Abstract
BACKGROUND
Elevated triglyceride-rich lipoprotein (TRL) and small-dense low-density lipoprotein (sdLDL) particles are hallmarks of atherogenic dyslipidemia, and their cholesterol content is hypothesized to drive atherosclerotic risk. Prospective epidemiological data pertaining to cholesterol content of TRLs and sdLDL in primary prevention populations are mostly limited to coronary heart disease.
OBJECTIVES
The purpose of this study was to prospectively evaluate whether triglyceride-rich lipoprotein cholesterol (TRL-C) and small-dense low-density lipoprotein cholesterol (sdLDL-C) concentrations associate with composite and individual incident cardiovascular disease (CVD) outcomes including myocardial infarction (MI), ischemic stroke (IS), and peripheral artery disease (PAD).
METHODS
In a prospective case-cohort study within the Women’s Health Study, TRL-C and sdLDL-C (mg/dl) were directly measured in baseline blood specimens of case subjects (n = 480) and the reference subcohort (n = 496). Risk associations were evaluated for total CVD (MI, IS, PAD, and CVD death), coronary and cerebrovascular disease (MI, IS, CVD death), and individual outcomes (MI, IS, and PAD). Models were adjusted for traditional risk factors, low-density lipoprotein cholesterol, and high-sensitivity C-reactive protein.
RESULTS
The risk of both composite outcomes significantly increased across quartiles of TRL-C and sdLDL-C. TRL-C was significantly associated with MI and PAD (MI hazard ratio [HR]Q4: 3.05 [95% confidence interval (CI): 1.46 to 6.39]; ptrend = 0.002; PAD HRQ4: 2.58 [95% CI: 1.18 to 5.63]; ptrend = 0.019), whereas sdLDL-C was significantly associated with MI alone (HRQ4: 3.71 [95% CI: 1.59 to 8.63]; ptrend < 0.001). Both markers weakly associated with IS. Association patterns were similar for continuous exposures and, for TRL-C, among subjects with low atherogenic particle concentrations (apolipoprotein B <100 mg/dl).
CONCLUSIONS
TRL-C strongly associates with future MI and PAD events, whereas sdLDL-C strongly associates with MI alone. These findings signal that the cholesterol content of TRLs and sdLDL influence atherogenesis independently of low-density lipoprotein cholesterol, and high sensitivity C-reactive protein, with potentially different potency across vascular beds. (Women’s Health Study; NCT00000479)
Keywords: atherogenesis, biomarkers, dyslipidemia, epidemiology, primary prevention, vascular disease
Therapies developed to reduce the cholesterol content of low-density lipoprotein particles (LDL-C) have been the mainstay of atherosclerosis prevention. Although LDL-C lowering has unequivocal benefit (1,2), efforts to identify other lipid targets remain a priority due to residual atherosclerotic risk (3). The atherogenic dyslipidemia complex, a state of reduced high-density lipoprotein cholesterol (HDL-C), elevations in triglyceride-rich lipoprotein (TRL), and small dense low-density lipoprotein (sdLDL) particles, is believed to be responsible for substantial residual risk (4–8). Nuclear magnetic resonance measures of TRL and sdLDL particle size and number are known to associate with incident coronary and cerebrovascular disease (CCVD) (9–11) and, more recently, incident peripheral artery disease (PAD) (12). Whether the cholesterol content of these lipoprotein fractions, analogous to cholesterol content of LDL, is atherogenic across vascular territories remains understudied.
Strong associations between triglyceride-rich lipoprotein cholesterol (TRL-C) (also referred to as remnant cholesterol and commonly estimated using the Friedewald formula) and small-dense low-density lipoprotein cholesterol (sdLDL-C) with incident coronary heart disease (CHD) have been previously described (13–19). However, prospective data for these cholesterol measures are sparse for stroke (20–22) and absent for PAD, with few studies utilizing clinical assays that directly measure TRL-C and sdLDL-C.
To evaluate whether cholesterol deriving from TRLs and sdLDL is atherogenic across different vascular beds among healthy subjects, we assessed baseline levels of directly measured TRL-C and sdLDL-C as they relate to multiple incident cardiovascular outcomes in the WHS (Women’s Health Study).
METHODS
STUDY PARTICIPANTS AND ENDPOINT ASCERTAINMENT
The WHS is a completed randomized trial of aspirin and vitamin E in the primary prevention of cardiovascular disease (CVD) and cancer among 39,876 female health professionals age ≥45 years. Enrollment began in 1992, and the trial completed in March 2004, at which time women were invited to participate in observational follow-up. In total, 27,552 participants provided adequate blood specimens at enrollment and comprised the source population for the sample analyzed in this study (23,24). All participants were continuously followed for the occurrence of incident myocardial infarction (MI), ischemic stroke (IS), death from cardiovascular causes, and peripheral artery disease (PAD) (intermittent-claudication and/or peripheral artery revascularization) via annual questionnaires. Adjudication of the study endpoints has been previously described (12,25). Cases were accrued during the initial trial period and observational follow-up period through April 2009.
LABORATORY ANALYSIS
Baseline blood samples were stored in vapor phase liquid nitrogen (−150°C to −180°C) and thawed at the time of analysis. Measurement methods of high-sensitivity C-reactive protein (hsCRP), total cholesterol, HDL-C, total LDL-C, apolipoprotein B (Apo B), and triglycerides (TG) have been previously described (12,26). TRL-C and sdLDL-C concentrations were directly measured using 2-stage, automated homogeneous assays developed by Denka Seiken Co. (Niigata, Japan) (27). The intra-assay coefficients of variation for TRL-C and sdLDL-C from blinded WHS duplicate samples were 6.1% and 4.3%, respectively.
OVERVIEW OF CASE-COHORT DESIGN
We conducted a prospective case-cohort study to efficiently evaluate multiple incident cardiovascular outcomes. These included MI, IS, PAD, a composite of coronary and cerebral vascular disease (CCVD) (nonfatal MI, nonfatal IS, death from CV causes), and total CVD (PAD + CCVD). Supplemental Figure 1 provides an overview of the creation of our case-cohort study population. Of the 27,552 women for whom blood samples were available, 914 incident cases of total CVD occurred in 900 women (nCCVD = 800; nPAD = 114 PAD) during the 15.7-year follow-up period. In total, 14 individuals developed both PAD and CCVD, of whom 8 had CCVD occur prior to PAD. Due to a low incidence of PAD in this cohort (28), we selected all PAD cases (n = 114) and a random sample of incident CCVD to achieve a combined case set of 500 women who developed total CVD. A random subcohort (n = 500) stratified by age at enrollment (≥65 years vs. <65 years) and baseline smoking status (smoker = yes or no) was frequency matched to the case set independent of follow-up case status. Specifically, subcohort subjects were randomly selected within each matching stratum (age ≥65 years and smoker; age ≥65 years and nonsmoker; age <65 years and smoker; age <65 years and nonsmoker) to approximately match the proportions of these characteristics in the total CVD case set. As expected in a case-cohort framework, some subjects (n = 25) were also previously selected as cases (29). A total of 20 subjects who developed hemorrhagic stroke were excluded from the case set used in the current analysis. After accounting for missing key exposure data, the final study sample comprised 480 women with incident total CVD (nCCVD = 380; nPAD = 114) and a subcohort of 496 women frequency-matched on age and smoking status.
STATISTICAL ANALYSIS
Baseline characteristics
Baseline characteristics were reweighted to the total WHS cohort and compared between case and subcohort control subjects using survey methods to account for study design. Subcohort characteristics were also summarized across quartiles of TRL-C and sdLDL-C using median regression (continuous variables) and logistic regression (categorical variables). Associations between continuous variables were estimated in the reference subcohort using Spearman correlation coefficients. All analyses of WHS baseline characteristics were adjusted for sampling stratification factors (age, smoking status).
