Abstract
An alarming disease caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) named COVID-19 has emerged as an unprecedented public health problem and ignited a world health crisis. As opposed to what was believed at the beginning of the pandemic, the virus has not only spread but persevere causing secondary waves and challenging the concept of herd immunity against viral infections. While the majority of SARS-CoV-2-infected individuals may remain asymptomatic, a fraction of individuals may develop low to high-grade severity signs and symptoms of COVID-19. The disease is multifactorial and can progress quickly, leading to severe complications and even death in a few days. Therefore, understanding the pre-existing factors for disease development has never been so pressing. In this scenario, the insights on the mechanisms underlying disease allied to the immune response developed during the viral invasion could shed light on novel predictive factors and prognostic tools for COVID-19 management and interventions. A recent genome-wide association study (GWAS) revealed several molecules that significantly impacted critically ill COVID-19 patients, leading to the core mechanisms of COVID-19 pathogenesis. Considering these findings and the fact that ACE-2 polymorphisms alone cannot explain disease progress and severity, this review aims at summarizing the most important and recent findings of the research and expert consensus of possible cytokine-related polymorphisms existing in the differential expression of paramount immune molecules that could be crucial for providing guidelines for decision-making and appropriate clinical management of COVID-19.
Keywords: SARS-CoV-2, Polymorphism, Cytokine storm, SARS
1. Background
SARS-CoV-2 gained notoriety when it spread in the city of Wuhan, China, in December of 2019 [20] and its continued dissemination led WHO to nominate it as a pandemic on 11 March 2020 [38-A). So far, it is estimated that around 134 million people were infected worldwide and about 2,9 million deaths occurred [16] in less than two years from its acknowledged viral circulation. SARS-CoV-2 pandemic was exceedingly uncontrolled and rapid progressive in contrast with other critical pandemics such as HIV, which is comprised of 38 million infected people worldwide accumulated over the last 40 years.
The majority of people infected with SARS-CoV-2 may remain asymptomatic and still contribute to viral spread and transmission, which renders the control of viral dissemination complicated. However, a fraction of SARS-CoV-2-infected patients develop COVID-19, which is characterized in mild cases by fever, myalgia or fatigue, cough, shortness of breath, headaches, and diarrhea ([19]. Statistically, the risk group is made up of people over 60, with diabetes, high blood pressure, and cardiovascular disease [4]. These people are more likely to develop the most severe form of the disease, characterized by complications such as pneumonia followed by severe respiratory distress syndrome (SARS), acute cardiac injury, and elevated levels of some cytokines [15,11].
Although the increase in inflammatory cytokines and chemokines is expected during infection for the coordinated fight by the immune system, their increase can cause a mismatch leading to a condition described as a cytokine storm. The cytokine storm is characterized by a picture of illness in multiple organs and hyper inflammation. In the context of COVID-19, this would manifest itself mainly, but not exclusively, in the lungs, arising as a result of the excessive release of cytokines and chemokines coming from an uncontrolled immune activation [14,39].
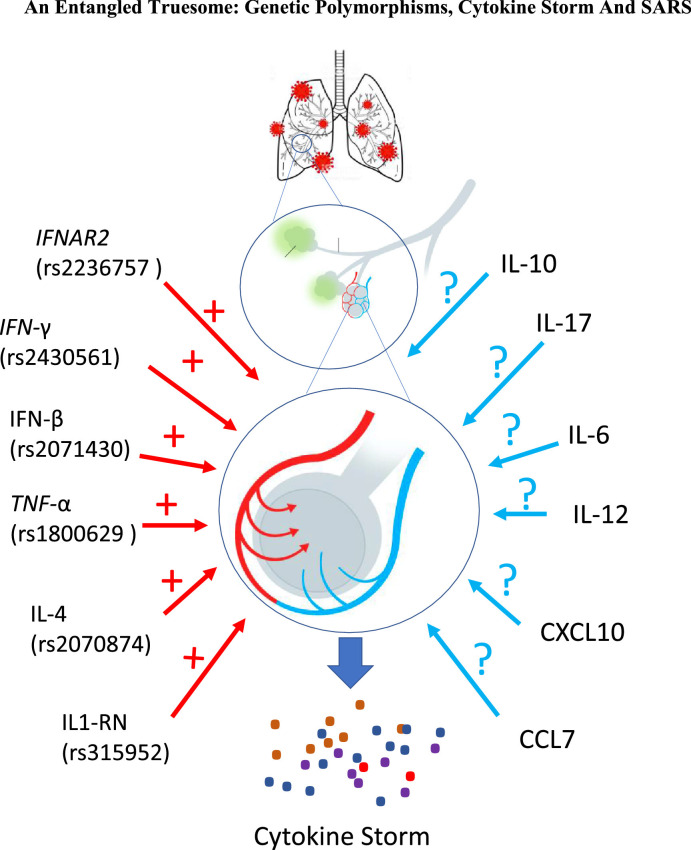
In this scenario, key polymorphisms in cytokine and chemokine genes have been described as associated with different pathogenic conditions, with a strong association between the severity of such responses and the level of their expression as illustrated in Fig. 1 .
