Abstract
Early life adversity has been linked to alterations of self-regulation outcomes, including neurobiological correlates of IC. We examined whether children’s ability to engage the parasympathetic nervous system impacts how adversity affects IC. Children ages 3–5 years completed two laboratory measures of IC while respiratory sinus arrhythmia (RSA) was measured as an index of parasympathetic activity. Children with greater adversity demonstrated lower IC; adversity also moderated associations between RSA reactivity and IC. For children with less adversity, greater RSA withdrawal during IC tasks was associated with better IC. In contrast, greater adversity was associated with poor IC, regardless of RSA withdrawal. Effects of adversity were significant both when considering cumulative risk exposure and household income alone. Results suggest that accumulation of early adverse experiences disrupts connectivity between physiological and behavioral components of self-regulation. Parasympathetic withdrawal to cognitive tasks may be a less adaptive response for developmental samples experiencing poverty-related stressors.
Keywords: inhibitory control, respiratory sinus arrhythmia, early adversity, individual differences, parasympathetic nervous system, cumulative risk, poverty
Children exposed to a range of early life stressors, such as poverty, maltreatment, and chaotic home environments, have been documented to exhibit deficits in inhibitory control (IC), that is, the ability to control impulsive or prepotent responses (Hostinar, Stellern, Schaefer, Carlson, & Gunnar, 2012; McDermott, Westerlund, Zeanah, Nelson, & Fox, 2012; Mueller et al., 2012; Pears, Fisher, Bruce, Kim, & Yoerger, 2010; Skowron, Cipriano-Essel, Gatzke-Kopp, Teti, & Ammerman, 2014). Characterizing biological processes underlying effective IC is particularly important in at-risk children exposed to multiple poverty-related stressors and in those who disproportionately experience negative health and well-being outcomes linked to IC deficits, such as academic failure, psychopathology, and risk-taking behavior (Evans & Kim, 2012). Measures of the autonomic nervous system may be critical for addressing this issue, given that early exposure to stressful experiences has profound effects on autonomic function, beginning as early as the prenatal environment (Propper & Holochwost, 2013). More specifically, activity of the parasympathetic branch of the autonomic nervous system has gained attention as a mechanism by which children flexibly engage and regulate their physiological state in the service of behavioral regulation (Graziano & Derefinko, 2013).
A growing body of research describes how regulation of peripheral physiology occurs via top-down influences on the parasympathetic nervous system (PNS) as a fast-acting mechanism to control heart rate (Berntson, Cacioppo, & Quigley, 1993). Flexible engagement and withdrawal of the PNS is theorized to help individuals rapidly alternate between states of engagement and disengagement in the context of environmental demands (Porges, 2001). When the PNS withdraws, cardiac output increases, and when the PNS is activated (the vagal brake is applied), heart rate and arousal are reduced. Respiratory sinus arrhythmia (RSA) reflects the measurement of parasympathetic influence on heart rate and serves as an index of physiological regulation (Berntson et al., 1993).
Measures of RSA taken in “resting” conditions, often while individuals sit passively and watch an affectively neutral film clip, have been proposed to reflect trait-like qualities of self-regulation (Beauchaine & Thayer, 2015). Higher resting RSA is commonly associated with better affective and behavioral regulation (e.g., Calkins, Graziano, & Keane, 2007; Gorka et al., 2013; Graziano & Derefinko, 2013; Hastings et al., 2008); however, a recent meta-analysis suggests this is a small effect with a high degree of heterogeneity and thus should be interpreted with caution (Zahn et al., 2016). Notably, adults who are more resilient to acute stressors show higher resting RSA (Lü, Wang, & You, 2016), raising the possibility that high resting RSA may be protective for individuals exposed to a greater number of stressful experiences earlier in life.
RSA reactivity reflects the extent to which individuals engage or disengage PNS activity relative to resting levels to meet the demands of a given context. Research that has examined how children’s RSA reactivity to cognitively challenging tasks supports behavioral regulation has typically found that RSA withdrawal supports higher cognitive function in children (for a review, see Graziano & Derefinko, 2013). In their meta-analysis, Graziano and Derefinko (2013) found that higher levels of RSA withdrawal were associated with better cognitive and academic performance. Taken together, these results suggest that, on average, it is helpful for children to withdraw vagal control of heart rate and increase physiological arousal in order to facilitate attentional control and successfully engage in assessments of cognitive function.
