Abstract
Background
Massive bleeding is a major preventable cause of early death in trauma. It often requires surgical and/or endovascular intervention. We aimed to describe the utilization of angioembolization in patients with abdominal and pelvic traumatic bleeding at a level 1 trauma center.
Methods
We conducted a retrospective analysis for all trauma patients who underwent angioembolization post-traumatic bleeding between January 2012 and April 2018. Patients’ data and details of injuries, angiography procedures and outcomes were extracted from the Qatar national trauma registry.
Results
A total of 175 trauma patients underwent angioembolization during the study period (103 for solid organ injury, 51 for pelvic injury and 21 for other injuries). The majority were young males. The main cause of injury was blunt trauma in 95.4% of the patients. The most common indication of angioembolization was evident active bleeding on the initial CT scan (contrast pool or blushes). Blood transfusion was needed in two-third of patients. The hepatic injury cases had higher ISS, higher shock index and more blood transfusion. Absorbable particles (Gelfoam) were the most commonly used embolic material. The overall technical and clinical success rate was 93.7% and 95%, respectively, with low rebleeding and complication rates. The hospital and ICU length of stay were 13 and 6 days, respectively. The median injury to intervention time was 320 min while hospital arrival to intervention time was 274 min. The median follow-up time was 215 days. The overall cohort mortality was 15%.
Conclusion
Angioembolization is an effective intervention to stop bleeding and support nonoperative management for both solid organ injuries and pelvic trauma. It has a high success rate with a careful selection and proper implementation.
Keywords: angioembolization, trauma, injury, bleeding, pelvic, solid organ
Introduction
Trauma is a leading cause of death and morbidity worldwide.1 It is considered the leading cause in the first four decades of life and the third across all the age groups.1 Massive bleeding is the most preventable cause of early death in trauma.2 It often requires surgical and/or endovascular intervention. The surgical intervention, whether with definitive intention or damage control, is well established.3 Nevertheless, surgery is not always the optimal solution, especially for arterial bleeding from pelvic fractures and solid organs injury.3 The management of trauma patients has evolved in recent decades, especially with advancements of imaging and endovascular interventions techniques like embolizations, stenting, filter placement, and resuscitative endovascular balloon occlusion of the aorta (REBOA).4–6 The computed tomography replaced diagnostic angiography to a great extent, but the best utilization of therapeutic angiography versus surgical intervention remains ill-defined in managing traumatic bleeding.3–5 Moreover, the associated complications of angioembolization, both early and late, are also of consideration. Angioembolization is challenging and region-specific; however, many factors influence its success such as the possible underlying anatomic variation, presence of multiple bleeding sources, institutional logistics, resources and physician experience and preferences. The ligation of bleeding vessels is a well-established option for surgical hemostasis. However, it might not be always possible or allowed; for all vessels primarily if they are end-arteries supplying a vital organ or in “difficult to access” areas or in the presence of abundant collaterals like the pelvic region.3 Such a non-selective control of bleeding should be used only as a last resort and on desperation. A more selective sort of control would make the best-case scenario for a safer and better outcome with preservation of organ function.6 The last points define the exact rationale behind the recommendation to use selective and superselective endovascular approaches.6
Arteriography with angioembolization is a useful adjunct to support the success of non-operative management (NOM) of many injuries.5 It helps to evaluate and potentially treat bleeding of solid organs and other injuries as well as control of pseudoaneurysm and traumatic arterio-venous fistulae (AVF).3–6 Angioembolization is appropriate in centers where experienced interventional radiologists are timely available. Prior studies showed that angioembolization in trauma patients increases the success rate of NOM.3–11
The present study aims to describe the utilization of angioembolization in patients with abdominal and pelvic traumatic bleeding at a level 1 trauma center. We hypothesized that angioembolization is an effective and integral option in the management of bleeding in trauma patients.
Methods
Study Design
A retrospective study was conducted on trauma patients who were admitted to Hamad Trauma Center (HTC); the only level 1 trauma center in the country and underwent angioembolization between January 2012 and April 2018. Inclusion criteria were adult patients who sustained abdominal or pelvic traumatic bleeding with subsequent angiography plus endovascular interventions. We excluded patients presented with cardiac arrest on arrival to the hospital or with incomplete data.
Data were obtained from a prospectively maintained trauma registry of the HTC. This national trauma registry is a mature database that participates in both the National Trauma Data Bank (NTDB) and the Trauma Quality Improvement Program (TQIP) of the American College of Surgeons-Committee on Trauma (ACS-COT). The HTC is the only tertiary facility in the country which admits around 1500–1700 trauma patients annually.
All patients were initially evaluated and resuscitated following Advance Trauma Life Support (ATLS) guidelines and by the trauma team attendance. The indications for angioembolization include the evidence of active extravasation (arterial blush), pseudoaneurysm and/or AVF on the initial admission CT scan. Also, subsequent scans after damage control surgery or repeat scans during the hospital stay as per our protocol for high-grade injuries in the case of solid organ injury (SOI). Hemoglobin drop/or blood transfusion in NOM of SOI and pelvic fractures is also an indication in some cases with high-grade injury suggestive of high risk of bleeding and failure of NOM. If needed, according to the Tiles classification and hemodynamic stability, patients with pelvic fracture are referred to the orthopedic team for further and definitive treatment.