Fig. 1.
The genetic polymorphisms in the cytokine encoding genes described and related to the cytokine storm are highlighted in red in the figure. In parentheses are the numbers for each Single Nucleotide polymorphism (SNP) for each polymorphic site. In blue are cytokines whose polymorphisms could explain the development of the cytokine storm in response to SARS-CoV-2. The positive sign (+) indicates the possible interference of the described cytokine polymorphism and the question mark (?) Indicates candidate polymorphism to be associated with the development of the cytokine storm.
An Entangled Truesome: Genetic Polymorphisms, Cytokine Storm And SARS
Understanding the role of genetic variants in the course of respiratory infections might help in the recognition of possible candidates for further analysis in patients affected by SARS-CoV-2, however, data related to polymorphism of cytokine genes in SARS-CoV-2 infection is very limited. Thus, this current review brings about important thoughts on how polymorphisms in cytokine genes could be essential for disease outcomes in COVID-19. For that, we discuss crucial findings and mechanistic aspects of polymorphism and cytokine gene expression and pinpoint some possible associations amongst cytokine gene polymorphisms, cytokine storm, and the risk of Severe Acute Respiratory Syndrome (Fig. 1).
2. Polymorphism of cytokine genes around the world
Although the genes encoding cytokines, as well as, their receptors are relatively conserved in their exons, non-silent mutations in the coding regions can result in loss, functional change, or overexpression of these proteins. Polymorphism can interfere with their expression through post-transcriptional modifications, at the transcriptional level and altering mRNA stability. For example, polymorphism in regulatory regions 5′ and 3′ can affect the entire gene transcription and since they can also alter the binding sites of transcription factors, it can therefore inhibit or over-stimulate gene expression [2].
The complex remodeling of the immune system resulting from infection by SARS-CoV-2, possibly related to polymorphisms in the cytokine and chemokine genes, may explain the pro-inflammatory status recognized by cell exhaustion and the cytokine storm characteristic of dysfunctional immunity and acute inflammation of respiratory syndrome. The progressive increase of cytokines and chemokines in pro-inflammatory status and its association with these polymorphisms needs to be clarified to gain a deeper understanding of disease progression in different parts of the world.
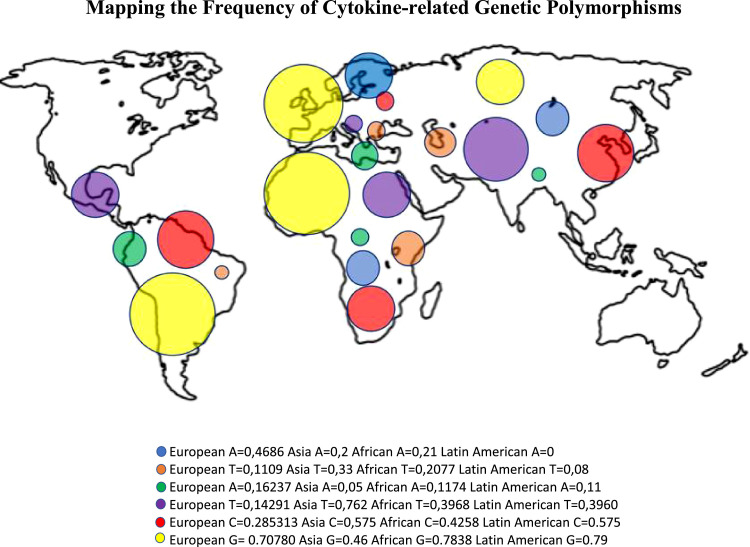
In order to map cytokine-related polymorphisms and see any association with the higher number of cases in the Americans, well-stablished polymorphisms on alleles coding for crucial cytokines such as IFNAR2, TNF, INF-a/b, IL-4 and IL-1RN were plotted in a globe map according to their frequency in European, Asian, African and American regions (Fig. 2 ).