COX PROPORTIONAL HAZARDS REGRESSION
We examined associations between baseline TRL-C and sdLDL-C quartiles with incident CVD outcomes by computing robust sandwich variance estimates using Cox proportional hazards models weighted by stratum-specific sampling frequencies (Barlow weighting) appropriate for a stratified case-cohort design (26,29–31). All models accounted for stratification factors (age, smoking status) and WHS treatment assignment (aspirin, vitamin E). Expanded models included conventional CVD risk factors (continuous body mass index, history of diabetes, history of hypertension, parental history of MI, menopausal hormonal therapy usage, and ≥1 exercise episode weekly vs. none [model 2]), total LDL-C (model 3), and hsCRP (model 4). Subjects without complete model covariate information were excluded from those analyses. Trend tests were performed after assigning a median value to each quartile. The potential impact of preceding CCVD events on PAD estimates was accounted for using cause-specific competing risk methods adapted for case-cohort study designs (32). To assess whether risk associations persist at both high and low atherogenic lipoprotein particle number levels, we repeated our analysis using median cutpoints of each biomarker among women with high and low total Apo B level (clinical threshold of 100 mg/dl). Additionally, we examined potential nonlinear relationships between TRL-C and sdLDL-C with the risk of incident cardiovascular outcomes nonparametrically using restricted cubic splines (33). Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. All analyses were performed using SAS version 9.4 for Unix (Cary, North Carolina).
RESULTS
BASELINE CHARACTERISTICS
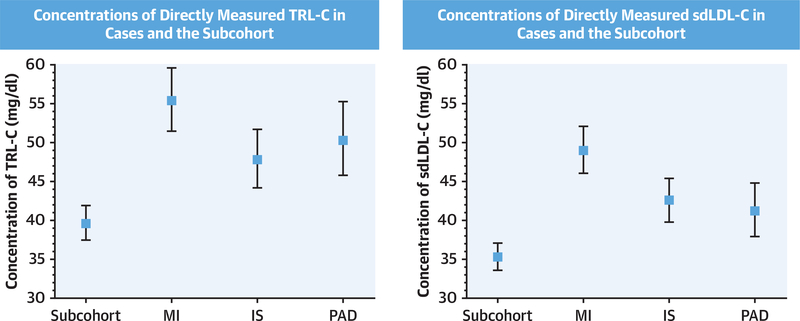
Baseline characteristics of women in the study population are summarized in Supplemental Table 1. Traditional cardiovascular risk factors were more prevalent among women who developed CVD compared with the subcohort control subjects. TRL-C and sdLDL-C concentrations in women with incident MI, IS, and PAD were found to be higher than those of women in the subcohort (geometric means: TRL-C: 55.4 mg/dl [95% confidence interval (CI): 51.5 to 59.6 mg/dl], 47.8 mg/dl [95% CI: 44.2 to 51.7 mg/dl], and 50.3 mg/dl [95% CI: 45.8 to 55.3 mg/dl] vs. 39.6 [95% CI: 37.5 to 41.9 mg/dl]; sdLDL-C: 49.0 mg/dl [95% CI: 46.1 to 52.1 mg/dl], 42.6 mg/dl [95% CI: 39.8 to 45.5 mg/dl], and 41.2 mg/dl [95% CI: 38.0 to 44.8 mg/dl] vs. 35.3 mg/dl [95% CI: 33.6 to 37.1 mg/dl]) (Figure 1).
FIGURE 1. Baseline Concentrations of TRL-C and sdLDL-C for Subjects With Incident CVD and the Subcohort.
Baseline concentrations of TRL-C (left) and sdLDL-C (right) for subjects in the reference subcohort and individual cardiovascular outcomes. Concentrations of TRL-C and sdLDL-C were significantly higher in each case group compared with the reference subcohort (p < 0.001 for all except PAD and sdLDL-C [p = 0.002]). Values shown are geometric means and 95% confidence interval. IS = ischemic stroke; MI = myocardial infarction; PAD = peripheral artery disease; sdLDL-C = small-dense low-density lipoprotein cholesterol; TRL-C = triglyceride-rich lipoprotein cholesterol.
Across quartiles of TRL-C and sdLDL-C (Table 1), the prevalence of modifiable risk factors for CVD trended upward, with more type 2 diabetes, hypertension, and current smoking among subjects in the highest quartile. Similarly, generally less favorable lipid profiles, elevated body mass index, and greater basal inflammation were noted in the higher quartiles. In the highest quartiles of both measures, TG and HDL-C concentrations approached the clinical thresholds that jointly define atherogenic dyslipidemia (Supplemental Figure 2). Consistent with biological expectations (6–8,34), TRL-C and sdLDL-C concentrations strongly correlated with each other (ρ = 0.72; p < 0.001), while strong correlations for both biomarkers were also observed with triglycerides and the TC to HDL-C ratio (Supplemental Table 2, Supplemental Figure 3).
TABLE 1.
Baseline Characteristics According to Quartile of Cholesterol Measures of Atherogenic Dyslipidemia Complex in the Reference Subcohort
| Cholesterol Measure | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|---|---|---|---|---|---|---|
| Number of subjects | TRL cholesterol† | n | 120 | 129 | 122 | 125 |
| nW | 7,384 | 6,815 | 6,765 | 6,354 | ||
| Small dense LDL cholesterol | n | 120 | 133 | 120 | 123 | |
| nW | 7,428 | 7,684 | 6,056 | 6,150 | ||
| Age, yrs | TRL cholesterol | 50.1 (1.00) | 53.2 (1.28) | 51.6 (1.09) | 54.4 (1.17) | |
| Small dense LDL cholesterol | 49.1 (0.94) | 51.8 (1.24) | 53.1 (1.04) | 55.2 (1.22) | ||
| Body mass index, kg/m2 | TRL cholesterol | 22.5 (0.56) | 24.6 (0.55) | 25.6 (0.58) | 26.6 (0.69) | |
| Small dense LDL cholesterol | 22.6 (0.54) | 25.5 (0.57) | 24.8 (0.59) | 26.4 (0.59) | ||
| History of diabetes mellitus | TRL cholesterol | 1.3 | 1.4 | 1.3 | 9.6 | |
| Small dense LDL cholesterol | 0.0 | 1.1 | 4.6 | 7.2 | ||
| History of hypertension | TRL cholesterol | 30.3 | 37.3 | 36.6 | 48.3 | |
| Small dense LDL cholesterol | 26.2 | 33.6 | 37.6 | 50.8 | ||
| Current smoker | TRL cholesterol | 9.0 | 11.4 | 10.1 | 14.9 | |
