Abstract
Study Objectives: Nonrapid eye movement sleep boosts hippocampus-dependent, long-term memory formation more so than wake. Studies have pointed to several electrophysiological events that likely play a role in this process, including thalamocortical sleep spindles (12–15 Hz). However, interventional studies that directly probe the causal role of spindles in consolidation are scarce. Previous studies have used zolpidem, a GABA-A agonist, to increase sleep spindles during a daytime nap and promote hippocampal-dependent episodic memory. The current study investigated the effect of zolpidem on nighttime sleep and overnight improvement of episodic memories. Methods: We used a double-blind, placebo-controlled within-subject design to test the a priori hypothesis that zolpidem would lead to increased memory performance on a word-paired associates task by boosting spindle activity. We also explored the impact of zolpidem across a range of other spectral sleep features, including slow oscillations (0–1 Hz), delta (1–4 Hz), theta (4–8 Hz), sigma (12–15 Hz), as well as spindle–SO coupling. Results: We showed greater memory improvement after a night of sleep with zolpidem, compared to placebo, replicating a prior nap study. Additionally, zolpidem increased sigma power, decreased theta and delta power, and altered the phase angle of spindle–SO coupling, compared to placebo. Spindle density, theta power, and spindle–SO coupling were associated with next-day memory performance. Conclusions: These results are consistent with the hypothesis that sleep, specifically the timing and amount of sleep spindles, plays a causal role in the long-term formation of episodic memories. Furthermore, our results emphasize the role of nonrapid eye movement theta activity in human memory consolidation.
Keywords: zolpidem, memory, sleep spindles, spindle, SO coupling, theta
Statement of Significance.
Sleep spindles have emerged as one of the key electrophysiological markers for memory consolidation during sleep, yet interventional studies that directly probe the causal relation are scarce. It also remains under-investigated if other oscillations, such as theta, contribute to memory consolidation during nonrapid eye movement sleep. Using a within-subject, double-blind, placebo-controlled paradigm, this study pharmacologically manipulated a range of spectra features over a night of sleep in order to identify the key mechanism of sleep-dependent memory consolidation. Our results suggest a functional role of both sigma and theta in which optimizing sleep spindles while preserving theta activity may be a goal of future sleep interventions to enhance memory consolidation.
Introduction
Converging evidence from both cellular and behavioral research suggests an essential role of sleep in memory consolidation [1–3]. For hippocampal-dependent episodic memories, studies have shown greater performance improvement following the first half of the night rich in slow-wave sleep (SWS), compared to the second half of the night with majority rapid eye movement (REM) sleep [4, 5]. In addition, neocortical slow oscillations (SOs, 0.5–1 Hz) and thalamocortical spindles (12–15 Hz) have emerged as two prominent features of non-REM (NREM) sleep associated with episodic memory consolidation [6]. Several studies have shown correlations between postsleep memory improvement and SOs, and causally increasing SOs using stimulation interventions have shown significant increases in hippocampal-dependent episodic memories in both animals [7] and humans [8, 9]. Studies investigating the role of sleep spindles in memory consolidation have also shown positive associations [10, 11]. Clemens et al. [12] reported correlations between spindles and overnight verbal memory retention, but not visual skill learning. In another study, spindle density in stage 2 sleep increased after episodic memory encoding but not after a nonlearning task where participants were instructed to count letters containing curved lines [13]. The important role of sleep spindles in sleep-dependent memory consolidation is further suggested in targeted memory reactivation (TMR) studies [14, 15]. Specifically, cues associated with memory reactivation optimally benefitted declarative memory when presented outside of spindle refractory periods [14], and TMR cues can increase fast spindle power [15]. In addition, Lustenberger et al. [16] have enhanced spindle activity using transcranial alternating current stimulation, which led to an increase in motor memory consolidation, but not declarative memory. In the current study, we used pharmacology to manipulate spindle activity and investigated the relationship between changes in memory performance and multiple sleep features.
More recently, the temporal coupling between spindles and SOs has been suggested to reflect hippocampal-thalamocortical communication during NREM sleep, and that this coordinated activity pattern may underlie the formation and protection of long-term memories [17, 18]. Accordingly, several studies suggest that coincident spindle–SO coupling leads to greater memory, optimally when spindle activity occurs during the SO up-state [19–22]. Optogenetically stimulating spindle activity during the up-state of SOs in rodents improved contextual fear-conditioning, compared with random or no stimulation [23]. A recent study examining the coupling of sigma and SO in relation to functional magnetic resonance imaging (fMRI) brain activity and memory performance in young and older adults [24] reported that spindle–SO coupling predicted postsleep episodic memory improvement in both age groups. However, older adults showed altered timing between spindle–SO coupling and decreased coupling over the frontal pole, which may contribute to decreases in overnight memory retention in older compared to young adults. Additionally, optimal spindle–SO timing may compensate for a lower total number of spindles and support memory [25]. Specifically, patients with schizophrenia showed reduced spindle number and density but similar spindles–SO coupling compared to healthy individuals, and magnitude of coordination between spindles and SOs was correlated with overnight improvement on a hippocampal-dependent memory task [25].
Experimental approaches to probe the causal nature of sleep spindles are scarce. Spindles can be pharmacologically increased with zolpidem (Ambien), a GABA-A agonist with a half-life of approximately 1.5–3.2 h and peak plasma concentration 1.6 h after ingestion [26]. Our group has shown that administering zolpidem during a morning nap increases spindle density compared with placebo and a comparison hypnotic, sodium oxybate [27]. Additionally, zolpidem improved episodic memory, but decreased perceptual learning, and had no effect on motor learning, compared with placebo [27]. Verbal memory performance was significantly correlated with spindle density in zolpidem and placebo, but not with sodium oxybate [27]. Furthermore, zolpidem increased the coincidence of the spindle–SO coupling measured by phase angle, which also correlated with memory improvement [21]. Similarly, eszopiclone, a GABA-based non-benzodiazepine, increased spindles and the association between spindles and motor learning [28]. Given the small number of studies that test the impact of directly manipulating spindles on memory, and that zolpidem has only been tested in a morning nap, more research is needed.
The current study investigated the impact of zolpidem on sleep physiology across a night of sleep, specifically on spindle activity and spindle–SO coupling, and overnight episodic memory consolidation of word-paired associates (WPA). In addition, zolpidem is known to reduce theta oscillation for both men and women [29], yet the effect on memory is unknown. Specifically, a study using zolpidem as a treatment for insomnia showed that zolpidem decreased theta power and increased sigma power with no change in SO in patients [30]. Another study showed that zolpidem decreased band power between 3.75 and 10 Hz for sleep-deprived participants, with theta power having the largest reduction [31]. Therefore, a secondary goal of the current study was to investigate a range of sleep features that could be altered by zolpidem, focusing on theta activity, but also including SO and delta, and their relation to memory performance. Taking into consideration the short half-life of zolpidem [26], we divided the night of sleep into four quartiles in order to assess the effect of zolpidem on sleep physiology during peak plasma periods, as well as across the whole night. We hypothesized that, compared to placebo, zolpidem would show increased performance on an episodic memory task, as well as greater spindle density and spindle–SO coupling during the first two quartiles of sleep due to the pharmacokinetics of zolpidem.