Data Collection
We recorded demographic information (age and gender), mechanisms of injuries, associated injuries including head, chest, abdomen, spine, upper and lower extremity; Glasgow Coma Score (GCS) at the emergency department, Abbreviated Injury Score (AIS), Injury Severity Score (ISS), initial vitals at ED such as systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR), shock index (SI), need for blood transfusion, number of blood units, massive transfusion protocol (MTP) activation, surgical intervention, angiography location, endovascular interventions (embolization and stenting), type of embolization (selective, non-selective, superselective, proximal or distal), the materials used and outcomes. Admission SI was defined as the initial HR divided by simultaneous SBP reading.12
Technical successful embolization was defined as cessation of vascular abnormality in post angioembolization (contrast medium extravasation, pseudoaneurysm and AVF) without any need for further endovascular or surgical interventions.6 The standard of care as per hospital policy dictates repeating CT scan in 48–72h in case of solid organ injury (SOI) and pelvis injury. Clinical success definition is radiologic and clinical evidence of bleeding control.6 Failure of angioembolization is defined as any unsuccessful arterial cannulation due to pathological or anatomical variations, failure of safe injection of embolic agents; re-bleeding in the same artery or a new bleeding in the same organ or territory. The procedure choices were left to interventional radiologist discretion, and technical demands. In general, left femoral artery access was the most prevailing. The vascular sheath is left for 24h considering the risk of rebleeding or need to repeat the angioembolization.
Complications Specific for the Angioembolization
Bleeding, ischemia, necrosis and contrast-induced nephropathy (CIN), coil migration, allergy and vascular access complications (hematoma, bleeding, pseudoaneurysm, infection, arteriovenous fistula, thrombosis, arterial dissection and injuries). The study outcomes included the success of angioembolization, complications, and mortality (in-hospital and during 1-year follow-up).
As this was a retrospective observational study with no direct contact with the patients, and data were kept anonymous and confidential and in compliance with the Declaration of Helsinki, the study was approved with a waiver of consent by the Institutional Review Board (IRB#MRC-01-18-125) at Medical Research Center, Hamad Medical Corporation Doha, Qatar. The study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for observational study.
Statistical Analysis
Data were presented as proportions, medians with interquartile range (IQR), or mean ± standard deviation, as appropriate. Differences in categorical and continuous variables were analyzed using χ2 and Student t-test, as necessary. Yates’ corrected chi-square was used for categorical variables if the expected cell frequencies were below 5. Data were expressed by the odds ratio (OR) and 95% confidence intervals (CIs). A two-tailed P value of <0.05 was considered to be statistically significant. All data analyses were carried out using the Statistical Package for the Social Sciences, version 18 (SPSS, Inc, Chicago, IL).
Results
During the 5-year study period, a total of 9000 patients who sustained traumatic injuries were admitted to the HTC. One hundred and seventy-five patients underwent angioembolization (1.9% of the total trauma admissions). The majority were males (90%), and the mean age was 32.6±12.2 years. The most common mechanism of injury was blunt trauma in 95.4% of the patients (Table 1).
Table 1.
Demographics, Associated Injuries and Clinical Characteristics of Trauma Patients Requiring Angioembolization (n=175)
| Variables | Values | Variables | Values |
|---|---|---|---|
| Age (mean±SD) | 32.6±12.2 | Arterial embolization | |
| Males | 158 (90.3%) | Facial | 3 (1.7%) |
| Mechanism of injury | Gastric | 1 (0.6%) | |
| Motor vehicle crash | 60 (34.3%) | Hepatic | 43 (24.9%) |
| Motor cycle crash | 8 (4.6%) | Splenic | 54 (31.2%) |
| All-terrain vehicle | 4 (2.3%) | Internal Iliac | 50 (28.9%) |
| Pedestrian | 36 (20.6%) | Intercostal | 2 (1.2%) |
| Fall from height | 49 (28.0%) | Lumber | 6 (3.5%) |
| Fall of heavy object | 10 (5.7%) | Renal | 6 (3.5%) |
| Others | 8 (4.6%) | Retroperitoneal | 1 (0.6%) |
| Injuries | Superior mesenteric artery | 1 (0.6%) | |
| Head | 54 (30.9%) | Subclavian artery | 1 (0.6%) |
| Chest | 135 (77.1%) | Sacral | 1 (0.6%) |
| Abdomen | 151 (86.3%) | Others | 4 (2.3%) |
| Injury severity score | 28.0±12.3 | Follow-up days | 101 (1–365) |
| ISS>15 | 154 (88.0%) | ||
| Initial vitals | |||
| Pulse rate | 96.0±25.2 | ||
| Systolic blood pressure | 117.4±26.9 | ||
| Diastolic blood pressure | 75.4±21.7 | ||
| Glasgow Coma Scale | 12±3.0 |
The average ISS was 28±12.3. The majority presented initially with pulse rate 96.0±25.0 beat/min and the mean SI was 0.86±0.31. The median follow-up period was 215 days.