Fig. 2.
Frequencies of polymorphisms in minor allelic genes encoding the cytokines according to the areas in the map with colored circles identifying: IFN-γ (rs2430561 – T/A single-nucleotide variation in the IFNG gene on chromosome 12) in dark blue, IFNβ (rs2071430 – G/T single-nucleotide variation in the MX1 gene on chromosome 21) in orange, TNF-α (rs1800629 - G/A single-nucleotide variation in the TNF gene on chromosome 6) in green, IL-4 (rs2070874 – C/T single-nucleotide variation in the IL4 gene on chromosome 5) in purple, IL1-RN (rs315952 – T/C single-nucleotide variation in the IL1RN gene on chromosome 2) in red and IFNAR2 (rs2236757 – A/G single-nucleotide variation in the IFNAR2 gene on chromosome 21) in yellow. The data presented are deposited in the NCBI [26] according to the SNP of each gene.
Mapping the Frequency of Cytokine-related Genetic Polymorphisms
Fig. 2 illustrates an interesting scenario in which the key polymorphisms selected are present in areas co-existing with SARS-CoV-2 infection. For example, polymorphisms in IFNAR2 (rs2236757 – A/G single-nucleotide variation in the IFNAR2 gene on chromosome 21) seem to be very frequent in Latin American and Europe, which presented elevated cases of SARS-CoV-2. Conversely, polymorphisms in IFNβ (rs2071430 – G/T single-nucleotide variation in the MX1 gene on chromosome 21) seem to be more frequent in Africa and Asia as opposed to European and American regions. A similar trend is observed for IL1-RN (rs315952 – T/C single-nucleotide variation in the IL1RN gene on chromosome 2) and for IL-4 (rs2070874 – C/T single-nucleotide variation in the IL4 gene on chromosome 5). These findings may indicate a relationship of polymorphisms of key cytokine genes in the course of COVID-19 as well as the SARS-CoV-2 resilience and predominance in specific areas of the globe. Therefore, genetic factors could contribute, along other social and economic factors, to the persistence of SARS-CoV-2 infection in the Americas, and regard should be taken on their frequency and distribution around the globe.
2.1. Genetic polymorphisms in cytokines involved in coronavirus-associated SARS
In acute respiratory infections caused by SARS-CoV-1 and SARS-CoV-2, the first consideration is to assume that polymorphisms associated to the ACE-2, the receptor that binds the viral spike protein are crucial during the course of disease. Nonetheless, SNPs on ACE-2 may vary significantly according to factors such as age, gender and origin/ethnicity. Therefore, data on ACE-2 SNPs may produce controversial findings amongst divergent populations. In addition, pre-existing non-infectious disorders as well as lifestyle and medications may have profound impact on ACE-2 gene expression, which may contribute to disease outcome. The intricacies and details on this matter are thoroughly discussed elsewhere [5].
Particularly, the genes encoding the cytokines IL-6, IL-1β, and CXCL8 (IL-8) show markedly high expression upon coronavirus infection, as has been described during the MERS-CoV outbreak [21]. In regard to the cytokine polymorphisms and their association to SARS-CoV-1 and SARS-CoV-2 infections, a study conducted by Lau and colleagues in 2009 confirmed the association between the IFN-γ polymorphism with the disease. In a recent study pioneered by Pairo-Castineira et al. based on Genome-Wide Association (GWAS) and conducted by the GenOMICC (Genetics of Mortality in Critical Care) initiative covered the entire genome of patients infected with SARS-CoV-2 in severe state, allowing for the discovery of completely novel pathophysiological mechanisms. In this investigation, a number of 2244 critically ill patients with COVID-19 admitted to the UK in therapy care (ICUs) were under scrutiny. Confirming the previous hypothesis, analysis of polymorphisms in genes related to antiviral responses pointed out and replicated the importance of interferon receptor IFNAR2 (chr21q22.1; rs2236757) and the 2′−5′-Oligoadenylate Synthetase (OAS) genes in COVID-19. The Mendelian randomization indicated that the presence of polymorphisms associated with an augmented expression of IFNAR2 decreased the chances of the development of severe life-threatening COVID-19 (Pairo-Castineira et al., 2020).