Methods
Participants
Thirty-six healthy adults (Mage = 21.00 ± 2.97 years, 19 females) with no history of neurological, psychological, or other chronic illnesses were recruited for the study. All participants signed informed consent, which was approved by the Western Institutional Review Board and the University of California, Riverside Human Research Review Board. Exclusion criteria included irregular sleep/wake cycles (defined as having a habitual bedtime after 02:00 am and a habitual wake time after 10:00 am), sleep disorder, personal or familial history of diagnosed psychopathology, substance abuse/dependence, loss of consciousness greater than 2 min or a history of epilepsy, current use of psychotropic medications, and any cardiac or respiratory illness that may affect cerebral metabolism, which was determined during an in-person psychiatric assessment with trained research personnel. Additionally, all participants underwent a medical history and physical appointment with a staff physician to ensure their physical well-being. All participants were naïve to or had limited contact with (<2 lifetime use and no use in last year) the medication used in the study. Participants received monetary compensation and/or course credit for participating in the study.
Procedure
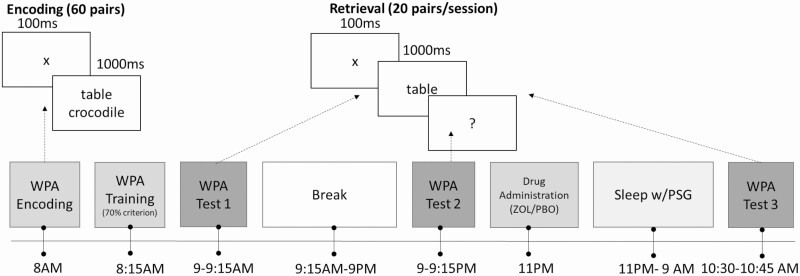
This study employed a double-blind, placebo-controlled, within-subject design, in which every participant experienced both zolpidem and placebo. The order of drug conditions was counterbalanced with at least a 1-week interval between each experimental visit to allow for drug clearance. Participants reported to the laboratory at 7:30 am and began encoding the paired associates verbal memory task at 8:00 am. This research was a part of a larger study that investigated the independent and combined effects of a psychostimulant (dextroamphetamine) and a hypnotic (zolpidem) on sleep and cognition. In this parent study, we tested the daytime administration of the stimulant, which is the reason participants encoded word-pairs in the morning and then tested three times across 24 h [32]. Participants returned to the laboratory at 9:00 pm at which point a second memory test was given. After testing, participants were prepared for nighttime sleep, which included a 32-channel polysomnography recording. Once in bed and directly before lights out, participants ingested either 10 mg of zolpidem or placebo. Participants were woken up at 9:00 am the next morning and provided a standardized breakfast. At 10:30 am, participants completed the memory task and were permitted to leave the laboratory after being cleared by study personnel (Figure 1).
Figure 1.
Study protocol. Participants encoded 60 word-pairs at 8:00 am and trained with 70% criterion, followed by an immediate test of 20 pairs. In the evening, participants returned to the laboratory and were tested on another set of 20 pairs. Before sleep, participants ingested a dose of zolpidem or placebo. In the morning, participants were tested on the remaining set of 20 pairs. Participants returned to the laboratory after 1-week washout period and performed the same protocol with a different drug. The order of the drug was counterbalanced between participants.
Task
The WPA task consisted of an encoding phase and three retrieval phases. Encoding consisted of viewing 60 pairs of words, each presented vertically stacked and shown twice in random order. Every word pair was presented for 1,000 ms followed by a fixation cross for 100 ms. We trained participants to criterion using a test in which participants were shown one word of the pair and were required to type in the missing word. Feedback was provided and participants had to achieve 70% accuracy to finish the training. For testing, the 60 word-pairs were divided into three sets of 20 pairs; one set was tested at each test session and the order was counterbalanced. Three retrieval tests were conducted: (1) immediately after encoding (09:00 am, test 1), (2) before sleep (09:00 pm, test 2), and (3) the morning after sleep (10:30 am, test 3). No feedback was provided during testing.
Sleep recording
EEG data were acquired using a 32-channel cap (EASEYCAP GmbH) with Ag/AgCI electrodes placed according to the international 10–20 System (Jasper, 1958). Twenty-two electrodes were scalp recordings and the remaining electrodes were used for electrocardiogram, electromyogram (EMG), electrooculogram (EOG), ground, an online common reference channel (at FCz location, retained after re-referencing), and mastoid (A1 & A2) recordings. The EEG was recorded with a 1,000 Hz sampling rate and was re-referenced to the contralateral mastoid (A1 & A2) postrecording (midline electrodes were re-referenced to the average of two mastoids [33]). Data were preprocessed using BrainVision Analyzer 2.0 (BrainProducts, Munich, Germany). Eight scalp electrodes (F3, F4, C3, C4, P3, P4, O1, and O2), the EMG, and EOG were used in the scoring of the nighttime sleep data. High-pass filters were set at 0.3 Hz and low-pass filters at 35 Hz for EEG and EOG. Raw data were visually scored in 30-s epochs into wake, stage 1, stage 2, SWS, and REM sleep according to the Rechtschaffen & Kales’ manual [34]. After staging, all epochs with artifacts and arousals were identified rejected by visual inspection before spectral analyses. Minutes in each sleep stage and sleep latencies (the number of minutes from lights out until the initial epoch of sleep, stage 2, SWS, and REM) were calculated. Additionally, wake after sleep onset (WASO) was calculated as total minutes awake after the initial epoch of sleep, and sleep efficiency (SE) was computed as total time spent asleep after lights out (~11:00 pm) divided by the total time spent in bed (~11:00 pm–9:00 am) × 100.
Spindle detection
For each electrode, we first found the peak frequency fp of stage 2 and SWS power spectrum within the 9–15 Hz band. For the electrodes with no power spectrum peak in this range, the average of peak frequencies from other EEG electrodes was considered as fp. Next, we calculated the time series of average EEG energy, E(t), after convolving the signals by complex Morlet wavelets ψt=Aexp(-t2/2σt2) exp (i2πf0t), where f0 is in range [fp − 1.5 fp +1.5] Hz with 0.1 Hz steps, A=σtπ-1/2, σt=1/2πσf, σ f=f0/10, i=-1, and wavelet duration is in range −5σt < t < 5σt. Spindles were detected at each EEG electrode by applying a thresholding algorithm on E2(t). The threshold was defined as four times the mean amplitude of E2(t) of all artifact-free 30-s epochs. A spindle event was identified whenever E2(t) exceeded the threshold for a minimum of 250 ms. Finally, the detected spindles were refined if the estimated frequency of each spindle fell in the range of 9–15 Hz. In order to estimate a spindle frequency, the zero-crossings of the high-passed filtered (2 Hz) version of EEG in the candidate spindle intervals were first detected. Then, the spindle frequency was estimated as fest=(N-1)/2∆T, where N is the number of zero-crossings and ΔT is the time difference between the last and first zero-crossings within a candidate spindle interval.
This method is based on Wamsley’s method [22, 35–39] which has been cited in a meta-analysis of spindle detectors as a high performer [40–42], with a few minor modifications. Specifically, Wamsley employed a fixed range of wavelets to filter the data for spindle activity across electrodes, while our method extracts a peak frequency in a possible range for spindle activity for each electrode. This allows a more flexible window to account for all spindle activities. After identifying candidate spindle events, we also do a refinement based on number of zero-crossings for candidate spindles.
Calculating spindle detection thresholds individually for both conditions risks the possibility that the threshold might differ significantly between conditions. If the spindle amplitudes were higher in one condition, a higher detection threshold would be used for that condition, biasing the density values to lower numbers. To address this issue, we have acquired individual threshold at each channel for each condition averaged across participants and performed paired t-test between the two conditions. No significant difference was found at any electrode between the two conditions (Supplementary Figure 2), suggesting spindle values detected from this method were not biased by drug condition. Another issue associated with the individual thresholds used in the spindle detector is the possibility to bias slow spindles as slower frequencies display higher spectral power. To address this issue, we acquired individual peak-frequencies at each channel for each condition averaged across participants and performed paired t-test between the two conditions. No significant difference between the two conditions was found at any channel, suggesting slow spindles were not biased by this detection method (Supplementary Figure 3).