Endovascular angioembolization was used in SOI (liver, spleen, kidney, pancreas and adrenals), for musculoskeletal injuries (pelvic, lumbar, retroperitoneal and others) and two cases of hollow viscus related bleeding (gastric and superior mesenteric artery). The most common involved arteries were the splenic (31%), internal iliac artery (29%), and hepatic artery (25%). The rest of the places were sporadic or just a few.
Table 2 shows details of arterial embolization, timing, indication, location, type of embolic agent, complications and outcomes. Seventy-three percent of cases had successful NOM. In contrast, the pre-surgery angioembolization used in 10% and post-surgery in 15% and 2% had angioembolization before and after surgical interventions. The primary indication of angioembolization was based on CT findings, ie, active bleeding (51.7%) and blush (19.1%), presence of pseudo-aneurysm (12.6%), and true aneurysm (1.7%) or intraoperative finding (active bleeding (14.9%).
Table 2.
Details of Arterial Embolization, Complications and Outcomes
| Variables | Values | Variables | Values |
|---|---|---|---|
| Timing of embolization | - Polyvinyl alcohol | 1 (0.6%) | |
| No surgical intervention | 128 (73.1%) | - Onyx | 1 (0.6%) |
| Embolization before surgery | 18 (10.3%) | Gel foam and coil (both) | 28 (16.2%) |
| Embolization after surgery | 26 (14.9%) | Stents | 1 (0.6%) |
| Before and after surgery | 3 (1.7%) | Number of sessions | 1 (IQR 1–2) |
| Indications for embolization* | Number of arteries/branches embolizedb | 1 (IQR 1–4) | |
| Active bleeding on CT | 90 (51.7%) | Technical success | 164 (93.7%) |
| Blush on CT scan | 33 (19.1%) | Clinical success | 166 (94.9%) |
| Pseudoaneurysm | 22 (12.6%) | Re-bleed | 9 (5.1%) |
| True aneurysm | 3 (1.7%) | Complications and management | 38 (21.7%) |
| Intraoperative active bleeding | 12 (6.9%) | Infarction needs surgical intervention | 6 (15.8%) |
| Active bleeding and pseudoaneurysm | 14 (8.0%) | Hepatic failure | 0 (0.0%) |
| Embolization type** | Abscess | 3 (7.9%) | |
| Failure of angioembolization | 1 (0.6%) | Infection | 4 (10.5%) |
| Non-selective | 39 (22.7%) | Gallbladder infarction needs cholecystectomy | 1 (2.6%) |
| Selective | 53 (30.8%) | Bowel Ischemia | 2 (5.3%) |
| Highly selective | 59 (34.3%) | Dislodgement of coil in the common femoral artery repaired by snare | 1 (2.6%) |
| Combination | 20 (11.6%) | Pseudoaneurysm of common femoral artery treated with thrombin injection | 1 (2.6%) |
| Location of embolization*** | |||
| No embolization due to technical failure | 5 (2.9%) | Exploratory laparotomy | 47 (26.9%) |
| Proximal | 79 (46.2%) | Damage control with open abdomen | 20 (42.6%) |
| Distal | 68 (39.8%) | MTP activation | 59 (33.7%) |
| Proximal and distal | 12 (7.0%) | Blood transfusion | 131 (74.9%) |
| Embolization to more than one area | 5 (2.9%) | Number of blood units | 8 (IQR 1–79) |
| Embolization to more than site in different organs | 2 (1.2%) | ICU length of stay | 6 (IQR 1–57) |
| Embolic agentsa | Ventilatory says | 9 (IQR 1–53) | |
| No embolization due to technical failure | 5 (2.9%) | Hospital length of stay | 13 (IQR 1–106) |
| Temporary | Mortality | 26 (15%) | |
| - Gel foam | 98 (56.6%) | Follow-up days | 215 (IQR 1–365) |
| Permanent | |||
| Coils (pushable/injectable/detachable) | 40 (23.1%) |
Notes: *Data available for 174 cases; **data available for 172 cases; ***data available for 171 cases; adata available for 173 cases; bdata available for 167 cases IQR: interquartile range.
Embolization Approaches
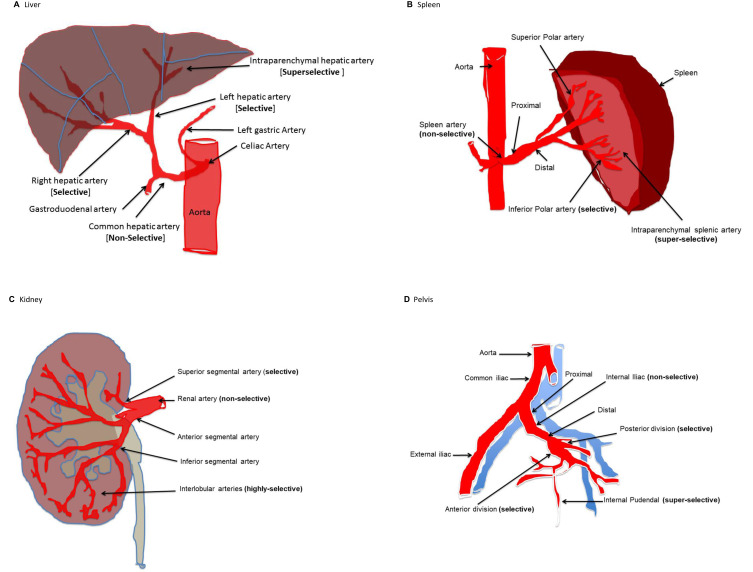
Non-selective embolization (catheter placed in the main trunk) in 23%, selective (catheter placed in first order) embolization of in 31%, superselective (catheter placed in second or third order) embolization in 34% and combined approach in 12%. In terms of the proximity to a given artery: proximal embolization was done in 46% and distal in 40% and both proximal and distal in 7%. Figure 1 shows examples of selectivity and proximity of angioembolization in different organs.