Additionally, the study showed evidence that supports a causal link between the evolution of severe COVID-19 cases with high expression of TYK2, a Janus kinase (Jak) family member important for IFN-γ and IL-12 pro-inflammatory cytokine production [18]. Thus, it is possible to affirm that increased signaling through IFNAR2 interferon receptor subunit and reduced signaling by pro-inflammatory TYK2-related pathway reduced the chances of severe COVID-19, which represents evidence of a protective role for IFNAR2 in COVID-19 (Pairo-Castineira et al., 2020). This study points out to significant and putative genetic markers associated with severe COVID-19. In agreement with these findings, the analysis of single-nucleotide polymorphisms (SNPs) for the IFN-γ + 874A/T, A/C genes in Chinese patients, suggests a genetic risk factor for the development of SARS. The role of IFN-γ in the antiviral response to SARS-CoV-1 has also been supported by studies showing that IFN-γ can inhibit its replication in combination with IFN-β in vitro [8]. However, the excessive production of this cytokine may bring about disproportionate pro-inflammatory responses that may lead to tissue damage. The relationship between the polymorphism on the IL-10, TNF-α, and IL-12 cytokine genes and SARS is yet to be described. More studies need to be performed to clearly tackle the association of these specific polymorphisms in the selected cytokine genes with SARS, once other SNPs in these genes may also be involved in gene expression regulation.
Mx gene encodes for GTPases, which present dynamin-like structures and play a crucial antiviral role by modulating the type I and type III interferon (IFN) systems. Mx1 is able to inhibit several different viruses by impeding the primary stages of the viral invasion and replication cycle [3]. Ching and colleagues in 2010 demonstrated that SNPs found on the minor alleles of Mx1 promoter, the −88 G/T and −123C/A, are associated with increased promoter activity and altered response to IFN-α and IFN-β treatment. The study demonstrated that the −123A minor allele provided a stronger binding affinity to nuclear proteins than the wild-type allele, whereas the −88T allele showed preferential nuclear protein binding after IFN-β stimulation. As it is widely known, endogenous IFN-α and IFN-β induction can be suppressed in coronavirus infection. A large case-control study confirmed that the −123A minor-allele carriers were significantly associated with a lower risk of infection, whereas the −88T minor-allele carriers were insignificant after adjustment for confounding effects [7].
Although Interferons usually take the central attention during viral infections, TNF-α is a crucial pro-inflammatory cytokine triggered by several respiratory viruses, such as Influenza virus, respiratory syncytial virus, and SARS-CoV-2 [25, 31,27,15]. The association between genetic polymorphisms of the TNF-α gene and susceptibility to SARS was conducted by Wang and colleagues [37] in a hospital-based case-control study, which included 75 SARS patients, 41 health care workers and 92 healthy controls. In this study, the TNF-1031T/C and TNF-863A/C genotypes may be risk factors of femoral head necrosis in discharged SARS patients even though SNPs on the promoter region of the TNF-α gene were not found to be associated with SARS-CoV-1 infection.
Interestingly, Azevedo and colleagues [1], showed a pattern of protection conferred by the TNF-308 G/A genotype against sepsis mortality and SARS in a pediatric population. On the other hand, an increased risk for the SARS outcome conferred by the TNF-863C/A genotype was detected. Although conflicting, these associations are potentially important, given the present scarcity of biomarkers with predictive value for the clinical evolution in pediatric sepsis, and the severity of the COVID-19 outcomes, which are closely associated with sepsis. The influences attributed to the TNF-308 G/A and TNF-863 G/A SNPs, that seem to work in opposite directions, could in fact reflect a different impact of these SNPs depending on age. As seen in the infant subgroup analysis, the protective effect of the TNF-308 G/A genotype against SARS is present, whereas the same was not observed for the risk effect of the TNF-863C/A genotype. The association between TNF-308 SNP and susceptibility to SARS has been addressed in a previous study, limited to the adult population, which reported a protective effect of the TNF-308A allele against SARS [10]. The different results regarding the risk effect of TNF-863C/A in infants and the full analysis could be related to a different effect of the SNP depending on the development and maturation of the immune system. Findings of Wilson and colleagues [36] indicate that TNF-308 G/A can be associated with higher levels of TNF-α, leading to speculation that the protection conferred by this polymorphism in SARS pediatric patients may be correlated not only to the canonical pro-inflammatory role of TNF-α, but also to its protective role both at the cellular and at lung tissue milieu by induction of apoptosis of viral-infected cells [34].
