job role, frequency of COVID-19 patient contact, patient-facing role, and workplace setting. The STATA code for the models of vaccine effectiveness is provided in appendix 1 (p 3). There was strong support for a significant variation between hospital trusts by both the likelihood ratio and Wald tests. The final model resulted in a mean χ2 estimate of 1·04. Since the mean of χ2 variable is its degree of freedom, comparing the mean χ2 with its mean value 1 provides a quick check model. The estimated χ2 value of 1·04 is not too far from 1, which implies that there is no evidence against the final model.
We calculated hazard ratios from 21 days after first dose and 7 days after second dose using a weighted average method, the point at which an immunological response to the vaccine dose should have been provoked. We calculated vaccine effectiveness as 1–adjusted hazard ratio (vaccinated vs unvaccinated).
We ran three models on different cohorts within the study population. The main model included the full study population and adjusted for cohort assignment. We also ran models on the two cohorts separately to provide estimates of vaccine effectiveness in the susceptible population (negative cohort) and the positive cohort with natural immunity following previous SARS-CoV-2 infection.
All participants meeting the inclusion criteria were included in the analysis, regardless of their testing frequency, with data censored accordingly. The category “unknown” was introduced for variables with missing values, such as symptom status or Index of Multiple Deprivation.
We used STATA software (version 15.1; StataCorp LLC, College Station, TX, USA) for all analyses.
This study is registered with ISRCTN, number ISRCTN11041050. The study follows the Strengthening the Reporting of Observational studies in Epidemiology guidelines.15
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
By Dec 7, 2020, 29 378 participants were enrolled and maintained in SIREN for the England cohort; 23 324 met the inclusion criteria and were included in this analysis from 104 hospitals, with a median age of 46·1 years (IQR 36·0–54·1). 8203 (35%) participants were assigned to the positive cohort and 15 121 (65%) were assigned to the negative cohort (table 1 ).
Table 1.
Characteristics of study participants and factors associated with vaccine coverage in multivariable logistic regression analysis (n=23 324)
| Not vaccinated | Vaccinated | OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Previous COVID-19 infection* | ||||||
| Negative | 1405 (9·3%) | 13 716 (90·7%) | 1 (ref) | <0·0001 | .. | <0·0001 |
| Positive | 1278 (15·6%) | 6925 (84·4%) | 0·56 (0·51–0·6) | .. | 0·59 (0·54–0·64) | .. |
| Gender | ||||||
| Male | 333 (9·2%) | 3270 (90·8%) | 1 (ref) | <0·0001 | .. | <0·0001 |
| Female | 2346 (11·9%) | 17 346 (88·1%) | 0·75 (0·67–0·85) | .. | 0·72 (0·63–0·82) | .. |
| Other | 4 (13·8%) | 25 (86·2%) | 0·64 (0·22–1·84) | .. | 0·94 (0·30–2·93) | .. |
| Age, years | ||||||
| <25 | 136 (16·1%) | 711 (83·9%) | 1 (ref) | <0·0001 | .. | <0·0001 |
| 25–34 | 886 (19·7%) | 3614 (80·3%) | 0·78 (0·64–0·95) | .. | 0·78 (0·64–0·96) | .. |
| 35–44 | 650 (11·5%) | 4998 (88·5%) | 1·47 (1·20–1·80) | .. | 1·45 (1·18–1·79) | .. |
| 45–54 | 600 (8·4%) | 6566 (91·6%) | 2·09 (1·71–2·56) | .. | 2·22 (1·80–2·73) | .. |
| 55–64 | 382 (8·0%) | 4412 (92·0%) | 2·21 (1·79–2·73) | .. | 2·31 (1·85–2·87) | .. |
| ≥65 | 29 (7·9%) | 340 (92·1%) | 2·24 (1·47–3·42) | .. | 2·19 (1·42–3·37) | .. |
| Ethnicity | ||||||
| White | 2119 (10·4%) | 18 305 (89·6%) | 1 (ref) | <0·0001 | .. | <0·0001 |
| Mixed race | 69 (19·4%) | 287 (80·6%) | 0·48 (0·37–0·63) | .. | 0·56 (0·43–0·75) | .. |
| Asian | 250 (15·8%) | 1337 (84·2%) | 0·62 (0·54–0·71) | .. | 0·65 (0·56–0·76) | .. |
| Black | 162 (34·9%) | 302 (65·1%) | 0·22 (0·18–0·26) | .. | 0·26 (0·21–0·32) | .. |
| Chinese | 17 (12·7%) | 117 (87·3%) | 0·80 (0·48–1·33) | .. | 0·73 (0·43–1·25) | .. |