| Small dense LDL cholesterol | 9.10 | 9.50 | 12.60 | 14.70 | ||
| Parental history of myocardial infarction | TRL cholesterol | 11.4 | 14.6 | 20.1 | 21.9 | |
| Small dense LDL cholesterol | 13.7 | 14.0 | 79.7 | 21.9 | ||
| Alcohol, ≥1 serving/week | TRL cholesterol | 51.8 | 49.8 | 36.3 | 37.7 | |
| Small dense LDL cholesterol | 47.1 | 49.4 | 39.0 | 39.7 | ||
| Exercise, ≥1 episode/week | TRL cholesterol | 43.8 | 36.3 | 37.2 | 39.6 | |
| Small dense LDL cholesterol | 46.0 | 35.8 | 39.4 | 36.6 | ||
| Menopausal hormone therapy | TRL cholesterol | 38.0 | 53.3 | 41.5 | 47.2 | |
| Small dense LDL cholesterol | 29.8 | 49.1 | 45.6 | 53.6 | ||
| Total cholesterol, mg/dl | TRL cholesterol | 191.0 (4.2) | 213.3 (5.3) | 211.0 (5.4) | 240.3 (4.7) | |
| Small dense LDL cholesterol | 181.7 (4.3) | 208.5 (4.1) | 225.3 (5.2) | 245.4 (3.9) | ||
| High-density lipoprotein cholesterol, mg/dl | TRL cholesterol | 58.6 (1.7) | 52.1 (1.7) | 45.2 (2.2) | 41.6 (1.6) | |
| Small dense LDL cholesterol | 56.2 (2.3) | 52.5 (2.1) | 45.9 (2.6) | 43.4 (2.1) | ||
| Low-density lipoprotein cholesterol, mg/dl | TRL cholesterol | 108.1 (4.5) | 125.7 (5.8) | 126.3 (5.3) | 148.4 (5.6) | |
| Small dense LDL cholesterol | 100.9 (3.9) | 118.9 (3.5) | 140.0 (4.3) | 152.5 (4.8) | ||
| Triglycerides, mg/dl | TRL cholesterol | 76.3 (5.5) | 101.7 (5.5) | 139.9 (6.6) | 204.9 (7.5) | |
| Small dense LDL cholesterol | 74.0 (6.7) | 95.0 (6.2) | 134.0 (8.7) | 196.0 (8.2) | ||
| High-sensitivity C-reactive protein, mg/l | TRL cholesterol | 1.57 (0.27) | 2.19 (0.50) | 3.32 (0.55) | 4.01 (0.61) | |
| Small dense LDL cholesterol | 1.37 (0.50) | 1.87 (0.44) | 2.76 (0.55) | 3.93 (0.54) | ||
| Small dense LDL cholesterol, mg/dl | TRL cholesterol | 24.9 (1.8) | 32.5 (1.7) | 38.4 (1.9) | 57.3 (2.2) | |
| TRL cholesterol, mg/dl | Small dense LDL cholesterol | 24.0 (2.5) | 39.0 (2.5) | 50.0 (3.2) | 72.0 (3.3) | |
Values are % or median (SE) adjusted for matching factors and reweighted to the source population using logistic regression and median regression, respectively. TRL-C quartiles: Q1, <28.0 mg/dl; Q2, 28.0–42.9 mg/dl; Q3, 43.0–60.9 mg/dl; Q4, ≥61.0 mg/dl. Small dense LDL cholesterol quartiles: Q1, <27.0 mg/dl; Q2, 27.0–37.9 mg/dl; Q3, 38.0–50.9 mg/dl; Q4, ≥51.0 mg/dl. nw = reweighted number of subjects. Reweighted nw represent the approximate number of subjects expected within each quartile of TRL cholesterol and small dense LDL cholesterol had the entire WHS blood cohort been analyzed.
LDL = low-density lipoprotein; TRL = triglyceride rich lipoprotein.
TRL-C, sdLDL-C, AND INCIDENT CVD
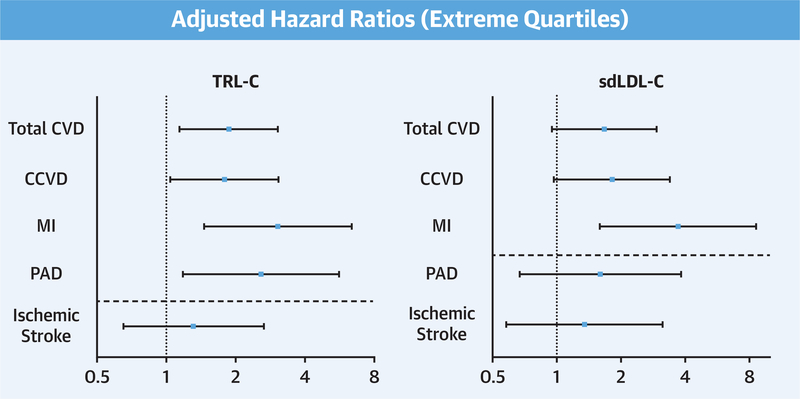
In base models, elevated levels of TRL-C and sdLDL-C were strongly associated with an increased risk of total CVD (model 1 HRQ4: TRL-C: 2.64 [95% CI: 1.76 to 3.97]; p < 0.001; sdLDL-C: 2.35 [95% CI: 1.58 to 3.52]; p<0.001) (Tables 2 and 3). These associations persisted in models adjusted for CVD risk factors, baseline total LDL-C, and hsCRP (TRL-C: HRQ4: 1.87 [95% CI: 1.14 to 3.06]; p = 0.013; sdLDL-C: HRQ4: 1.67 [95% CI: 0.95 to 2.94]; p = 0.025) (Figure 2). Both cholesterol measures remained strongly associated with incident CCVD. The extreme quartile HRs estimated in adjusted models were 1.79 (95% CI: 1.04 to 3.07; p = 0.036) for TRL-C and 1.82 (95% CI: 0.97 to 3.39; p = 0.021) for sdLDL-C. However, when endpoints were evaluated separately, elevations in TRL-C and sdLDL-C were stronger predictors of incident MI and not significantly associated with incident IS (for MI: TRL-C HRQ4: 3.05 [95% CI: 1.46 to 6.39]; p = 0.002; sdLDL-C HRQ4: 3.71 [95% CI: 1.59 to 8.63]; p < 0.001; for IS: TRL-C HRQ4: 1.31 [95% CI: 0.65 to 2.65]; p = 0.45; sdLDL-C HRQ4: 1.35 [95% CI: 0.58 to 3.13]; p = 0.31) (Central Illustration).
TABLE 2.
Incident Cases of Cardiovascular Outcomes Among Initially Healthy Women According to Concentration of TRL-C
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p Value for Trend | |
|---|---|---|---|---|---|
| Median (range), mg/dl | 21.0 (<28.0) | 35 (28.0–42.9) | 51 (43.0–60.9) | 76 (≥61.0) | |
| Total cardiovascular disease | |||||
| Incident cases (n) | 62 | 93 | 139 | 186 | |
| Incident cases, reweighted (nw)* | 110 | 165 | 250 | 328 | |
| Model 1 | 1 | 1.25 (0.81–1.92) | 2.24 (1.47–3.41) | 2.64 (1.76–3.97) | <0.001 |
| Model 2 | 1 | 1.22 (0.78–1.90) | 1.92 (1.23–3.00) | 1.97 (1.26–3.08) | 0.002 |
| Model 3 | 1 | 1.18 (0.75–1.86) | 1.83 (1.16–2.90) | 1.83 (1.13–2.97) | 0.011 |
| Model 4 | 1 | 1.25 (0.78–1.99) | 1.94 (1.21–3.10) | 1.87 (1.14–3.06) | 0.013 |
| Coronary and cerebrovascular disease | |||||
| Incident cases (n) | 47 | 72 | 112 | 148 | |
| Incident cases, reweighted (nw)* | 93 | 142 | 224 | 289 | |
| Model 1 | 1 | 1.29 (0.81–2.06) | 2.42 (1.55–3.79) | 2.83 (1.83–4.37) | <0.001 |
| Model 2 | 1 | 1.22 (0.75–1.98) | 2.00 (1.23–3.24) | 1.93 (1.18–3.14) | 0.006 |
| Model 3 | 1 | 1.17 (0.71–1.92) | 1.89 (1.15–3.10) | 1.76 (1.04–3.00) | 0.030 |
| Model 4 | 1 | 1.23 (0.74–2.05) | 1.99 (1.20–3.30) | 1.79 (1.04–3.07) | 0.036 |
| Myocardial infarction | |||||
| Incident cases (n) | 14 | 24 | 53 | 74 | |
| Incident cases, reweighted (nw)* | 28 | 48 | 106 | 144 | |
| Model 1 | 1 | 1.53 (0.75–3.14) | 3.98 (2.06–7.66) | 5.01 (2.65–9.44) | <0.001 |