In contrast to the group average effects reported in the meta-analytic study, a growing number of findings suggests that this pattern of RSA withdrawal during cognitive challenge may not facilitate performance for all children. For example, Conradt and colleagues (2016) found that greater RSA withdrawal at age 1 month predicted greater behavioral dysregulation at age 3 years in children exposed to higher degrees of early adversity; however, a significant relationship between RSA reactivity and later behavioral dysregulation was not observed in children exposed to less adversity. Amongst the same sample, Conradt and colleagues (2014) reported a similar pattern of increasing RSA withdrawal, assessed in yearly longitudinal measurements at ages 3 to 6 years, associated with more disinhibited behavior problems later in life (ages 8–14) for children exposed to greater early adversity. Conversely, for children with less exposure to early adversity, increases in RSA withdrawal from ages 3 to 6 were associated with fewer disinhibited behavior problems later in life (Conradt et al., 2014). A separate study of maltreatment-exposed children found that lower levels of RSA during challenging parent–child dyadic interaction tasks were associated with worse performance on two separate measures of IC (Skowron et al., 2014). The reverse pattern was observed in children not exposed to child maltreatment: Higher RSA levels during challenging tasks with a parent were associated with better IC.
One theoretical explanation for these findings is that children who are more physiologically reactive may be more sensitive to environmental effects, such that a biological profile of greater parasympathetic reactivity may exacerbate the impact of exposure to a high degree of daily stressors on self-regulation abilities (Conradt et al., 2016). An alternative, and perhaps complementary, explanation is that children with higher cumulative risk have less-coordinated activity amongst biological systems underlying self-regulation (El-Sheikh et al., 2009). Given that control of parasympathetic function is largely related to neural networks involving prefrontal cortical activity (Thayer, Åhs, Fredrikson, Sollers, & Wager, 2012), the activity of which is strongly implicated in behavioral control, it may be that cumulative stress exposure disrupts the ability to effectively leverage parasympathetic activity for concurrent cognitive demands. For example, children with relatively lower cumulative risk may have fewer “bottom-up” impulsive arousal demands to control; thus, releasing vagal control over heart rate may facilitate speeded responding without increased distraction from other bottom-up impulses linked to chronically heightened arousal.
We suggest that it is critical to establish the extent to which profiles of PNS function characterized by RSA withdrawal during cognitively challenging tasks reflect resiliency for all children (Obradović, 2012). In particular, it is unknown if cumulative risk may moderate links between RSA reactivity to cognitive challenge and regulated behaviors such as IC. Research in this area is particularly important because interventions are increasingly looking to biological markers as indexes of intervention efficacy, and increased RSA withdrawal may not reflect an adaptive profile for children with the highest levels of adversity.
Present Study
The first aim of the present study was to examine links between parasympathetic function and IC performance in a large sample of preschool-age children from a wide range of socioeconomic backgrounds. We were particularly interested in how RSA reactivity during cognitively challenging IC tasks supports effective IC performance in those same tasks. This is a notable design strength compared with those of previous studies that have measured RSA and cognitive function at separate time points, given that our design allowed for assessment of how physiological regulation supports concurrent behavioral regulation. In characterizing the link between parasympathetic function and IC behavior, we were interested in both resting RSA (indexing baseline function) and RSA reactivity (indexing regulatory flexibility). Consistent with previous research, it was hypothesized that higher baseline RSA would predict better IC. It was also expected that, overall, greater RSA withdrawal would be predictive of better IC.
The second study aim was to examine the role of early adversity in understanding the RSA and IC relationship. We examined early adversity both as a comprehensive measure of cumulative risk and as a simpler measure based solely on household income. Building on previous research (Evans & Kim, 2007; Evans, Li, & Whipple, 2013), we conceptualized cumulative risk as an umbrella term for several known risk factors, including sociodemographic influences (i.e., single-parent status, household income, mother’s educational attainment), psychosocial influences (i.e., family turmoil, child separation from family, exposure to violence), and physical environmental influences (i.e., residential crowding, noise, housing problems). Greater early adversity, measured both as higher cumulative risk and lower household income, was hypothesized to predict lower IC performance, as has been shown by previous research. Moderation analyses were specified to examine the interrelationship between RSA reactivity and IC performance as a function of early adversity. Based on previous findings (Conradt et al., 2014; Conradt et al., 2016), we hypothesized that early adversity would moderate the relationship between RSA reactivity and IC, such that patterns of RSA reactivity would be differentially predictive of regulated behavior on the basis of degree of early adversity experienced. Specifically, we were interested in whether patterns of RSA withdrawal are similarly facilitative of behavioral regulation (indexed by IC) for children who have experienced lower and higher levels of early adversity.
Methods
Participants
Mother–child dyads were recruited for participation as part of a larger study of parenting and family processes (for more details, see Skowron, Cipriano-Essel, Benjamin, Pincus, & Van Ryzin, 2013). Dyads qualified for participation if mothers were age 18 years or older, spoke fluent English, and were living with their preschool-age children. Participants were 184 preschool children ranging in age from 3 to 5 years (M = 3.81, SD = .76). The majority of participants were female (54.5%), White (80.1%), lived in two-parent households (58.2%), and had mothers who had obtained at least a high school diploma (75.2%). A majority of families reported annual household incomes at or below $30,000 (86.5%). Of the sample, 41.8% had no documented child maltreatment present, and the remaining had experienced physical abuse or neglect to varying degrees of severity, based on documentation from Child Protective Services.