| Other ethnic group | 56 (17·8%) | 258 (82·2%) | 0·53 (0·40–0·71) | .. | 0·54 (0·39–0·73) | .. |
| Prefer not to say | 10 (22·2%) | 35 (77·8%) | 0·41 (0·20–0·82) | .. | 0·30 (0·14–0·65) | .. |
| Pre-existing medical condition† | ||||||
| No medical condition | 2060 (11·8%) | 15 390 (88·2%) | 1 (ref) | 0·0710 | .. | .. |
| Immunosuppression | 56 (11·7%) | 421 (88·3%) | 1·01 (0·76–1·33) | .. | .. | .. |
| Chronic respiratory conditions | 305 (10·4%) | 2619 (89·6%) | 1·15 (1·01–1·31) | .. | .. | .. |
| Chronic non-respiratory conditions | 262 (10·6%) | 2211 (89·4%) | 1·13 (0·99–1·29) | .. | .. | .. |
| Household size | ||||||
| Just you | 283 (12·1%) | 2063 (87·9%) | 1 (ref) | <0·0001 | .. | .. |
| Two to four | 2080 (11·2%) | 16 494 (88·8%) | 1·09 (0·95–1·24) | .. | .. | .. |
| More than four | 297 (12·7%) | 2037 (87·3%) | 0·94 (0·79–1·12) | .. | .. | .. |
| Prefer not to say | 23 (32·9%) | 47 (67·1%) | 0·28 (0·17–0·47) | .. | .. | .. |
| Index of Multiple Deprivation | ||||||
| 5 (least deprived) | 507 (9·0%) | 5107 (91·0%) | 1 (ref) | <0·0001 | .. | <0·0001 |
| 4 | 534 (9·7%) | 4947 (90·3%) | 0·92 (0·81–1·04) | .. | 1·02 (0·89–1·16) | .. |
| 3 | 591 (11·1%) | 4731 (88·9%) | 0·79 (0·70–0·90) | .. | 0·92 (0·81–1·05) | .. |
| 2 | 577 (14·1%) | 3512 (85·9%) | 0·60 (0·53–0·69) | .. | 0·78 (0·69–0·90) | .. |
| 1 (most deprived) | 436 (16·6%) | 2198 (83·4%) | 0·50 (0·44–0·57) | .. | 0·75 (0·65–0·87) | .. |
| Unknown | 38 (20·7%) | 146 (79·3%) | 0·38 (0·26–0·55) | .. | 0·53 (0·36–0·78) | .. |
| Region | ||||||
| Yorkshire and the Humber | 239 (11·5%) | 1832 (88·5%) | 1 (ref) | <0·0001 | .. | 0·4171 |
| East Midlands | 248 (10·1%) | 2200 (89·9%) | 1·16 (0·96–1·40) | .. | 1·14 (0·80–1·62) | .. |
| East of England | 299 (10·8%) | 2462 (89·2%) | 1·07 (0·90–1·29) | .. | 1·12 (0·80–1·56) | .. |
| London | 444 (15·5%) | 2416 (84·5%) | 0·71 (0·60–0·84) | .. | 1·00 (0·73–1·37) | .. |
| North East | 53 (9·7%) | 496 (90·3%) | 1·22 (0·89–1·67) | .. | 1·31 (0·76–2·26) | .. |
| North West | 350 (12·7%) | 2403 (87·3%) | 0·90 (0·75–1·07) | .. | 0·96 (0·70–1·32) | .. |
| South East | 247 (9·1%) | 2462 (90·9%) | 1·30 (1·08–1·57) | .. | 1·24 (0·91–1·71) | .. |
| South West | 464 (9·7%) | 4335 (90·3%) | 1·22 (1·03–1·44) | .. | 1·11 (0·82–1·49) | .. |
| West Midlands | 339 (14·3%) | 2035 (85·7%) | 0·78 (0·66–0·93) | .. | 0·87 (0·63–1·19) | .. |
| Staff group | ||||||
| Administrative or executive | 377 (10·5%) | 3223 (89·5%) | 1 (ref) | <0·0001 | .. | <0·0001 |
| Nursing or health-care assistant | 1275 (13·0%) | 8551 (87·0%) | 0·78 (0·69–0·89) | .. | 0·96 (0·84–1·09) | .. |
| Doctor | 189 (7·5%) | 2332 (92·5%) | 1·44 (1·20–1·73) | .. | 1·82 (1·49–2·22) | .. |
| Midwife | 88 (15·5%) | 478 (84·5%) | 0·64 (0·49–0·82) | .. | 0·74 (0·57–0·97) | .. |
| Specialist staff | 156 (11%) | 1262 (89·0%) | 0·95 (0·78–1·15) | .. | 1·28 (1·04–1·57) | .. |
| Estates, porters, or security | 38 (17·1%) | 184 (82·9%) | 0·57 (0·39–0·82) | .. | 0·61 (0·42–0·90) | .. |
| Pharmacist | 35 (10·0%) | 316 (90·0%) | 1·06 (0·73–1·52) | .. | 1·59 (1·09–2·33) | .. |
| Health-care scientist | 91 (11·1%) | 729 (88·9%) | 0·94 (0·74–1·19) | .. | 1·16 (0·90–1·49) | .. |
| Other | 434 (10·8%) | 3566 (89·1%) | 0·96 (0·83–1·11) | .. | 1·13 (0·97–1·31) | .. |
| Occupation setting‡ | ||||||
| Offices and laboratory (lower risk) | 932 (11·2%) | 7384 (88·8%) | 1 (ref) | <0·0001 | .. | .. |
| Patient facing non-clinical | 112 (12·9%) | 757 (87·1%) | 0·85 (0·69–1·05) | .. | .. | .. |
| Outpatient | 469 (11·6%) | 3590 (88·4%) | 0·97 (0·86–1·09) | .. | .. | .. |
| Inpatient wards and ambulance | 498 (14·0%) | 3069 (86·0%) | 0·78 (0·69–0·87) | .. | .. | .. |
| Intensive care (higher risk) | 157 (13·0%) | 1053 (87·0%) | 0·85 (0·71–1·01) | .. | .. | .. |
| Other | 515 (9·7%) | 4788 (90·3%) | 1·17 (1·05–1·31) | .. | .. | .. |
| Contact with patients or working in patient-facing areas | ||||||