On the other hand, Skoog and colleagues [32] have shown an association between the TNF-863C/A polymorphism and reduced levels of TNF-α. In comparison with adults, healthy children produce lower levels of cytokines such as IFN-α, which could synergize with lower levels of TNF-α and represent an impaired ability to deal with respiratory infections. This age-related immune impairment in antiviral and pro-inflammatory cytokine production in children support the increased risk of SARS in pediatric patients with this profile (Lilic et al., 1997). In particular, the lower levels of TNF-α and other pro-inflammatory cytokines in children could be associated with protection from SARS during severe COVID-19, which are less common in the pediatric population. It is important to state that elevated plasma levels of TNF-α for COVID-19 patients have been associated with disease severity in adults, and assessment of the local immunological profile in the airway of COVID-19 patients in an age-dependent manner is crucial for the establishment of the polymorphisms here described and possible disease outcome [15]. Genetic effects on viral-stimulated cytokine storms in the lung are further discussed elsewhere (Forbester & Humphreys, 2020).
Regarding the modulatory cytokines, a study conducted by Patarčić and collaborators [28], performed meta-analyses of studies on tuberculosis, influenza, respiratory syncytial virus, SARS-CoV-1, and pneumonia. One single-nucleotide polymorphism from the IL4 +1059 C/T gene was significant for pooled respiratory infections (rs2070874). IL4 has previously been described to have a pivotal role in shaping the nature of the immune response, by promoting and stimulating both T-cell and B-cell differentiation and modulation. It provides a balance between Th1 and Th2 response, biased to the latter, and therefore alteration of its function may substantially affect immune response. Most commonly reported such alteration is associated with an increased risk of atopy and allergies [28].
In 2013, Meyer and colleagues [24] analyzed a total of 12 SNPs in genes encoding cytokines, which were associated with SARS. The rs315952 in the IL1RN gene encoding IL-1 receptor antagonist (IL1RA) replicated its association with reduced risk of SARS in the early stages. Plasma IL1RA level was associated with rs31595 in a subset of critically ill subjects. The effect of rs315952 was independent of the tandem repeat variant in IL1RN. The IL1RN SNP rs315952C is associated with decreased risk of SARS in three populations with heterogeneous risk factors, and with increased plasma IL1RA response. IL1RA can thus mitigate the risk of SARS.
The study about the GG genotype of the interleukin IL-10 promoter polymorphism in position −1082 G/G has been associated with increased IL-10 production [9]. The authors hypothesized that the −1082 G/G genotype is associated with the development of SARS outcomes. The −1082 G/G genotype was associated with decreased severity of illness on admission, lower daily organ dysfunction scores, and lower 60-day mortality. In conclusion, the high IL-10 producing −1082 G/G genotype may be associated with variable odds for SARS development in an age-dependent fashion. Among those with severe acute respiratory distress syndrome, the −1082 G/G genotype is associated with lower mortality and organ failure. More recently, Mahallawi and colleagues (2018) have also reported elevated levels of IL-10 in patients infected with MERS-CoV and SARS-CoV-1 [23]. These results show the importance of the study of polymorphisms in the context of COVID-19, which may be divergent from other SARS-associated viral or non-viral infections.
2.2. Future analyzes of possible genetic cytokine polymorphisms and their consequences in the SARS-CoV-2 response
Other interleukins have increased dramatically in coronavirus-related SARS. An immune signature profiling on coronavirus-related SARS showed that the cytokine storm served a critical role in the infection process [14,39,21]. As the cytokine polymorphisms genes possibly related to the differential response to SARS-CoV-2 and mentioned earlier, other possible polymorphisms are involved in the differential expression of these cytokines. It still remains unknown the role of these polymorphisms in the different levels and biases of the immune response, even those involved in the exhaustion of the immune system and the cytokine storm associated with the severe forms of SARS-CoV-2. An example is interleukin IL-6, which appears to be one of the most important cytokines of this storm, characterizing severity, and hyper inflammation [14,39]. Other markers associated with this syndrome may be IL-2RA (RA – receptor antagonist, also called CD25) (associated with severity), IFN-γ (gamma interferon, elevated when compared to healthy controls), IL-1β (variable elevation according to severity), CXCL9 (C-X-C motif ligand 9, induced by IFN-γ; useful in monitoring), CXCL8, IL-10, C-reactive protein (produced by hepatic tissue and it is released in response to IL-6; associated with severity), TNF-α (tumor necrosis factor α) [14,29].