Measures
Early adversity.
Early adversity was examined both as a composite measure of cumulative risk and as a single measure of household income. Cumulative risk was calculated as an unweighted additive scale comprising sociodemographic, psychosocial, and maltreatment risk factors, with seven potential risk factors included in this study. Each risk factor was coded dichotomously as 1 for present or 0 for not present. Three measures of sociodemographic risk included low income (<$30,000), single-parent status, and maternal high school dropout status. In addition, there were three measures of psychosocial risk: family turmoil, child separation from home, and exposure to community violence. Family turmoil was assessed using the Life Experiences Survey (LES; Sarason, Johnson, & Siegel, 1978), which is a 60-item questionnaire that aims to assess mothers’ perceptions of positive and negative life events experienced during the previous year. Scores ranged from 0 to 17, and respondents in the upper quartile (at or above a score of 4) were coded as 1 (risk present). Child separation from home was assessed according to whether or not the child had been placed in foster care (1 = yes, 0 = no). Exposure to violence was assessed via a parent-reported Children’s Exposure to Community Violence (CECV) survey (Richters & Saltzman, 1990), a 54-item scale that is used to assess the frequency at which a child has been victimized by, witnessed, or heard about 20 forms of violence and violence-related activities in their community. As with family turmoil, respondents in the upper quartile (at or above a score of 8) were coded as 1 (risk present). Last, risk in the form of child maltreatment (CM) was included, with two separate measures: 1 point given for the presence of CM, and another point given for a severity score of 3 or above (severity ranged on a 5-point scale from 1 to 5, with 1 being least severe). Composite scores of cumulative risk (with a total of seven possible risk factors) were available for 184 participants (M = 2.58, SD = 1.47; range, 1 to 7). Overall descriptive statistics for cumulative risk responses are shown in Table 1.
Table 1.
Descriptive Statistics for Components of Cumulative Risk
| M (SD) | N (%) | |
|---|---|---|
| Sociodemographic risk | 1.27 (.63) | |
| Low income (< $30,000) | 88 (47.8) | |
| Maternal high school dropout | 22 (12.0) | |
| Single-mother household | 125 (67.9) | |
| Psychosocial risk | .67 (.80) | |
| Family turmoil (upper quartile) | 63 (34.2) | |
| Child placed in foster care | 12 (6.5) | |
| Exposure to violence (upper quartile) | 49 (26.6) | |
| Child maltreatment risk | .63 (.61) | |
| Maltreatment history positive | 90 (48.9) | |
| Severe maltreatment | 13 (7.1) |
Inhibitory control.
IC was measured as a composite performance score from two separate Stroop-like tasks administered by a researcher. First, children performed the Shapes Stroop task (Kochanska, Murray, & Coy, 1997), followed by the more difficult Day/Night Stroop task (Gerstadt, Hong, & Diamond, 1994). During the Shapes task, children were shown pictures of three large fruits and three small fruits, then shown three pictures of a small fruit embedded within a different large fruit (e.g., a small orange embedded in a large banana). Children were asked to point to each of the small fruits embedded within larger fruits (e.g., “show me the little banana”). For each trial, scores ranged from 0 (incorrect), to 1 (incorrect, followed by spontaneous self-correction), and 2 (correct on first response). Final scores ranged from 0 to 6 (M = 4.80, SD = 1.72). During the Day/Night task, children completed 16 trials during which they were shown a series of cards with pictures of a sun or moon and instructed to say “day” whenever they saw a card with a moon on it and to say “night” when they saw a card with a sun on it. For each trial, scores ranged from 0 (incorrect), to 1 (incorrect followed by spontaneous self-correction), and 2 (correct on first response). Final scores ranged from 0 to 32 (M = 16.20, SD = 10.01). Scores were standardized and summed across the two tasks to yield a single index of IC performance, with higher scores indexing better IC.
Respiratory Sinus Arrhythmia (RSA).
Electrocardiogram (ECG) data were acquired through the use of Ag/AgCl electrodes placed on each participant’s chest in a modified Lead II placement, on the distal end of the clavicle, lower left rib cage, and lower abdomen. The ECG signal was recorded with Mindware Technologies ambulatory electrocardiograph (MW1000A; Gahanna, OH) at a sampling rate of 500 Hz and transmitted via a wireless signal to a computer monitored by a research assistant. The ECG signal was synchronized at acquisition with video recordings of behavior to enable identification of task onset and offset. ECG data were processed offline using Mindware HRV software. Trained research assistants visually inspected 30-second epochs for erroneously identified heartbeats. The resulting interbeat interval time series was then subjected to a fast-Fourier transformation, and power in the respiratory frequency band common to children in this age group (0.24–1.04 Hz) was derived from the spectral density function to estimate RSA values. Child RSA values were averaged across 30-second epochs to create a single score for the baseline period (RSAbase) and one for each of the IC tasks (RSAday-night, RSAshapes). RSAbase data were available for 184 participants (M = 6.09, SD = 1.39; range, 1.61–9.88). RSAday-night and RSAshapes were averaged together to create a single measure of RSA during IC tasks (RSAtask) and were available for 151 participants (M = 6.03, SD = 1.31; range, 2.40–9.65). RSA reactivity was then calculated for each participant by subtracting RSAbase from RSAtask values. Using this subtraction method, negative RSA reactivity values indicate that RSA levels decreased from baseline to task (i.e., RSA withdrawal), and positive values reflect RSA increases from baseline to task. Outlier values with Z-scores exceeding ±2.73 were winsorized while preserving rank-order.