| No | 330 (10·1%) | 2940 (89·9%) | 1 (ref) | 0·0056 | .. | .. |
| Yes | 2353 (11·7%) | 17 701 (88·3%) | 0·84 (0·75–0·95) | .. | .. | .. |
| Frequency of contact with COVID-19 patients in the workplace | ||||||
| Never | 793 (9·6%) | 7484 (90·4%) | 1 (ref) | <0·0001 | .. | .. |
| Daily | 871 (15·4%) | 4777 (84·6%) | 0·58 (0·52–0·64) | .. | .. | .. |
| Weekly | 448 (10·8%) | 3688 (89·2%) | 0·87 (0·77–0·99) | .. | .. | .. |
| Monthly | 239 (11·3%) | 1883 (88·7%) | 0·83 (0·72–0·97) | .. | .. | .. |
| Less than monthly | 332 (10·6%) | 2809 (89·4%) | 0·90 (0·78–1·03) | .. | .. | .. |
Data are n (%) unless otherwise stated. The multivariable model included and adjusted for site (as a random effect) and fixed effects: prior infection status, age, gender, ethnicity, Index of Multiple Deprivation, region, and staff group. OR=odds ratio.
Determined by a positive antibody or PCR test as of Dec 7, 2020.
Pre-existing medical condition categories: immunosuppression (cancers affecting the immune system in the past 5 years, rheumatological or autoimmune conditions and on immunosuppressive therapy, organ or bone marrow transplantation, and asplenia), Chronic respiratory conditions (asthma and chronic respiratory disease), chronic non-respiratory conditions (diabetes, obesity, chronic heart disease, chronic kidney disease, chronic liver disease, other cancers, dementia, other neurological disorder, and HIV) and no reported medical conditions. If participants reported multiple conditions, they were assigned to a category dependent on which condition was considered by the study team to be the most severe.
Occupation setting categories were: 1: office, laboratory, or estates; 2: community pharmacy, hospital pharmacy, communal areas open to the public, or mobile across areas (porters); 3: outpatient, radiology, day ward, general practice, or renal dialysis unit; 4: inpatient ward, theatres, emergency department, maternity unit or labour ward, or ambulance; 5: intensive care; and 6: other.
Although recruitment of participants from Scotland and Northern Ireland began before Dec 31, 2020, their testing and vaccination data were not available for linkage by the study team at the time of this analysis so they were excluded. Recruitment of Welsh participants began in 2021.
Most participants were female (19 692 [84%]), of White ethnicity (20 424 [88%]), in a patient-facing role (20 054 [86%]), and in a clinical discipline (15 502 [66%]; table 1). 5874 (25%) participants had a reported medical condition, with asthma (n=2893), obesity (n=1988), and diabetes (n=677) being the most frequent.
The total follow-up time in this analysis was 2 calendar months, 1 106 905 participant person-days, 710 587 person-days unvaccinated, and 396 318 person-days vaccinated. Participants were followed-up for a maximum of 59 days post-first dose (median 21, IQR 13–31) and 39 days post-second dose (23, 17–28). Total person-days of follow-up was 711 135 in the negative cohort and 395 770 in the positive cohort. 49 740 PCR tests were done in the unvaccinated follow-up period and 38 071 PCR tests in the vaccinated follow-up period, with a test interval of 14·3 days per test in the unvaccinated period and 10·4 days per test in the vaccinated period.
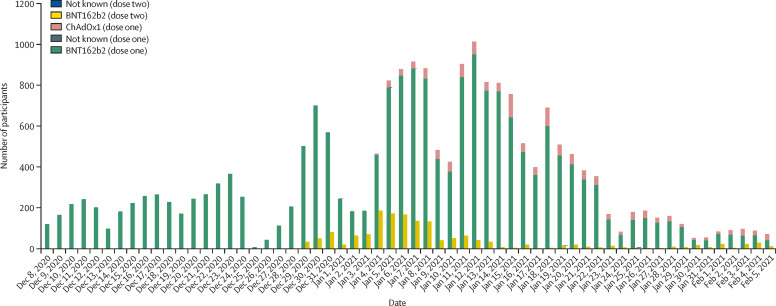
At least one dose of vaccine was given to 20 641 (89%) participants by Feb 5, 2021; 19 384 (94%) received the BNT162b2 vaccine and 1252 (6%) received the ChAdOx1 vaccine. Roll-out of the first dose of vaccine in this cohort peaked on Jan 12, 2021 (figure 1 ). Two doses of vaccine were given to 1607 (8%) participants by Feb 5, 2021, 1605 (99·9%) of whom received the BNT162b2 vaccine and two (0·1%) received the ChAdOx1 vaccine. The median length of time between first dose and second dose was 23 days (IQR 21–26; range 19–28). 14 participants had an unreliable date of a second dose; their follow-up time was censored at the date of the suspect second dose.