SO detection
SO events were detected based on the algorithm developed by Massimini et al. [43]. The EEG signals were first filtered (zero-phase bandpass, 0.1–4 Hz). Then, the SO events were detected based on a set of criteria for down- to up-state amplitude (>140 µV), down-state amplitude (<−80 µV), and duration of down-state (between 0.3 and 1.5 s) and up-state (<1 sec) (see the study of Dang-Vu et al. [44] for more details).
SO–spindle modulation index
Coupling between the phase of SO and amplitude of sigma power (12–15 Hz) during stage 2 and SWS was measured by modulation index (MI) as described by Canolty et al. [45] and adapted by our group [21]. First, we based the MI analysis on detected SO events (described in the SO detection section) in order to eliminate spurious EEG coupling from the entire sleep stage. In addition, we narrowed our analysis to frontal electrodes (F3 and F4), which have been reported to show the strongest SO activity [43]. To calculate the MI, we applied a Hilbert Transformation to SO and sigma power within the SO event windows to construct the composite complex-valued signal of the amplitude of sigma power and the phase of the SO: Z[n]=asigma[n]expi∅SOn. The normalized mean of this composite vector across trials is the raw MI. Higher MI values indicate less variability in the timing between spindle amplitude peak and a certain phase of the SO. To account for overestimation of MI due to noise, a normalized MI was calculated. A distribution of surrogate MI values was generated randomly, with mean μ and standard deviation σ and the normalized MI was computed as MIraw−μ/σ.
We also measured the phase angle of the composite signal Z[n], which is the SO phase at which the amplitude tends to peak. For each SO event, the SO phase during the peak sigma amplitude was calculated. A value of zero for SO phase (∅SO = 0) represents the negative peak of the oscillation (SO trough), and a positive value suggests the up-state of the SO. Here, we set the phase angle of zero as the trough of the SO, in contrast to Niknazar et al. [21], where the phase angle of zero was the positive peak of the SO. MI and phase angle were computed for zolpidem and placebo separately to determine the consistency and preference of the temporal relationship between the phase of SO and the amplitude of spindles. If MI or phase angle variables were significantly different between drug conditions, we computed Pearson’s r between memory performance and the variable to determine if such a difference plays a role in behavioral change.
Power spectrum estimation
To examine whether other sleep frequency bands might account for memory changes, we analyzed the following sleep frequency bands: sigma (11–15 Hz), theta (4–7 Hz), delta (1–4 Hz), and SO (0–1 Hz). The EEG epochs that were contaminated by muscle and/or other artifacts were rejected using a simple out-of-bounds test (with a ±200 µV threshold) on high-pass filtered (0.5 Hz) version of the EEG signals. The EEG power spectra were computed using the Welch method (4 s Hanning windows with 50% overlap) on the artifact-free 30-s epochs. Then, the estimated power spectra were averaged within each participant/sleep condition/stage/quartile/electrode.
Statistical analysis
Data reduction
Eight participants (6 females) did not complete both visits due to scheduling conflicts, which resulted in 28 participants being included in the analyses.
Behavioral data
To assess the impact of zolpidem on memory, we examined memory performance using two difference scores (24-h retention: test 3 − test 1, and overnight retention: test 3 − test 2). We conducted a two-sample paired t-test for each difference score comparing placebo and zolpidem conditions. Our primary hypothesis was that the improvement in memory in the zolpidem condition compared to placebo is correlated with a corresponding increase in spindle-related activity. To test this hypothesis, we calculated a change score for spindle density from zolpidem to placebo for each electrode, within each sleep stage, averaged within each quartile. We then calculated Pearson’s r between the memory difference scores and spindle difference scores and the sleep frequency bands. Benjamini–Hochberg correction with false discovery rate set as 5% was used to control for multiple comparisons.
Power spectrum estimation
To examine the effect of zolpidem on the sleep frequency bands, we performed paired t-tests on the estimated power spectra averaged among all the electrodes for zolpidem and placebo from 0 to 30 Hz in 0.5 frequency bins, corrected by Benjamini–Hochberg correction test [46]. To investigate the spatial distribution of sleep frequency bands, we then performed paired t-tests for each sleep frequency band at each electrode. To control for multiple comparison, we performed Benjamini–Hochberg correction test [46] and cluster-based permutation [47].
Cluster-based permutation
Cluster-based permutation tests have been widely used in the field of fMRI studies to control for multiple comparison problems [48]. Maris and Oostenveld [47] developed a method to incorporate cluster-based permutation tests in EEG data, which is used in the current analysis. This technique increases the statistical power to find a drug effect, while sacrificing spatial specificity (i.e. we cannot say which electrode had the significant effect). To test a significant drug effect within each sleep stage and each quartile, we performed a paired t-tests on each electrode site “sample” pair. Clusters were identified if one or more than one adjacent electrode reached significance level (p < 0.05) in the same direction. Within each cluster, t-statistics were summed, and the max of the summed t-stats across all samples was calculated thereby creating the “real” cluster-level statistic. Then, the assigned drug condition to each electrode data point was randomly shuffled, and a “permuted” cluster-level statistic was calculated using the same above procedure. We repeatedly shuffled and calculated the “permuted” cluster-level statistic 2,000 times to get the expected distribution of the cluster-level statistic if there was no drug effect (permutation distribution). The real cluster-level statistic was then compared with permutation distribution and drug was considered to have a significant effect when it was larger than 98.75% of the shuffled t-values after correcting for multiple comparisons (i.e. 100 – 5/number of quartiles). The reported statistic is the number of times the real cluster-level statistic happens in the 2,000 permutations.
Results
Behavioral data
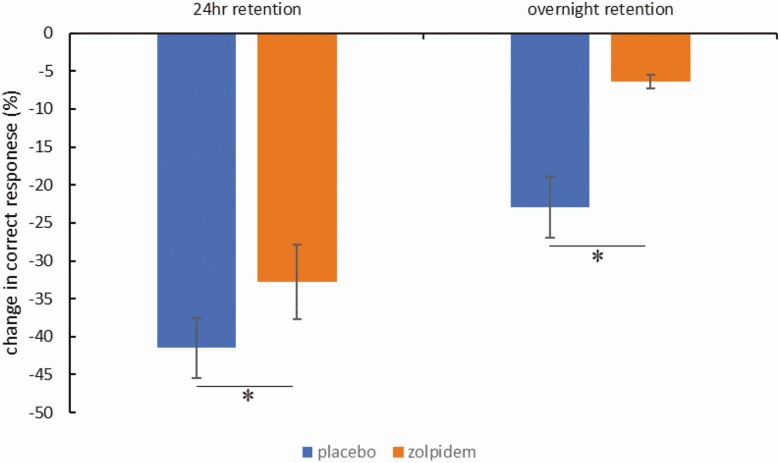
For the placebo condition, participants took 2.89 ± 1.37 trials to reach the 70% criterion. For the zolpidem condition, participants took 2.61 ± 1.03 trials to reach the 70% criterion. Participants took similar amount of trials to reach the criterion between conditions, t27 = 1.31, p = 0.20. Two conditions did not differ in performance during test 1 (t27 = −1.09, p = 0.29) or test 2 (t27 = −1.00, p = 0.32), suggesting a comparable baseline (Supplementary Figure 1). Similar to prior reports [24], memory recollection was improved in the zolpidem group compared to the placebo group. Specifically, participants in zolpidem condition had better verbal memory retention both at the 24-h retention (t27=2.40, p = 0.02) and overnight retention (t27 = 2.64, p = 0.01) tests (Figure 2).
