Abstract
Background:
The cingulum bundle (CB), specifically the dorsal anterior portion of the CB, plays an important role in psychiatric illnesses; however, its role during early development is unclear. This study investigated whether neonatal white matter microstructure in the CB and its subregions is associated with subsequent preterm behavioral phenotype symptoms (internalizing, inattention, and social deficits) in very preterm (VPT) children.
Method:
Diffusion MRI data were obtained on a 3T scanner in 138 sleeping, non-sedated neonates, including 55 full-term (FT) neonates (gestational age (GA) ≥36 weeks) and 83 VPT neonates (GA <30 weeks). The CB was tracked using probabilistic tractography and split into anterior and posterior portions. At 5 years of age, parents (n=80) and teachers (n=63) of VPT children completed questionnaires of preterm behavioral phenotype symptoms. Linear regression models were used to relate measures of neonatal CB microstructure and childhood preterm behavioral phenotype symptoms (n=56 parent report, n=45 teacher report).
Results:
Mean diffusivity in the anterior and posterior CB was increased in VPT neonates compared to FT neonates. Increased fractional anisotropy and decreased mean diffusivity in the right anterior CB, but not the posterior CB, were related to increased preterm behavioral phenotype symptoms as reported by parents and teachers in VPT children.
Conclusion:
Aberrations in the anterior portion of the right CB may underlie the early development of the preterm behavioral phenotype. This finding provides the foundation for future mechanistic and therapeutic investigations into the role of the anterior cingulum in the development of psychopathology in VPT infants.
Keywords: Neonatal, DTI, Preterm, Anxiety, Autism, ADHD
Introduction
Premature birth remains a major public health issue in the United States, with approximately 1 in 10 infants born prior to 37 weeks gestation (1). Preterm infants can experience a range of adverse outcomes, including psychiatric impairments such as internalizing disorders, attention deficit hyperactivity disorder (ADHD), and autism (2,3). Numerous studies have shown that preterm children demonstrate specific impairments in three key domains of psychopathology – emotional processing, attention, and social-communication – with rates of impairment three-to-four times higher in preterm children compared to full-term (FT) children (2,4). Furthermore, preterm infants born at earlier gestational ages have greater risks of psychiatric illnesses, with very preterm infants (VPT; born <32 weeks gestation) demonstrating greater risk than moderate-to-late preterm infants (5,6). The consistent pattern of elevated rates of internalizing disorders, ADHD, and autism (without concurrently elevated rates of conduct disorder) has been recognized as the ‘preterm behavioral phenotype’ (2,3,7,8).
Preterm infants have also been shown to demonstrate altered structural and functional brain connectivity compared to their full-term peers. Multiple studies using conventional and diffusion MRI have demonstrated global gray and white matter alterations in VPT infants at term-equivalent age (9–12), with differences persisting into adolescence and adulthood (13,14). Diffusion tensor imaging (DTI) specifically has been used to assess these white matter abnormalities and link aberrant white matter microstructure with later psychiatric impairments. In VPT infants, neonatal white matter abnormalities qualitatively graded based on overall severity have been associated with lower socioemotional competence scores at age 2 years (15), with additional evidence demonstrating that the increased FA in the cingulum bundle (CB) may play a key role during early development of socio-emotional competence (16).
The CB is a white matter tract that connects the anterior cingulate, dorsolateral prefrontal, medial prefrontal, and orbitofrontal cortices to the insula and amygdala ventrally and to the posterior cingulate, parietal, hippocampal, and parahippocampal cortices posteriorly. The CB can first be observed at approximately 19–20 weeks GA, with myelination beginning postnatally and continuing throughout the first 24 months of life (17,18). The postnatal myelination of the CB may make it particularly susceptible to the effects of preterm birth and other forms of early life adversity (19–21). While the CB has typically been characterized as an anterior-to-posterior white matter tract, it also contains many short-range, lateral fibers (22). Histological and animal studies have divided the cingulum bundle into distinct segments: the subgenual, which connects the amygdala, insula, orbitofrontal, and medial prefrontal cortices; the dorsal anterior, which connects the frontal pole, dorsolateral prefrontal, anterior cingulate, and midcingulate cortices; the dorsal posterior, which connects the midcingulate, retrosplenial, and posterior cingulate cortices; and the temporal segments, which connect the medial parietal, lateral parietal, amygdala, hippocampal, and parahippocampal cortices (22,23), based on differences in anatomical properties and cytoarchitecture (22,23).
Prior research in children and adults has discovered specific functions for each of the segments of the CB. The dorsal and anterior subregions of the cingulum bundle have been associated with psychiatric illnesses, including ADHD (24,25), internalizing disorders (26–30), and autism (31–33), whereas the parahippocampal and posterior subregions have been associated with memory deficits (22). The anterior portion of the CB may be particularly important in psychiatric illnesses because of its connections within the prefrontal, orbitofrontal, and anterior cingulate cortices. These frontal regions – and their connections to the amygdala – are thought to support emotion processing, emotion regulation, and executive control (34–40) and to be involved in internalizing disorders, ADHD, and autism (16,24,34,40–55). However, it is unknown whether the subregions of the CB play a similar role in the early development of psychopathology in FT or VPT neonates. Prior research associating the dorsal CB with social competence in VPT infants indicates that either the dorsal anterior and/or dorsal posterior CB may play a role in early psychopathology (16). Defining the role of the dorsal anterior and dorsal posterior CB in early psychopathology is critical for guiding future mechanistic studies and clinical interventions, which are especially needed in VPT populations given the high risk of psychiatric impairments.
Subsequently, the aims of this study are to [1] examine whether VPT neonates have altered white matter microstructure in the dorsal anterior CB and the dorsal posterior CB compared to FT neonates, and [2] investigate the extent to which neonatal regional microstructural alterations in the CB relate to subsequent preterm behavioral phenotype symptoms as rated by both parents and teachers at age 5 years in a longitudinal cohort of VPT children. We hypothesized that increased FA and decreased MD, AD, and RD in the dorsal anterior CB, but not dorsal posterior CB, will be associated with preterm behavioral phenotype symptoms at age 5 years.
Methods and Materials
Sample and Procedure
This study used a prospective, longitudinal design that included neonatal neuroimaging and behavioral follow-up at age 5 years in a cohort of VPT and FT infants. VPT infants (born at <30 weeks GA, range=23–30 weeks; n=83) were recruited prospectively from the St. Louis Children’s Hospital level III Neonatal Intensive Care Unit. Singleton FT infants (born at ≥36 weeks GA, range=36–41 weeks; n=55) were recruited from the adjoining maternity ward at Barnes-Jewish Hospital. Exclusion criteria for both groups included chromosomal abnormalities, congenital infection, high-grade intraventricular hemorrhage, cystic periventricular leukomalacia, moderate-severe cerebellar hemorrhage, and/or deep nuclear gray matter lesions. Additional exclusion criteria for FT infants included a positive maternal urine drug screen or neonatal acidosis on cord blood gas (pH < 7.20).
DTI data were obtained as part of a multimodal acquisition on a Siemens Tim Trio 3T scanner (Erlangen, Germany) using an infant-specific quadrature head coil (Advanced Imaging Research, Cleveland, OH, USA). All infants were imaged without sedation during natural sleep or while resting quietly. FT infants were scanned within the first 48–72 hours of life and VPT infants were scanned at term-equivalent age (35 – 42 weeks GA). Medical personnel were present in the scanner room throughout the scan acquisition and monitored the infant’s heart rate and oxygen saturation continuously.