| Model 2 | 1 | 1.45 (0.70–3.03) | 3.28 (1.65–6.54) | 3.47 (1.73–6.94) | <0.001 |
| Model 3 | 1 | 1.36 (0.65–2.85) | 3.02 (1.51–6.05) | 2.99 (1.45–6.17) | 0.001 |
| Model 4 | 1 | 1.42 (0.66–3.02) | 3.15 (1.54–6.46) | 3.05 (1.46–6.39) | 0.002 |
| Ischemic stroke | |||||
| Incident cases (n) | 25 | 35 | 45 | 56 | |
| Incident cases, reweighted (nw)* | 49 | 69 | 90 | 111 | |
| Model 1 | 1 | 1.13 (0.62–2.05) | 1.84 (1.04–3.25) | 1.94 (1.10–3.40) | 0.007 |
| Model 2 | 1 | 1.04 (0.56–1.94) | 1.48 (0.81–2.72) | 1.29 (0.68–2.44) | 0.379 |
| Model 3 | 1 | 1.04 (0.56–1.94) | 1.48 (0.79–2.78) | 1.29 (0.65–2.57) | 0.416 |
| Model 4 | 1 | 1.11 (0.58–2.12) | 1.58 (0.82–3.04) | 1.31 (0.65–2.65) | 0.453 |
| Peripheral artery disease | |||||
| Incident cases (n) | 16 | 23 | 31 | 44 | |
| Model 1 | 1 | 1.44 (0.69–2.99) | 2.22 (1.11–4.47) | 2.58 (1.33–5.01) | 0.002 |
| Model 2 | 1 | 1.54 (0.72–3.31) | 2.26 (1.09–4.66) | 2.50 (1.23–5.10) | 0.010 |
| Model 3 | 1 | 1.53 (0.71–3.30) | 2.23 (1.07–4.64) | 2.45 (1.15–5.23) | 0.019 |
| Model 4 | 1 | 1.67 (0.75–3.72) | 2.44 (1.14–5.22) | 2.58 (1.18–5.63) | 0.019 |
Values are hazard ratio (95% confidence interval) unless otherwise indicated. Model 1: age, smoking status, original WHS treatment assignments. Model 2: model 1 covariates + body mass index, hypertension, diabetes mellitus, parental history of myocardial infarction, HRT use, exercise frequency (weekly vs. none). Model 3: model 2 + low-density lipoprotein cholesterol. Model 4: model 3 + high-sensitivity C-reactive protein. Hazard ratios and 95% confidence intervals were obtained from Cox proportional hazards models using the robust sandwich variance estimator and Barlow weighting method.
Cases reweighted to the source population.
n represent the crude number of cases in the sample analyzed. Reweighted nw represent the approximate number of cases that would be expected had the entire WHS blood cohort been analyzed. All peripheral artery disease cases were sampled for this analysis; thus, no reweighting was applied for these cases. HRT = hormone replacement therapy.
TABLE 3.
Incident Cases of Incident Cardiovascular Outcomes Among Initially Healthy Women According to Concentration of sdLDL-C
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p Value for Trend | |
|---|---|---|---|---|---|
| Median (range), mg/dl | 22.0 (<27.0) | 32 (27.0–37.9) | 44 (38.0–50.9) | 64 (≥51.0) | |
| Total cardiovascular disease | |||||
| Incident cases (n) | 65 | 113 | 105 | 197 | |
| Incident cases, reweighted (nw)* | 116 | 201 | 188 | 349 | |
| Model 1 | 1 | 1.26 (0.83–1.90) | 1.38 (0.90–2.09) | 2.35 (1.57–3.52) | <0.001 |
| Model 2 | 1 | 1.14 (0.73–1.77) | 1.07 (0.67–1.70) | 1.84 (1.18–2.88) | 0.003 |
| Model 3 | 1 | 1.10 (0.69–1.75) | 1.01 (0.60–1.72) | 1.71 (0.98–2.99) | 0.019 |
| Model 4 | 1 | 1.09 (0.68–1.74) | 0.98 (0.57–1.66) | 1.67 (0.95–2.94) | 0.025 |
| Coronary and cerebrovascular disease | |||||
| Incident cases (n) | 48 | 91 | 72 | 168 | |
| Incident cases, reweighted (nw)* | 95 | 180 | 144 | 328 | |
| Model 1 | 1 | 1.38 (0.88–2.15) | 1.31 (0.83–2.09) | 2.71 (1.76–4.17) | <0.001 |
| Model 2 | 1 | 1.23 (0.76–1.98) | 1.00 (0.60–1.66) | 2.00 (1.23–3.26) | 0.002 |
| Model 3 | 1 | 1.19 (0.71–1.97) | 0.94 (0.53–1.69) | 1.86 (1.01–3.46) | 0.016 |
| Model 4 | 1 | 1.18 (0.71–1.96) | 0.92 (0.51–1.65) | 1.82 (0.97–3.39) | 0.021 |
| Myocardial infarction | |||||
| Incident cases (n) | 13 | 33 | 32 | 87 | |
| Incident cases, reweighted (nw)* | 26 | 65 | 64 | 171 | |
| Model 1 | 1 | 1.95 (0.97–3.91) | 2.30 (1.14–4.64) | 5.77 (3.03–11.00) | <0.001 |
| Model 2 | 1 | 1.71 (0.84–3.51) | 1.67 (0.80–3.49) | 3.98 (1.99–8.00) | <0.001 |
| Model 3 | 1 | 1.67 (0.78–3.55) | 1.61 (0.72–3.61) | 3.78 (1.63–8.79) | <0.001 |
| Model 4 | 1 | 1.67 (0.78–3.56) | 1.56 (0.70–3.51) | 3.71 (1.59–8.63) | <0.001 |
| Ischemic stroke | |||||
| Incident cases (n) | 25 | 40 | 37 | 59 | |
| Incident cases, reweighted (nw)* | 50 | 79 | 74 | 116 | |
| Model 1 | 1 | 1.12 (0.63–2.00) | 1.24 (0.68–2.26) | 1.73 (0.97–3.07) | 0.035 |
| Model 2 | 1 | 0.98 (0.53–1.83) | 0.92 (0.48–1.78) | 1.33 (0.69–2.55) | 0.265 |
| Model 3 | 1 | 1.00 (0.52–1.95) | 0.96 (0.44–2.08) | 1.39 (0.61–3.21) | 0.274 |
| Model 4 | 1 | 0.99 (0.51–1.94) | 0.91 (0.42–1.99) | 1.35 (0.58–3.13) | 0.313 |
| Peripheral artery disease | |||||
| Incident cases (n) | 18 | 24 | 35 | 37 | |
| Model 1 | 1 | 0.99 (0.49–1.99) | 1.67 (0.87–3.23) | 1.75 (0.92–3.33) | 0.031 |
| Model 2 | 1 | 0.95 (0.46–1.97) | 1.45 (0.71–2.93) | 1.66 (0.83–3.33) | 0.062 |
| Model 3 | 1 | 0.95 (0.45–1.99) | 1.43 (0.65–3.14) | 1.64 (0.69–3.89) | 0.146 |
| Model 4 | 1 | 0.92 (0.43–1.96) | 1.37 (0.62–3.02) | 1.60 (0.67–3.83) | 0.154 |
Values are hazard ratio (95% confidence interval) unless otherwise indicated. Model 1: age, smoking status, original WHS treatment assignments. Model 2: model 1 covariates + body mass index, hypertension, diabetes mellitus, parental history of myocardial infarction, HRT use, exercise frequency (weekly vs. none). Model 3: model 2 + LDL-C. Model 4: model 3 + hsCRP. Hazard ratios and 95% confidence intervals were obtained from Cox proportional hazards models using the robust sandwich variance estimator and Barlow weighting method.