Figure 2.
Behavioral results. Participants performed better after taking zolpidem than placebo both for 24-h retention (t27=2.40, p = 0.02) and overnight retention (t27 = 2.64, p = 0.01). *Statistically significant at p < 0.05
Sleep data
No differences in sleep architecture were detected except for zolpidem showing more SWS and less REM sleep (Table 1), similar to our prior finding [27]. Specifically, total sleep time, WASO, SE, and time spent in stage 1 and stage 2 were not significantly different between two conditions (p > 0.05). Zolpidem condition had significant more SWS (p < 0.05) and less REM sleep (p < 0.05) compared to placebo. When examined by quartile (Supplementary Table 1), we found that the placebo condition had significantly more stage 2 (p < 0.05) and less SWS (p < 0.05) during quartile 1 as well as more REM during quartile 2 (p < 0.05).
Table 1.
Sleep Architecture
| Sleep stage | Placebo | Zolpidem | p |
|---|---|---|---|
| TST (min) | 536.18 (47.92) | 537.71 (39.15) | 0.83 |
| N1 (min) | 14.46 (8.44) | 13.32 (11.73) | 0.59 |
| N1 (%) | 2.74 (1.62) | 2.55 (2.28) | 0.63 |
| N2 (min) | 283.66 (53.44) | 288.61 (46.66) | 0.44 |
| N2 (%) | 52.87 (8.61) | 53.75 (8.19) | 0.40 |
| N3 (min) | 110.09 (37.99) | 121.66 (41.68) | 0.02 |
| N3 (%) | 20.64 (7.32) | 22.58 (7.46) | 0.02 |
| REM (min) | 127.95 (32.07) | 113.66 (29.06) | 0.01 |
| REM (%) | 23.76 (5.26) | 21.01 (4.58) | 0.00 |
| WASO | 31.30 (27.60) | 25.73 (26.00) | 0.11 |
| SE | 92.39 (5.64) | 93.26 (4.89) | 0.18 |
Means ± SD. TST, total sleep time; N1, stage 1 sleep; N2, stage 2 sleep; N3, stage 3 sleep/slow-wave sleep; REM, nonrapid eye movement sleep; WASO, wake after sleep onset; SE, sleep efficiency.
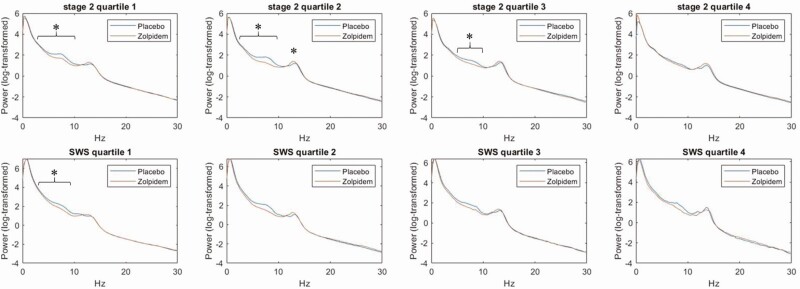
As shown in Figure 3, when averaged across all electrodes, zolpidem showed (1) decreases in power in theta frequencies in stage 2 (quartiles 1 and 2) and SWS (quartile 1) and (2) increases in sigma frequencies in stage 2 (quartiles 2), after correcting for multiple comparisons. Importantly, expected peak plasma concentrations occur during quartiles 1 and 2.
Figure 3.
Power spectrum for zolpidem and placebo in stage 2 and SWS (slow wave sleep) divided into four quartiles, averaged across electrodes. *Statistically significant for the mean across all electrodes following Benjamini–Hochberg correction for multiple comparisons (p < 0.05).
Sigma
Electrode-based power spectrum estimation
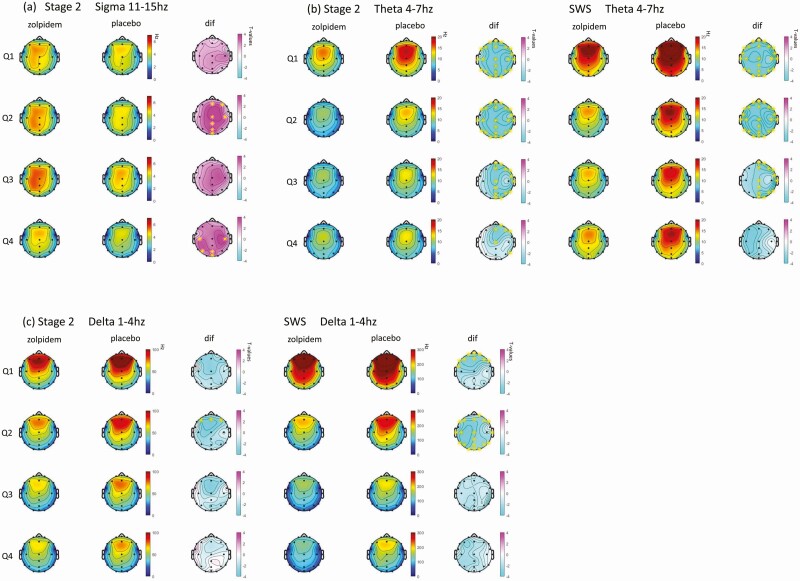
Zolpidem shows significantly greater sigma activity in stage 2 (33% of electrodes) and SWS (10% of electrodes), compared to placebo. After correction for multiple comparisons across electrodes using the Benjamini–Hochberg correction, seven electrodes remained significant in quartile 2 and five electrodes in quartile 4 in stage 2, as shown in Figure 4, A. The range of increase is between 14% and 16%.
Figure 4.
Topographic plots of the estimated marginal mean difference in spectral power by 4 quartiles between zolpidem and placebo for (a) sigma frequency (11-15Hz) at stage 2; (b) theta frequency (4-7Hz) at stage 2 and SWS; (c) delta frequency (1-4Hz) at stage 2 and SWS. Q1: quartile 1; Q2: quartile 2; Q3: quartile 3; Q4: quartile 4; dif: difference in the estimated marginal mean between zolpidem and placebo. *Statistically significant at this electrode following Benjamini–Hochberg correction for multiple comparisons.
Cluster-based permutation for power spectrum estimation
Cluster-based permutation tests confirmed the individual electrode analysis, where the zolpidem group exhibited significantly greater sigma in stage 2 quartile 1 (p = 13/2,000), quartile 2 (p < 1/2,000), quartile 3 (p < 1/2,000), and quartile 4 (p < 1/2,000). Significance was also detected in SWS quartile 2 (p = 2/2,000) and quartile 3 (p = 42/2,000).
Correlation between EEG activity and performance
No significant correlations emerged between sigma activity and performance change in either the cluster-based permutation or individual electrode site after Benjamini–Hochberg correction.
Spindle density
Electrode-based estimation
Spindle density was correlated with sigma power (r = 0.36, p < 0.001), and zolpidem showed increases in spindle density in stage 2 (4% electrodes were significant) and SWS (5% electrodes were significant), compared with placebo. However, no comparisons survived Benjamini–Hochberg correction (Supplementary Table 2).
Cluster-based permutation for power spectrum estimation
No drug effect was detected for spindle density.