At age 5 years (mean=5.6 years; range=4.7–6.5 years), the parents of a subset of the VPT infants with neonatal neuroimaging (n=80) completed questionnaires to assess children’s psychiatric symptoms. Teachers of the children who participated at age 5 were contacted and asked to complete behavioral questionnaires that were analogous to the parent questionnaires. The demographic characteristics of this sample are displayed in Tables 1 (birth) and 2 (age 5). Written informed consent was obtained from primary caregivers prior to participation in the study. All study procedures were approved by Washington University Institutional Review Board.
Table 1.
Neonatal White Matter Microstructure Differences in the Cingulum Bundle
| Term (M ± SD) N=55 | Preterm (M ± SD) N=69 | p | |
|---|---|---|---|
| Gestational Age at Birth (weeks) | 38.9 ± 1.3 | 26.7 ± 1.8 | <.001 |
| Gestational Age at Scan (weeks) | 38.7 ± 1.2 | 37.5 ± 1.4 | <.001 |
| FA of Right CB | .184 ± .02 | .181 ± .02 | .715 |
| FA of Right Anterior CB | .168 ± .02 | .154 ± .02 | .060 |
| FA of Right Posterior CB | .219 ± .03 | .222 ± .02 | .479 |
| FA of Left CB | .194 ± .02 | .187 ± .01 | .724 |
| FA of Left Anterior CB | .175 ± .02 | .162 ± .02 | .155 |
| FA of Left Posterior CB | .223 ± .03 | .230 ± .02 | .278 |
| MD of Right CB | 1.35 ± .06 | 1.40 ± .06 | .002 |
| MD of Right Anterior CB | 1.37 ± .07 | 1.42 ± .08 | .002 |
| MD of Right Posterior CB | 1.32 ± .06 | 1.36 ± .06 | .005 |
| MD of Left CB | 1.34 ± .05 | 1.41 ± .07 | <.001 |
| MD of Left Anterior CB | 1.36 ± .06 | 1.43 ± .08 | .001 |
| MD of Left Posterior CB | 1.32 ± .06 | 1.37 ± .06 | <.001 |
| Male/Female | 24/31 | 29/40 | .857 |
| African American/Caucasian | 40/15 | 29/34* | .003 |
4 Asian & 2 biracial VPT participants
Bold represents p-values that passed FDR correction
Measures
DTI
DTI data were collected using a diffusion weighted sequence (TR=13300ms; TE=112ms; FoV=128mm, voxel size=1.2×1.2×1.2mm3, bandwidth=1266 Hz/Px, and 48b amplitudes and directions). The diffusion signal attenuation curve was modeled as a monoexponential function plus a constant, and diffusion parameters were established using Bayesian probability theory (56). Maps of fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) were generated. Region of interest masks were placed in the front and back of the dorsal CB with a forced waypoint in the middle. The dorsal CB (Figure 1A) and a control tract, the corpus callosum (See Supplement), were tracked in native space using Bayesian probabilistic tractography with FDT in FSL (FMRIB’s Software Library v6) (57,58). Due to technical limitations, the subgenual portion of the CB was not tracked.
Figure 1:
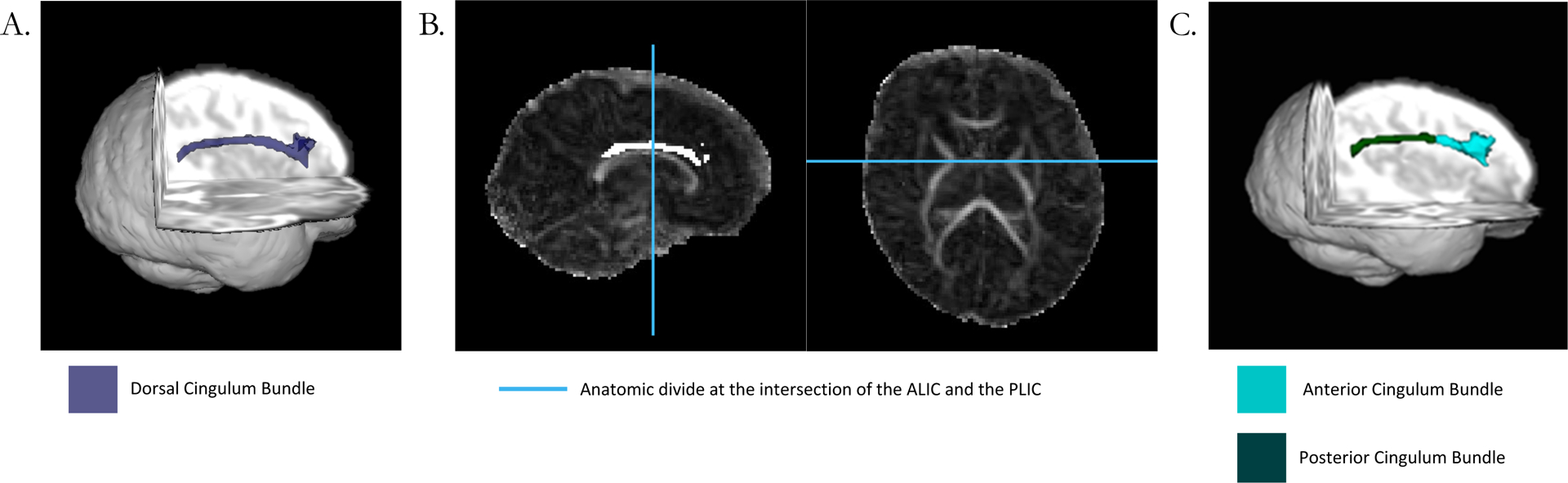
Panel A demonstrates the dorsal CB in a representative single subject. Panel B demonstrates the anterior-posterior divide, which was placed at the intersection between the anterior limb of the internal capsule and the posterior limb of the internal capsule for each subject. Panel C shows the dorsal anterior CB and dorsal posterior CB after the division. Diffusion measures were extracted from the tracts in panel A and panel C.
The dorsal CB was manually segmented into anterior and posterior sections using the intersection of the anterior portion of the internal capsule and the posterior portion of the internal capsule as an anatomical landmark by a single rater (R.B. with oversight from C.S. and J.S.; Figure 1B). This landmark was chosen based on the anatomical landmarks corresponding to previous segmentations of the CB in non-human primates (23). Measures of FA, MD, RD, and AD were extracted from the CB. Extreme outlier values (>3 SD) for each DTI metric within each tract segment were excluded from analyses.