Procedures
All procedures used in this study were approved and monitored by the Office for Research Protections. Mother–child dyads completed a 2.5-hour laboratory visit to assess physiological function and parent–child interaction, conducted by a team of two trained experimenters. Upon arrival to the laboratory, participants first gave informed consent, then electrodes for ECG monitoring were applied to mother and child (only child data are reported here). Then, a baseline physiological recording was taken during a 5-minute resting period while mother and child were seated together on a couch in a room with dimmed lights, viewing a relaxing video depicting slow-moving animals with soothing music. Following the baseline data collection, children, accompanied by their mothers, participated in four tasks lasting approximately 40 minutes (not reported here) and a short snack break, then completed the Shapes and Day/Night tasks while physiology was monitored. Families were paid $150 to complete the protocol and were provided transportation, snacks, and small toys for the children.
Results
Zero-order correlations between all variables of interest are shown in Table 2. Child age was positively correlated with IC performance, r(176) = .43, p < .001, and sex was associated with RSA reactivity [males, M = .05, SD = .91; females, M = −.27, SD = .98; r(149) = .16, p = .043]; therefore, age and sex were used as covariates for all the following analyses. Cumulative risk was not significantly correlated with either baseline RSA or RSA reactivity. However, cumulative risk was significantly associated with children’s IC scores, r(176) = −.23, p = .002, such that greater risk exposure was associated with worse IC performance. Baseline RSA was also correlated with IC, r(176) = .16, p = .032. Children with higher baseline levels of RSA showed better IC performance.
Table 2.
Zero-order Correlations Between All Variables of Interest
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Child age | – | ||||||
| 2. Child sex | .072 | – | |||||
| 3. Cumulative risk | −.097 | .062 | – | ||||
| 4. Baseline RSA | −.004 | −.008 | −.073 | – | |||
| 5. Task RSA | .134 | .132 | −.068 | .689** | – | ||
| 6. RSA reactivity | .135 | .165* | .020 | −.441** | .332** | – | |
| 7. Inhibitory control performance | .430** | .030 | −.230** | .161* | .132 | .132 | – |
p < .05
p < .01
Structural Equation Modeling
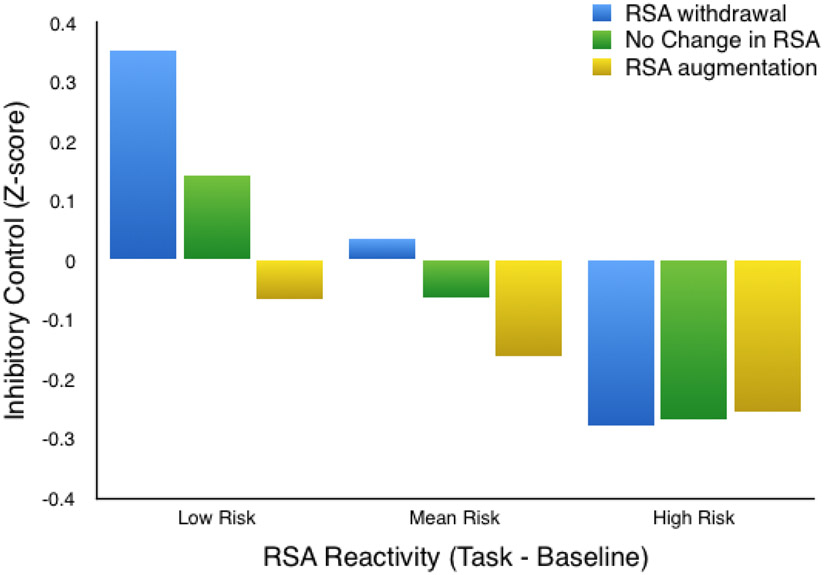
Structural equation modeling (SEM) was conducted to examine the relative contributions of a composite measure of early adversity (i.e., cumulative risk), baseline RSA, RSA reactivity, and child age to IC performance. We further examined interactions between RSA and risk to determine if there were physiological profiles for children with different levels of risk exposure. The model fit the data well, χ2(3) = .012, p > .999, comparative fit index (CFI) = 1.00, Tucker-Lewis Index (TLI) = 1.147, root mean square error of approximation (RMSEA) = .000. Results indicate that greater cumulative risk was associated with lower IC scores (Estimate = −.099, SE = .039, p = .011). Age was also significantly associated with IC scores in the expected direction (Estimate = .515, SE = .078, p < .001). Of particular interest, RSA reactivity showed a significant interaction with cumulative risk in predicting IC (Estimate = .108, SE = .050, p = .030). As shown in Figure 1, greater RSA withdrawal during the task from baseline levels was associated with better IC scores, and this effect increased as a function of diminishing cumulative risk. For children of high cumulative risk, RSA reactivity was not related to IC performance.