Comprehensive studies from the Human Functional Genomics Project have shown that distinct immune mediators such as cytokines may have different levels of genetic influence [6]. This is a crucial concept for the host defense and cytokine-associated disease pathogenesis, as these molecules are fundamental in orchestrating immunological and immunopathological events during diseases such as COVID-19 (Chaim, 2020). Recently, it was proposed that the IL-1β/IL-6 axis would need the finest tuning and regulation triggered by genetic factors [22]. In agreement with this hypothesis, a preliminary study using RNAseq approach compared the expression levels of these cytokines, as well as other immune modulators, in Caucasian Americans and African Americans. The authors have demonstrated that IL-1β and IL-18 receptor 1 (IL18R1), IL12Rβ1, TLR7, and TLR9 were significantly higher in African and Americans, suggesting perhaps a tendency in the later to develop higher inflammatory cytokine responses [33]. Therefore, expression of cytokine receptor genes may be a crucial pathway by which cytokines and their functional effect is modulated.
In regard to the expression of cytokine receptors, in silico recent analysis using dbSNP database, which is available on the National Biotechnology Information Center website (HTTP: //www.ncbi.nlm.nih.gov/SNPS) [26], 16 SNPs for genes coding for both cytokines and 21 SNPs for the respective cytokine receptor genes were found to be elevated in patients with COVID-19. This analysis demonstrated that 3′-UTR polymorphism in genes encoding for cytokines or their receptors was found in higher levels in patients with COVID-19. Among these, rs1126579 (in CXCR2), rs1061624 (in TNFRSF1B), and rs8178562 (in IL10RB) polymorphisms are particularly noteworthy. These SNPs would result in a break on the miRNA-mRNA binding sites specific for miR-516a-3p, miR-720, and miR-328, which are important for regulating CXCR2, TNFR and IL10RB, respectively. It has been suggested that the main cause of lung tissue grievance during the response against SARS-CoV-2 is an increase in these chemokine and cytokine receptors [17]. Although IL-10 is a regulatory cytokine, its receptor subunit is also utilized in the assembly of the receptor for IFN-λ, which could exert an antiviral as well as pro-inflammatory activity during severe and unbalanced COVID-19 immune response.
Ramos-Lopez and colleagues [30] conducted a comprehensive survey to explore the possible genetic polymorphisms associated with clinical results during SARS-CoV-2 infection in humans. From this review, 27 polymorphisms in genes encoding for cytokines were identified as associated with susceptibility/resistance to SARS-CoV-2 infection, disease severity and possible clinical outcomes in the Asian population. Thus, some genes implicated in important pathophysiological processes have been described, such as the mechanisms related to the entry of the virus in the cell and the antiviral immune/inflammatory responses. Among the polymorphisms described, for example, SNP G/T stands out at position −88 in the promoter region of the MxA, which was associated with a high risk of worsening the disease in the Chinese population [12], while the SNP −123 C/A in the promoter region of the same gene seems to protect against development of SARS in the same population. As described before, Mx proteins are known by their ability to inhibit RNA viruses and exerting modulatory effects on the INF gene family ([3]; Ching et al., 2020).
Specifically for SARS-CoV-2, increased levels of IL-10 in the serum of COVID-19 patients have been reported in association with IL-6, placing these two cytokines as possible biomarkers regarding disease severity [13]. In this context, and in view of the remarkable role of IL-6 in triggering the associated pathology, several therapeutic proposals have been evaluated in order to block the pathways of induction of this cytokine. Preliminary studies indicate that Tocilizumab (a recombinant humanized monoclonal antibody) would be able to immediately improve the clinical outcome in critically ill patients with COVID-19, due to the blockage of the febrile and inflammatory response triggered by IL-6, being an effective treatment for reducing mortality [39]. Tocilizumab, an IL-6 inhibitor, was used in the study of Xu and colleagues in the treatment of 21 patients with COVID-19 in a critical condition. All patients presented a high rate of IL-6 before the intervention. Most of them showed clinical improvement of symptoms such as hypoxia and changes in computed tomography (CT) opacity immediately after treatment, suggesting that this approach focused on IL-6 could be an efficient therapy strategy for the treatment of COVID-19.