Behavioral Phenotype
Parent Report:
Parents completed the Child Behavior Checklist for ages 1.5 to 5 (CBCL) (59), Conners Rating Scale-Revised (Conners) (60), and the Social Responsiveness Scales-2 (SRS-2) (61) in 80 VPT children. Internalizing problems were measured using the internalizing symptoms score on the CBCL, which is comprised of four subscales: anxiety/depression, withdrawn, somatic complaints, and emotional reactivity. Autism symptoms including social-communication impairments and restrictive and repetitive behaviors were assessed using the SRS-2 total score (61). ADHD symptoms including hyperactivity/impulsivity, inattention, and cognitive problems were measured using the Conners ADHD Index (62). The CBCL, SRS-2, and Conners ADHD Index have all been well validated for use in diverse samples of children, including those from low socio-economic status (SES) backgrounds and racial/ethnic minorities (63–66). The SRS-2 and Conners were chosen to assess autism and ADHD symptoms, respectively, because they assess multiple domains within each diagnostic category and are more specific than the CBCL subscales. On all measures, higher scores indicated greater symptom severity. The clinical cut-off score is 65 for the CBCL and 60 for the SRS-2 and Conners (59–61). In this cohort, previous research has shown that VPT children have increased psychiatric symptoms compared to FT children (3). Full Scale IQ was also assessed as a negative control (See Supplement).
Teacher Report:
Teacher-reported data, including the Teacher Response Form (TRF), SRS-2, and Conners, were obtained for 72 VPT children. The TRF is a parallel assessment completed by teachers that assesses the same domains as the CBCL, including internalizing symptoms. The TRF has been well-validated with diverse samples of children including those from low SES backgrounds and racial/ethnic minorities (64,67,68). The clinical cut-off score for the TRF is 65 (59). Analogous to the parent report data, the SRS-2 total score and the Conners ADHD Index score were used for the teacher report measures of autism symptoms and ADHD symptoms, respectively.
Data Analysis
All statistics were run in SPSS statistics software version 25 (IBM Corporation, NY, USA). Outliers in the data were identified for each behavioral measure (> 3 SDs) and removed. Data was only included for participants with either a complete set of parent questionnaires after multiple imputation or a complete set of teacher questionnaires. Multiple imputation was used to address missing data in parent reported questionnaires, but not teacher questionnaires due to >15% data missing (69,70) (see Supplement).
As the aim of the study was to examine whether the CB is associated with common shared variance across the three domains of the preterm behavioral phenotype, a principal component analysis (PCA) was performed to assess the degree of variance that was explained by a composite measure of the preterm behavioral phenotype and to identify the amount of common variance in parent and teacher ratings (see Supplement). In the initial PCA, parent and teacher ratings loaded onto separate factors and, since the sample size was reduced when both parent and teacher variables were used in the model, separate PCAs were performed for parent and teacher variables. Both PCAs produced single factor models explaining 80% of the variance with factor loadings between 0.89 and 0.92 for all variables, which eliminated the need to rotate the solution. These principal component factors were subsequently used in behavioral analyses labelled as the “parent-rated preterm behavioral phenotype score” and “teacher-rated preterm behavioral phenotype score.”
To address the first aim of the study, the microstructure of the dorsal anterior and dorsal posterior portions of the CB within each hemisphere in VPT neonates was compared to FT neonates. Linear Mixed Models were used to account for multiple births (twins and triplets) in the VPT population that were not present in the FT cohort. These models were also controlled for gestational age at scan and race because these factors differed between the VPT and FT groups.
To address the second aim of the study, we first examined the relationship between neonatal white matter microstructure of the dorsal CB and preterm behavioral phenotype symptoms. Then, we investigated whether the dorsal anterior and/or the dorsal posterior portions of the CB were responsible for any significant associations between the dorsal CB and psychiatric outcome measures. Linear regression models adjusted for age at scan, sex, and medical risk were performed in VPT children (n=56 parent-rated; n=45 teacher-rated). Medical risk is a composite measure of perinatal clinical factors (see Supplement) that has been previously validated in the literature (71,72). Age at scan, sex, and medical risk were included in the model because they were related to the cingulum bundle or outcome measures (see Supplement). Social risk was not included in the models because it was not related to any variables of interest for VPT children (see Supplement). Separate linear regression models were run for each diffusion measure (e.g. FA, MD, AD, RD). Cronbach’s alpha (α) was set at 0.05. Multiple comparisons were accounted for using a Benjamini-Hochberg procedure for False Discovery Rate (FDR) correction across all the statistical tests in the paper (73).
Results
White Matter Microstructure of the CB in VPT vs. FT neonates
Diffusion measures in the whole, right, and left CB differed between VPT (n=69) and FT (n=55) infants. Specifically, VPT infants had increased MD, AD, and RD in the CB compared to FT infants (Table 1; Supplementary Table 2). FA of the CB did not differ between VPT and FT neonates (Table 1). In both the anterior and posterior portions of the CB, VPT neonates had greater MD, AD, and RD than FT neonates, but similar FA values (Table 1; Supplementary Table 2).
Neonatal CB and Parent-Rated Preterm Behavioral Phenotype
Increased FA of the right CB in the neonatal period was associated with greater parent-rated preterm behavioral phenotype symptoms at age 5 years in VPT children (Figure 2). In contrast, there was no association with the FA of the left CB (Table 3). Similarly, MD, AD, and RD of the right and left CB were not associated with the parent-rated preterm behavioral phenotype scores (Table 3). To investigate whether the association between FA of the right CB and the parent-rated preterm behavioral phenotype score was regionally specific, we examined subregions of the CB and found that increased FA of the right anterior, but not right posterior CB, was associated with greater parent-rated preterm behavioral phenotype symptoms (Figure 2). Additional supplemental analyses supported the specificity of these findings as the FA and MD of the CC were not related to the parent-rated preterm behavioral phenotype scores and the FA and MD of the CB were not related to Full-Scale IQ (see Supplement).
Figure 2:
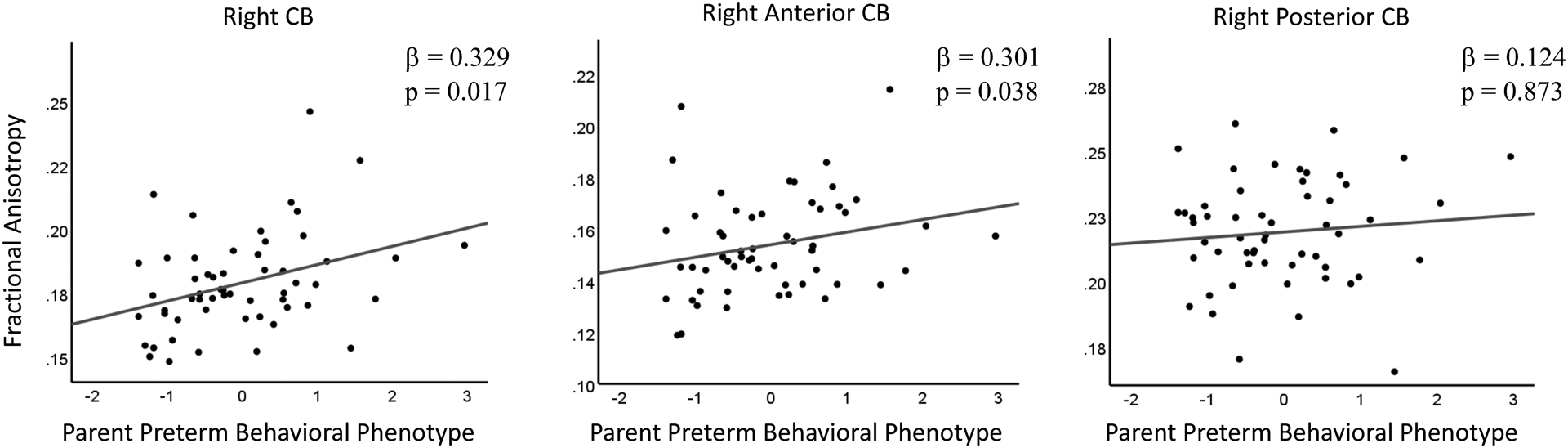
The left panel shows the significant association between the FA of the right CB and the parent-rated preterm behavioral phenotype. The middle panel shows the significant association between the FA of the right anterior CB and the parent-rated preterm behavioral phenotype. The right panel shows the non-significant association between the FA of the right posterior CB and the parent-rated preterm behavioral phenotype.