Figure 1.
Number of vaccinated SIREN participants by dose, manufacturer, and day, Dec 8, 2020 to Feb 5, 2021 (n=20 641)
In multivariable analysis, after controlling for all other risk factors and given site, having a previous infection, gender, age, ethnicity, IMD score, and staff group remained significantly associated with vaccine coverage (table 1). Participants were less likely to have been vaccinated if they had a previous infection (adjusted odds ratio [aOR] 0·59, 95% CI 0·54–0·64), were female (0·72, 0·63–0·82), were aged under 35 (0·78, 0·64–0·96), were from Black, Asian or minority ethnic groups, especially if they were Black (0·26, 0·21–0·32), lived in areas of higher deprivation (IMD 1 [most] vs 5 [least] aOR 0·75, 0·65–0·87), or worked as a porter, security, or in estates (0·61, 0·42–0·90) or midwife (0·74, 0·57–0·97).
There were 977 new infections during 710 587 person-days of follow-up in the unvaccinated group, an incidence density of 14 infections per 10 000 person-days of follow-up (table 2 ). In the vaccinated group, 21 days after the first dose, there were 71 new infections (incidence density of eight per 10 000 person-days of follow-up) and nine new infections 7 days after the second dose (incidence density of four per 10 000 person-days of follow-up).
Table 2.
Effectiveness of the BNT162b2 COVID-19 vaccine, between Dec 7, 2020, and Feb 5, 2021 (n=23 324)
| Total person-time, days | Number of PCR positives | Incidence density per 10 000 person-days | Unadjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | |
|---|---|---|---|---|---|
| Full cohort | |||||
| Unvaccinated | 710 587 | 977 | 14 | 1 (ref) | 1 (ref) |
| Dose 1 | 87 278 | 71 | 8 | 0·43 (0·23–0·64) | 0·30 (0·15–0·45) |
| Dose 2 | 20 978 | 9 | 4 | 0·23 (0·06–0·40) | 0·15 (0·04–0·26) |
| Negative cohort | |||||
| Unvaccinated | 442 605 | 902 | 20 | 1 (ref) | 1 (ref) |
| Dose 1 | 59 748 | 66 | 11 | 0·33 (0·17–0·49) | 0·28 (0·14–0·42) |
| Dose 2 | 14 746 | 8 | 5 | 0·18 (0·04–0·31) | 0·14 (0·03–0·24) |
| Positive cohort | |||||
| Unvaccinated | 267 982 | 75 | 3 | .. | .. |
| Dose 1 | 27 530 | 5 | 2 | .. | .. |
| Dose 2 | 6232 | 1 | 2 | .. | .. |
We calculated cumulative vaccine effectiveness after suitable intervals (21 days post-first dose and 7 days post-second dose) to focus on infections acquired since vaccination after a sufficient interval for biological protection. Unadjusted includes vaccine effect (period) only. The full model was adjusted for site as a random effect, period, and eight fixed effects: age, gender, ethnicity, comorbidities, job role, frequency of contact with COVID-19 patients, employed in a patient facing role, and occupational exposure. There was insufficient information to model the positive cohort separately so stratified hazard ratios are not available for the positive cohort.
Looking at symptom status on the date, or in the preceding 14 days, of a positive PCR test, among the unvaccinated participants infected, 140 (14%) were asymptomatic, 543 (56%) had typical COVID-19 symptoms (fever, cough, or change or loss of taste or smell), 133 (14%) had other symptoms, and 161 (16%) did not complete a questionnaire in the time period. In the vaccinated group (n=80), 21 days after first dose or 7 days after second dose, 15 (19%) were asymptomatic, 29 (36%) had typical COVID-19 symptoms, 12 (15%) had other symptoms, and 24 (30%) did not complete the questionnaire. Using a longer window for symptoms associated with a PCR infection, within 14 days before and after the date of a positive test, 51 (5%) participants in the unvaccinated group were asymptomatic, 620 (63%) had typical COVID-19 symptoms, 139 (14%) had other symptoms, and 167 (17%) did not complete the symptom status questionnaire. In the vaccinated group in the same period, 10 (13%) were asymptomatic, 32 (40%) had typical COVID-19 symptoms, 13 (16%) had other symptoms, and 25 (31%) did not complete the questionnaire.
After controlling for the other risk factors, cohort, and at a given site, vaccine effectiveness against infection 21 days after the first dose of BNT162b2 vaccine in the overall study population was 70% (95% CI 55–85) and increased to 85% (74–96) 7 days after the second dose (table 2). Point estimates of protection were marginally higher when the negative cohort was modelled separately, and after adjustment for the other risk factors and at a given site, vaccine effectiveness was 72% (95% CI 58–86) 21 days after first dose and 86% (76–97) 7 days after the second dose. There was insufficient information to separately model vaccine effectiveness in the positive cohort at this analysis timepoint. The overall model showed that the positive cohort had 90% immune protection (95% CI 88–92) compared with the negative cohort after their natural infection (appendix 2).