Correlation between EEG activity and performance
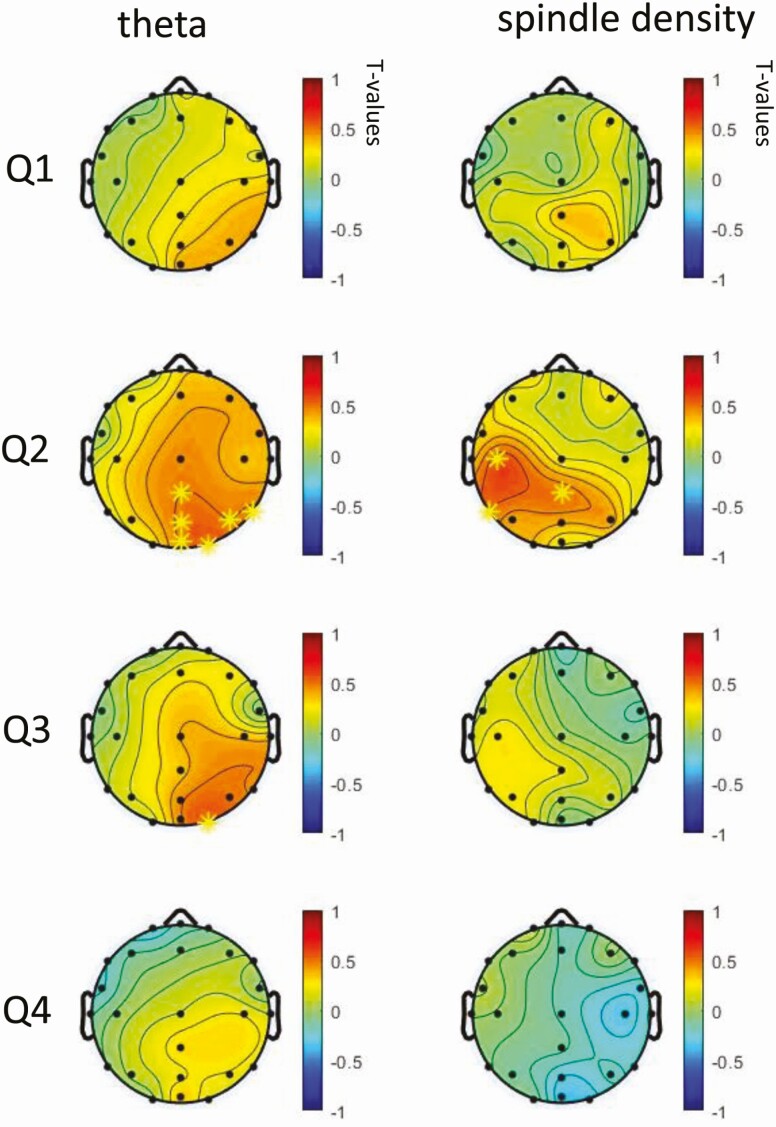
For spindle density, three electrodes located at central occipital and left temporal areas displayed a positive correlation with overnight retention at quartile 2 in stage 2 after Benjamini–Hochberg correction (Figure 5). Similarly, overnight retention and cluster-based permutation on spindle density were significantly correlated during stage 2 quartile 2 (p = 33/2,000).
Figure 5.
Topographic plots of Pearson’s r in spectral power change (zolpidem minus placebo) and performance change (zolpidem minus placebo) for overnight retention. *Statistically significant at this electrode following Benjamini–Hochberg correction for multiple comparisons.
Theta
Electrode-based power spectrum estimation
When each electrode was considered separately, there was a general decrease in theta power in the zolpidem condition compared to placebo in stage 2 (68% electrodes were significant) and SWS (40% electrodes were significant). As shown in Figure 4, B, after correction for multiple comparisons using Benjamini–Hochberg correction, 23 electrodes remained significant in quartiles 1 and 2, 14 electrodes located in the right hemisphere in quartile 3, and 6 electrodes located in central frontal in quartile 4 for stage 2 remained significant. For SWS, 23 electrodes remained significant in quartiles 1 and 2 and 14 electrodes located in the right hemisphere in quartile 3.
Cluster-based permutation for power spectrum estimation
The zolpidem group exhibited significantly lower theta in stage 2 (p = 98/2,000). All four quartiles showed significantly decreased theta for zolpidem compared to placebo in stage 2 (p < 1/2,000 for quartiles 1–4) and quartiles 1 (p < 1/2,000) and 2 (p < 1/2,000) in SWS.
Correlations between EEG activity and memory performance
The correlation between differences in overnight memory performance and theta power was significant during stage 2 quartile 2 (p = 37/2,000) from cluster-based permutation. As shown in Figure 5, after Benjamini–Hochberg correction for multiple comparisons, six electrodes located at right occipital lobe displayed a positive correlation with overnight retention at quartile 2 in stage 2, one electrode remained significant at quartile 3 stage 2, suggesting increased theta power has a positive association with better memory retention.
Delta
Electrode-based power spectrum estimation
When each electrode was considered separately, there was a general decrease in delta power in the zolpidem condition compared to placebo in stage 2 (23% electrodes were significant) and SWS (18% electrodes were significant). After correction for multiple comparisons using Benjamini–Hochberg correction, three frontal electrodes remained significant in stage 2 quartile 2. Nine frontal electrodes were significant in quartile 1 SWS and 19 electrodes for quartile 2 SWS, as shown in Figure 4, C.
Cluster-based permutation for power spectrum estimation
The zolpidem group exhibited significantly lower delta in stage 2 quartile 1 (p = 4/2,000), quartile 2 (p < 1/2,000), and quartile 3 (p = 7/2,000), as well as quartiles 1 (p < 1/2,000) and 2 (p < 1/2,000) in SWS.
Correlations between EEG activity and memory performance
The correlation between overnight retention and delta power change was significant in stage 2 quartile 2 using the cluster-based permutation analysis (p < 42/2,000). However, there was no significant correlation between delta power and performance at individual electrodes after Benjamini–Hochberg correction.
Slow oscillation
Electrode-based power spectrum estimation
When each electrode was considered separately, changes in SO did not survive Benjamini–Hochberg correction.
Cluster-based permutation for power spectrum estimation
No drug effect was detected for SO.
Correlations between EEG activity and memory performance
No correlation was detected between SO and performance by cluster-based permutation or individual electrode analysis after Benjamini–Hochberg correction.