Cases reweighted to the source population.
n represent the crude number of cases in the sample analyzed. Reweighted nw represent the approximate number of cases that would be expected had the entire WHS blood cohort been analyzed. All PAD cases were sampled for this analysis; thus, no reweighting was applied for these cases.
FIGURE 2. Extreme Quartile Adjusted Hazard Ratios for Associations With Incident CVD Outcomes.
Estimates above the dashed horizontal had a statistically significant trend across quartiles (ptrend <0.05), whereas those below did not (ptrend >0.05). All analyses adjusted for model 4 covariates. CCVD = coronary and cerebrovascular disease; CVD = cardiovascular disease; other abbreviations as in Figure 1.
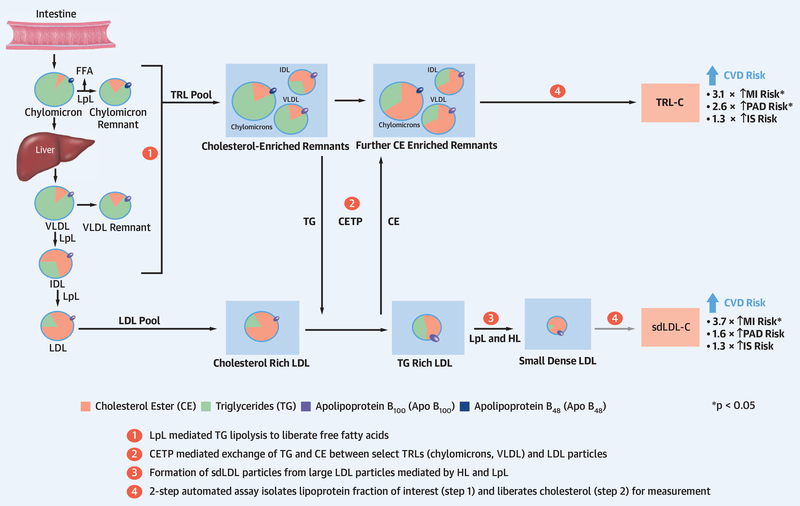
CENTRAL ILLUSTRATION. Cholesterol Content of Triglyceride-Rich Lipoproteins and Small-Dense Low-Density Lipoprotein.
Cholesterol-esterase transfer protein exchange of triglycerides and cholesterol esters occurs between triglyceride-rich lipoproteins (TRL) and low-density lipoprotein particles in hypertriglyceridemia, while hepatic lipase activity promotes conversion of large low-density lipoprotein particles to small dense low-density lipoprotein (sdLDL) particles. The result is an increase in cholesterol-enriched TRL particles and in sdLDL particles of varying triglyceride and cholesterol content. The cholesterol content of TRL and sdLDL is associated with an increased risk of cardiovascular disease outcomes of varying magnitude across vascular territories. Apo = apolipoprotein; CE = cholesteryl ester; CETP = cholesterylester transfer protein; FFA = free fatty acids; HL = hepatic lipase; IDL = intermediate-density lipoprotein; IS = ischemic stroke; LDL = low-density lipoprotein; LpL = lipoprotein lipase; MI = myocardial infarction; PAD = peripheral artery disease; TG = triglycerides; VLDL = very-low-density lipoprotein.
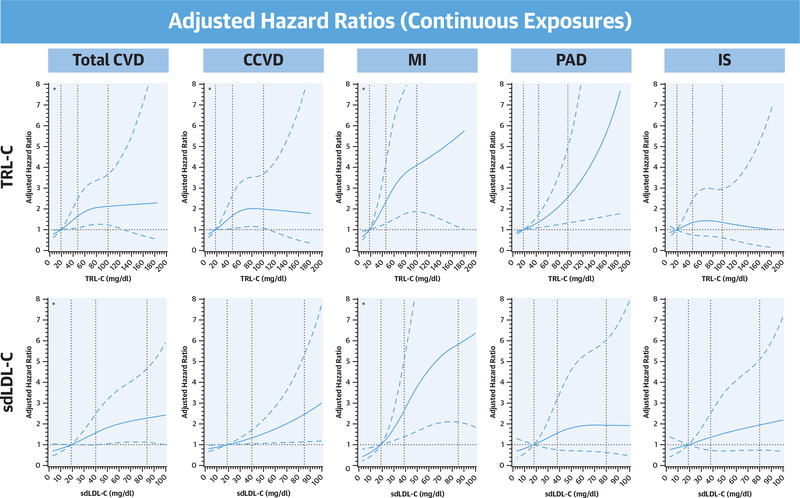
In PAD models, TRL-C strongly associated with an increased risk of incident PAD in the base model (model 1 HRQ4: 2.58 [95% CI: 1.33 to 5.01]; p = 0.002) and in sequentially adjusted models (model 4 HRq4: 2.58 [95% CI: 1.18 to 5.63]; p = 0.019). In contrast, sdLDL-C was weakly associated with incident PAD in the base model and not significantly associated after adjustment for traditional CVD risk factors alone (model 2), with further attenuation after adjustment for total LDL-C (model 3) and hsCRP (model 4 HRq4: 1.60 [95% CI: 0.67 to 3.83]; p = 0.15). We additionally found no difference in our competing risk analysis evaluating these associations in the presence of a preceding CCVD event (data not shown). When evaluated on a continuous scale, there was no material difference in our findings (Figure 3). Nonlinear associations were identified for TRL-C with total CVD, CCVD, and MI (pnonlinearity <0.001 for CVD and MI, pnonlinearity = 0.001 for CCVD) and for sdLDL-C with total CVD and MI (pnonlinearity <0.001 for both). Associations of TRL-C with PAD and sdLDL-C with CCVD were most consistent with a linear relationship.
FIGURE 3. Spline Analyses for Associations With Incident CVD Outcomes.
For each plot, the first knot identified is used as a reference point (hazard ratio: 1.00). *Nonlinear relationships. TRL-C had a nonlinear relationship with incident total CVD (pnonlinearity <0.001; knots at 19.0, 48.0, and 100 mg/dl) as did sdLDL-C (pnonlinearity <0.001; knots at 19.0, 40.0, and 84.0 mg/dl). Associations with CCVD were found to be nonlinear for TRL-C and linear for sdLDL-C (TRL-C pnonlinearity = 0.001; sdLDL-C pnonlinearity = 0.19; plinearity <0.001). Both associations with MI were nonlinear (pnonlinearity <0.001 for both). TRL-C and PAD association was more consistent with linearity (pnonlinearity = 0.17; plinearity = 0.002), whereas sdLDL-C and PAD were not significantly associated. Neither marker significantly associated incident IS. All analyses adjusted for model 4 covariates. Abbreviations as in Figures 1 and 2.