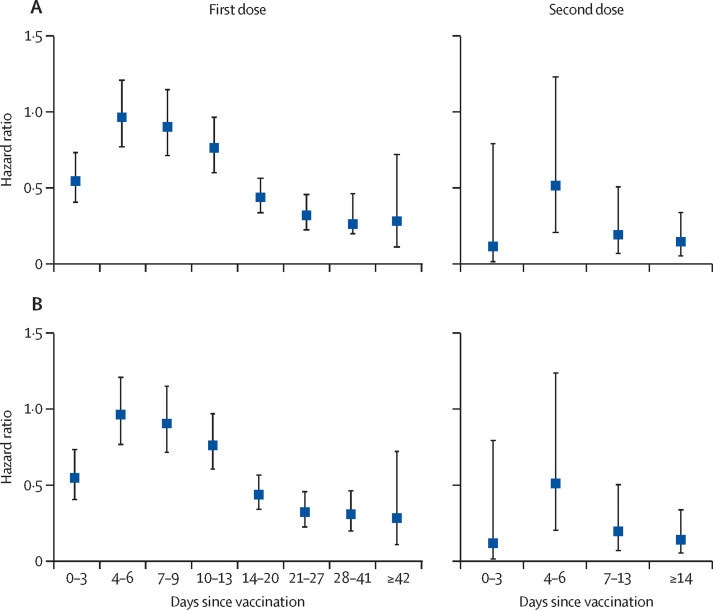
Vaccinated individuals had a reduced risk of infection immediately (days 0–3) after their first dose; there was no significant effect between days 4 to 9, with substantial protection from infection increasing from day 10 onwards and plateauing after 21 days (figure 2 ). A similar pattern was seen after the second dose. Hazard ratios (adjusted and unadjusted) for each timepoint post-vaccination in the full cohort and the negative cohort are provided in appendix 1 (pp 4–5).
Figure 2.
Adjusted hazard ratios at post-vaccination intervals in the (A) full cohort (n=23 324) and (B) negative cohort (n=15 121), Dec 7, 2020 to Feb 5, 2021
Hazard ratios were adjusted for site as a random effect, period, and eight fixed effects: age, gender, ethnicity, comorbidities, job role, frequency of contact with COVID-19 patients, employed in a patient facing role, and occupational exposure.
Discussion
Our follow-up of this large cohort of 23 324 health-care workers, whose SARS-CoV-2 infection history is known 2 months after vaccine roll-out provides unique real-world data on the short-term vaccine effectiveness of the BNT162b2 vaccine against both symptomatic and asymptomatic infection. Regular PCR-testing of participants, regardless of symptom status, allowed for the detection of asymptomatic infection, an important proxy for reduction in transmission. 2 months after vaccine roll-out started, 89% of our cohort had received at least one dose of COVID-19 vaccine and 8% had received two doses. We detected modest variability in coverage, with lower coverage in participants with previous infection, from Black, Asian and minority ethnic backgrounds, working as a midwife, porter, security, or in estates, and living in areas of higher deprivation. We estimated the vaccine effectiveness against infection for the BNT162b2 vaccine to be 70% 21 days after the first dose, increasing to 85% 7 days after the second dose in our study population. Given the dominance of the B.1.1.7 variant in England during the study period, which accounted for 50% or more positive tests in Pillar 2 laboratories since the beginning of December, 2020, in the South East, London, and East of England, and in all regions by early January 2021 (with Yorkshire and the Humber the last region), our findings suggest that the BNT162b2 is effective against the B1.1.7.16
It is possible that we missed infections during follow-up, depending on the timing of the infection relative to PCR testing or PCR sensitivity. The testing frequency in our vaccinated population was slightly higher than in the unvaccinated population (10·4 days per test vs 14·3 days per test), making us more likely to pick up infections among the vaccinated, and, therefore, biasing our vaccine effectiveness results towards the null hypothesis. However, given the frequency of testing in our cohort, with asymptomatic PCR testing every 14 days within SIREN, and many participants also undergoing twice weekly lateral flow testing with PCR confirmation, we believe most infections during this period were detected. We believe that testing a health-care worker cohort of this size more frequently would not be feasible or a prudent use of resources. Given this cohort undergo regular serological testing using both N and S assays, future analyses will include seroconversions after infection, capturing any investigations that could have been missed through PCR testing. Conversely, it is also possible that infections detected at a short interval after vaccination were acquired before vaccination, and, therefore, were misclassified as events within vaccinated follow-up, biasing vaccine effectiveness to the null hypothesis. This possibility informed our decision to focus on events 21 days after first dose and 7 days after the second dose, which a suitable interval for the vaccine to take immunological effect and to avoid counting infections transmitted before vaccination.
Although we have detected both asymptomatic and symptomatic infections during follow-up, the small number of asymptomatic infections detected to date means we do not have the power to do a stratified analysis of vaccine effectiveness by symptom status yet. We also recognise that, given our symptom status is ascertained by self-completed questionnaires sent at 14-day intervals, there is the possibility of recall bias. We do not believe this bias would have affected symptom reports by vaccination status, in particular as we have excluded infections occurring in the time period, chiefly 48 hours after vaccination, where reactogenicity might have resulted in differential symptom reporting.
Given the high vaccine coverage and small proportion of participants remaining unvaccinated, the characteristics and exposures of this group might become sufficiently different from the vaccinated cohort to undermine the validity of future analyses. However, given the short follow-up period for this analysis, with all participants contributing follow-up time to the unvaccinated group, we do not believe this would have introduced substantial bias at this stage.