Table 3.
Regression Models Predicting the Preterm Behavioral Phenotype
| Parent | Teacher | |||
|---|---|---|---|---|
| β | p | β | p | |
| Fractional Anisotropy | ||||
| Right CB | .329 | .017 | .334 | .029 |
| Right Anterior CB | .301 | .038 | .263 | .072 |
| Right Posterior CB | .124 | .400 | .084 | .581 |
| Left CB | −.022 | .873 | .106 | .481 |
| Mean Diffusivity | ||||
| Right CB | −.191 | .232 | −.354 | .024 |
| Right Anterior CB | −.078 | .506 | −.426 | .003 |
| Right Posterior CB | .007 | .940 | −.141 | .379 |
| Left CB | −.081 | .583 | −.192 | .212 |
| Axial Diffusivity | ||||
| Right CB | .001 | .932 | −.245 | .134 |
| Right Anterior CB | −.011 | .911 | −.493 | .001 |
| Right Posterior CB | .134 | .414 | −.140 | .395 |
| Left CB | −.105 | .488 | −.185 | .251 |
| Radial Diffusivity | ||||
| Right CB | −.280 | .363 | −.396 | .011 |
| Right Anterior CB | −.155 | .300 | −.455 | .002 |
| Right Posterior CB | −.042 | .790 | −.089 | .575 |
| Left CB | −.093 | .527 | −.163 | .292 |
Bold represents p-values that passed FDR correction
Neonatal CB and Teacher-Rated Preterm Behavioral Phenotype
Increased FA and decreased MD of the right CB in the neonatal period were associated with greater teacher-rated preterm behavioral phenotype symptoms at age 5 years (Figure 3). Decreased RD of the right CB was related to greater teacher-rated preterm behavioral phenotype symptoms, but decreased AD of the right CB was not (Table 3). The left CB was not related to the teacher-rated preterm behavioral phenotype symptoms on any diffusion measures (Table 3). When evaluating subregions of the CB, decreased MD, AD, and RD of the right anterior CB were associated with greater teacher-rated preterm behavioral phenotype symptoms (Figure 3). The right posterior CB was not related to any teacher-rated preterm behavioral phenotype scores (Figure 3). Additional supplemental analyses supported the specificity of these findings as the FA and MD of the CC were not related to the teacher-rated preterm behavioral phenotype scores and the FA and MD of the CB were not related to Full-Scale IQ (see Supplement).
Figure 3:
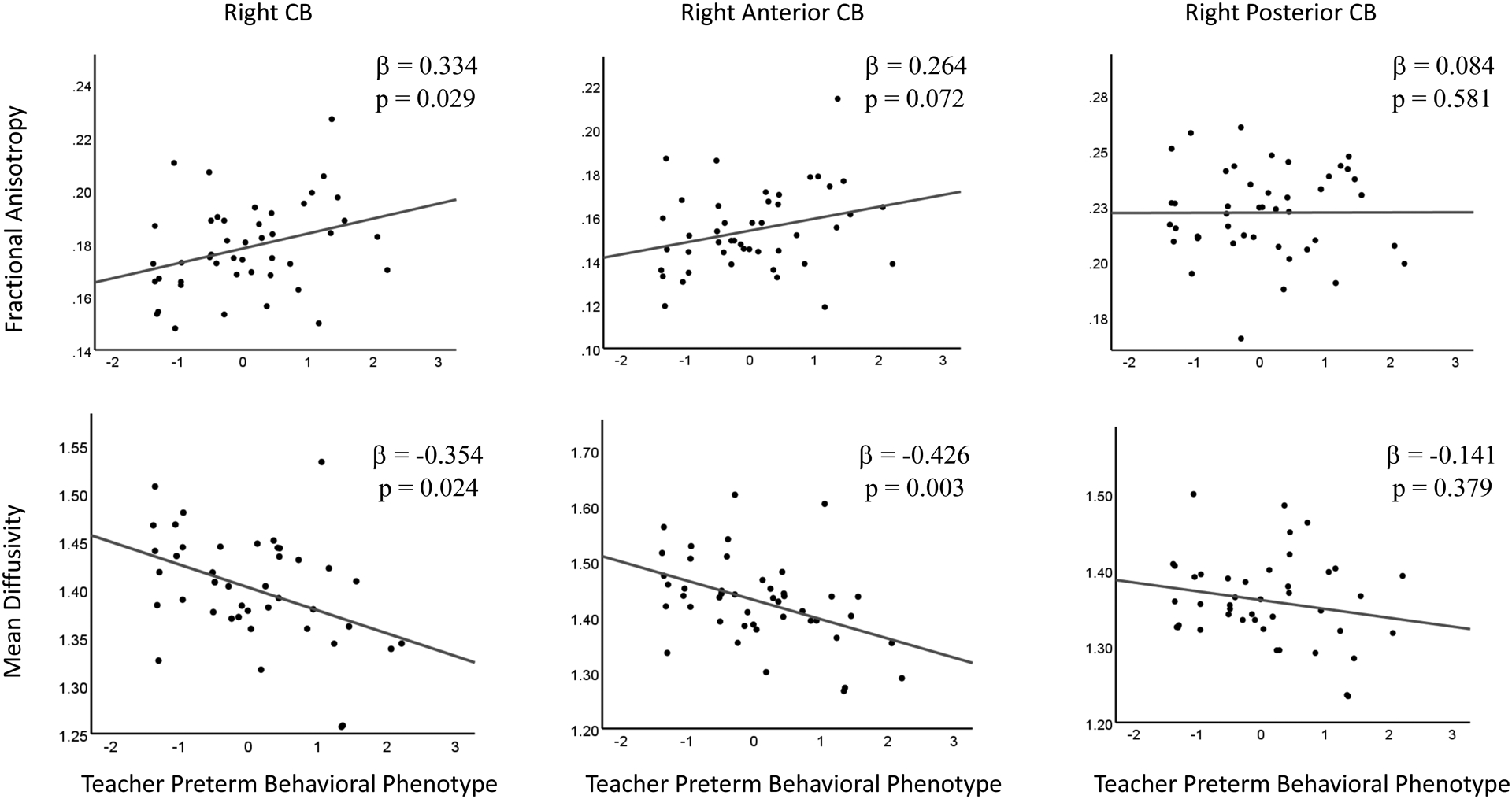
The top row of Figure 3 demonstrates the association of the FA of the right CB (left), the right anterior CB (middle), and the right posterior CB (right) with the teacher-rated preterm behavioral phenotype. The bottom row of Figure 3 shows the association of the MD of the right CB (left), the right anterior CB (middle) and right posterior CB (right) with the teacher-rated preterm behavioral phenotype. The relationships displayed in the left and middle columns were significant whereas the associations displayed in the right column were not significant.