Figure 1.
Inhibitory control scores as a function of RSA reactivity and cumulative risk.
Next, we re-ran the model but with a single measure of cumulative risk, household income. The model fit the data well, χ2(3) = .786, p = .853, CFI = 1.00, TLI = 1.105, RMSEA = .000. A highly similar pattern of effects was seen, as in the previously described model. Lower household income was associated with lower IC scores (Estimate = .202, SE = .095, p = .034). Age was positively associated with IC (Estimate = .508, SE = .080, p < .001). The interaction between RSA reactivity and household income was also significant in predicting IC (Estimate = −.172, SE = .086, p = .045). Greater RSA withdrawal during the task from baseline levels was associated with better IC scores, and this effect increased as a function of greater household income. For children from lower income homes, RSA reactivity was not significantly associated with IC performance.
Discussion
The goal of this study was to characterize the links between PNS function and IC performance in a sample of preschool-age children with heterogeneous exposure to early adversity. We were particularly interested in the extent to which measures of early adversity would affect the association between PNS activity and IC performance in children. Building on previous research, we hypothesized that (a) baseline RSA would be associated with better IC, (b) children who had experienced greater levels of early adversity would have lower IC, and (c) parasympathetic reactivity would be more predictive of IC for lower risk children. Results reported here support all three hypotheses and thus provide further evidence that, in young children, the presence of early environmental risk is associated with a diminished capacity for self-regulation (Fisher, Leve, Delker, Roos, & Cooper, 2016) and altered links between physiological and behavioral self-regulatory processes (Conradt et al., 2014; Conradt et al., 2016; Skowron et al., 2014).
The chaotic nature of early environments characterized by high cumulative risk or poverty may help explain the links between adversity and IC impairment (Evans et al., 2013). Specifically, IC development is believed to be supported by environmental factors, such as responsive caregiving (Kochanska, Murray, & Harlan, 2000), low household chaos (Vernon-Feagans, Willoughby, & Garrett-Peters, 2016), and positive maternal mental health (Crandall, Deater-Deckard, & Riley, 2015). Each of these factors is more likely to be limited in households characterized by high cumulative adversity or poverty (Evans et al., 2013). Future research should systematically assess intrinsic and extrinsic factors such as these to identify more causal mechanisms in the association between early adversity and IC (e.g., Fox & Calkins, 2003). Recent studies by Lengua and colleagues (2007, 2014, 2015) have also found that proximal parenting may function as a mediator between cumulative risk and its effects on child development; in other words, positive parenting may even serve to attenuate the harmful impact of contextual risk on development.
Notably, the link between IC and physiological regulation, indexed by RSA reactivity, was also moderated by early adversity. Specifically, the oft-reported finding that greater RSA withdrawal is linked to higher IC (Graziano & Derefinko, 2013) was observed in our study sample only for children with lower levels of adversity. These results contribute to the growing body of literature that chronicles the varied associations between PNS function and behavior, based on early life adversity, in higher risk samples (Conradt et al., 2014; Conradt et al., 2016; Skowron et al., 2014). We suggest that the heterogeneous links between RSA reactivity and IC, in particular the small effect sizes noted in meta-analyses (Graziano & Derefinko, 2013; Zahn et al., 2016), may be attributable to the moderation of variables such as early life adversity, or may be a consequence of a sampling bias in psychological research toward lower risk samples. It is also important to note that resting RSA was associated with IC scores for the sample overall but did not interact with early adversity in predicting behavior, as seen with RSA reactivity. These findings are consistent with reports of resting RSA as a trait-like marker of self-control (Beauchaine & Thayer, 2015), as well as reports in much younger samples among whom RSA reactivity in the first months of life predicts greater sensitivity to the negative effects of early adversity on later behavioral problems (Conradt et al., 2016).
A final notable aspect of results from our study is the consistency in findings, whether early adversity is conceptualized as basic household income or a more comprehensive measure of cumulative risk. A challenge in the early adversity research literature is the widely varied conceptualizations of risk, which can include disparate measures such as poverty, cumulative maltreatment, and other risk factors across more than eight psychosocial domains. Thus, although diverse types of adverse experiences may be represented, a potential tradeoff exists in terms of the specificity of theoretical mechanisms to be targeted by translational scientists (Evans et al., 2013). It may be useful in future research to examine risk by using multiconstruct measures alongside single indicators that are relevant and show sufficient between-subject variability across samples.