Figure 1.
Examples of selectivity and proximity of angioembolization in different injured organs (A: liver, B: spleen, C: kidney, D: pelvis).
Table 2 shows the materials used for angioembolisation. Temporary material was most commonly used in 57% (Gelfoam), while permanent materials such as coils were used in 23.1%.
The technical success rate was 93.7% correspond to the clinical success of 94.9% with a rebleeding rate of 5% (in 9 cases).
The angioembolization complications included infarction (extensive necrosis) in six patients (16%) and required surgical debridement, infection in seven cases (three had an abscess), one case developed gall bladder necrosis and gangrene demanded subsequent cholecystectomy, and two cases had bowel gangrene (Table 2). An algorithm for the management of bleeding in pelvic and abdominal solid organ injuries is shown in Figure 2.
Figure 2.
Algorithm for angioembolization in solid organ injury and pelvic injury.
Massive transfusion protocol was activated in 34% of the cases, while blood transfusion use was reported in 75% of the cases, with an average of 8 (1–79) units transfused. The average length of stay in ICU was 6 (1–57) days and in hospital was 13 (1–106) days. The overall mortality was 15% (26 cases), and there was no reported angiography-related mortality. The majority of deaths occurred due to multiorgan failure (15 patients) followed by the associated head injury (7 patients), gastrointestinal bleeding and pulmonary embolism (1 patient), pulmonary embolism (1 patient), gastrointestinal bleeding (1 patient), and cardiac arrest (1 patient) (Table 3).
Table 3.
Cause of Death
| Overall (n=26) | Mortality ≤30 Days (n=14) | Mortality >30 Days (n=12) | |
|---|---|---|---|
| Multiorgan failure post-cardiac arrest before arrival to the emergency department | 10 (38.5%) | 7 (50.0%) | 3 (25.0%) |
| In-hospital multiorgan failure | 4 (15.4%) | 3 (21.4%) | 1 (8.3%) |
| Head injury | 7 (26.9%) | 3 (21.4%) | 4 (33.3%) |
| Multiorgan failure/preexisting Non-Hodgkin lymphoma/Hepatitis C virus with liver cirrhosis | 1 (3.8%) | 0 (0.0%) | 1 (0.0%) |
| Gastrointestinal bleeding and pulmonary embolism | 1 (3.8%) | 0 (0.0%) | 1 (8.3%) |
| Gastrointestinal bleeding | 1 (3.8%) | 0 (0.0%) | 1 (8.3%) |
| Pulmonary embolism | 1 (3.8%) | 1 (7.2%) | 0 (0.0%) |
| Cardiac arrest | 1 (3.8%) | 0 (0.0%) | 1 (8.3%) |
Tables 4 and 5 compare the demography, clinical characteristics, and outcomes based on the anatomical arteries angio-embolized (hepatic, splenic, renal and pelvic). The hepatic cases had higher ISS, higher need for surgery (laparotomy) and blood transfusion. Also, the hepatic group was more likely to be embolized prior to surgery and had prolonged ICU and hospital stay in comparison to other groups (p=0.001). On the other hand, one-third of the patients in renal group underwent embolization after surgery (p=0.001). Figure 3 shows the time of angioembolization in relation to surgical intervention such as exploratory laparotomy. There was no preperitoneal pelvic packing but intrapelvic packing.
Table 4.