Discussion
Summary of Key Findings
The critical finding in this prospective, longitudinal study was that neonatal white matter microstructure alterations in the right anterior CB are related to the severity of preterm behavioral phenotype symptoms at age 5 as rated by both parents and teachers. This result advances the field by showing that the white matter alterations in the anterior CB seen in adults with psychiatric illnesses (24,25,33) may be identifiable during the neonatal period as early as term-equivalent age. Specifically, we found that increased FA of the right anterior CB in the neonatal period was related to greater parent-rated preterm behavior phenotype symptoms at 5 years in VPT children. Decreased MD, RD, and AD of the right anterior CB were related to greater teacher-rated preterm behavioral phenotype symptoms. Importantly, this work suggests that white matter alterations in the anterior CB are more likely to contribute to the emergence of psychiatric symptoms than be a downstream consequence of psychiatric illnesses, identifying the CB microstructure as a potentially viable therapeutic target.
The Anterior CB’s Role in Internalizing Disorders, Autism, and ADHD
In older children and adults, the anterior CB has been associated with internalizing disorders, autism, and ADHD, which comprise the preterm behavioral phenotype. Many studies show reduced FA in the dorsal and anterior portions of the CB in patients with internalizing disorders (26–30). Similar studies in autistic populations show decreased FA, increased MD, and increased RD in the anterior and dorsal segments of the CB (31–33). Additionally, Makris et al. (2008) and Konrad et al. (2010) demonstrated that there was decreased FA and increased MD in the right anterior CB in adults with ADHD (24,25). Across these investigations, right hemisphere specific findings were thought to occur because the right prefrontal cortex, directs attention (74) and contributes to emotional-attentional modulation (75). Previous studies also supported the specificity of this association as patients with anterior cingulotomies did not have lasting cognitive deficits (76,77). Our study further demonstrates this specificity by showing that early variance in right anterior CB microstructure is not associated with full-scale IQ. This may indicate that white matter microstructure disruption in the CB plays a specific role in the early development of psychiatric illnesses.
The white matter of the CB may be particularly important in the early development of psychiatric illnesses because it connects key socioemotional brain regions. The CB connects the anterior cingulate, dorsolateral prefrontal, medial prefrontal, and orbitofrontal cortices to the insula and amygdala ventrally and to the posterior cingulate, parietal, hippocampus, and parahippocampal cortices posteriorly (22). These regions play important roles in social and emotional functioning (22,34) with the prefrontal, orbitofrontal, and anterior cingulate cortices thought to be particularly important. These frontal regions – and their connections to the amygdala – are thought to support emotion processing, emotion regulation, and executive control (34–40) and to be involved in internalizing disorders, ADHD, and autism (16,24,34,40–55). Disruptions in the anterior CB may be associated with psychiatric symptoms because of the multitude of short-range connections between prefrontal areas and anterior cingulate cortex, as well as long-range connections to the amygdala (22).
Microstructural Differences between Neonates and Older Individuals
The prior literature linking the dorsal anterior CB to psychiatric illnesses aligns with our results, except for a developmental shift in directionality. In adults, lower FA and increased MD, which are thought to represent decreased myelination and reduced axonal strength, have been associated with greater psychiatric impairment; however, in neonates, higher FA and lower MD have been associated with increased symptomatology (78–80). This change in directionality is thought to occur because late-developing white matter tracts, such as the CB, are incompletely myelinated at term-equivalent age (17,81). In the newborn brain, increases in FA and decreases in MD may reflect reduced fiber branching, smaller axonal diameters, or fewer crossing fibers (82). This is especially true for VPT infants who have reduced myelination compared to FT infants due to disruptions in late oligodendrocyte progenitor cells and sub-plate neurons (19,83,84), which are selectively vulnerable to oxidative stress and excitotoxicity during development (20,85–87). Therefore, comparisons between VPT and FT infants may reflect differences in myelination (10,12,88), whereas comparisons within VPT populations may be more sensitive to reduced fiber branching, smaller axonal diameters, or fewer crossing fibers.
Unexpected brain-behavior relationships in VPT children may also be attributable to the fact that DTI relies upon average measures within a single voxel, which make crossing fibers particularly difficult to delineate in the neonatal brain. If the vectors of crossing fibers point in different directions within a voxel, the average of the vectors will have less directionality (low FA) and greater diffusivity (high MD) (56,89). Prior anatomical investigations show that the CB contains many short-range cortico-cortical association fibers that cross the medial portions of the frontal, parietal, and temporal lobes (23,79,90,91). These beneficial crossing fibers may be absent in VPT infants with greater preterm behavioral phenotype symptoms. A previous analysis conducted in this VPT cohort showed that higher FA in the cingulum bundle was related to increased autism symptoms at age 2 (78). Similar associations regarding higher FA have also been reported in other samples of VPT infants as well as infants at risk for ASD in childhood, which suggests a developmentally specific relationship between neonatal DTI values and later psychiatric illnesses (80,92).
Comparison of Results across Parents and Teachers
Our findings linking the microstructure of the CB to parent and teacher reports of the preterm behavioral phenotype were largely similar; however, teacher reports were associated with more diffusion measures than parent reports. These differences in brain-behavior relationships were not unexpected given that multiple studies report parent-teacher disagreements, especially in preterm populations (72,93,94). The behavioral demands placed on children at school and in the home may differ in a context-dependent manner. Teachers are able to observe children in relation to a large peer group and may be better poised to identify social-communication difficulties as well as classroom inattention (95). Parents, on the other hand, may be more aware of internalizing problems because they spend more one-on-one time with their children (96). Differences in associations between the CB and the parent-rated and teacher-rated preterm behavioral phenotype likely stem from atypical behaviors that are more observable in one context than another. In support of this interpretation, diffusion measures in the right anterior CB were related to teacher, but not parent, ratings of autism symptoms (see Supplement), which would be expected if social communications deficits are more observable at school than at home. Each rater’s biases and expectations may also play a role.
Limitations
The first limitation of this study is that the subgenual portion of the CB was not assessed due to technical limitations. While the dorsal portion of the CB is thought to be more important for psychopathology in older populations, the subgenual portion has many connections within limbic regions. If these subgenual, intra-limbic connections are important during development, this study may underestimate the importance of the CB in later psychopathology. The second limitation of this study was our inability to differentiate crossing fibers due to the limited dimensionality of the DTI data. Additionally, due to sample size limitations, we were unable to assess the relationship between the neonatal CB and psychiatric outcomes in FT infants. Even though FT infants do not exhibit the preterm behavioral phenotype, examining FT infants could help determine whether the link between white matter disruptions in the CB and psychiatric impairments are specific to prematurity. Future studies with higher DTI dimensionality, novel computational approaches, and larger sample sizes may allow for assessment of the subgenual CB and crossing fibers in both VPT and FT neonates.