Spindle–SO coupling
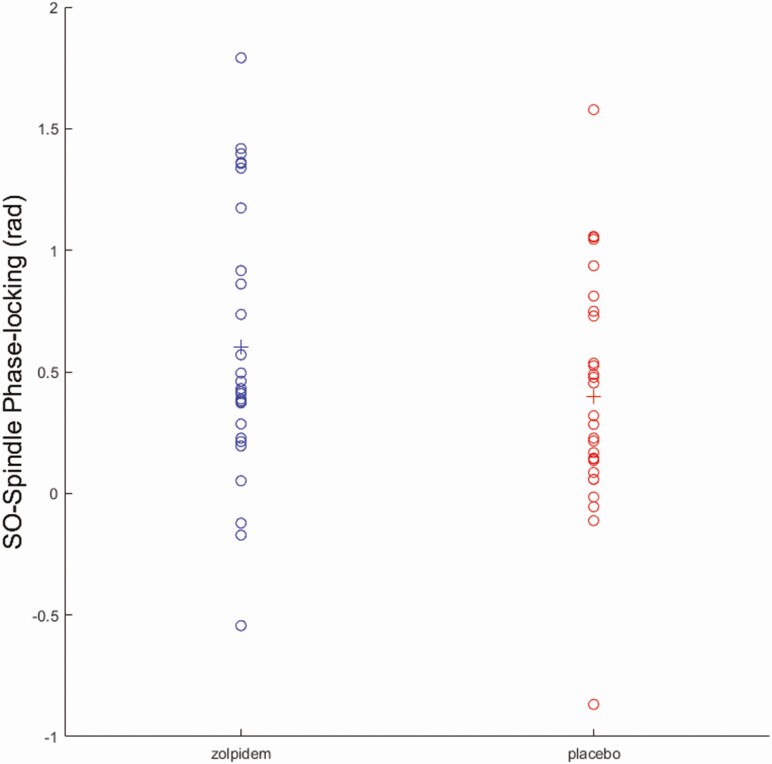
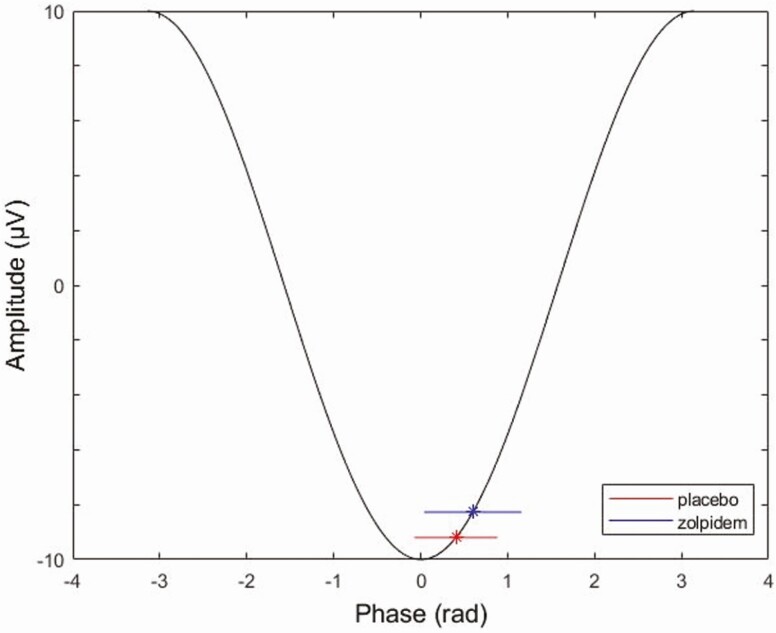
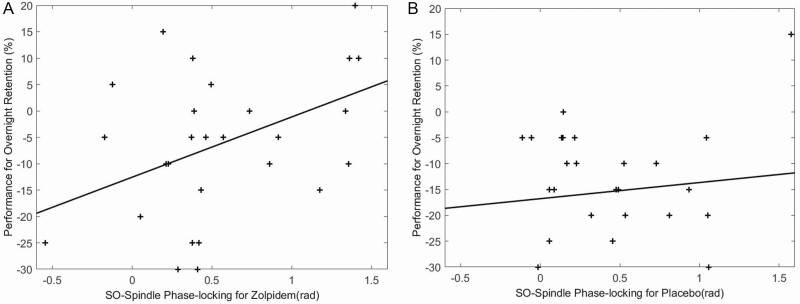
Similar to Niknazar et al. [21], we found significantly higher phase angle measures for zolpidem (0.60 ± 0.56) compared to placebo (0.40 ± 0.48) at F4 (t27 = −2.18, p = 0.04), but not at F3 (p > 0.05) (Figure 6). Higher phase angle measures indicate the spindles were clustered in the up-state of the SO phase closer to the positive peak (Figure 7). Furthermore, a positive relationship was observed between phase angle and memory performance in zolpidem (r = 0.46, p = 0.01) but not placebo (r = 0.11, p > 0.50) at F4, as shown in Figure 8. These findings are similar to Niknazar et al. [21], which showed spindles clustered in the up-state closer to the positive peak of the SO in the zolpidem condition compared to placebo, as well as the significant correlation between phase angle and memory in the zolpidem condition and only marginal correlation in placebo. A higher positive phase angle and the positive correlation between phase values and memory performance in zolpidem suggest that spindles occurring during the up-state of the SO and closer to the positive peak may be optimal as this phase-locking was associated with better memory enhancement. There was no difference in MI between zolpidem and placebo for F3 or F4 (p > 0.05), nor was there a correlation between MI and memory improvement.
Figure 6.
The phase angle of SO–spindle coupling for placebo and zolpidem. Individual phase angle is marked in circles while the average value for each condition is marked by a plus sign. Compared to placebo, zolpidem condition had a shifted phase angle at F4 (t27 = −2.18, p = 0.04).
Figure 7.
Mean and standard deviation of SO–spindle coupling phase angle for zolpidem and placebo on a schematic SO.
Figure 8.
Memory performance improvement and SO–spindle coupling: phase angle and memory performance are positively associated in zolpidem (r = 0.46, p = 0.01) but not placebo (r = 0.11, p > 0.50) at F4.
Discussion
The current study showed that zolpidem led to higher memory retention after a night of sleep compared to placebo, which adds valuable information regarding the effect of zolpidem on memory. Zolpidem also led to increased sigma power and decreased theta and delta power. Overnight retention in the zolpidem condition was associated with increased spindle density, replicating prior work [27], and theta power, a novel finding. Studies investigating the effect of hypnotics on sleep-dependent memory consolidation have shown mixed results. While our group report a positive effect of zolpidem on declarative memory consolidation here and in a prior study [27], others have observed no effect [49] or even a negative effect [50]. Conflicting findings may be due to methodological differences between studies. Specifically, Hall-Porter et al. [50] used the extended release version of zolpidem with 6–8 h of action and found decreased memory performance after drug administration, while zolpidem used in our study has a half-life of approximately 1.5–3.2 h. Meléndez et al. [49] used the same version and dosage of zolpidem as our study and found no memory differences between zolpidem and the control condition. However, they investigated item-memory while we probed associative memory, which has been shown to engage the hippocampus to a greater extent [51]. The current study builds on this literature by showing that zolpidem administered over a full night of sleep enhances associative memory, replicating a prior result using 90-min daytime naps [27].
The positive correlation between theta power and memory performance suggests that even though zolpidem leads to a decrease in theta power globally, participants who had the least reduction in theta tended to have a better memory retention. Even though we did not find significant increases in spindle density in the zolpidem condition compared to placebo, this relationship has been consistently shown in previous studies [21, 27, 29]. Discrepancies between prior results and the current data may be due in part to algorithm-based spindle detection used here while prior studies used visual inspection to hand count spindles [42, 52]. A positive association between spindle density and memory improvement is consistent with previous findings, adding more support to the theory that sleep spindles are critical for memory consolidation [23]. Even though we did not find a correlation between SO and memory improvement, the coupling of SO and spindles was associated with memory, which is consistent with prior literature [21, 53]. Current models of sleep-dependent memory consolidation may help clarify the role of spindles and spindle–SO coupling in this process.
One of predominate views of long-term memory consolidation is the active system consolidation theory, which indicates that memories are consolidated during sleep by reactivating memory traces associated with learning and redistributing them in the neocortex [54]. The three oscillations that are hallmarks of memory reactivation include sharp wave ripples, spindles, and SOs [20, 55]. Sharp wave ripples have been found to be nested into the troughs of faster spindle oscillations [18, 53], and spindles occur to a great extent in the up-state of the SO [9, 17, 23], emphasizing the interdependent role of these three oscillations in relaying information from hippocampus to neocortex [23]. Indeed, the simultaneous occurrence of all three oscillations naturally or by experimental intervention leads to great memory consolidation, compared with the features occurring out of phase with one another [23]. In addition to facilitating the thalamocortical communication [54], spindles have been shown to induce long-term potentiation (LTP), which is a key process in long-term memory consolidation [56, 57]. Interestingly, LTP was only inducible in synchronous pre- and postsynaptic spindles but not asynchronous spindles [56], suggesting that the timing between spindles and occurrence of pre- and postsynaptic events is crucial for memory consolidation [57]. Similar results have been shown in humans where memory improvement was observed when auditory stimuli were applied in phase but not out of phase with SO and spindles [9], and both the study of Niknazar et al. [21] and the current study showed that hippocampus-dependent memory improvement is associated with the coupling of SO and spindles during the up-state of the SO. It has been proposed that the up-state of neocortical SOs leads to depolarization and provides an opportunity for reactivation [6]. In short, the positive association between spindle density, spindle–SO coupling, and declarative memory improvement supports the notion that sleep benefits hippocampus-dependent memory by transferring the information from hippocampus to neocortex, as well as inducing synaptic plasticity.