3. Final remarks: the eye of the storm
The impact of polymorphisms on the differential expression of cytokines, chemokines, and immune molecules in the different clinical manifestations of COVID-19 is currently unknown. It also remains to be understood if the already known SNP polymorphisms to immune molecules could be associated with the exhaustion of the immune system associated with the overexpression or suppression of cytokines discussed here. It is considered that, in the case of SARS-CoV-2, the infection triggers an important imbalance in the eye of the storm, which is composed of immune cells. Innate and adaptive programmed cell subsets are the main sources of cytokines and immune molecules. Monocytes, macrophages, dendritic cells, and T cells are protagonists of the immune system, with close attention to the CD4+ T-lymphocytes that could produce Interferon and TNF abundantly.
In parallel, T, NK, cytotoxic and regulatory lymphocytes also appear to be either reduced in severe cases or may be related to the exhaustion of the immune system. In either asymptomatic and severe cases, SARS-CoV-2-specific memory CD8+ and CD4+ T-cell subsets were also found [29, 39,40,35]. The decrease in subpopulations of regulatory T cells may be one of the factors favorable to the cytokine storm or aggravate the effects of the infection itself [29]. Thus, differential expression of cytokines caused by point mutations in their coding genes - genetic polymorphisms, can interfere in the evolution of the immune response and in the different clinical variables crucial in the context of COVID-19 and could direct the use of therapies and the development of vaccines to combat COVID-19.
Funding
JGACdR received support from ADRC early-career research support program (09/2019 - UFMG). FGF and EFBS thank the CNPq for the PQ fellowships program. The authors thank the Programa de Pós-graduação em Microbiologia (PPGM/ICB/UFMG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for masters, doctoral and post-doctoral fellowships to AAOP (PhD), ALR (Ms), DSODeS (PostDoc), LAFA (PostDoc), TFSM (PhD).
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors thank all the members of the Laboratory of Basic and Applied Virology that have encouraged and contributed to the critical review of this article.
References
- 1.Azevedo Z.M., Moore D.B., Lima F.C., et al. Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA) single nucleotide polymorphisms: importance in ARDS in septic pediatric critically ill patients. Hum Immunol. 2012;73(6):661–667. doi: 10.1016/j.humimm.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Bali V., Bebok Z. Decoding mechanisms by which silent codon changes influence protein biogenesis and function. Int. J. Biochem. Cell Biol. 2015;64:58–74. doi: 10.1016/j.biocel.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bizzotto J., Sanchis P., Abbate G., et al. SARS-CoV-2 infection boosts MX1 antiviral effector in COVID-19 patients. iScience. 2020:23. doi: 10.1016/j.isci.2020.101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhary M. COVID-19 susceptibility: potential of ACE2 polymorphisms. Egypt J Med Hum Genet. 2020;21:54. doi: 10.1186/s43042-020-00099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob C O. On the genetics and immunopathogenesis of COVID-19. Clin. Immunol. 2020;220 doi: 10.1016/j.clim.2020.108591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ching J.C., Chan K.Y., Lee E.H., et al. Significance of the myxovirus resistance A (Mx1) gene -123C a single-nucleotide polymorphism in suppressed interferon-beta induction of severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2010;201(12):1899–1908. doi: 10.1086/652799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong W.P., Ip W.K., Tso G.H., et al. The interferon-gamma gene polymorphism +874 A/T is associated with severe acute respiratory syndrome. BMC Infect. Dis. 2006;6:82. doi: 10.1186/1471-2334-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong M.N., Thompson B.T., Williams P.L., et al. Interleukin-10 polymorphism in position -1082 and acute respiratory distress syndrome. Eur Respir J. 2006;27(4):674–681. doi: 10.1183/09031936.06.00046405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong M.N., Zhou W., Williams P.L., et al. 308GA and TNFB polymorphisms in acute respiratory distress syndrome. Eur. Respir. J. 2005;26(3):382–389. doi: 10.1183/09031936.05.00000505. [DOI] [PubMed] [Google Scholar]
- 11.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamano A., Hijikata M., Itoyama S., et al. Polymorphisms of interferon-inducible genes OAS-1 and MxA associated with SARS in the Vietnamese population. Biochem. Bio- phys. Res. Commun. 2005;329(4):1234–1239. doi: 10.1016/j.bbrc.2005.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han H., Ma Q., Li C., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson L.A., Canna S.W., Schulert G.S., et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthr. Rheumatol. 2020 doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johns Hopkins University&Medicine, Coronavirus Resource Center, 2016. https://coronavirus.jhu.edu/.