Demographics, Clinical Characteristics and Outcome Based on Anatomical Location and Timing of Arterial Angioembolization*
| Variables | Hepatic (n=43) | Splenic (n=54) | Pelvic (n=51) | Renal (n=6) | P value |
|---|---|---|---|---|---|
| Age | 30.4±10.9 | 32.3±13.6 | 33.6±11.2 | 24.5±5.6 | 0.02 |
| Males | 40 (93.0%) | 53 (98.1%) | 40 (78.4%) | 6 (100%) | 0.007 |
| Injury severity score | 31.3±10.7 | 24.2±11.2 | 30.2±12.2 | 29.1±10.6 | 0.03 |
| Shock index ≥0.80 | 63% | 32% | 58.5% | 50% | 0.04 |
| Exploratory laparotomy | 27 (62.8%) | 2 (3.7%) | 12 (23.5%) | 2 (33.3%) | 0.001 |
| MTP Activation | 21 (48.8%) | 2 (3.7%) | 25 (49.0%) | 3 (50.0%) | 0.001 |
| Blood transfusion | 38 (88.4%) | 22 (40.7%) | 49 (96.1%) | 5 (83.3%) | 0.001 |
| Number of blood units | 10 (1–73) | 2 (1–18) | 10 (1–42) | 13 (2–26) | 0.001 |
| ICU length of stay | 14 (2–57) | 2 (1–41) | 6.5 (1–47) | 5 (2–15) | 0.001 |
| Hospital length of stay | 25 (2–102) | 8 (4–106) | 22 (1–102) | 8.5 (2–52) | 0.001 |
| Surgical intervention | |||||
| No surgical intervention | 19 (44.2%) | 51 (94.4%) | 37 (72.5%) | 4 (66.7%) | 0.001 for all |
| Embolization before surgery | 9 (20.9%) | 3 (5.6%) | 4 (7.8%) | 0 (0.0%) | |
| Embolization after surgery | 12 (27.9%) | 0 (0.0%) | 10 (19.6%) | 2 (33.3%) | |
| Both (pre and post-surgical intervention) | 3 (6.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Follow-up (days) | 126 (1–346) | 109 (6–342) | 102 (1–365) | 6 (2–154) | 0.24 |
| Mortality | 7 (16.3%) | 1 (1.9%) | 10 (19.6%) | 2 (33.3%) | 0.008 |
Note: *Other sporadic vessels were excluded from this comparative analysis (n=19 cases).
Table 5.
Timing, Indication, Type and Location of Embolization Based on Anatomical Location*
| Hepatic (n=43) | Splenic (n=54) | Pelvic (n=51) | Renal (n=6) | P value | |
|---|---|---|---|---|---|
| Indications for embolization | |||||
| Active bleeding on CT | 17 (39.5%) | 21 (38.9%) | 36 (72.0%) | 4 (66.7%) | 0.001 for all |
| Blush on CT scan | 9 (20.9%) | 14 (25.9%) | 6 (12.0%) | 0 (0.0%) | |
| Pseudoaneurysm | 8 (18.6%) | 11 (20.4%) | 1 (2.0%) | 0 (0.0%) | |
| True aneurysm | 0 (0.0%) | 3 (5.6%) | 0 (0.0%) | 0 (0.0%) | |
| Intraoperative active bleeding | 6 (14.0%) | 0 (0.0%) | 6 (12.0%) | 0 (0.0%) | |
| Active bleeding and pseudoaneurysm | 3 (7.0%) | 5 (9.3%) | 1 (2.0%) | 2 (33.3%) | |
| Embolization type | |||||
| Non-selective | 6 (14.3%) | 26 (49.1%) | 3 (5.9%) | 2 (33.3%) | 0.001 for all |
| Selective | 12 (28.6%) | 4 (7.5%) | 24 (47.1%) | 2 (33.3%) | |
| Highly selective | 18 (42.9%) | 12 (22.6%) | 22 (43.1%) | 1 (16.7%) | |
| Combination | 5 (11.9%) | 11 (20.8%) | 2 (3.9%) | 1 (16.7%) | |
| Location of embolization | |||||
| Proximal | 13 (31.7%) | 22 (41.5%) | 30 (58.8%) | 2 (33.3%) | 0.005 for all |
| Distal | 18 (43.9%) | 21 (39.6%) | 20 (39.2%) | 3 (50.0%) | |
| Proximal and distal both | 3 (7.3%) | 8 (15.1%) | 1 (2.0%) | 0 (0.0%) | |
| Embolization to more than one area | 3 (7.3%) | 1 (1.9%) | 0 (0.0%) | 0 (0.0%) | |
| Embolization to more than site in different organs | 1 (2.4%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | |
| No embolization due to technical failure | 3 (7.1%) | 1 (1.9%) | 0 (0.0%) | 0 (0.0%) | 0.001 for all |
| Temporary agent | 25 (59.5%) | 14 (26.4%) | 45 (88.2%) | 4 (66.7%) | |
| Permanent agent | 4 (9.5%) | 31 (58.5%) | 1 (2.0%) | 0 (0.0%) | |
| Both agents | 10 (23.8%) | 7 (13.2%) | 5 (9.8%) | 2 (33.3%) | |
| Stents | 0 (0.0%) | 1 (1.9%) | 0 (0.0%) | 0 (0.0%) | 0.68 |
| Number of sessions | 1 (1–2) | 1 (1–2) | 1 (1–1) | 1 (1–2) | 0.02 |
| Number of embolized vessel | 1 (1–4) | 1 (1–3) | 1 (1–2) | 1.5 (1–3) | 0.71 |
| Technical success | 37 (86.0%) | 51 (94.4%) | 51 (100%) | 5 (83.3%) | 0.06 |
| Clinical success | 37 (86.0%) | 53 (98.1%) | 51 (100%) | 5 (83.3%) | 0.01 |
| Re-bleed | 6 (14.0%) | 2 (3.7%) | 0 (0.0%) | 1 (16.7%) | 0.01 |
Note: *Other sporadic vessels were excluded from this comparative analysis (n=19 cases).
Figure 3.
Time of angioembolisation in relation to surgical intervention.