The inhibitory effect of zolpidem on theta power has been previously reported [29–31], and such an effect has been suggested to result from binding to GABA receptors [58]. Specifically, mice with insensitive alpha1GABAA receptors and controls both had decreased sleep latency after taking zolpidem, but only the wild type and not the mutants showed significant power reduction encompassing a broad power band of more than 5 Hz, which suggests that alpha1GABAA receptor is responsible for decreased EEG power while the other two are responsible for promoting sleep [58]. The current study showed that theta power was positively associated with memory improvement, suggesting that engineering zolpidem to preserve theta could potentially create an optimal environment for memory consolidation. Prior studies indicate candidate mechanisms by which theta preservation might be achieved. For example, histaminergic neurons in the hypothalamus are known to promote wakefulness, and increased GABA activity in these areas promotes sleep [59]. Increasing GABA activity only on histamine neurons using zolpidem promoted sleep in mice without reducing EEG power [60]. Specifically, zolpidem-insensitive mice were genetically manipulated to be selectively sensitive to zolpidem in histaminergic neurons in the tuberomammillary nucleus of the hypothalamus, and they experienced the sleep promoting effect of zolpidem without having reduced EEG power [60]. Further pharmacological research may be useful in optimizing the memory-enhancing effects of sleep.
Theta oscillation during wakefulness is essential for episodic memory consolidation [61, 62] and has been proposed to integrate information between hippocampus and neocortex [63]. It has been speculated that theta during sleep might have similar functionality as during wakefulness [64]. Specifically, theta oscillation tends to occur in close temporal proximity to synaptic and neuronal changes after memory encoding [65], signaling its role in memory consolidation. Animal studies show that theta peaks signal the depolarization phase of the cell membrane, which leads to increased neuron reactivity to inputs [65, 66] and facilitates the induction of LTP during wakefulness [67]. Such a neural firing pattern is replayed during REM sleep [68], supporting theta’s role in memory consolidation during sleep. Specifically, Poe et al. [68] found that hippocampal cells that were active when animals were in novel places tended to fire during theta peaks in REM sleep, whereas cells that were associated with familiar places tended to fire during the trough of the theta oscillation. Therefore, theta oscillation is implicated in memory consolidation through neural replay during REM sleep [69]. Although the theta that can be measured via scalp EEG in humans is unlikely the same as the hippocampal theta measured in rodents, human memory studies have shown a relation between theta EEG and emotional memory consolidation during REM sleep [70, 71]. In addition, a growing number of TMR studies has explored the role of theta on memory consolidation during NREM sleep [64, 72, 73]. Specifically, Schreiner et al. [72, 74] reported that induced theta power during NREM sleep signals successful memory reactivation. They further showed that the theta power triggered by memory cues shared similar neural signatures between wakefulness and NREM sleep, suggesting a role for theta in stabilizing reactivated memories in both wakefulness and sleep [64, 73]. Our positive correlation between theta and memory suggests that pharmacological modulation of theta power during NREM sleep might also play a role in hippocampal-dependent memory consolidation.
The current study has limitations. Even though our discussion on GABA receptor subtypes provides a plausible mechanism and intervention to increase spindles while preserving theta pharmacologically, alternative explanations about how decreased theta contribute to memory retention are possible. For example, it might be the case that as the number of spindles increased, theta power decreased as a simple side effect of the fact that a greater portion of each 30-s epoch was taken up by sigma-frequency activity. In this scenario, participants with “preserved” theta might be those for whom sigma power increased as a result of an increase in the amplitude of spindles, rather than the number or duration of spindles. Here, we showed that spindle density, but not sigma power, is positively correlated with memory, suggesting that the number of spindles contribute to memory, which weakens the possibility that spindle amplitude contributes to memory. However, a specific investigation of how theta and sigma independently and collectively contribute to memory is warranted. Other limitations include small sample size and that the dosage is not based on mg/kg. Factors like sex and BMI could influence the metabolism of zolpidem, which would increase the individual variability of the drug effect [75]. In addition, we were not able to tease apart the specific and potentially independent roles that theta and spindles may play in memory consolidation. Recent findings by Kim et al. [76] have demonstrated that SO and delta activity may support different and even opposing aspects of the memory process. It would be interesting to investigate how theta and spindles may contribute to different aspects of the memory process using a more complex memory task. For example, a memory task that distinguishes sensory-rich content from non-sensory content to investigate the possibility that theta during NREM preferentially enhances sensory-rich information [77, 78].
Taken together, this study demonstrates a positive role of zolpidem on overnight memory performance as well as suggests a role for spindle density and theta frequency power in these performance improvements. Furthermore, it provides additional support for the critical role of sleep spindles as well as the coupling between SO and spindles in memory consolidation. Future studies are needed to tease apart mechanisms behind the role of NREM theta power and spindle–SO coupling on memory consolidation.
Supplementary Material
Acknowledgments
We thank undergraduate research assistants in the laboratory for assistance with data collection.
The work was performed at the University of California, Riverside.
Funding
This work was supported by the Office of Naval Research (grant N00014-14-1-0513) and the National Institutes of Health (NIH) (grant R01 AG046646).
Financial disclosure: None.
Nonfinancial disclosure: None.
Conflict of interest statement. None declared.