- 17.Karakas Celik S., Cakmak Genc G., Dursun A. A bioinformatic approach to investigating cytokine genes and their receptor variants concerning COVID-19 progression. Int. J. Immunogenet. 2020;00:1–8. doi: 10.1111/iji.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaghiosoff M., Neubauer H., Lassnig C., Kovarik P., Schindler H., Pircher H., McCoy B., Bogdan C., Decker T., Brem G., Pfeffer K., Müller M. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13(4):549–560. doi: 10.1016/s1074-7613(00)00054-6. OctPMID: 11070173. [DOI] [PubMed] [Google Scholar]
- 19.Kimball A., Hatfield K.M., Arons M., et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing Facility - King County, Washington, March 2020. MMWR Morb. Mortal Wkly Rep. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau S.K.P., Lau C.C.Y., Chan K.H., et al. Delayed induction of pro-inflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J. Gen. Virol. 2013;94(Pt 12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Oosting M., Smeekens S.P., et al. A functional genomic approach to understand variation in cytokine production. Cell. 2016;167(4):1099–1110. doi: 10.1016/j.cell.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer N.J., Feng R., Li M., et al. IL1RN coding variant is associated with lower risk of acute respiratory distress syndrome and increased plasma IL-1 receptor antagonist. Am. J. Respir. Crit. Care Med. 2013;187(9):950–959. doi: 10.1164/rccm.201208-1501OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monteerarat Y., Sakabe S., Ngamurulert S., et al. Induction of TNF-alpha in human macrophages by avian and human influenza viruses. Arch. Virol. 2010;155(8):1273–1279. doi: 10.1007/s00705-010-0716-y. [DOI] [PubMed] [Google Scholar]
- 26.NCBI - National Center for Biotechnology Information, 2021. https://www.ncbi.nlm.nih.gov.
- 27.Neuzil K.M., Tang Y.W., Graham B.S. Protective Role of TNF-alpha in respiratory syncytial virus infection in vitro and in vivo. Am. J. Med. Sci. 1996;311(5):201–204. doi: 10.1097/00000441-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Pairo-Castineira Patarčić I., Gelemanović A., Kirin M., et al. The role of host genetic factors in respiratory tract infectious diseases: systematic review, meta-analyses, and field synopsis. Sci. Rep. 2015;5:16119. doi: 10.1038/srep16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos-Lopes O., Daimiel L., et al. Exploring host genetic polymorphisms involved in SARS-CoV infection outcomes: implications for personalized. Int. J. Genomics. 2020;8 doi: 10.1155/2020/6901217. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo S.H., Webster R.G. Tumor necrosis factor-alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J. Virol. 2002;76(3):1071–1076. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skoog T., van't Hooft F.M., Kallin B., et al. A common functional polymorphism (C-A substitution at position -863) in the promoter region of the tumor necrosis factor-alpha (TNF-alpha) gene associated with reduced circulating levels of TNF-alpha. Hum. Mol. Genet. 1999;8(8):1443–1449. doi: 10.1093/hmg/8.8.1443. [DOI] [PubMed] [Google Scholar]
- 33.Tal Y., Adini A., Eran A., Adini I. Racial disparity in Covid-19 mortality rates - a plausible explanation. Clin. Immunol. 2020:217. doi: 10.1016/j.clim.2020.108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wada H., Saito K., Kanda T., et al. Tumor necrosis factor-α (TNF-α) plays a protective role in acute viral myocarditis in mice. Aha J. 2001;103:743–749. doi: 10.1161/01.cir.103.5.743. [DOI] [PubMed] [Google Scholar]
- 35.Wang W., Su B., Pang L., et al. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell Mol. Immunol. 2020;17(6):650–652. doi: 10.1038/s41423-020-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson A.G., Symons J.A., McDowell T.L., McDevitt H.O., Duff G.W. Effects of a polymorphism in the human tumor necrosis factor-alpha promoter on transcriptional activation. Proc. Natl. Acad. Sci. U S A. 1997;94(7):3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S., Wei M., Han Y., et al. Roles of TNF-α gene poly- morphisms in the occurrence and progress of SARS-Cov infection: a case-control study. BMC Infect. Dis. 2008;8(1):p27. doi: 10.1186/1471-2334-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report-51.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 [Google Scholar]
- 39.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng M., Gao Y., Wang G., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]