Conclusions
This prospective, longitudinal, dual-informant study provides the first evidence that alterations in the anterior CB in neonates relate to specific psychiatric outcomes five years later. The results show that increased FA and decreased MD, AD, and RD of the right anterior CB at term-equivalent age is related to greater internalizing, autism, and ADHD symptoms at age 5 years in VPT children. In addition, these results show that subregions of the CB have early functional specificity in the context of prematurity. Taken together, these findings highlight the importance of studying the role of the CB and its subregions in the emergence of the preterm behavioral phenotype. Early assessment of the CB in preterm neonates may be useful for the prognostication of later psychiatric disease and help clinicians better allocate resources and services prior to the onset of symptoms. Crucially, this work provides the foundation for future studies of the CB that both advance our understanding of the pathophysiology underlying psychiatric diseases in the context of prematurity and may lead to new therapeutic interventions for vulnerable premature infants.
Supplementary Material
Table 2.
Preterm Behavioral Phenotype Symptoms at Age 5 in VPT children
| Parent Report (M ± SD) | Teacher Report (M ± SD) | |
|---|---|---|
| N=80 | N=63 | |
| Gestational Age at Birth (weeks) | 26.5 ± 1.8 | 26.5 ± 1.8 |
| Age at 5-year follow-up (months) | 67.2 ± 5.0 | 67.0 ± 5.0 |
| CBCL/TRF Internalizing T-Score | 49.1 ± 14 | 51.8 ± 12.7 |
| % of Children with Clinical Impairment (T-Score >65) | 13.7 | 15.9 |
| SRS-2 Total T-Score | 54.8 ± 12 | 56.2 ± 12.7 |
| % of Children with Clinical Impairment (T-Score >60) | 24.7 | 34.9 |
| Conners ADHD Index T-Score | 57.4 ± 14 | 59.8 ± 13.6 |
| % of Children with Clinical Impairment (T-Score >60) | 39.1 | 44.4 |
| Male/Female | 36/45 | 25/38 |
| African American/Caucasian | 32/41a | 23/35b |
In addition, there were 4 Asian participants and 3 biracial very preterm participants.
In addition, there were 4 Asian participants and 1 biracial participant.
Acknowledgements
Research reported in this publication was supported by the National Institutes of Health (T32-EB014855, R01-HD057098, R01-MH113570, K02-NS089852, UL1-TR000448, K23-MH105179, R01-HD061619), Intellectual and Developmental Disabilities Research Center at Washington University (U54-HD087011), Cerebral Palsy International Research Foundation, The Dana Foundation, The Child Neurology Foundation, The Doris Duke Charitable Foundation, and the Washington University Medical Scientist Training Program. The authors would like to thank the Washington University Neonatal Developmental Research Group and the families involved with the study. The cingulum bundle diffusion data and parent-rated preterm behavioral phenotype was previously presented as an abstract and poster at the Organization for Human Brain Mapping conference in 2019.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.March of Dimes (n.d.): Preterm Birth United States | PeriStats | March of Dimes. Retrieved February 11, 2019, from https://www.marchofdimes.org/Peristats [Google Scholar]
- 2.Johnson S, Marlow N (2011): Preterm Birth and Childhood Psychiatric Disorders. Pediatr Res 69: 22–28. [DOI] [PubMed] [Google Scholar]
- 3.Lean RE, Lessov-Shlaggar CN, Gerstein ED, Smyser TA, Paul RA, Smyser CD, Rogers CE (2020): Maternal and family factors differentiate profiles of psychiatric impairments in very preterm children at age 5-years. J Child Psychol Psychiatry 61: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N (2010): Psychiatric Disorders in Extremely Preterm Children: Longitudinal Finding at Age 11 Years in the EPICure Study. J Am Acad Child Adolesc Psychiatry 49: 453–463.e1. [PubMed] [Google Scholar]
- 5.Platt MJ (2014): Outcomes in preterm infants. Public Health 128: 399–403. [DOI] [PubMed] [Google Scholar]
- 6.Voigt B, Pietz J, Pauen S, Kliegel M, Reuner G (2012): Cognitive development in very vs. moderately to late preterm and full-term children: Can effortful control account for group differences in toddlerhood? Early Hum Dev 88: 307–313. [DOI] [PubMed] [Google Scholar]
- 7.Johnson S, Marlow N (2014): Growing up after extremely preterm birth: Lifespan mental health outcomes. Semin Fetal Neonatal Med 19: 97–104. [DOI] [PubMed] [Google Scholar]
- 8.Johnson S, Waheed G, Manktelow BN, Field DJ, Marlow N, Draper ES, Boyle EM (2018): Differentiating the Preterm Phenotype: Distinct Profiles of Cognitive and Behavioral Development Following Late and Moderately Preterm Birth. J Pediatr 193: 85–92.e1. [DOI] [PubMed] [Google Scholar]
- 9.Smyser TA, Smyser CD, Rogers CE, Gillespie SK, Inder TE, Neil JJ (2016): Cortical Gray and Adjacent White Matter Demonstrate Synchronous Maturation in Very Preterm Infants. Cereb Cortex 26: 3370–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Counsell SJ (2006): Axial and Radial Diffusivity in Preterm Infants Who Have Diffuse White Matter Changes on Magnetic Resonance Imaging at Term-Equivalent Age. PEDIATRICS 117: 376–386. [DOI] [PubMed] [Google Scholar]
- 11.Hintz SR, Barnes PD, Bulas D, Slovis TL, Finer NN, Wrage LA, et al. (2015): Neuroimaging and Neurodevelopmental Outcome in Extremely Preterm Infants. 135: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ (2007): Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage 35: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 13.Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, et al. (2003): Preterm Children Have Disturbances of White Matter at 11 Years of Age as Shown by Diffusion Tensor Imaging. Pediatr Res 54: 672–679. [DOI] [PubMed] [Google Scholar]
- 14.Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, et al. (2008): Prematurely Born Children Demonstrate White Matter Microstructural Differences at 12 Years of Age, Relative to Term Control Subjects: An Investigation of Group and Gender Effects. PEDIATRICS 121: 306–316. [DOI] [PubMed] [Google Scholar]
- 15.Spittle AJ, Treyvaud K, Doyle LW, Roberts G, Lee KJ, Inder TE, et al. (2009): Early Emergence of Behavior and Social-Emotional Problems in Very Preterm Infants. J Am Acad Child Adolesc Psychiatry 48: 909–918. [DOI] [PubMed] [Google Scholar]
- 16.Rogers CE, Lean RE, Wheelock MD, Smyser CD (2018): Aberrant structural and functional connectivity and neurodevelopmental impairment in preterm children. J Neurodev Disord 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinney HC, Ann brody B, Kloman AS, Gilles FH (1988): Sequence of Central Nervous System Myelination in Human Infancy. II. Patterns of Myelination in Autopsied Infants: J Neuropathol Exp Neurol 47: 217–234. [DOI] [PubMed] [Google Scholar]
- 18.Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D (2006): Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: A feasibility study using quantitative diffusion tensor imaging and tractography. NeuroImage 30: 1121–1132. [DOI] [PubMed] [Google Scholar]
- 19.Miller SP, Ferriero DM (2009): From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci 32: 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, et al. (2002): Selective Vulnerability of Late Oligodendrocyte Progenitors to Hypoxia–Ischemia. J Neurosci 22: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ (1998): Maturation-Dependent Vulnerability of Oligodendrocytes to Oxidative Stress-Induced Death Caused by Glutathione Depletion. J Neurosci 18: 6241–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bubb EJ, Metzler-Baddeley C, Aggleton JP (2018): The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev 92: 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heilbronner SR, Haber SN (2014): Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders. J Neurosci 34: 10041–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. (2008): Attention and Executive Systems Abnormalities in Adults with Childhood ADHD: A DT-MRI Study of Connections. Cereb Cortex 18: 1210–1220. [DOI] [PubMed] [Google Scholar]