The splenic artery cases were the larger group but with lower ISS, only two needed laparotomy, 40% received blood and only 2 (4%) needed MTP. The pelvic group were the second larger group, older in age, had lower male percentage compared to others, laparotomy needed in 12 (23.5%), MTP was needed in 49% and blood transfusion was used in 96%.
While the renal embolization was performed in six young male patients with higher blood unit usage, active bleeding on CT scan was the only indication for the angioembolization with higher mortality (33%) among the groups.
Technical failure reported in 3 of the hepatic (7.3%), and one of the splenic (1.9%), non reported in the pelvic or renal angioembolized patients. Shock index ≥0.80 was more evident with hepatic (63%), pelvic (58.5%), renal (50%) and splenic group (32%); p=0.04.
Single session of angioembolization was the most common in the cohort. The Absorbable embolic agent (Gelfoam) was the most commonly used material for embolization.
The technical and clinical success were 86% for hepatic cases, 100% for pelvic and 83.3% for renal injury cases. Few cases had a rebleed mainly in the hepatic group (6 cases), two patients in the splenic and one in the renal group.
Time to angioembolization in SOI and pelvic injury is given in Table 6.
Table 6.
Time to Angioembolization
| SOI (n=56) | Pelvis (n=32) | P value | |
|---|---|---|---|
| Hospital arrival to intervention time (min) | 273 (46–1259) | 220 (79–997) | 0.12 |
| Hospital arrival to intervention time <180 | 15 (27.3%) | 10 (31.3%) | 0.69 for all |
| Hospital arrival to intervention time ≥180 | 40 (72.7%) | 22 (68.8%) | |
| Injury to intervention time (min) | 418 (96–1397) | 275.5 (168–1057) | 0.01 |
| Injury to intervention time <180 | 4 (9.8%) | 3 (13.6%) | 0.64 for all |
| Injury to intervention time ≥180 | 37 (90.2%) | 19 (86.4%) | |
| Intervention time (min) | 67 (11–185) | 59 (29–165) | 0.67 |
Overall complications were rare; one case had femoral pseudoaneurysm and the procedure was well tolerated. Infarction demanding surgical intervention was noticed in four hepatic cases and one splenic case. All infarcted cases had a sort of infection and one of them had liver abscess. Only one case of hepatic angioembolization had gall bladder necrosis and needed open cholecystectomy and one case had bowel ischemia following mesenteric angioembolization. The open abdomen (damage control surgery; DCS) approach was used in nine of the hepatic cases, eight of the pelvic cases and two of the renal while the splenic group had zero DCS. During the follow-up period (median 215 days), there was no reported mortality.
Discussion
This is the first report on the use of angioembolization from the national trauma center of Qatar.
The overall frequancy of angioembolization was 1.9% among the total trauma admission in the present study. The majority were young male, and had blunt poly-trauma with high ISS. The most common aniogioembolized organ was the spleen followed by pelvis and liver. Previous studies showed that shock index ≥0.80 after injury can be used to predict the early need for MTP, laparotomy and mortality.12 The current study showed that the mean shock index for the cohort was greater than 0.80 and this figure was more likely seen post-hepatic (SI=0.91) and pelvic (SI=0.90) injury.
The majority of patients in this study had initial CT scan based on the hemodynamic status. The indication for angioembolization as part of the NOM was considered according to the CT findings in the majority of cases. Some cases with hemodynamic instability underwent DCS followed with angioembolization based on the intraoperative findings. The blush as an indication for angioembolization is still debatable. However, it remains a high-risk factor for failure of NOM for SOI.13–25 However, Diamond et al; reported that nearly half of patients may not need any interventions especially in the retroperitoneum and the pelvis areas regardless of the size or volume of the bleed.26,27
Selection of embolic agents in trauma patients is guided by the size of the vessels to be occluded and permanence of the desired occlusion. Coils are also commonly used for trauma patients, most often when permanent occlusion is desired. The most common technique was the use of a micro-catheter system to achieve the superselective or the selective approaches in our cohort. Sclafani et al introduced the concept of proximal splenic embolization with a high success rate of 97%.28 This was preferably proximal to achieve hemostasis with clotting and decrease pressure while preserving the tissues and function through collaterals and decrease complications but to restrict the subsequent superselective embolization for re-bleeding cases. The superselective approach which is more demanding from a skill perspective and time consuming represents a technical challenge and explains in part the lower percentage of this approach in unstable cases. Our overall success rate was 95% which relatively higher than Velmahos et al series in Los Angeles who reported 91% success rate but with more pelvic injury cases.29 Overall, the high success rates (clinical and technical) correspond to the reported range (77–100%) with an average of 93%.30,31 Recurrent bleeding after the initial attempt at angioembolization can be treated successfully with repeated angioembolization. We have relatively low complication rates like re-bleeding, necrosis, and infection. The overall mortality after angioembolization ranges between 16% and 50% and often related to the associated injuries.31
Limitations
We acknowledge the boundaries of our study. The retrospective study design and single trauma center experience are limitations with possible selection bias and missing information; however, it is a representative nationwide data that also have regular internal and external validation. Although time to intervention is important, it was lacking in many cases. Recent studies showed that the utility, priority and timing of angioembolization in patients with pelvic fractures remain not well-settled and not protocolized in many centers.31 Details of surgical interventions for pelvic injury were not fully described; however, our experience in this regard was published before.32,33
Conclusions
Angioembolization is an effective intervention to stop bleeding and support nonoperative management for both solid organ injuries as well as pelvic fracture. It has a high success rate with a careful selection and proper implementation.