In terms of more-clinical applications, multiple interventions (e.g., Tools of the Mind, Kids in Transition to School) designed for children with early adversity exposure (low-income homes, maltreatment) have been documented to improve children’s IC or closely related aspects of self-regulation (Blair & Diamond, 2008; Pears, Kim, & Fisher, 2012). Self-regulation-focused and parent-focused interventions, such as Parent–Child Interaction Therapy (PCIT), are also strong candidates for improving children’s IC (e.g., Fisher & Skowron, 2016). Future research would do well to examine the pathways through which these interventions improve IC and how measuring children’s underlying biology can help understand intervention-related IC improvements. One possibility is that interventions such as these strengthen the association between PNS function and IC for at-risk children, resulting in PNS–IC links that are similar in children from lower risk environments. Another possibility is that the extent to which PNS function is supportive of (or at least associated with) IC is “programmed” relatively early in life, and interventions help at-risk children develop more effective IC through alternate pathways, such as changes in goal-directed motivation supported by sympathetic nervous system engagement (Brenner & Beauchaine, 2011). Studies have increasingly reported a link between blunted sympathetic nervous system activity and reduced behavioral regulation during development (Beauchaine et al., 2013; Hinnant, Erath, Tu, & El-Sheikh, 2016). Interactions between the sympathetic nervous system and the PNS may be critical in that changes in sympathetic nervous system function might free up a greater dynamic range for parasympathetic reactivity in terms of autonomic space (Berntson, Cacioppo, Quigley, & Fabro, 1994), thus enabling more dynamic physiological engagement in fluctuating environmental demands.
It is necessary to note several limitations of this study. First, the data are cross-sectional and cannot lend themselves to causal interpretations; while results support an inverse relationship between cumulative risk exposure and the degree of association between IC and RSA reactivity, it cannot be concluded that the former causes the latter. However, there is some evidence that adversity experienced during the prenatal environment has a causal effect on RSA reactivity (Kaplan, Evans, & Monk, 2008; Monk et al., 2004), and that a profile of greater RSA reactivity in infancy is associated with later deficits in self-regulation during early childhood (Conradt et al., 2016). In addition, the study sample was predominantly White, of lower socioeconomic status, and from rural communities, so findings may be limited in their generalizability. In terms of cumulative risk, this study relied upon self-report measures by parents of their child’s early environment, without including physical measures of household risks, such as noise or housing problems, that have been included in previous reports on cumulative risk (Evans & Kim, 2007). Thus, measures of cumulative risk in our study may have underestimated the extent of risk exposure for individual children.
Taken together, study results replicate previous findings, that exposure to early adversity is associated with deficits in children’s self-regulation abilities and that successful child self-regulation is associated with parasympathetic reactivity (i.e., RSA withdrawal). We extended these findings by showing that parasympathetic reactivity is not predictive of self-regulation abilities for children who have experienced a high degree of early adversity, whether measured as a greater degree of exposure to an array of life stressors or of being raised in a household with a lower annual income. This raises the possibility that deficits in childhood self-regulation may have to do with the effectiveness with which children engage their PNS, and that this process might be altered by early experiences of adversity. These results add to a growing evidence base for intervention and prevention science efforts aimed at improving childhood self-regulation in samples of children often exposed to higher levels of early adversity.
Acknowledgments
We thank the members of the Penn State Family Systems Lab for assistance with data collection, transcription, and coding. This project was supported by National Institutes of Health Research grant R01 MH079328 to E.A. Skowron and funded by the National Institute of Mental Health and Administration for Children and Families/Children’s Bureau of the Administration on Children, Youth, and Families as part of the Federal Child Neglect Research Consortium.