Speculation of high levels of vaccine hesitancy in health-care workers are not supported by the findings of our cohort study, with almost 90% receiving at least one dose of vaccination within 2 months of vaccine roll-out.17 Although we recognise that this high coverage might not be generalisable to health-care workers in the rest of the UK or the general population, as those who have self-selected to participate in a research study might not be representative of health-care workers in the UK or the population more generally, three recent single-centre studies in health-care workers in Israel and the UK reported vaccine coverage of 79%, 90%, and 65%, respectively.18, 19, 20, 21 Whilst differences in vaccine coverage by demographic and occupational factors were modest, our findings of differences by age, gender, ethnicity, residential deprivation, and occupation have been reported in other studies.17, 20, 21 The finding that these differences are present among SIREN participants, a self-selected and engaged population, underscores the importance of continued efforts nationally to ensure equitable vaccination roll-out.
Our analysis identified a reduced risk of infection in vaccinated individuals immediately (days 0–3) after the first dose, which cannot be plausibly explained by the immune response to the vaccine; this effect could be explained by deferral effect bias, where individuals who are symptomatic, currently PCR positive, or have been recently exposed to a COVID-19 case might defer their vaccination and be under-represented in accordance with national guidance.22
We found vaccine effectiveness, at a given site, to be 70% overall (72% in the negative cohort) against both asymptomatic and symptomatic infection, from 21 days post-first dose of the BNT162b2 vaccine. With fewer of the cohort vaccinated with the ChAdOx1 nCoV-19 vaccine, and the later roll-out resulting in less follow-up time accrued, we are unable to investigate the effectiveness of the ChAdOx1 nCoV-19 vaccine within this analysis. Our estimates are comparable with a single-centre Israeli health-care worker cohort study reporting vaccine effectiveness of BNT162b2 vaccine against infection of 75% (95% CI 72–84) 15–28 days after first dose and 85% for symptomatic COVID-19 15–28 days after dose 1 (95% CI 71–92),18 and a large Israeli study that reported vaccine effectiveness of 60% (53–66) at 21–27 days after dose one and 92% (88–95) at 7 days after dose two.23
Variability in estimates of vaccine effectiveness between studies could result from differences in study design, testing protocols, and study populations, with most relying on symptomatic testing rather than structured asymptomatic testing as in SIREN, some excluding health-care workers,23 and some excluding individuals with previous infection.18, 24 The timing of the post-vaccination follow-up was also variable and, as our results show, although good protection was seen at earlier intervals, it plateaued from 21 days onwards. Therefore, estimates of vaccine effectiveness at earlier intervals, such as 51% at 13–24 days after first dose, understandably would give lower results.25, 6 Notably, to date, most data on vaccinated individuals in the UK are from people aged >75 years old, in whom vaccine effectiveness can be lower due to immunosenesence.26 Given the SIREN cohort is composed of working-age adults, the findings are more relevant for the general adult population, although the healthy worker effect bias might underestimate the disease impact compared with the general population.27
To date, little has been published on the effect of vaccination on reducing asymptomatic infection or transmission globally. A substudy of the Oxford–AstraZeneca COVID-19 vaccine did weekly swabbing and reported reduced viral load and PCR positivity in vaccinated participants; a signal that transmission might be reduced by this vaccine.28 With our structured, frequent asymptomatic screening in SIREN, we are well placed to investigate vaccine effectiveness against asymptomatic infection in future analyses. Through our detailed investigation of new infections 21 days after first dose or second dose, including repeat swabbing and viral culture, we will investigate potential impacts on transmission. Further investigations will include serological assessment after vaccination: duration of antibody response, impact of previous infection on antibody response, and factors affecting post-vaccination antibody response. We also aim to sequence all PCR-positive infections within SIREN and will, therefore, be able to detect and monitor the proportion of novel variants among vaccinated participants over time.
The SIREN study shows that the BNT162b2 mRNA vaccine does not prevent all cases of infection and, therefore, health-care workers should continue to wear personal protective equipment while caring for all patients, observe physical distancing and other non-pharmaceutical measures in and outside work, and continue regular asymptomatic testing (especially as typical symptoms were reduced post-vaccination) until COVID prevalence is considerably lower.
Data sharing
The metadata will be available through the Health Data Research UK CO-CONNECT platform and available for secondary analysis once the SIREN study has completed reporting.
Declaration of interests
All authors declare no competing interests. The Immunisation and Countermeasures Division at PHE has provided vaccine manufacturers (including Pfizer) with post-marketing surveillance reports on pneumococcal and meningococcal infection which the companies are required to submit to the UK Licensing authority in compliance with their Risk Management Strategy. A cost recovery charge is made for these reports.
Acknowledgments
Acknowledgments
This study was funded by the UK Department of Health and Social Care and PHE, with contributions from the Scottish, Welsh, and Northern Irish governments. Funding is also provided by the National Institute for Health Research (NIHR) as an Urgent Public Health Priority Study. SH and VJH are supported by the NIHR Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance at the University of Oxford in partnership with PHE (NIHR200915). We thank all staff supporting study delivery at participating sites and all participants for their ongoing commitment and contributions to this study. We also thank the PHE COVID-19 Vaccine Coverage team and the COVID-19 data store team for their support establishing linkage to the National Immunisation Management System, and all colleagues from PHE, NHS England & Improvement, and NHS Digital involved in developing the National Immunisation Management System.