Acknowledgment
The authors thank all the staff of the trauma registry database at the trauma surgery section.
Abbreviations
ISS, injury severity score; NOM, non-operative management; DCM, damage control management.
Data Sharing Statement
All data were shown in the study analysis and tables.
Ethics Approval and Consent to Participate
As this was a retrospective observational study with no direct contact with the patients, and data were kept anonymous and confidential and in compliance with the Declaration of Helsinki, the study was approved with a waiver of consent by the Institutional Review Board (IRB#MRC-01-18-125) at Medical Research Center, Hamad Medical Corporation Doha, Qatar.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Papakostidis C, Kanakaris N, Dimitriou R, Giannoudis PV. The role of arterial embolization in controlling pelvic fracture haemorrhage: a systematic review of the literature. Eur J Radiol. 2012;81(5):897‐904. doi: 10.1016/j.ejrad.2011.02.049 [DOI] [PubMed] [Google Scholar]
- 2.Geeraedts LM Jr, Kaasjager HA, van Vugt AB, Frölke JP. Exsanguination in trauma: a review of diagnostics and treatment options. Injury. 2009;40(1):11‐20. doi: 10.1016/j.injury.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 3.Zealley IA, Chakraverty S. The role of interventional radiology in trauma. BMJ. 2010;340(feb08 2):c497. doi: 10.1136/bmj.c497 [DOI] [PubMed] [Google Scholar]
- 4.Nicodemo A, Decaroli D, Pallavicini J, Sivieri R, Aprato A, Massè A. A treatment protocol for abdomino-pelvic injuries. J Orthop Traumatol. 2008;9(2):89‐95. doi: 10.1007/s10195-008-0003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoff WS, Holevar M, Nagy KK, et al. Practice management guidelines for the evaluation of blunt abdominal trauma: the East practice management guidelines work group. J Trauma. 2002;53(3):602‐615. doi: 10.1097/00005373-200209000-00038 [DOI] [PubMed] [Google Scholar]
- 6.Ierardi AM, Duka E, Lucchina N, et al. The role of interventional radiology in abdominopelvic trauma. Br J Radiol. 2016;89(1061):20150866. doi: 10.1259/bjr.20150866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnüriger B, Inaba K, Konstantinidis A, Lustenberger T, Chan LS, Demetriades D. Outcomes of proximal versus distal splenic artery embolization after trauma: a systematic review and meta-analysis. J Trauma. 2011;70(1):252‐260. doi: 10.1097/TA.0b013e3181f2a92e [DOI] [PubMed] [Google Scholar]
- 8.Quencer KB, Smith TA. Review of proximal splenic artery embolization in blunt abdominal trauma. CVIR Endovasc. 2019;6(1):11. doi: 10.1186/s42155-019-0055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salcedo ES, Brown IE, Corwin MT, Galante JM. Angioembolization for solid organ injury: a brief review. Int J Surg. 2016;33(Pt B):225‐230. doi: 10.1016/j.ijsu.2015.10.030 [DOI] [PubMed] [Google Scholar]
- 10.Gheju I, Beuran M. The role of angiography and embolization in blunt splenic trauma. Chirurgia (Bucharest, Romania: 1990). 2014;109(4):433‐438. [PubMed] [Google Scholar]
- 11.Pryor JP, Braslow B, Reilly PM, Gullamondegi O, Hedrick JH, Schwab CW. The evolving role of interventional radiology in trauma care. J Trauma. 2005;59(1):102‐104. doi: 10.1097/01.TA.0000171455.66437.DE [DOI] [PubMed] [Google Scholar]
- 12.El-Menyar A, Jabbour G, Asim M, Abdelrahman H, Mahmood I, Al-Thani H. Shock index in patients with traumatic solid organ injury as a predictor of massive blood transfusion protocol activation. Inj Epidemiol. 2019;6(1):41. doi: 10.1186/s40621-019-0218-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goslings JC, van Delden OM. Angiografie en embolisatie van bloedingen na stomp buik- of bekkenletsel [Angiography and embolisation to control bleeding after blunt injury to the abdomen or pelvis]. Ned Tijdschr Geneeskd. 2007;151(6):345‐352. [PubMed] [Google Scholar]
- 14.Gamanagatti S, Rangarajan K, Kumar A. Blunt abdominal trauma: imaging and intervention. Curr Probl Diagn Radiol. 2015;44(4):321‐336. doi: 10.1067/j.cpradiol.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 15.Charbit J, Manzanera J, Millet I, et al. What are the specific computed tomography scan criteria that can predict or exclude the need for renal angioembolization after high-grade renal trauma in a conservative management strategy? J Trauma. 2011;70(5):1219‐1228. doi: 10.1097/TA.0b013e31821180b1 [DOI] [PubMed] [Google Scholar]