References
- 1. Diekelmann S. Sleep for cognitive enhancement. Front Syst Neurosci. 2014;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mednick SC, et al. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34(10):504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–1278. [DOI] [PubMed] [Google Scholar]
- 4. Fowler MJ, et al. Sleep and memory. Science. 1973;179(4070):302–304. [DOI] [PubMed] [Google Scholar]
- 5. Plihal W, et al. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9(4):534–547. [DOI] [PubMed] [Google Scholar]
- 6. Diekelmann S, et al. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. [DOI] [PubMed] [Google Scholar]
- 7. Binder S, et al. Transcranial slow oscillation stimulation during NREM sleep enhances acquisition of the radial maze task and modulates cortical network activity in rats. Front Behav Neurosci. 2013;7:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. [DOI] [PubMed] [Google Scholar]
- 9. Ngo HV, et al. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78(3):545–553. [DOI] [PubMed] [Google Scholar]
- 10. Lustenberger C, et al. Triangular relationship between sleep spindle activity, general cognitive ability and the efficiency of declarative learning. PLoS One. 2012;7(11):e49561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schabus M, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. [DOI] [PubMed] [Google Scholar]
- 12. Clemens Z, et al. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132(2):529–535. [DOI] [PubMed] [Google Scholar]
- 13. Gais S, et al. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22(15):6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antony JW, et al. Sleep spindle refractoriness segregates periods of memory reactivation. Curr Biol. 2018;28(11):1736–1743.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cairney SA, et al. Memory consolidation is linked to spindle-mediated information processing during sleep. Curr Biol. 2018;28(6):948–954.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lustenberger C, et al. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Biol. 2016;26(16):2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mölle M, et al. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Staresina BP, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18(11):1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gais S, et al. Declarative memory consolidation: mechanisms acting during human sleep. Learn Mem. 2004;11(6):679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mölle M, et al. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci. 2009;29(5):1071–1081. [DOI] [PubMed] [Google Scholar]
- 21. Niknazar M, et al. Coupling of thalamocortical sleep oscillations are important for memory consolidation in humans. PLoS One. 2015;10(12):e0144720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sattari N, et al. Does working memory improvement benefit from sleep in older adults? Neurobiol Sleep Circadian Rhythms. 2019;6:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Latchoumane C-FV, et al. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron. 2017;95(2):424–435.e6. [DOI] [PubMed] [Google Scholar]
- 24. Helfrich RF, et al. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron. 2018;97(1):221–230.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Demanuele C, et al. Coordination of slow waves with sleep spindles predicts sleep-dependent memory consolidation in schizophrenia. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drover DR. Comparative pharmacokinetics and pharmacodynamics of short-acting hypnosedatives: zaleplon, zolpidem and zopiclone. Clin Pharmacokinet. 2004;43(4):227–238. [DOI] [PubMed] [Google Scholar]
- 27. Mednick SC, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33(10):4494–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wamsley EJ, et al. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013;36(9): 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dijk DJ, et al. Sex differences and the effect of gaboxadol and zolpidem on EEG power spectra in NREM and REM sleep. J Psychopharmacol. 2010;24(11):1613–1618. [DOI] [PubMed] [Google Scholar]
- 30. Lundahl J, et al. EEG spectral power density profiles during NREM sleep for gaboxadol and zolpidem in patients with primary insomnia. J Psychopharmacol. 2012;26(8): 1081–1087. [DOI] [PubMed] [Google Scholar]
- 31. Landolt HP, et al. Zolpidem and sleep deprivation: different effect on EEG power spectra. J Sleep Res. 2000;9(2):175–183. [DOI] [PubMed] [Google Scholar]
- 32. Whitehurst LN, et al. The impact of psychostimulants on sustained attention over a 24-h period. Cognition. 2019;193:104015. [DOI] [PubMed] [Google Scholar]
- 33. Choi SI, et al. Corrigendum: Effects of different re-referencing methods on spontaneously generated Ear-EEG. Front Neurosci. 2019;13:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rechtschaffen A, et al. A Manual of Standardized Terminology, Techniques, and Scoring System for the Sleep Stages of Human Subjects. Bethesda, MD: U.S. National Institute of Neurological Diseases and Blindness, Neurological Information Network. [Google Scholar]
- 35. Malerba P, et al. Spatio-temporal structure of sleep slow oscillations on the electrode manifold and its relation to spindles. Sleep. 2018;42(1). doi: 10.1093/sleep/zsy197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mylonas D, et al. Naps reliably estimate nocturnal sleep spindle density in health and schizophrenia. J Sleep Res. 2019;00:e12968. doi:10.1111/jsr.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naji M, et al. Coupling of autonomic and central events during sleep benefits declarative memory consolidation. Neurobiol Learn Mem. 2019;157:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schapiro AC, et al. Sleep benefits memory for semantic category structure while preserving exemplar-specific information. Sci Rep. 2017;7(1):14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wamsley EJ, et al. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71(2):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lacourse K, et al. A sleep spindle detection algorithm that emulates human expert spindle scoring. J Neurosci Methods. 2019;316:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsanas A, et al. Stage-independent, single lead EEG sleep spindle detection using the continuous wavelet transform and local weighted smoothing. Front Hum Neurosci. 2015;9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Warby SC, et al. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods. 2014;11(4):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Massimini M, et al. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24(31):6862–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dang-Vu TT, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A. 2008;105(39):15160–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Canolty RT, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313(5793):1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benjamini Y, et al. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 47. Maris E, et al. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. [DOI] [PubMed] [Google Scholar]
- 48. Woo CW, et al. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meléndez J, et al. Zolpidem and triazolam do not affect the nocturnal sleep-induced memory improvement. Psychopharmacology (Berl). 2005;181(1):21–26. [DOI] [PubMed] [Google Scholar]
- 50. Hall-Porter JM, et al. The effect of two benzodiazepine receptor agonist hypnotics on sleep-dependent memory consolidation. J Clin Sleep Med. 2014;10(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davachi L, et al. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88(2):982–990. [DOI] [PubMed] [Google Scholar]
- 52. Lacourse K, et al. Massive Online Data Annotation (MODA): crowdsourcing to generate high-quality sleep spindle annotations from EEG data (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clemens Z, et al. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci. 2011;33(3):511–520. [DOI] [PubMed] [Google Scholar]
- 54. Rasch B, et al. Reactivation and consolidation of memory during sleep. Curr Dir Psychol Sci. 2008;17(3): 188–192. [Google Scholar]
- 55. Paller KA, et al. Memory reactivation and consolidation during sleep. Learn Mem. 2004;11(6):664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rosanova M, et al. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25(41):9398–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ulrich D. Sleep spindles as facilitators of memory formation and learning. Neural Plast. 2016;2016:1796715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kopp C, et al. Sleep EEG changes after zolpidem in mice. Neuroreport. 2004;15(14):2299–2302. [DOI] [PubMed] [Google Scholar]
- 59. Sherin JE, et al. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18(12):4705–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Uygun DS, et al. Bottom-up versus top-down induction of sleep by zolpidem acting on histaminergic and neocortex neurons. J Neurosci. 2016;36(44):11171–11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klimesch W, et al. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91(6):428–441. [DOI] [PubMed] [Google Scholar]
- 62. Nyhus E, et al. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev. 2010;34(7):1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lega BC, et al. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22(4):748–761. [DOI] [PubMed] [Google Scholar]
- 64. Schreiner T, et al. Theta phase-coordinated memory reactivation reoccurs in a slow-oscillatory rhythm during NREM sleep. Cell Rep. 2018;25(2):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. [DOI] [PubMed] [Google Scholar]
- 66. Fox SE, et al. Hippocampal theta rhythm and the firing of neurons in walking and urethane anesthetized rats. Exp Brain Res. 1986;62(3):495–508. [DOI] [PubMed] [Google Scholar]
- 67. Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29(2–3):169–195. [DOI] [PubMed] [Google Scholar]
- 68. Poe GR, et al. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855(1):176–180. [DOI] [PubMed] [Google Scholar]
- 69. Poe GR. Sleep is for forgetting. J Neurosci. 2017;37(3):464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hutchison IC, et al. The role of REM sleep theta activity in emotional memory. Front Psychol. 2015;6:1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nishida M, et al. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19(5):1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schreiner T, et al. Boosting vocabulary learning by verbal cueing during sleep. Cereb Cortex. 2015;25(11):4169–4179. [DOI] [PubMed] [Google Scholar]
- 73. Schreiner T, et al. The beneficial role of memory reactivation for language learning during sleep: a review. Brain Lang. 2017;167:94–105. [DOI] [PubMed] [Google Scholar]
- 74. Schreiner T, et al. Auditory feedback blocks memory benefits of cueing during sleep. Nat Commun. 2015;6:8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Greenblatt DJ, et al. Gender differences in pharmacokinetics and pharmacodynamics of zolpidem following sublingual administration. J Clin Pharmacol. 2014;54(3):282–290. [DOI] [PubMed] [Google Scholar]
- 76. Kim J, et al. Competing roles of slow oscillations and delta waves in memory consolidation versus forgetting. Cell. 2019;179(2):514–526.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Karakaş S, et al. Oscillatory responses representing differential auditory processing in sleep. Int J Psychophysiol. 2007;65(1):40–50. [DOI] [PubMed] [Google Scholar]
- 78. Fuentemilla L, et al. Theta oscillations orchestrate medial temporal lobe and neocortex in remembering autobiographical memories. NeuroImage. 2014;85:730–737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.