Contributors
SH conceived this study, commented on the draft protocol, supervised the study, and drafted and edited the final manuscript. JL-B, NA, and VJH wrote the first draft of the protocol and analysis plan for the vaccine effectiveness substudy. SF and VJH cleaned and analysed data. VJH and BO did the literature search and drafted the manuscript. AS did the statistical modelling of vaccine effectiveness, supervised by NA and AC. VJH, SF, AS, and AC verified the data in the study. All authors contributed to the study design, contributed to drafting the protocol, revised the manuscript for important intellectual content, had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Contributor Information
SIREN Study Group:
N Andrews, A Atti, H Aziz, T Brooks, CS Brown, D Camero, C Carr, MA Chand, A Charlett, H Crawford, M Cole, J Conneely, S D'Arcangelo, J Ellis, S Evans, S Foulkes, N Gillson, R Gopal, L Hall, VJ Hall, P Harrington, S Hopkins, J Hewson, K Hoschler, D Ironmonger, J Islam, M Kall, I Karagiannis, O Kay, J Khawam, E King, P Kirwan, R Kyffin, A Lackenby, M Lattimore, E Linley, J Lopez-Bernal, L Mabey, R McGregor, S Miah, EJM Monk, K Munro, Z Naheed, A Nissr, AM O'Connell, B Oguti, H Okafor, S Organ, J Osbourne, A Otter, M Patel, S Platt, D Pople, K Potts, M Ramsay, J Robotham, S Rokadiya, C Rowe, A Saei, G Sebbage, A Semper, M Shrotri, R Simmons, A Soriano, P Staves, S Taylor, A Taylor, A Tengbe, S Tonge, A Vusirikala, S Wallace, E Wellington, M Zambon, D Corrigan, M Sartaj, L Cromey, S Campbell, K Braithwaite, L Price, L Haahr, S Stewart, ED Lacey, L Partridge, G Stevens, Y Ellis, H Hodgson, C Norman, ED Lacey, B Larru, S Mcwilliam, A Roynon, J Northfield, S Winchester, P Cieciwa, A Pai, P Bakker, C Loughrey, A Watt, F Adair, A Hawkins, A Grant, R Temple-Purcell, J Howard, N Slawson, C Subudhi, S Davies, A Bexley, R Penn, N Wong, G Boyd, A Rajgopal, A Arenas-Pinto, R Matthews, A Whileman, R Laugharne, J Ledger, T Barnes,, C Jones, N Osuji, N Chitalia, T Bailey, S Akhtar, G Harrison, S Horne, N Walker, K Agwuh, V Maxwell, J Graves, S Williams, A O'Kelly, P Ridley, A Cowley, H Johnstone, P Swift, J Democratis, M Meda, S Brake, J Gunn, A Selassi, S Hams, V Irvine, B Chandrasekaran, C Forsyth, J Radmore, C Thomas, K Brown, S Roberts, P Burns, K Gajee, T Lewis, TM Byrne, F Sanderson, S Knight, E Macnaughton, BJL Burton, H Smith, R Chaudhuri, J Aeron-Thomas, K Hollinshead, RJ Shorten, A Swan, RJ Shorten, C Favager, J Murira, S Baillon, S Hamer, A Shah, J Russell, D Brennan, A Dave, A Chawla, F Westwell, D Adeboyeku, P Papineni, C Pegg, M Williams, S Ahmad, A Horsley, C Gabriel, K Pagget, P Cieciwa, G Maloney, J Ashcroft, I Del Rosario, R Crosby-Nwaobi, D Flanagan, D Dhasmana, S Fowler, E Cameron, L Prentice, C Sinclair, V Irvine, V Bateman, K McLelland-Brooks, A Ho, M Murphy, A Cochrane, A Gibson, M Patel, K Black, K Tempeton, S Donaldson, L Coke, N Elumogo, J Elliott, D Padgett, A Cross, M Mirfenderesky, S Joyce, I Sinanovic, M Howard, T Lewis, P Cowling, M Brazil, E Hanna, A Abdelrazik, S Brand, EA Sheridan, B Wadams, A Lloyd, J Mouland, J Giles, G Pottinger, H Coles, M Joseph, M Lee, S Orr, H Chenoweth, D Browne, C Auckland, R Lear, T Mahungu, A Rodger, S Warren, D Brooking, S Pai, R Druyeh, E Smith, S Stone, S Meisner, D Delgado, E Underhill, L Keen, M Aga, P Domingos, S Gormley, C Kerrison, S Birch, T DeSilva, L Allsop, S Ambalkar, M Beekes, S Jose, J Tomlinson, Sharen Painter, C Price, J Pepperell, K James, T Trinick, L Moore, J Day, A Boulos, I Knox, E Defever, D McCracken, K Brown, K Gray, A Houston, T Planche, R Pritchard Jones, Diane Wycherley, S Bennett, J Marrs, K Nimako, B Stewart, SC Bain, N Kalakonda, S Khanduri, A Ashby, M Holden, N Mahabir, J Harwood, B Payne, K Court, N White, R Longfellow, LE Hughes, ME Green, M Halkes, P Mercer, A Roebuck, E Wilson-Davies, L Gallego, R Lazarus, N Aldridge, L Berry, F Game, T Reynolds, C Holmes, M Wiselka, A Higham, M Booth, C Duff, J Alderton, D Hilton, J Powell, A Jackson, AJ Plant, N Ahmed, T Chin, MZ Qazzafi, AM Moody, RE Tilley, T Donaghy, M O'Kane, K Shipman, R Sierra, C Parmar, G Mills, D Harvey, YWJ Huang, J Birch, L Robinson, S Board, A Broadley, C Laven, N Todd, DW Eyre, K Jeffery, S Dunachie, C Duncan, P Klenerman, L Turtle, H Baxendale, and JL Heeney
Supplementary Materials
References
- 1.WHO WHO Coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int/
- 2.Office for National Statistics Coronavirus (COVID-19) latest insights. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19/latestinsights#deaths