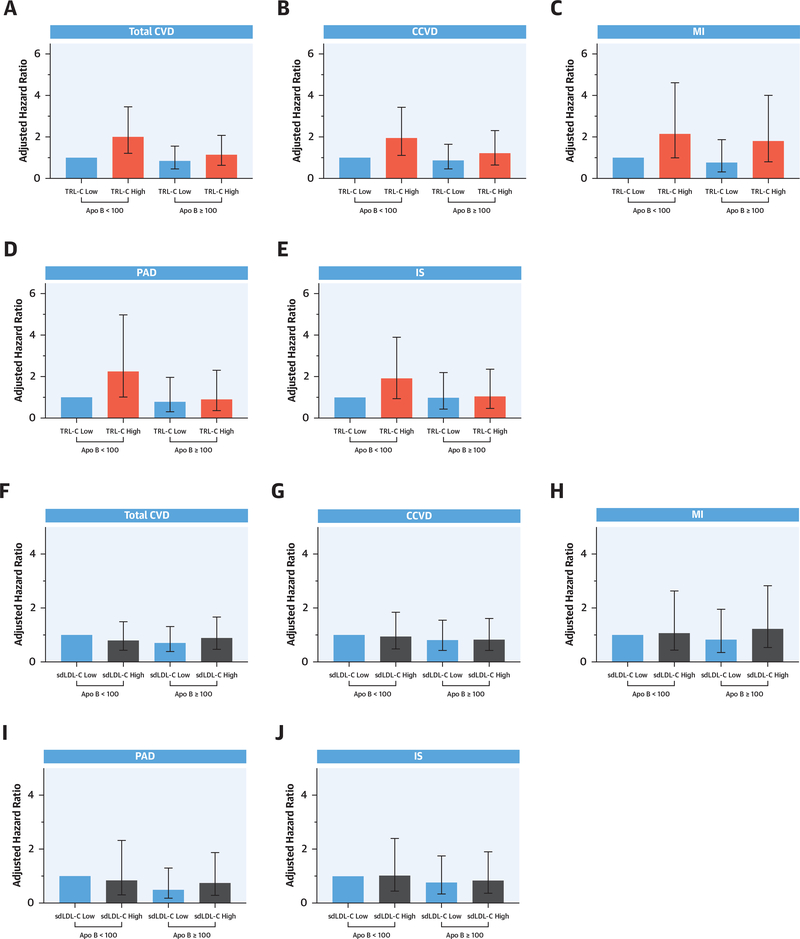
The results of our analyses stratified by concentration of Apo B are shown in Figure 4. Elevated TRL-C (top panels) appeared to confer the greatest risk for each outcome among women with discordantly low atherogenic particle number (most pronounced for MI and PAD). A similar pattern was not observed for sdLDL-C (bottom panels). No evidence of statistical interaction was present.
FIGURE 4. Effects of TRL-C and sdLDL-C Stratified by Apolipoprotein B.
Adjusted cardiovascular risk associations stratified by Apolipoprotein B (<100 mg/dl or ≥100 mg/dl) are shown for TRL-C (A to E) and sdLDL-C (F to J). Panels for total CVD (A, F), CCVD (B, G), MI (C, H), PAD (D, I), and IS (E, J). All analyses adjusted for model 4 covariates. Abbreviations as in Figures 1 and 2.
DISCUSSION
In these prospective analyses, we report that the cholesterol content of lipoprotein fractions elevated in the atherogenic dyslipidemia complex associate with incident cardiovascular outcomes, including total CVD, CCVD, MI, and PAD, among otherwise apparently healthy women (Central Illustration). Elevated levels of TRL-C (also referred to as remnant cholesterol) were strongly associated with a roughly 2-fold increased risk of total CVD and CCVD, 3-fold increased risk of MI, and 2.5-fold increased risk of PAD. In contrast, sdLDL-C was not significantly associated with PAD or IS in multivariable models despite being strongly correlated with TRL-C (Supplemental Figure 3). sdLDL-C associations with increased risk of CCVD and total CVD appeared largely driven by a 3.7-fold increased risk of MI and were independent of traditional risk factors, total LDL-C, and hsCRP. We found similar patterns when TRL-C and sdLDL-C were treated as continuous variables. Our findings thus suggest potential heterogeneity in associations between atherogenic dyslipidemia markers and vascular risk according to vascular bed. Our data also suggest that the influence of elevated cholesterol content of TRL particles may be more important when total Apo B particle concentrations are within the clinically normal range.
ATHEROGENESIS AND TRL-C
TRL atherogenicity is thought to be largely due to TRL conversion to lipoprotein remnants, transcytosis into the arterial intima, and subsequent deposition of cholesterol in arterial walls (35–37). Due to larger size and cholesterol content, preferential retention of cholesterol-enriched remnants in the intima compared to LDL may result in more efficient cholesterol deposition (35,38–41). Differences in the mechanisms of foam cell formation mediated by LDL and TRLs may also be important. Unlike LDL particles, which require oxidative modification before phagocytosis in the arterial intima, TRL remnants are directly phagocytized by macrophages in foam cell formation (42,43). Thus, more efficient cholesterol deposition by TRLs coupled with more direct atherogenicity may account for linkage of this marker to incident MI and PAD, the latter because higher total plaque volume may be required for development of peripheral atherosclerotic lesions. Associations between Friedewald-estimated TRL-C (remnant cholesterol) and ischemic stroke have been reported in Danish cohorts (20). However, these findings were not replicated in our study or another using direct assays (21).
PLAQUE HETEROGENEITY AND sdLDL
Atherosclerotic plaque instability and vulnerability to rupture may be relevant to our findings regarding sdLDL-C. Thin fibrous caps and large lipid or necrotic cores are key features of unstable plaques known as fibroatheromas, and such features are known drivers of acute rupture and erosion leading to subsequent vascular events (44,45). Thin-capped fibroatheromas have been identified as the dominant plaque phenotype in coronary artery disease, with a mixture of thin- and thick-capped fibroatheroma in atherosclerotic cerebrovascular disease (46). In contrast, fibrocalcific plaques are more stable, are less prone to rupture (45), and are the main plaque phenotype in subjects with clinically diagnosed PAD (46,47). Compared with LDL, sdLDL particles are more susceptible to oxidative modification and more readily engulfed by macrophages (48,49), with greater levels of oxidized-LDL particles being linked to plaque instability in coronary (50) and carotid (51,52) artery disease. These experimental observations may explain why elevated sdLDL-C concentrations in the current study correspond with development of unstable, rupture-prone plaque phenotypes characteristic of MI rather than the more stable plaque phenotypes of claudicants with PAD. Additionally, knowledge that smaller LDL particle size has previously been associated with PAD in the WHS (12) suggests that measured sdLDL-C concentration, similar to measured total LDL-C concentration in our prior work, does not adequately reflect particle number (5,53). In aggregate, our data from the WHS suggest that LDL particle number but not cholesterol content are pathogenic in PAD.
ASSOCIATIONS WITH IS
To our knowledge, prospective epidemiological data linking the cholesterol content of sdLDL particles to incident IS have not been previously reported. Current evidence suggests that total LDL-C concentrations in women may be less important in development of IS than CHD (54,55). When present, positive associations with LDL-C have been identified with development of large vessel atherosclerotic subtypes of IS but not with cardioembolic subtypes (56). We are unable to distinguish stroke subtypes in our dataset. However, a recent Mendelian randomization study including over 12,000 cases of ischemic stroke found that elevated LDL-C concentrations were not associated with either total IS or IS subtype, but were strongly associated with incident CHD (57). As noted in the previous text, associations of TRL-C (or its subfractions) with IS have also varied across studies (20,21), and further investigation is needed to determine why cholesterol measures that consistently predict CHD are less reliable predictors of future IS.
RELATIONSHIP WITH Apo B
Also relevant to our findings, recent evidence from a large study of LPL and LDLR gene variants suggests that the total Apo B particle concentration may be more influential in the development of CHD than either the triglyceride or cholesterol content of TRL and LDL particles (58). The protective effects against coronary atherosclerosis associated with lower triglyceride and LDL-C observed in that study were nearly identical and proportional to differences in Apo B concentration, suggesting that Apo B–containing particles are equally atherogenic. However, most participants in that study did not have elevated triglycerides. Thus, these findings may not apply to patients with hypertriglyceridemia, in whom the atherogenic potential and cardiovascular risk per TRL particle may be greater than per LDL particle. We observed that elevated TRL-C concentrations appeared most important when Apo B levels are normal or low. Specifically, subjects with clinically normal Apo B levels remain at high risk of total CVD, CCVD, MI, PAD, and IS when TRL-C levels are high (Figures 4A to 4E). These findings support the concept that fewer TRL particles with greater cholesterol content (i.e., large particles double-enriched with triglycerides and cholesterol) confer greatest risk for coronary and peripheral atherosclerosis. The triglyceride content of TRLs, although not studied in this analysis, may also contribute to vulnerable atherosclerotic plaques and heightened cardiovascular risk, as elevated triglycerides have been associated with low-grade inflammation in observational and genetic Mendelian randomization studies (15,59).