References
- Beauchaine TP, Gatzke-Kopp L, & Mead HK (2007). Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology, 74, 174–184. doi: 10.1016/j.biopsycho.2005.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Neuhaus E, Chipman J Reid MJ, & Webster-Stratton C (2013). Sympathetic- and parasympathetic-linked cardiac function and prediction of externalzing behavior, emotion regulation, and prosocial behavior among preschooers treated for ADHD. Journal of Consulting and Clinical Psychology, 81(3), 481–492. doi: 10.1037/a0032302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, & Thayer JF (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2), 338–350. doi: 10.1016/j.ijpsycho.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993). Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30, 183–196. doi: 10.1111/psyp.1993.30.issue-2 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, & Fabro VT (1994). Autonomic space and psychophysiological response. Psychophysiology, 31, 44–61. doi: 10.1111/j.1469-8986.1994.tb01024.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen Y-J, & Cacioppo JT (2004). Where to Q in PEP. Psychophysiology, 41(2), 333–337. doi: 10.1111/j.1469-8986.2004.00156.x [DOI] [PubMed] [Google Scholar]
- Blair C, & Diamond A (2008). Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology, 20(03), 899–911. doi: 10.1017/S0954579408000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner SL, & Beauchaine TP (2011). Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: A pilot study. Psychophysiology, 48(11), 1588–1596. doi: 10.1111/j.1469-8986.2011.01230.x [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, & Keane SP (2007). Cardiac vagal regulation differentiates among children at risk for behavior problems, Biological Psychology, 74, 144–153. doi: 10.1016/j.biopsycho.2006.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Beauchaine T, Abar B, Lagasse L, Shankaran S, Bada H, … Lester B (2016). Early caregiving stress exposure moderates the relation between respiratory sinus arrhythmia reactivity at 1 month and biobehavioral outcomes at age 3. Psychophysiology, 53(1), 83–96. doi: 10.1111/psyp.12569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, DeGarmo D, Fisher P, Abar B, Lester BM, Lagasse LL, … Hammond JA (2014). The contributions of early adverse experiences and trajectories of respiratory sinus arrhythmia on the development of neurobehavioral disinhibition among children with prenatal substance exposure. Development and Psychopathology, 26(4pt1), 901–916. doi: 10.1017/S095457941400056X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall A, Deater-Deckard K, & Riley AW (2015). Maternal emotion and cognitive control capacities and parenting: A conceptual framework. Developmental Review, 36, 105–126. doi: 10.1016/j.dr.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, & Staton L (2009). Marital conflict and children’s externalizing behavior: Pathways involving interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for research in Child Development, 74(1), vii–79. doi: 10.1111/j.1540-5834.2009.00501.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2012). Childhood poverty and young adults’ allostatic load: The mediating role of childhood cumulative risk exposure. Psychological Science, 23(9), 979–983. doi: 10.1177/0956797612441218 [DOI] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2007). Cumulative risk exposure and stress dysregulation. Psychological Science, 18(11), 953–958. doi: 10.1111/j.1467-9280.2007.02008.x [DOI] [PubMed] [Google Scholar]
- Evans GW, Li D, & Whipple SS (2013). Cumulative risk and child development. Psychological Bulletin, 139(6), 1342–1396. doi: 10.1037/a0031808 [DOI] [PubMed] [Google Scholar]
- Fisher PA, Leve LD, Delker B, Roos LE, & Cooper B (2016). A developmental psychopathology perspective on foster care research. In Cicchetti D (Ed.), Developmental psychopathology (3rd ed., pp. 513–548). New York, NY: Wiley. [Google Scholar]
- Fisher P, & Skowron E (2016). Social-learning parenting intervention research in the era of translational neuroscience. In van IJzendoorn M (Ed.), Current opinion in psychology. Amsterdam, Netherlands: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, & Calkins SD (2003). The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion, 27(1), 7–26. doi: 10.1023/A:1023622324898 [DOI] [Google Scholar]
- Gerstadt CL, Hong YJ, & Diamond A (1994). The relationship between cognition and action: Performance of children 3 1/2–7 years old on a Stroop-like day-night test. Cognition, 53(2), 129–153. doi: 10.1016/0010-0277(94)90068-X [DOI] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Sarapas C, Campbell M, Lewis GF, Bishop JR, & Shankman SA (2013). Relation between respiratory sinus arrythymia and startle response during predictable and unpredictable threat. Journal of Psychophysiology, 27(2), 95–104. doi: 10.1027/0269-8803/a000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P, & Derefinko K (2013). Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology, 94(1), 22–37. doi: 10.1016/j.biopsycho.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, & Sullivan C (2008). Applying the polyvagal theory to children’s emotion regulation: Social context, socialization, and adjustment. Biological Psychology, 79, 299–306. doi: 10.1016/j.biopsycho.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Hinnant JB, Erath SA, Tu KM, & El-Sheikh M (2016). Permissive parenting, deviant peer affiliations, and delinquent behavior in adolescence: the moderating role of sympathetic nervous system reactivity. Journal of Abnormal Child Psychology, 44(6), 1071–1081. doi: 10.1007/s10802-015-0114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Stellern SA, Schaefer C, Carlson SM, & Gunnar MR (2012). Associations between early life adversity and executive function in children adopted internationally from orphanages. Proceedings of the National Academy of Sciences, 109(S2), 17208–17212. doi: 10.1073/pnas.1121246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan LA, Evans L, & Monk C (2008). Effects of mothers’ prenatal psychiatric status and postnatal caregiving on infant biobehavioral regulation: Can prenatal programming be modified? Early Human Development, 84(4), 249–256. doi: 10.1016/j.earlhumdev.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Murray K, & Coy KC (1997). Inhibitory control as a contributor to conscience in childhood: From toddler to early school age. Child Development, 68(2), 263–277. doi: 10.2307/1131849 [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, & Harlan ET (2000). Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology, 36(2), 220–232. doi: 10.1037/0012-1649.36.2.220 [DOI] [PubMed] [Google Scholar]