- 3.Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medicines and Healthcare Regulatory Authority MHRA guidance on coronavirus (COVID-19) March 19, 2020. https://www.gov.uk/government/collections/mhra-guidance-on-coronavirus-covid-19
- 5.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Department of Health and Social Care UK COVID-19 vaccine uptake plan. Feb 13, 2021. https://www.gov.uk/government/publications/covid-19-vaccination-uptake-plan/uk-covid-19-vaccine-uptake-plan
- 8.Department of Health and Social Care Optimising the COVID-19 vaccination programme for maximum short-term impact. Jan 26, 2021. https://www.gov.uk/government/publications/prioritising-the-first-covid-19-vaccine-dose-jcvi-statement/optimising-the-covid-19-vaccination-programme-for-maximum-short-term-impact
- 9.Our World in Data Coronavirus (COVID-19) vaccinations. 2021. https://ourworldindata.org/covid-vaccinations
- 10.Hall V, Foulkes S, Charlett A, et al. Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? Large multi-centre prospective cohort study (the SIREN study), England: June to November 2020. medRxiv. 2021 doi: 10.1101/2021.01.13.21249642. published online Jan 15. (preprint). [DOI] [Google Scholar]
- 11.Wallace S, Hall V, Charlett A, et al. SIREN protocol: Impact of detectable anti-SARS-CoV-2 on the subsequent incidence of COVID-19 in 100,000 healthcare workers: do antibody positive healthcare workers have less reinfection than antibody negative healthcare workers? medRxiv. 2020 doi: 10.1101/2020.12.15.20247981. published online Dec 18. (preprint). [DOI] [Google Scholar]
- 12.Peto T & UK COVID-19 Lateral Flow Oversight Team COVID-19: rapid antigen detection for SARS-CoV-2 by lateral flow assay: a national systematic evaluation for mass-testing. medRxiv. 2021 doi: 10.1101/2021.01.13.21249563. published online Jan 26. (preprint). [DOI] [Google Scholar]
- 13.Holford TR. Life tables with concomitant information. Biometrics. 1976;32:587–597. [PubMed] [Google Scholar]
- 14.Holford TR. The analysis of rates and of survivorship using log-linear models. Biometrics. 1980;36:299–305. [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Public Health England Investigation of SARS-CoV-2 variants of concern in England. Technical briefing 6. Feb 13, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961299/Variants_of_Concern_VOC_Technical_Briefing_6_England-1.pdf
- 17.Galanis PA, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Intention of health care workers to accept COVID-19 vaccination and related factors: a systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.12.08.20246041. published online Dec 11. (preprint). [DOI] [Google Scholar]
- 18.Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin CA, Marshall C, Patel P, et al. Association of demographic and occupational factors with SARS-CoV-2 vaccine uptake in a multi-ethnic UK healthcare workforce: a rapid real-world analysis. medRxiv. 2021 doi: 10.1101/2021.02.11.21251548. published online Feb 18. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacKenna B, Curtis HJ, Morton CE, et al. Trends, regional variation, and clinical characteristics of COVID-19 vaccine recipients: a retrospective cohort study in 23·4 million patients using OpenSAFELY. medRxiv. 2021 doi: 10.1101/2021.01.25.21250356. published online Jan 27. (preprint). [DOI] [Google Scholar]
- 22.Public Health England Chapter 14a: COVID-19, the Green Book. Feb 12, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961287/Greenbook_chapter_14a_v7_12Feb2021.pdf
- 23.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101765. published online Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Israeli Ministry of Health Vaccine safety and effectiveness. 2021. https://govextra.gov.il/ministry-of-health/covid19-vaccine/en-covid19-vaccine-faqs/
- 25.Chodick G, Tene L, Patalon T, et al. The effectiveness of the first dose of BNT162b2 vaccine in reducing SARS-CoV-2 infection 13–24 days after immunization: real-world evidence. medRxiv. 2021 doi: 10.1101/2021.01.27.21250612. published online Jan 29. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiello A, Farzaneh F, Candore G, et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah D. Healthy worker effect phenomenon. Indian J Occup Environ Med. 2009;13:77–79. doi: 10.4103/0019-5278.55123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) Vaccine Against SARS-CoV-2 VOC 202012/01 (B.1.1.7) Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The metadata will be available through the Health Data Research UK CO-CONNECT platform and available for secondary analysis once the SIREN study has completed reporting.