- 16.Bhullar IS, Frykberg ER, Tepas JJ, Siragusa D, Loper T, Kerwin AJ. At first blush: absence of computed tomography contrast extravasation in Grade IV or V adult blunt splenic trauma should not preclude angioembolization. J Trauma Acute Care Surg. 2013;74(1):105‐112. doi: 10.1097/TA.0b013e3182788cd2 [DOI] [PubMed] [Google Scholar]
- 17.Burlew CC, Kornblith LZ, Moore EE, et al. Blunt trauma induced splenic blushes are not created equal. World J Emerg Surg. 2012;7(1):8. doi: 10.1186/1749-7922-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Post R, Engel D, Pham J, Barrios C. Computed tomography blush and splenic injury: does it always require angioembolization? Am Surg. 2013;79(10):1089‐1092. [PubMed] [Google Scholar]
- 19.Olthof DC, van der Vlies CH, Joosse P, et al. Consensus strategies for the nonoperative management of patients with blunt splenic injury: a Delphi study. J Trauma Acute Care Surg. 2013;74(6):1567‐1574. doi: 10.1097/TA.0b013e3182921627 [DOI] [PubMed] [Google Scholar]
- 20.Bansal S, Karrer FM, Hansen K, Partrick DA. Contrast blush in pediatric blunt splenic trauma does not warrant the routine use of angiography and embolization. Am J Surg. 2015;210(2):345‐350. doi: 10.1016/j.amjsurg.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 21.Hotaling JM, Sorensen MD, Smith TG, Rivara FP, Wessells H, Voelzke BB. Analysis of diagnostic angiography and angioembolization in the acute management of renal trauma using a national data set. J Urol. 2011;185(4):1316‐1320. doi: 10.1016/j.juro.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu CY, Wu SC, Chen RJ, et al. Evaluation of need for angioembolization in blunt renal injury: discontinuity of Gerota’s fascia has an increased probability of requiring angioembolization. Am J Surg. 2010;199(2):154‐159. doi: 10.1016/j.amjsurg.2008.12.023 [DOI] [PubMed] [Google Scholar]
- 23.Asensio JA, Petrone P, García-Núñez L, Kimbrell B, Kuncir E. Multidisciplinary approach for the management of complex hepatic injuries AAST-OIS grades IV-V: a prospective study. Scand J Surg. 2007;96(3):214‐220. doi: 10.1177/145749690709600306 [DOI] [PubMed] [Google Scholar]
- 24.Letoublon C, Morra I, Chen Y, Monnin V, Voirin D, Arvieux C. Hepatic arterial embolization in the management of blunt hepatic trauma: indications and complications. J Trauma. 2011;70(5):1032‐1037. doi: 10.1097/TA.0b013e31820e7ca1 [DOI] [PubMed] [Google Scholar]
- 25.Fang JF, Chen RJ, Wong YC, et al. Classification and treatment of pooling of contrast material on computed tomographic scan of blunt hepatic trauma. J Trauma. 2000;49(6):1083‐1088. doi: 10.1097/00005373-200012000-00018 [DOI] [PubMed] [Google Scholar]
- 26.Diamond IR, Hamilton PA, Garber AB, et al. Extravasation of intravenous computed tomography scan contrast in blunt abdominal and pelvic trauma. J Trauma. 2009;66(4):1102‐1107. doi: 10.1097/TA.0b013e318174f13d [DOI] [PubMed] [Google Scholar]
- 27.Bhakta A, Magee DS, Peterson MS, O’Mara MS. Angioembolization is necessary with any volume of contrast extravasation in blunt trauma. Int J Crit Illn Inj Sci. 2017;7(1):18‐22. doi: 10.4103/IJCIIS.IJCIIS_125_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sclafani SJ, Shaftan GW, Scalea TM, et al. Nonoperative salvage of computed tomography-diagnosed splenic injuries: utilization of angiography for triage and embolization for hemostasis. J Trauma. 1995;39(5):818‐827. doi: 10.1097/00005373-199511000-00004 [DOI] [PubMed] [Google Scholar]
- 29.Velmahos GC, Chahwan S, Falabella A, Hanks SE, Demetriades D. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J Surg. 2000;24(5):539‐545. doi: 10.1007/s002689910087 [DOI] [PubMed] [Google Scholar]
- 30.Green CS, Bulger EM, Kwan SW. Outcomes and complications of angioembolization for hepatic trauma: a systematic review of the literature. J Trauma Acute Care Surg. 2016;80(3):529‐537. doi: 10.1097/TA.0000000000000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaidya R, Waldron J, Scott A, Nasr K. Angiography and embolization in the management of bleeding pelvic fractures. J Am Acad Orthop Surg. 2018;26(4):e68–e76. doi: 10.5435/JAAOS-D-16-00600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdelrahman H, El-Menyar A, Keil H, et al. Patterns, management, and outcomes of traumatic pelvic fracture: insights from a multicenter study. J Orthop Surg Res. 2020;15(1):249. doi: 10.1186/s13018-020-01772-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Menyar A, Abdelrahman H, Alhammoud A, et al. Prognostic role of shock index in traumatic pelvic fracture: a retrospective analysis. J Surg Res. 2019;243:410–418. doi: 10.1016/j.jss.2019.05.062 [DOI] [PubMed] [Google Scholar]