NOVEL THERAPEUTICS
Our findings are relevant to completed and ongoing clinical trials using novel therapeutic interventions to reduce hypertriglyceridemia, a surrogate for elevated TRLs and TRL-C (60). As recently demonstrated in REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial), icosapent ethyl was associated with a 25% reduction in the major cardiovascular events in patients enrolled on the basis of hypertriglyceridemia (61). Whether levels of TRL-C and sdLDL-C are influenced by icosapent ethyl is currently unknown and warrants further investigation. Notably, icosapent ethyl did not significantly reduce the incidence of lower-extremity amputation, although detailed analyses of limb-related outcomes have not been published. As previously shown in the FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study, fibrate therapy reduced lower-extremity minor amputation attributable to diabetic microvascular disease in the absence of large-vessel atherosclerotic disease (i.e., PAD) (62). As this effect was not observed when large-vessel disease was present, the role of fibrates in PAD amputations requires furthers investigation.
Recent evidence related to the efficacy of pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator (63), may also be informative. In phase 2 trials of subjects with residual dyslipidemia on background statin therapy, pemafibrate was associated with a 20% reduction in the cholesterol content of sdLDL particles, resulting in a shift toward increased cholesterol content of larger LDL particles (64). Pemafibrate therapy also resulted in a 50% reduction in the cholesterol content of TRL remnants while also lowering triglycerides. The clinical efficacy and safety of this approach to lipid modification is being evaluated in the ongoing PROMINENT (Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in patients with Diabetes) study (65). The primary endpoint is a composite of fatal and nonfatal coronary and stroke events. However, incident PAD is adjudicated as a key secondary endpoint, and the trial will inform whether treatment of atherogenic dyslipidemia will reduce all 3 major vascular endpoints.
STUDY LIMITATIONS AND STRENGTHS
First, our findings may not be generalizable to demographic groups not included in our study population. The use of a homogenous study population with a low prevalence of risk factors associated with the key exposures does, however, strengthen the internal validity of our findings and diminish the potential for residual confounding. Second, interindividual variation and longitudinal changes in TRL-C and sdLDL-C could not be assessed in our study. However, we leveraged the efficiency of the case-cohort design to evaluate risk associations between baseline measures of 2 markers of atherogenic dyslipidemia with multiple incident cardiovascular outcomes, including PAD, all validated by a cardiovascular endpoint committee.
CONCLUSIONS
Baseline levels of TRL-C and sdLDL-C measured in our study associated with future cardiovascular events differently despite similar correlations with traditional CV risk factors and traditional indexes of atherogenic dyslipidemia, and strong correlations with each other. The cholesterol content of TRL particles appears to confer atherosclerotic risk mainly through MI and PAD events, even among subjects with clinically normal total Apo B particle levels. Recommendations in future lipid guidelines to measure TRL-C when Apo B is within normal limits may help to identify subjects who are at persistent risk of future atherosclerotic events. The cholesterol content of sdLDL particles, however, appears to influence the development of total cardiovascular events largely through increased risk of fatal and nonfatal MI. Our data are relevant to understanding residual cardiovascular risk beyond LDL-C and to drug-development programs exploring novel targets for atherosclerosis prevention.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
TRL-C and sdLDL-C are associated with other indexes of dyslipidemia and with incident MI independent of conventional risk factors, LDL-C, and inflammation. TRL-C is also associated with PAD.
TRANSLATIONAL OUTLOOK
Further studies of TRL-cholesterol content and sdLDL-cholesterol content may improve understanding of the pathogenesis of atherosclerosis and open new avenues for primary and secondary prevention in patients with dyslipidemia.
ACKNOWLEDGMENTS
The authors thank statistical programmers Eunjung Kim and M. Vinayaga Moorthy for their efforts
This work was supported by the National Institutes of Health under Award Number 5T32HL007575-33 (Dr. Duran) and K12HL133117 (Dr. Aday). The Women’s Health Study was funded by grants CA047988, HL043851, HL099355, HL080467, and UM1 CA182913. Additional funding was provided by Denka-Seiken and Kowa Research Institute for TRL-C and sdLDL-C measurements. Dr. Ridker has received research funding support from multiple not-for-profit entities including the National Heart, Lung, and Blood Institute, the National Cancer Institute, the American Heart Association, the Doris Duke Charitable Foundation, the Leducq Foundation, and the Donald W. Reynolds Foundation; has received investigator-initiated research support from AstraZeneca, Novartis, Pfizer, and Kowa; is listed as a coinventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease that have been licensed to Siemens and AstraZeneca; has served during the past year as a research consultant to Iqvia, Novartis, Corvidia, Inflazome, Amgen, Merck, Sanofi, Civibio, and Janssen; and has served as co-principal investigator of the PROMINENT trial. Dr. Pradhan has received investigator-initiated research support from Kowa Research Institute; has served as co-principal investigator of the PROMINENT trial; and has served as a consultant for OptumCare. Assays and research support for this study were provided by Kowa Denka Company Ltd. and Kowa Research Europe Ltd.
ABBREVIATIONS AND ACRONYMS
- CCVD
coronary and cerebrovascular disease
- CVD
cardiovascular disease
- HR
hazard ratio
- hsCRP
high-sensitivity C-reactive protein
- IS
ischemic stroke
- MI
myocardial infarction
- PAD
peripheral artery disease
- sdLDL-C
small-dense low-density lipoprotein cholesterol
- TRL-C
triglyceride-rich lipoprotein cholesterol
- WHS
Women’s Health Study
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For supplemental tables and figures, please see the online version of this paper.