- 25.Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, et al. (2010): Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur J Neurosci 31: 912–919. [DOI] [PubMed] [Google Scholar]
- 26.de Diego-Adeliño J, Pires P, Gómez-Ansón B, Serra-Blasco M, Vives-Gilabert Y, Puigdemont D, et al. (2014): Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med 44: 1171–1182. [DOI] [PubMed] [Google Scholar]
- 27.Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, et al. (2010): Altered White Matter Microstructure in Adolescents With Major Depression: A Preliminary Study. Adolesc PSYCHIATRY 49: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Zhang Y, Li L, Li Z, Li W, Ma N, et al. (2011): Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J Affect Disord 133: 294–299. [DOI] [PubMed] [Google Scholar]
- 29.Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A (2013): Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: A diffusion tensor imaging study. Psychiatry Res Neuroimaging 214: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SJ, Jeong D-U, Sim ME, Bae SC, Chung A, Kim MJ, et al. (2006): Asymmetrically Altered Integrity of Cingulum Bundle in Posttraumatic Stress Disorder. Neuropsychobiology 54: 120–125. [DOI] [PubMed] [Google Scholar]
- 31.Shukla DK, Keehn B, Müller R-A (2011): Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder: Tract-specific patterns of white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry 52: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jou RJ, Jackowski AP, Papademetris X, Rajeevan N, Staib LH, Volkmar FR (2011): Diffusion Tensor Imaging in Autism Spectrum Disorders: Preliminary Evidence of Abnormal Neural Connectivity. Aust N Z J Psychiatry 45: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikuta T, Shafritz KM, Bregman J, Peters BD, Gruner P, Malhotra AK, Szeszko PR (2014): Abnormal cingulum bundle development in autism: A probabilistic tractography study. Psychiatry Res Neuroimaging 221: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kujawa A, Wu M, Klumpp H, Pine DS, Swain JE, Fitzgerald KD, et al. (2016): Altered Development of Amygdala-Anterior Cingulate Cortex Connectivity in Anxious Youth and Young Adults. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeDoux J (2003): The Emotional Brain, Fear, and the Amygdala. Cell Mol Neurobiol 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price JL (2006): Comparative Aspects of Amygdala Connectivity. Ann N Y Acad Sci 985: 50–58. [DOI] [PubMed] [Google Scholar]
- 37.LeDoux JE (n.d.): Emotion Circuits in the Brain. 31. [DOI] [PubMed] [Google Scholar]
- 38.Montagna A, Nosarti C (2016): Socio-Emotional Development Following Very Preterm Birth: Pathways to Psychopathology. Front Psychol 7. 10.3389/fpsyg.2016.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy DP, Adolphs R (2012): The social brain in psychiatric and neurological disorders. Trends Cogn Sci 16: 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He N, Li F, Li Y, Guo L, Chen L, Huang X, et al. (2015): Neuroanatomical deficits correlate with executive dysfunction in boys with attention deficit hyperactivity disorder. Neurosci Lett 600: 45–49. [DOI] [PubMed] [Google Scholar]
- 41.Andreescu C, Mennin D, Tudorascu D, Sheu LK, Walker S, Banihashemi L, Aizenstein H (2015): The many faces of anxiety-neurobiological correlates of anxiety phenotypes. Psychiatry Res Neuroimaging 234: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ (2011): Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cereb Cortex 21: 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpenter KLH, Angold A, Chen NK, Copeland WE, Gaur P, Pelphrey K, et al. (2015): Preschool anxiety disorders predict different patterns of amygdala-prefrontal connectivity at school-age. PLoS ONE 10: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamm LL, Jacobs RH, Johnson MW, Fitzgerald DA, Fitzgerald KD, Langenecker SA, et al. (2014): Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol Mood Anxiety Disord 4: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hannesdottir DK, Doxie J, Bell MA, Ollendick TH, Wolfe CD (2010): A longitudinal study of emotion regulation and anxiety in middle childhood: Associations with frontal EEG asymmetry in early childhood. Dev Psychobiol 52: 197–204. [DOI] [PubMed] [Google Scholar]
- 46.Kalin NH (2017): Mechanisms underlying the early risk to develop anxiety and depression: A translational approach. Eur Neuropsychopharmacol 27: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams LE, Oler JA, Fox AS, McFarlin DR, Rogers GM, Jesson MAL, et al. (2015): Fear of the Unknown: Uncertain Anticipation Reveals Amygdala Alterations in Childhood Anxiety Disorders. Neuropsychopharmacology 40: 1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agam Y, Joseph RM, Barton JJS, Manoach DS (2010): Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage 52: 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ibrahim K, Eilbott JA, Ventola P, He G, Pelphrey KA, McCarthy G, Sukhodolsky DG (2019): Reduced Amygdala–Prefrontal Functional Connectivity in Children With Autism Spectrum Disorder and Co-occurring Disruptive Behavior. Biol Psychiatry Cogn Neurosci Neuroimaging S2451902219300230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holiga Š, Hipp JF, Chatham CH, Garces P, Spooren W, D’Ardhuy XL, et al. (2019): Patients with autism spectrum disorders display reproducible functional connectivity alterations. Sci Transl Med 11: eaat9223. [DOI] [PubMed] [Google Scholar]
- 51.Müller R-A, Fishman I (2018): Brain Connectivity and Neuroimaging of Social Networks in Autism. Trends Cogn Sci 22: 1103–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castellanos FX, Proal E (2012): Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. 16: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baroni A, Castellanos FX (2015): Neuroanatomic and cognitive abnormalities in attention-deficit/hyperactivity disorder in the era of “high definition” neuroimaging. Curr Opin Neurobiol 30: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cubillo A, Halari R, Smith A, Taylor E, Rubia K (2012): A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex J Devoted Study Nerv Syst Behav 48: 194–215. [DOI] [PubMed] [Google Scholar]
- 55.Pliszka S, Glahn D (2006): Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Of. Retrieved from http://psychiatryonline.org/article.aspx?articleid=96693 [DOI] [PubMed] [Google Scholar]
- 56.Kroenke CD, Bretthorst GL, Inder TE, Neil JJ (2006): Modeling water diffusion anisotropy within fixed newborn primate brain using Bayesian probability theory. Magn Reson Med 55: 187–197. [DOI] [PubMed] [Google Scholar]
- 57.Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. (2003): Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50: 1077–1088. [DOI] [PubMed] [Google Scholar]
- 58.Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage 34: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Achenbach TM, Rescorla LA (2000): Manual for the ASEBA preschool forms and profiles. Burlington: University of Vermont. Res Cent Child Youth Fam. [Google Scholar]