- Lengua LJ, Honorado E, & Bush NR (2007). Contextual risk and parenting as predictors of effortful control and social competence in preschool children. Journal of Applied Developmental Psychology, 28(1), 40–55. doi: 10.1016/j.appdev.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Kiff C, Moran L, Zalewski M, Thompson S, Cortes R, & Ruberry E (2014). Parenting mediates the effects of income and cumulative risk on the development of effortful control. Social Development, 23(3), 631–649. doi: 10.1111/sode.12071 [DOI] [Google Scholar]
- Lengua LJ, Moran L, Zalewski M, Ruberry E, Kiff C, & Thompson S (2014). Relations of growth in effortful control to family income, cumulative risk, and adjustment in preschool-age children. Journal of Abnormal Child Psychology, 43(4), 705–720. doi: 10.1007/s10802-014-9941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü W, Wang Z, & You X (2016). Physiological responses to repeated stress in individuals with high and low trait resilience. Biological Psychology, 120, 46–52. doi: 10.1016/j.biopsycho.2016.08.005 [DOI] [PubMed] [Google Scholar]
- McDermott JM, Westerlund A, Zeanah CH, Nelson CA, & Fox NA (2012). Early adversity and neural correlates of executive function: Implications for academic adjustment. Developmental Cognitive Neuroscience, 2, S59–S66. doi: 10.1016/j.dcn.2011.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, … Fifer WP (2004). Fetal heart rate reactivity differs by women’s psychiatric status: An early marker for developmental risk? Journal of the American Academy of Child & Adolescent Psychiatry, 43(3), 283–290. doi: 10.1097/00004583-200403000-00009 [DOI] [PubMed] [Google Scholar]
- Mueller SC, Hardin MG, Korelitz K, Daniele T, Bemis J, Dozier M, … Ernst M (2012). Incentive effect on inhibitory control in adolescents with early-life stress: An antisaccade study. Child Abuse & Neglect, 36(3), 217–225. doi: 10.1016/j.chiabu.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović J (2012). How can the study of physiological reactivity contribute to our understanding of adversity and resilience processes in development?. Development and psychopathology, 24(02), 371–387. doi: 10.1017/S0954579412000053 [DOI] [PubMed] [Google Scholar]
- Pears KC, Fisher PA, Bruce J, Kim HK, & Yoerger K (2010). Early elementary school adjustment of maltreated children in foster care: The roles of inhibitory control and caregiver involvement. Child Development, 81(5), 1550–1564. doi: 10.1111/j.1467-8624.2010.01491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears KC, Kim HK, & Fisher PA (2012). Effects of a school readiness intervention for children in foster care on oppositional and aggressive behaviors in kindergarten. Children and Youth Services Review, 34(12), 2361–2366. doi: 10.1016/j.childyouth.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42(2), 123–146. doi: 10.1016/S0167-8760(01)00162-3 [DOI] [PubMed] [Google Scholar]
- Propper CB, & Holochwost SJ (2013). The influence of proximal risk on the early development of the autonomic nervous system. Developmental Review, 33(3), 151–167. doi: 10.1016/j.dr.2013.05.001 [DOI] [Google Scholar]
- Richters JE, & Saltzman W (1990). Survey of exposure to community violence: Parent Report version. JE Richters. [Google Scholar]
- Sarason I, Johnson J, & Siegel J (1978). Assessing the impact of life changes: Development of the Life Experiences Survey. Journal of Consulting and Clinical Psychology, 46, 932–946. doi: 10.1037/0022-006X.46.5.932 [DOI] [PubMed] [Google Scholar]
- Skowron EA, Cipriano-Essel E, Benjamin LS, Pincus AL, & Van Ryzin MJ (2013). Cardiac vagal tone and quality of parenting show concurrent and time-ordered associations that diverge in abusive, neglectful, and non-maltreating mothers. Couple & Family Psychology, 2(2), 95–115. doi: 10.1037/cfp0000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron EA, Cipriano-Essel E, Gatzke-Kopp LM, Teti DM, & Ammerman RT (2014). Early adversity, RSA, and inhibitory control: Evidence of children’s neurobiological sensitivity to social context. Developmental Psychobiology, 56(5), 964–978. doi: 10.1002/dev.21175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron EA, Loken E, Gatzke-Kopp LM, Cipriano-Essel EA, Woehrle PL, Van Epps JJ, & Ammerman RT (2011). Mapping cardiac physiology and parenting processes in maltreating mother-child dyads. Journal of Family Psychology, 25(5), 663–674. doi: 10.1037/a0024528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. doi: 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Vernon-Feagans L, Willoughby M, & Garrett-Peters P (2016). Predictors of behavioral regulation in kindergarten: Household chaos, parenting, and early executive functions. Developmental Psychology, 52(3), 430–441. doi: 10.1037/dev0000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn D, Adams J, Krohn J, Wenzel M, Mann CG, Gomille, … Kubiak T (2016). Heart rate variability and self-control—A meta-analysis. Biological Psychology, 115, 9–26. doi: 10.1016/j.biopsycho.2015.12.007 [DOI] [PubMed] [Google Scholar]