REFERENCES
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction in J Am Coll Cardiol 2019;73:3237-41]. J Am Coll Cardiol 2019;73: e285–350. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, Emberson J, et al. , for the Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fruchart JC, Sacks FM, Hermans MP, et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diab Vasc Dis Res 2008;5: 319–35. [DOI] [PubMed] [Google Scholar]
- 4.Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF. Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL Ccholesterol. Diabetes 2016; 65:1767–78. [DOI] [PubMed] [Google Scholar]
- 5.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol 2002;90:22i–9i. [DOI] [PubMed] [Google Scholar]
- 6.Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans 2003;31:1066–9. [DOI] [PubMed] [Google Scholar]
- 7.Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008; 28:1225–36. [DOI] [PubMed] [Google Scholar]
- 8.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 2002;43:1363–79. [DOI] [PubMed] [Google Scholar]
- 9.Mora S, Szklo M, Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007;192:211–7. [DOI] [PubMed] [Google Scholar]
- 10.Lawler PR, Akinkuolie AO, Chu AY, et al. Atherogenic lipoprotein determinants of cardiovascular disease and residual risk among individuals with low low-density lipoprotein cholesterol. J Am Heart Assoc 2017;6:e005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S, Pradhan AD. Lipoprotein particle profiles, standard lipids, and peripheral artery disease incidence. Circulation 2018;138:2330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease [published correction in J Am Coll Cardiol 2019;73:987–8]. J Am Coll Cardiol 2013;61:427–36. [DOI] [PubMed] [Google Scholar]
- 14.Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther 2014;141:358–67. [DOI] [PubMed] [Google Scholar]
- 15.Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013;128: 1298–309. [DOI] [PubMed] [Google Scholar]
- 16.Varbo A, Freiberg JJ, Nordestgaard BG. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin Chem 2015;61:533–43. [DOI] [PubMed] [Google Scholar]
- 17.Varbo A, Nordestgaard BG. Remnant cholesterol and ischemic heart disease. Curr Opin Lipidol 2014;25:266–73. [DOI] [PubMed] [Google Scholar]
- 18.Hoogeveen RC, Gaubatz JW, Sun W, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol 2014; 34:1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varbo A, Freiberg JJ, Nordestgaard BG. Remnant cholesterol and myocardial infarction in normal weight, overweight, and obese individuals from the copenhagen general population study. Clin Chem 2018;64:219–30. [DOI] [PubMed] [Google Scholar]
- 20.Varbo A, Nordestgaard BG. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol 2019;85:550–9. [DOI] [PubMed] [Google Scholar]
- 21.Saeed A, Feofanova EV, Yu B, et al. Remnantlike particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol 2018;72:156–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallejo-Vaz AJ, Fayyad R, Boekholdt SM, et al. Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT Trial. Circulation 2018; 138:770–81. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–304. [DOI] [PubMed] [Google Scholar]
- 24.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA 2005;294:56–65. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Manson JE, Buring JE, Shih J, Matias M, Hennekens CH. Homocysteine and risk of cardiovascular disease among postmenopausal women. JAMA 1999;281:1817–21. [DOI] [PubMed] [Google Scholar]
- 26.Duran EK, Cook NR, Bobadilla M, et al. Plasma placental growth factor concentrations are elevated well in advance of type 2 diabetes mellitus onset: prospective data from the WHS. J Am Heart Assoc 2019;8:e012790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito Y, Fujimura M, Ohta M, Hirano T. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem 2011; 57:57–65. [DOI] [PubMed] [Google Scholar]
- 28.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation 2008;117: 823–31. [DOI] [PubMed] [Google Scholar]
- 29.Therneau TM, Li H. Computing the Cox model for case cohort designs. Lifetime Data Anal 1999; 5:99–112. [DOI] [PubMed] [Google Scholar]
- 30.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics 1994;50: 1064–72. [PubMed] [Google Scholar]
- 31.Langholz B, Jiao J. Computational methods for case-cohort studies. Computational Statistics & Data Analysis 2007;51:3737–48. [Google Scholar]
- 32.Wolkewitz M, Palomar-Martinez M, Olaechea-Astigarraga P, Alvarez-Lerma F, Schumacher M. A full competing risk analysis of hospital-acquired infections can easily be performed by a case-cohort approach. J Clin Epidemiol 2016;74: 187–93. [DOI] [PubMed] [Google Scholar]
- 33.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8: 551–61. [DOI] [PubMed] [Google Scholar]
- 34.Adiels M, Taskinen MR, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 2006;49:755–65. [DOI] [PubMed] [Google Scholar]
- 35.Mamo JC, Proctor SD, Smith D. Retention of chylomicron remnants by arterial tissue; importance of an efficient clearance mechanism from plasma. Atherosclerosis 1998;141 Suppl 1:S63–9. [DOI] [PubMed] [Google Scholar]
- 36.Zilversmit DB. A proposal linking atherogenesis to the interaction of endothelial lipoprotein lipase with triglyceride-rich lipoproteins. Circ Res 1973;33:633–8. [DOI] [PubMed] [Google Scholar]
- 37.Mahley RW, Innerarity TL, Rall SC Jr., Weisgraber KH. Lipoproteins of special significance in atherosclerosis. Insights provided by studies of type III hyperlipoproteinemia. Ann N Y Acad Sci 1985;454:209–21. [DOI] [PubMed] [Google Scholar]
- 38.Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol 1995;15:534–42. [DOI] [PubMed] [Google Scholar]
- 39.Nordestgaard BG. The vascular endothelial barrier–selective retention of lipoproteins. Curr Opin Lipidol 1996;7:269–73. [DOI] [PubMed] [Google Scholar]
- 40.Rutledge JC, Mullick AE, Gardner G, Goldberg IJ. Direct visualization of lipid deposition and reverse lipid transport in a perfused artery: roles of VLDL and HDL. Circ Res 2000;86:768–73. [DOI] [PubMed] [Google Scholar]
- 41.Shaikh M, Wootton R, Nordestgaard BG, et al. Quantitative studies of transfer in vivo of low density, Sf 12–60, and Sf 60–400 lipoproteins between plasma and arterial intima in humans. Arterioscler Thromb 1991;11:569–77. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi S, Sakai J, Fujino T, et al. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atheroscler Thromb 2004;11:200–8. [DOI] [PubMed] [Google Scholar]
- 43.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013;13:709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen YC, Huang AL, Kyaw TS, Bobik A, Peter K. Atherosclerotic plaque rupture: identifying the straw that breaks the camel’s back. Arterioscler Thromb Vasc Biol 2016;36:e63–72. [DOI] [PubMed] [Google Scholar]
- 45.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–66. [DOI] [PubMed] [Google Scholar]
- 46.Matsuo Y, Takumi T, Mathew V, et al. Plaque characteristics and arterial remodeling in coronary and peripheral arterial systems. Atherosclerosis 2012;223:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polonsky TS, Liu K, Tian L, et al. High-risk plaque in the superficial femoral artery of people with peripheral artery disease: prevalence and associated clinical characteristics. Atherosclerosis 2014;237:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation 2004;109 Suppl 23:III2–7. [DOI] [PubMed] [Google Scholar]
- 49.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988;260:1917–21. [PubMed] [Google Scholar]
- 50.Ehara S, Ueda M, Naruko T, et al. Pathophysiological role of oxidized low-density lipoprotein in plaque instability in coronary artery diseases. J Diabetes Complications 2002;16:60–4. [DOI] [PubMed] [Google Scholar]
- 51.Nishi K, Itabe H, Uno M, et al. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol 2002;22: 1649–54. [DOI] [PubMed] [Google Scholar]
- 52.Sigala F, Kotsinas A, Savari P, et al. Oxidized LDL in human carotid plaques is related to symptomatic carotid disease and lesion instability. J Vasc Surg 2010;52:704–13. [DOI] [PubMed] [Google Scholar]
- 53.Otvos J Measurement of triglyceride-rich lipoproteins by nuclear magnetic resonance spectroscopy. Clin Cardiol 1999;22 Suppl 6: II21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Everett BM, Kurth T, Buring JE, Ridker PM. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol 2006;48:2235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willey J, Gonzalez-Castellon M. Cholesterol level and stroke: a complex relationship. JAMA Intern Med 2013;173:1765–6. [DOI] [PubMed] [Google Scholar]
- 56.Imamura T, Doi Y, Arima H, et al. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: the Hisayama study. Stroke 2009;40: 382–8. [DOI] [PubMed] [Google Scholar]
- 57.Valdes-Marquez E, Parish S, Clarke R, et al. Relative effects of LDL-C on ischemic stroke and coronary disease: a Mendelian randomization study. Neurology 2019;92:e1176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ference BA, Kastelein JJP, Ray KK, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA 2019;321:364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen SEJ, Madsen CM, Varbo A, Nordestgaard BG. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: a study of more than 115000 individuals from the general population. Clin Chem 2019;65:321–32. [DOI] [PubMed] [Google Scholar]
- 60.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res 2016;118:547–63. [DOI] [PubMed] [Google Scholar]
- 61.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380: 11–22. [DOI] [PubMed] [Google Scholar]
- 62.Rajamani K, Colman PG, Li LP, et al. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet 2009;373:1780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fruchart JC. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor alpha modulator for management of atherogenic dyslipidaemia. Cardiovasc Diabetol 2017;16:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arai H, Yamashita S, Yokote K, et al. Efficacy and safety of K-877, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), in combination with statin treatment: Two randomised, doubleblind, placebo-controlled clinical trials in patients with dyslipidaemia. Atherosclerosis 2017; 261:144–52. [DOI] [PubMed] [Google Scholar]
- 65.Pradhan AD, Paynter NP, Everett BM, et al. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J 2018;206:80–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.