- 60.Conners CK (1989): Manual for Conners’ Rating Scales: Conners’ Teaching Rating Scales, Conners’ Parent Rating Scales. Multi-Health Systems, Incorporated. [Google Scholar]
- 61.Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. (2003): Validation of a Brief Quantitative Measure of Autistic Traits: Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview-Revised. J Autism Dev Disord 33: 427–433. [DOI] [PubMed] [Google Scholar]
- 62.Conners CK, Sitarenios G, Parker JDA, Epstein JN (1998): Revision and Restandardization of the Conners Teacher Rating Scale (CTRS-R): Factor Structure, Reliability, and Criterion Validity. J Abnorm Child Psychol 26: 279–291. [DOI] [PubMed] [Google Scholar]
- 63.Konold TR, Hamre BK, Pianta RC (2003): Measuring Problem Behaviors in Young Children. Behav Disord 28: 111–123. [Google Scholar]
- 64.Achenbach TM, Becker A, Döpfner M, Heiervang E, Roessner V, Steinhausen H-C, Rothenberger A (2008): Multicultural assessment of child and adolescent psychopathology with ASEBA and SDQ instruments: research findings, applications, and future directions. J Child Psychol Psychiatry 49: 251–275. [DOI] [PubMed] [Google Scholar]
- 65.Goyette CH, Conners CK, Ulrich RF (1978): Normative data on Revised Conners Parent and Teacher Rating Scales. J Abnorm Child Psychol 6: 221–236. [DOI] [PubMed] [Google Scholar]
- 66.Bölte S, Poustka F, Constantino JN (2008): Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS). Autism Res 1: 354–363. [DOI] [PubMed] [Google Scholar]
- 67.Liu J, Cheng H, Leung PWL (2011): The Application of the Preschool Child Behavior Checklist and the Caregiver–Teacher Report Form to Mainland Chinese Children: Syndrome Structure, Gender Differences, Country Effects, and Inter‐Informant Agreement. J Abnorm Child Psychol 39: 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tehrani-Doost M, Shahrivar Z, Pakbaz B, Rezaie A, Ahmadi F (2011): Normative Data and Psychometric Properties of the Child Behavior Checklist and Teacher Rating Form in an Iranian Community Sample. Iran J Pediatr 21: 12. [PMC free article] [PubMed] [Google Scholar]
- 69.Barnard J, Rubin D (1999): Small-sample degrees of freedom with multiple imputation. Biometrika 8. [Google Scholar]
- 70.Scheffer J (2002): Dealing with Missing Data. Res Lett Inf Math Sci 3: 8. [Google Scholar]
- 71.Lean RE, Paul RA, Smyser CD, Rogers CE (2018): Maternal intelligence quotient (IQ) predicts IQ and language in very preterm children at age 5 years. J Child Psychol Psychiatry 59: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lean RE, Lessov-Shlaggar CN, Gerstein ED, Smyser TA, Paul RA, Smyser CD, Rogers CE (2019): Maternal and family factors differentiate profiles of psychiatric impairments in very preterm children at age 5-years. J Child Psychol Psychiatry jcpp.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benjamini Y, Hochberg Y (1995): Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol 57: 289–300. [Google Scholar]
- 74.Heilman KM, Van Den Abell T (1980): Right hemisphere dominance for attention: The mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology 30: 4. [DOI] [PubMed] [Google Scholar]
- 75.Mesulam M-M (1990): Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28: 597–613. [DOI] [PubMed] [Google Scholar]
- 76.Cohen R, Kaplan R, Moser D, Jenkins M, Wilkinson H (1999): Impairments of attention after cingulotomy. Neurology 53: 819–819. [DOI] [PubMed] [Google Scholar]
- 77.Kim C-H, Chang JW, Koo M-S, Kim JW, Suh HS, Park IH, Lee HS (2003): Anterior cingulotomy for refractory obsessive-compulsive disorder. Acta Psychiatr Scand 107: 283–290. [DOI] [PubMed] [Google Scholar]
- 78.Rogers CE, Smyser T, Smyser CD, Shimony J, Inder TE, Neil JJ (2016): Regional White Matter Development in very preterm infants: perinatal predictors and early developmental outcomes. Pediatr Res 79: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, Wedeen VJ (2007): Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130: 630–653. [DOI] [PubMed] [Google Scholar]
- 80.Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. (2012): Differences in White Matter Fiber Tract Development Present From 6 to 24 Months in Infants With Autism. Am J Psychiatry 169: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli RC, et al. (1988): Normal Brain in Human Newborns: Apparent Diffusion Coefficient and Diffusion Anisotropy Measured by Using Diffusion Tensor MR Imaging. Radiology 209: 57–66. [DOI] [PubMed] [Google Scholar]
- 82.Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15: 435–455. [DOI] [PubMed] [Google Scholar]
- 83.Volpe JJ (2009): Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu X-B, Shen Y, Plane JM, Deng W (2013): Vulnerability of premyelinating oligodendrocytes to white-matter damage in neonatal brain injury. Neurosci Bull 29: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM (2003): Selective Vulnerability of Subplate Neurons after Early Neonatal Hypoxia-Ischemia. J Neurosci 23: 3308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Follett PL (2004): Glutamate Receptor-Mediated Oligodendrocyte Toxicity in Periventricular Leukomalacia: A Protective Role for Topiramate. J Neurosci 24: 4412–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE (2000): NBQX Attenuates Excitotoxic Injury in Developing White Matter. J Neurosci 20: 9235–9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rose SE, Hatzigeorgiou X, Strudwick MW, Durbridge G, Davies PSW, Colditz PB (2008): Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magn Reson Med 60: 761–767. [DOI] [PubMed] [Google Scholar]
- 89.Assaf Y, Pasternak O (2008): Diffusion Tensor Imaging (DTI)-based White Matter Mapping in Brain Research: A Review. J Mol Neurosci 34: 51–61. [DOI] [PubMed] [Google Scholar]
- 90.Beevor CE (1890): On the Course of Fibres of the Cingulum and the Posterior Parts of the Corpus Callosum and Fornix in the Marmoset Monkey.
- 91.Yakovlev PI, Locke S, Koskoff D, Patton R (1960): Limbic Nuclei of Thalamus and Connections of Limbic Cortex. 22. [DOI] [PubMed] [Google Scholar]
- 92.Giménez M, Miranda MJ, Born AP, Nagy Z, Rostrup E, Jernigan TL (2008): Accelerated cerebral white matter development in preterm infants: A voxel-based morphometry study with diffusion tensor MR imaging. NeuroImage 41: 728–734. [DOI] [PubMed] [Google Scholar]
- 93.O’Meagher S, Norris K, Kemp N, Anderson P (2019): Parent and teacher reporting of executive function and behavioral difficulties in preterm and term children at kindergarten. Appl Neuropsychol Child 1–12. [DOI] [PubMed] [Google Scholar]
- 94.Youngstrom E, Loeber R, Stouthamer-Loeber M (2000): Patterns and Correlates of Agreement Between Parent, Teacher, and Male Adolescent Ratings of Externalizing and Internalizing Problems. J Consult Clin Psychol 68: 1038–1050. [DOI] [PubMed] [Google Scholar]
- 95.Bora S, Pritchard VE, Moor S, Austin NC, Woodward LJ (2011): Emotional and behavioural adjustment of children born very preterm at early school age: Very preterm birth and behavioural sequelae. J Paediatr Child Health 47: 863–869. [DOI] [PubMed] [Google Scholar]
- 96.Hinshaw SP, Han SS, Erhardt D, Huber A (1992): Internalizing and Externalizing Behavior Problems in Preschool Children: Correspondence Among Parent and Teacher Ratings and Behavior Observations. J Clin Child Psychol 21: 143–150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


